Abstract
Background
Mobile health (mHealth) technologies have the potential to facilitate self-monitoring and self-management for individuals with substance use disorders (SUD). S-Health is a bilingual smartphone application based on cognitive behavioral principles and is designed to support recovery from drug addiction by trigger recognition so as to allow practice in-the-moment coping to prevent relapse.
Method
For this pilot randomized controlled study, 75 participants were recruited from methadone maintenance treatment clinics and the social worker consortium in Shanghai, China. Participants in the control group (N=25) received text messages from S-Health (e.g., HIV prevention and other educational materials). Participants in the intervention group (N=50) received both text messages and daily surveys on cravings, affects, triggers, responses to triggers, and social contexts.
Results
At the end of the 1-month study trial, 26.2% of the intervention group and 50% of the control group had positive urine test results (p=0.06). Also, the number of days using drug in the past week was significantly lower among participants in the intervention group (Mean= 0.71, SD=1.87) relative to the control group (Mean=2.20, SD=3.06) (p<.05). The two groups did not differ in slopes (i.e., rates of change in outcomes measured weekly) based on the mixed effects model. Participants in the intervention group also preferred answering questions on the cellphone (46.8%) relative to in-person interviews (36.2%).
Conclusions
This pilot demonstrated the feasibility and potential benefits to deliver mobile health intervention among participants with SUD. Further research with larger samples over a longer period of time is needed to test the effectiveness of S-Health as a self-monitoring tool supporting recovery from addiction.
Keywords: Mobile health technologies, Substance use disorders, Self-monitoring
1. Introduction
Substance use disorders (SUD) can be chronic conditions, with high potential for relapse (McLellan, 2002). According to the Chronic Care Model (CCM), patients themselves are the principal caregiver, and in addition to professional help, their own monitoring and management of chronic conditions is also crucial to improve outcomes and reduce costs (Bodenheimer, Lorig, Holman, & Grumbach, 2002; Bodenheimer, Wagner, & Grumbach, 2002a, 2002b; Wagner et al., 2001). Nevertheless, self-monitoring and self-management can be challenging if patients are not supported by appropriate skills, tools, and assessments. This is particularly problematic in countries or areas with limited access to supportive services. The mHealth offers an option that may be promising.
1.1. Treatment options for SUD in Asian countries
Asian countries have the largest overall populations with opioid dependence and amphetamine dependence (Degenhardt et al., 2013). In China and many other Asian countries, heroin has been the primary drug of addiction (Hser, Liang, Lan, Vicknasingam, & Chakrabarti, 2016). More recently, an increasing number of people are using amphetamine-type stimulants (ATS), ketamine, and buprenorphine (Ho, Chen, Broekman, & Mak, 2009; Ho & Zhang, 2016). In Western developed countries, a variety of pharmacologic and behavioral treatments are delivered in many different settings for patients with SUD. However, Asian countries traditionally take punitive measures toward individuals using illicit drugs (e.g., incarcerating individuals using illicit drugs) (Sullivan & Wu, 2007). The HIV epidemics have pushed these countries to gradually embrace harm reduction approaches, as people who inject drugs (PWID) are at high risk for HIV and usually lack resources for testing and treating SUD and HIV (Hser, et al., 2016; Nguyen et al 2017).
In the case of China, the methadone maintenance treatment (MMT) program, which was initially one of the most important components of China’s harm reduction strategies, has now become one voluntary treatment option for individuals using illicit drugs, in addition to other voluntary detoxification and rehabilitation services in healthcare facilities (Sullivan & Wu, 2007; Wu, Wang, Detels, & Bulterys, 2015). Individuals may also be enrolled into community-based treatment and rehabilitation programs, or be admitted into compulsory detoxification and rehabilitation facilities, depending on the severity of drug use behaviors. Those who are released from compulsory programs need also to participate in community-based rehabilitation programs afterwards. In most settings mentioned above, though pharmacological treatments are usually available, psycho-therapeutic services are very scant (Tang, Zhao, Zhao, & Cubells, 2006; Zhang et al., 2013). According to China’s Mental Health Law, psycho-therapeutic services can only be provided in healthcare facilities, such as a specialty mental health hospital or the mental health department in a general hospital (Chen et al., 2012). However, healthcare facilities in two-thirds of counties in China do not have any mental health providers, and counseling services outside healthcare facilities are often very expensive and only available in metropolitan areas. The barriers to professional psycho-therapeutic services are not likely to be resolved in the short term (Gao et al., 2010).
