Abstract
Sexual differentiation of the malaria parasite is a pre-requisite for transmission from humans to the mosquito vector and has emerged as a target for intervention in eradication efforts. In this issue of Cell, a study from Marti, Clardy and colleagues (Brancucci et al., 2017) describes a host-derived lipid lysophosphatidylcholine (LysoPC) that regulates sexual commitment.
Malaria continues to ravage much of the developing world with over one hundred countries affected and half the world’s population living in areas at risk of malaria transmission. Malaria is caused by single-celled eukaryotic parasites of the genus Plasmodium, which demonstrate a complex lifecycle in humans and female mosquito hosts. The lifecycle initiates with the bite of a mosquito, which delivers Plasmodium sporozoites that go on to establish a liver-stage infection. A small number of infected hepatocytes will eventually release thousands of merozoites, which invade red blood cells and initiate the cyclic intraerythrocytic stage (Figure 1). The clinical symptoms of malaria (including fever, aches, and malaise) result from waves of asexual blood-stage infection, which in severe cases progress to anemia, acidosis, multiple organ failure and ultimately death if left untreated. Onward transmission of the parasite to the mosquito vector requires differentiation into male and female sexual forms during blood-stage development (Figure 1). Curiously, amongst P. falciparum strains, and even clones, the rate of gametocyte conversion varies but is generally low with less than 10% of parasites producing gametocytes in each 48-hour intraerythrocytic cycle (Kafsack et al., 2014). A mechanistic understanding of this cellular transformation process has been lacking and is therefore the subject of intense investigation. In the elegant study led by Matthias Marti, Jon Clardy and colleagues in this issue of Cell, the authors provide an important initial step toward understanding how malaria parasites may be sensing and responding to changing host conditions to initiate sexual differentiation (Brancucci et al., 2017).
Figure 1. Lysophosphatidylcholine (LysoPC) tips the balance away from sexual differentiation.
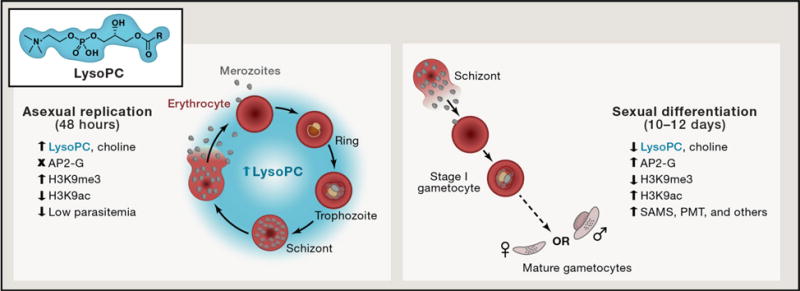
The balance between asexual parasite replication and sexual differentiation is explored in every 48-hour cycle of the human malaria parasite Plasmodium falciparum’s developmental cycle. Brancucci and Gerdt et al. have identified lysophosphatidylcholine (LysoPC) as an important molecule whose presence (at low parasitemia) represses conversion to the sexual gametocyte form required for transmission to the mosquito host. LysoPC is a host-derived lipid that gets metabolized by the parasite into critical membrane phospholipids. Decreased levels of LysoPC result in transcriptional activation of key enzymes required to replenish phospholipid stores via S-adenosylmethioine (SAM) – mediated reactions including S-adenosylmethioine synthetase (SAMS) and phosphoethanolamine methyltransferase (PMT). This diversion of SAM suggests a potential interplay of metabolic adjustments that likely result in a decrease in H3K9me3 and a concomitant de-repression of the gene encoding the critical AP2-G transcription factor (Kafsack et al., 2014) required to drive gametocyte commitment and differentiation.