In summary, patients in Asian countries in general have limited access to professional SUD treatment services. The limited availability of professional SUD services in these countries further highlights the importance of patients’ self-management.
1.2. Smartphone as a tool for self-management support in relapse prevention
As smartphone users increase rapidly over the world, smartphones, which are important vehicles for mobile health (mHealth) technologies, have the potential to provide self-management support at low-cost to populations having barriers to professional psychotherapeutic services (Gibbons et al., 2011). In 2016, 58% of the total adult population and 85% of those aged 18-34 owned a smartphone in China (Poushter, 2016). Smartphones’ short messaging service (SMS) and more complex applications are often the core elements of mHealth programs. For a variety of chronic physical and mental conditions including SUD, mHealth has been found to be effective in supporting self-management in North America (Davis, DiClemente, & Prietula, 2016; Gustafson et al., 2014; Monney, Penzenstadler, Dupraz, Etter, & Khazaal, 2015; Weaver, Horyniak, Jenkinson, Dietze, & Lim, 2013). In Asia, mHealth has also been designed and used to offer psychoeducation and early intervention for substance use, especially alcohol use (Zhang et al 2014; Zhang et al 2015; Zhang et al 2016a; Zhang et al 2016b; Zhang et al 2017a; Zhang et al 2017b).
1.3. The present study
Few studies have examined the efficacy or effectiveness of smartphone applications in the field of illicit drug use (Linas et al., 2015; Monney, Penzenstadler, Dupraz, Etter, & Khazaal, 2015). To our knowledge none has been reported from Asian countries (Brian & Ben-Zeev, 2014; Li et al., 2014).
We developed a smartphone application, S-Health, to support the self-management of addictive symptoms and recovery for SUD patients with limited access to mental health services (see Figure 1). S-Health prompts daily surveys and self-initiated surveys to support self-management in real time and natural environments. Surveys in S-Health are designed to help patients better identify triggers (e.g., emotions, places, people), recognize strategies for dealing with those situations, and monitor substance use and deal with cravings, based on the cognitive-behavioral model of many evidence-based relapse prevention therapies (see Appendix) (Larimer, Palmer, & Marlatt, 1999; Marlatt & George, 1984; Marlatt & Donovan, 2005). Development of S-Health was reported previously (Schulte et al., 2016). This present article reports finding based on a pilot trial which tests the feasibilities and outcomes of S-Health among individuals using illicit drugs in China.
Figure 1.
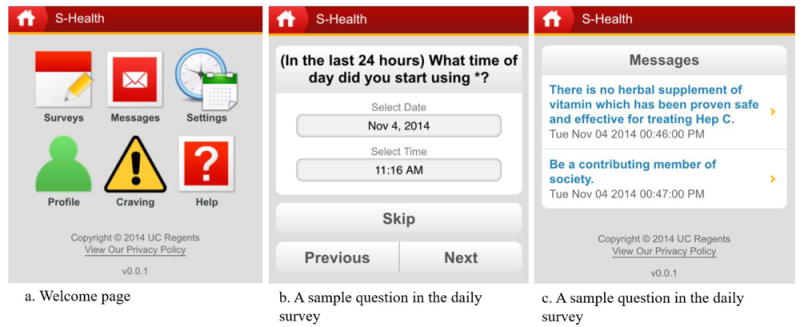
Screenshots of S-Health
2. Methods
2.1. Study setting
We recruited participants from the MMT clinics and the network of social workers in Shanghai, China. As the largest and most developed city in China, Shanghai has approximately 24 million residents and more than 80,000 registered individuals using illicit drugs. Among the 16 districts in Shanghai, 14 districts have a MMT clinic. There is also a social worker consortium (Shanghai Zi-Qiang consortium) with more than 1000 social workers who specialize in helping individuals using illicit drugs in the community. Our participants were recruited from the MMT clinics in three districts (Yangpu, Xuhui, Hongkou) and the Shanghai Zi-Qiang consortium.
2.2. Study participants
Eligible participants for this study were adult individuals who had used heroin or other substances in the past 30 days, and had a smartphone. Participants were excluded if he or she had (1) dependence on an illicit substance for which medical detoxification was imminently needed, or (2) presence of clinically significant psychiatric symptoms as assessed by Mini International Neuropsychiatric Interview (MINI), such as psychosis, acute mania, or suicide risk that would require immediate treatment or make study compliance difficult. From June 2015 to April 2016, 75 participants were recruited for this pilot study, with 58 from MMT clinics (10 from Xuhui district, 26 from Hongkou district, and 22 from Yangpu district) and 17 from the Shanghai Zi-Qiang consortium.
2.3. Study design and procedures
This study was a randomized controlled trial. Eligible participants who agreed to participate in the study were randomly assigned, with 50 participants in the intervention group and 25 participants in the control group1. We oversampled the intervention group in order to maximize the possibility to identify barriers and problems associated with smartphone interventions. All participants downloaded S-Health to their personal phone, completed a training on how to use the phone application, and were asked to carry the phone with them at all times during the trial. The study was approved by institutional review boards in Shanghai Mental Health Center and University of California, Los Angeles2.
2.3.1 Control group
Participants in the control group only received text messages from S-Health (e.g., HIV prevention and other educational materials). Participants could set the time of receiving messages.
2.3.2 Intervention group
Participants in the intervention group received both surveys and text messages from S-Health. Participants were asked to complete daily surveys at a time of their choosing, and they could also initiate a survey at any time or frequency by themselves. In daily surveys, participants were asked to report their cravings, affects, triggers, responses to triggers, and social context: 1) Cravings: participants were asked to rate their cravings for substances on a scale from 0 to 10 (0=no craving, 10= maximum craving); 2) Affect was assessed with the International Positive and Negative Affect Schedule Short-form (I-PANAS-SF) (Thompson, 2007); 3) Trigger thoughts, places, and situations: participants were asked to input their own text or choose from a list of common triggers associated with relapse (e.g. “I am bored”; “I feel sad”) (Larimer, Palmer, & Marlatt, 1999); 4) Responses to triggers: participants were asked to input their own text or choose from a list of common coping responses (e.g., “I called and talked this over with my friend”); 5) Social context: participants were asked if use behaviors occur alone or with a romantic partner, family, friends, gang, coworkers, classmates, or others. For safety and privacy considerations, all information submitted through daily surveys and self-initiated surveys were transmitted to the server at UCLA after encryption and were no longer accessible on participants’ phones.
Additionally, the I-PANAS-SF was assessed at the baseline to determine which emotional group they belong to. Participants were then categorized into the “positive” or “negative” emotional group, in order to tailor text messages for them.
2.4. Study measures
During the study period, data were obtained from face-to-face interviews and urine tests at baseline and end of each week over the 4-week trial.
Urine test
Five drugs (morphine, methamphetamine, ketamine, 3,4-methylenedioxy-methamphetamine (MDMA), and marijuana) were examined in urine tests, using the 5-panel drug test kit produced by Venture Biotechnology Co., Ltd (Shanghai, China). For participants recruited from MMT clinics, urine tests were conducted by clinicians in the clinic, while for participants recruited from the social worker consortium, urine tests were conducted by social workers. The drug test kit was discarded after the test to protect participants’ privacy. A urine test result was coded as positive if any drug was tested positive using the 5-panel drug test kit. Participants took urine tests at baseline and at the end of each week during the study period.
Timeline follow-back (TLFB) survey
For participants recruited from MMT clinics, physicians conducted the baseline and weekly interviews. For other participants recruited from social workers, social workers conducted the baseline and weekly interviews. In the baseline TLFB survey, participants self-reported their primary drug by answering the following question: “what is the primary drug used by the participant?” Participants were then asked about their drug use (heroin use, marijuana use, methamphetamine/amphetamine use, and cocaine use) and life events since the year before the first use of their primary drug. At the end of each week during the study period, participants were assessed by the weekly TLFB survey to report daily drug use in the past 7 days: heroin use, marijuana use, methamphetamine/amphetamine use, and cocaine use.
Addiction Severity Index
Participants’ baseline use status and demographic information were measured by the Addiction Severity Index (ASI) (McLellan, et al., 1980; 1992). ASI is a structured interview that assesses problem severity in seven areas: alcohol use, drug use, employment, family and social relationships, legal, psychological, and medical status. For each area, an ASI composite score is calculated according to the ASI Composite Score Manual (McGahan, Griffith, Parente, & McLellann, 1986). The ASI is the most widely used instrument in the substance abuse field both in the U.S. and internationally. The ASI has been examined among Chinese patients (Luo et al., 2007; Zhao et al., 2004). Zhao’s study (2004) based on heroin addicts in a Chinese rehabilitation center indicates that ASI has good reliability and validity for Chinese drug-dependent patients.