Numerous reports have suggested that factors which “stress” asexually replicating parasites in the blood-stage, such as exposure to drugs and growth in spent medium can induce gametocytogenesis (Baker, 2010). In terms of the key molecular drivers of gametocytogenesis, what we do know is that a transcription factor called AP2-G is absolutely required for malaria parasites to commit to sexual development (Kafsack et al., 2014; Sinha et al., 2014). In the majority of asexually replicating parasites, the ap2-g locus is silenced by histone H3 lysine9 trimethylation (H3K9me3) and heterochromatin protein 1 (HP1) (Josling and Llinas, 2015). Although the details regarding upstream activation of the ap2-g gene are still under investigation, recent studies have demonstrated that depletion of HP1 as well as histone deacetylase 2 (HDA2) can unsilence the ap2-g locus, enabling sexual differentiation to proceed.
Brancucci and Gerdt et al. identify lysophosphatidylcholine (LysoPC) as a critical molecule found in human serum that significantly impacts gametocyte formation. Building on a previous study in which they found that parasites grown in the presence of spent serum had increased gametocyte production (Brancucci et al., 2015), they use fractionation coupled with mass spectrometry to identify serum components that lead to decreased gametocyte formation. LysoPC is exclusively present in fractions that block commitment to gametocytogenesis. The authors go on to further demonstrate that there is a critical window between 34 and 38 hours post red blood cell invasion (hpi) where LysoPC depletion leads to irreversible commitment to sexual differentiation.
During the intraerythrocytic stage of development, each malaria parasite develops into a schizont that produces 16 to 32 new merozoites. Most of these parasites will re-invade new red blood cells and develop asexually, while merozoites from sexually committed (34-38 hpi) schizonts will reinvade and mature to become either all male or all female mature parasites (Figure 1) (Silvestrini et al., 2000; Smith et al., 2000). Schizogony requires that parasites produce a large number of phospholipids. These include phosphatidylcholine, the most abundant membrane phospholipid in P. falciparum, and phosphatidylethanolamine, which are made through the consumption of serine, choline, and ethanolamine from the host serum. Brancucci and Gerdt et al. use metabolic tracing experiments to demonstrate that exogenous LysoPC from the serum can cross several membranes to reach the parasite. Once inside the parasite it is metabolized into choline and converted via the Kennedy pathway into phosphatidylcholine, an essential component of membranes. The importance of the Kennedy pathway is exemplified by their demonstration that exogenously supplied choline alone (albeit not at nonphysiologically high levels) could also block gametocyte conversion.
One of the most intriguing aspects of the current study comes out of the dissection of gene expression changes triggered by differential availability of LysoPC (Figure 1). When LysoPC is depleted, the AP2-G transcription factor is activated along with upregulation of phosphoethanolamine methyltransferase (PMT), S-adenosylmethionine (SAM) synthetase and other factors which intersect with this metabolic pathway. This cellular response suggests that LysoPC depletion likely results in increased production of SAM as a means to metabolically adjust to maintain an active Kennedy pathway. The upregulation of PMT is consistent with previous studies demonstrating that PMT is down-regulated at both the transcriptional and protein level in the presence of choline and plays a critical role in the formation of mature gametocytes (Bobenchik et al., 2013; Pessi et al., 2004). In addition to the genes involved in metabolism, transcription of a number of genes involved in cell cycle regulation and differentiation such as the sirtuin sir2a, histone deacetylase 1 (hda1), lysine-histone demethylase 1 (lsd1), and the gametocyte development protein 1 (gdv1) are also impacted by LysoPC metabolism.
Although metabolic depletion of LysoPC is clearly critical to the process of gametocyte formation, we are still left pondering the mechanistic aspects of how the levels of LysoPC trigger a cascade of events that result in ap2-g activation and downstream sexual differentiation. One tantalizing possibility is that under serum-rich conditions, phospholipid production relies on exogenous sources of choline, with minimal reliance on SAM-dependent enzymes. In serum-rich conditions, cellular pools of SAM would be more readily available in the nucleus where it serves as a methyl donor for a number of histone methyltransferases, which in turn repress expression via deposition of H3K9me3. In the converse situation, LysoPC- or serum-depleted conditions may lead to increased utilization of SAM by PMT and other cytoplasmic enzymes, switching methyltransferase activity out of the nucleus. Consequently, this may lead to a decreased availability of SAM in the nucleus and downstream loss of repressive H3K9me3 (Figure 1). This would suggest that the key link between depletion of LysoPC, transcriptional activation of AP2-G and enhanced gametocyte production is likely to be modulation of SAM levels, and consequently, histone methylation.