2.5. The Usability Items
To examine the feasibility and usability of S-Health, participants in the intervention group were asked to rate the following statements on a scale from 1 to 5 (1=strongly agree, 5= strongly disagree) at the end of the 4-week trial: “(1) these questions were easy to understand; (2) I was comfortable answering these questions about my alcohol, tobacco, and drug use; (3) I was able to remember the number of days or frequency I used alcohol, tobacco, or drugs in the past week; (4) I would be willing to answer questions like these in the annual survey; (5) the smartphone screen was easy to use; (6) I would prefer that a person ask me these questions instead of answering them myself on the cellphone; (7) I would prefer to answer these questions myself on a cellphone instead of having a person ask me” (Kelly et al., 2014). The participants in the control group were also asked to rate the statements above based on their experiences with in-person interviews (e.g., TLFB surveys) and receiving text messages from S-Health. Analytic approach
The t-tests (for continuous measures) and chi-square tests (for categorical measures) were used for comparing baseline characteristics and drug use status of participants measured by urine tests and TLFB surveys in the intervention group and the control group. Generalized linear equations (GEE) with binomial distribution and exchangeable covariance structure were used to estimate the impacts of intervention on having positive urine test results and primary drug use over the study period. Mixed-effects regression modeling with unstructured covariance structure (growth curve modeling) was used to model the number of days using primary drug according to TLFB results. All hypotheses were tested using a significance level of α=0.05.
In all regression models, a variable indicating the number of weeks was included to account for the time trends, and the product term of time and treatment was included to capture the potential interaction between treatment and time. Other covariates adjusted in all regression models included the ASI drug composite score at baseline, the type of primary drug, and demographics (age, gender, education, marital status, and employment status in the past three years). All categorical variables were transformed into a series of dichotomous indicators with an omitted reference category. Data were managed and analyzed using Stata 14.2.
3. Results
3.1. Baseline characteristics and respondents’ daily survey
Among all 75 participants, only 4 participants (5.3%) were lost to follow-up: two were not willing to participate after randomization; one dropped out because his/her cellphone did not work; and one was arrested during the study (see Figure 2). Participants’ ASI Composite Scores and demographic characteristics did not significantly differ between the experiments group and the control group (Table 1). The primary drug reported by 79.5% of participants was heroin, but the type of primary drug did not differ between the two groups.
Figure 2.
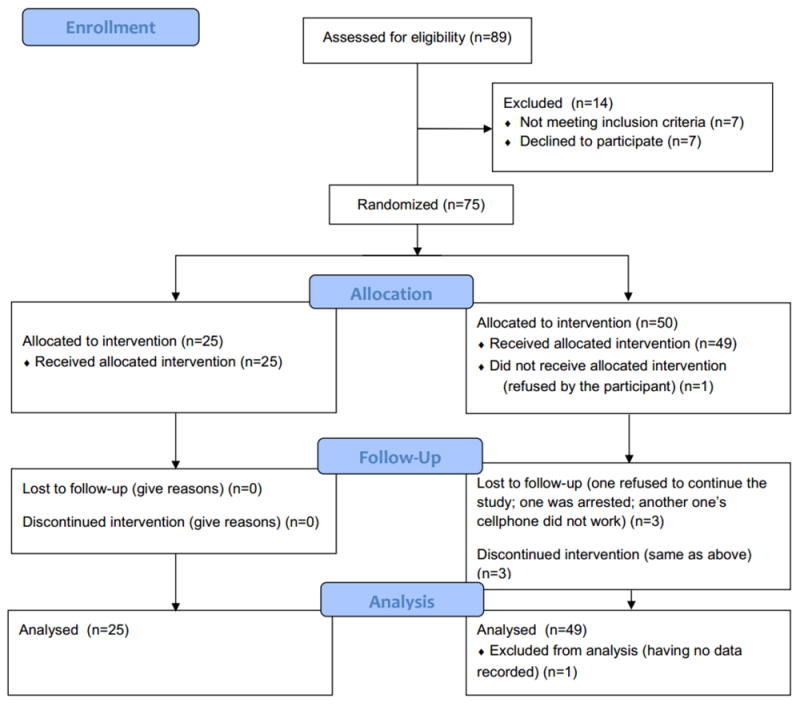
Consort Flow Diagram
Table 1.