Future avenues of investigation will be needed to answer a number of remaining questions. For instance, upon LysoPC removal, why don’t all asexual parasites convert to gametocytes? This implies that the epigenetic silencing of the ap2-g locus is only removed in a subset of cells and that other factors are involved. Single cell analyses of Plasmodium parasites are needed to examine the effect of LysoPC in individual cells and recently Poran, et al. 2017 have demonstrated that such studies are feasible and very informative to dissect transcriptional changes associated in gametocyte-committed cells. Along these lines, although this work reveals that the depletion of LysoPC results in relatively equivalent increases in gametocyte production across strains when comparing Pf2004, HB3, and NF54 strains of P. falciparum, previous studies have demonstrated that gametocyte production is strain- and clone- dependent (Brancucci et al., 2015; Kafsack et al., 2014). Perhaps the differences in gametocyte formation may indicate genetic variation has arisen that results in differential responses to LysoPC.
It is now clear that LysoPC is a critical metabolite that is sensed by the parasite and acts upstream of any previously described event of sexual differentiation. However, this study also highlights that there is still much to be learned to clarify our understanding of the mechanisms underlying sexual commitment and differentiation. Future studies are critical since targeted therapies aimed at interfering with gametocyte formation are an important component of ongoing efforts to eliminate malaria globally by blocking its transmission.
References
- Baker DA. Malaria gametocytogenesis. Mol Biochem Parasitol. 2010;172:57–65. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobenchik AM, Witola WH, Augagneur Y, Nic Lochlainn L, Garg A, Pachikara N, Choi JY, Zhao YO, Usmani-Brown S, Lee A, et al. Plasmodium falciparum phosphoethanolamine methyltransferase is essential for malaria transmission. Proc Natl Acad Sci U S A. 2013;110:18262–18267. doi: 10.1073/pnas.1313965110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancucci NM, Goldowitz I, Buchholz K, Werling K, Marti M. An assay to probe Plasmodium falciparum growth, transmission stage formation and early gametocyte development. Nat Protoc. 2015;10:1131–1142. doi: 10.1038/nprot.2015.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancucci NMB, Gerdt JP, Wang C, De Niz M, Philip N, Adapa SR, Zhang M, Hitz E, Niederwieser I, Boltryk SD, et al. Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell. 2017 doi: 10.1016/j.cell.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josling GA, Llinas M. Sexual development in Plasmodium parasites: knowing when it's time to commit. Nat Rev Microbiol. 2015;13:573–587. doi: 10.1038/nrmicro3519. [DOI] [PubMed] [Google Scholar]
- Kafsack BF, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE, Drought LG, Kwiatkowski DP, Baker DA, et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014;507:248–252. doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessi G, Kociubinski G, Mamoun CB. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc Natl Acad Sci U S A. 2004;101:6206–6211. doi: 10.1073/pnas.0307742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini F, Alano P, Williams JL. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology. 2000;121(Pt 5):465–471. doi: 10.1017/s0031182099006691. [DOI] [PubMed] [Google Scholar]
- Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, Religa AA, Bushell E, Graham AL, Cameron R, et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507:253–257. doi: 10.1038/nature12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TG, Lourenco P, Carter R, Walliker D, Ranford-Cartwright LC. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology. 2000;121(Pt 2):127–133. doi: 10.1017/s0031182099006265. [DOI] [PubMed] [Google Scholar]