Baseline characteristics of participants
| Total (N=74) | Intervention group (N=49) | Control group (N=25) | |
|---|---|---|---|
| Age, mean(SD), years | 41.6 (8.0) | 41.7 (8.7) | 41.3 (6.8) |
| Male, n (%) | 53 (70.7) | 36 (72.0) | 17 (68.0) |
| Ethnicity, n (%) | |||
| Han | 74 (100.0) | 49 (100.0) | 25 (100.0) |
| Education, n (%) | |||
| Less than high school | 30 (41.1) | 21 (43.8) | 9 (36.0) |
| High school (10-12 grade) | 35 (48.0) | 22 (45.8) | 13 (52.0) |
| More than high school | 8 (11.0) | 5 (10.4) | 3 (12.0) |
| Marital status, n (%) | |||
| Currently married | 32 (44.4) | 20 (42.6) | 12 (48.0) |
| Employment status in the 3 years prior to the baseline interview, n (%) | |||
| Employed | 44 (60.3) | 33 (67.4) | 11 (45.8) |
| Unemployed | 24 (32.9) | 14 (28.6) | 10 (41.7) |
| Incarcerated | 5 (6.9) | 2 (4.1) | 3 (12.5) |
| ASI composite scores, mean (SD) | |||
| Medical status | 0.10 (0.22) | 0.08 (0.17) | 0.14 (0.30) |
| Employment status | 0.27 (0.19) | 0.24 (0.18) | 0.32 (0.21) |
| Alcohol use | 0.22 (0.14) | 0.23 (0.16) | 0.20 (0.10) |
| Drug use | 0.16 (0.10) | 0.15 (0.09) | 0.19 (0.08) |
| Legal status | 0.23 (0.09) | 0.23 (0.08) | 0.24 (0.10) |
| Family/ social status | 0.10 (0.12) | 0.08 (0.09) | 0.14 (0.16) |
| Psychiatric status | 0.06 (0.13) | 0.05 (0.11) | 0.09 (0.16) |
| Self-reported primary drug, n (%) | |||
| Heroin | 58 (79.5) | 38 (79.2) | 20 (80.0) |
| ATS | 11 (15.1) | 9 (18.8) | 2 (8.0) |
| Heroin and ATS | 4 (5.5) | 1 (2.1) | 3 (12.0) |
Note:
p<0.05,
p<0.01,
p<0.001 when compared to the control group
During the study period, the number of daily surveys submitted by the 50 participants in the intervention group ranged from 0 to 28, resulting in a mean of 13.8 (SD: 10.4) and a median of 13.5. Five participants in the intervention group did not submit any daily survey over the study period. The percentage of participants who submitted daily survey in S-Health each day ranged from 35.4% to 66.7%.
3.2. Drug use during the trial
The intervention group and the control group did not differ significantly in having positive urine test results at each of the 4 weeks according to chi-square test (Table 2 & Figure 1). However, the intervention group reported significantly fewer days of using primary drug each of the 4 weeks (Table 2).
Table 2.
Participants’ drug use status over time
| Urine Test Results (%) | Days of Using Primary Drugs Each Week (TLFB) (mean (SD)) | |||
|---|---|---|---|---|
|
| ||||
| Intervention Group (N=43)† | Control Group (N=20) | Intervention Group (N=48) | Control Group (N=25) | |
| Week 1 | 55.8 | 70.0 | 1.33* (2.48) | 3.08 (3.37) |
| Week 2 | 48.8 | 60.0 | 0.92** (1.84) | 2.68 (3.18) |
| Week 3 | 35.7 | 60.0 | 0.56** (1.29) | 1.88 (2.67) |
| Week 4 | 26.2 | 50.0 | 0.71* (1.87) | 2.20 (3.06) |
Note:
p<0.05,
p<0.01,
p<0.001 when compared to the control group
In week 3 and week 4, the number of participants who had urine test results was 42.
Results of GEE models are shown in Table 3. Compared to the control group, the intervention group was less likely to have positive urine tests (Odds Ratio: 0.57, 95% CI: 0.11 – 2.84, p=0.49) and report primary drug use in TLFB surveys (Odds Ratio: 0.29, 95% CI: 0.06 – 1.44, p=0.13), although differences were not statistically significant, after controlling for baseline ASI drug composite score and demographic characteristics. During the study period, participants from both groups were less likely to have positive urine tests over time (Odds Ratio: 0.71, 95% CI: 0.49 – 1.04, p=0.08) and were also less likely to report primary drug use over time (Odds Ratio: 0.70, 95% CI: 0.48 – 1.04, p=0.08); but these differences were not statistically significant. The coefficient for the treatment-time product term was not statistically significant as well.
Table 3.
Mixed effects regression analysis results
| Urine Tests OR (95% CI) | Weekly Primary Drug Use OR (95% CI) | Days of Using Primary Drugs Each Week Coefficients (95% CI) | |
|---|---|---|---|
| Treatment | 0.57 (0.11, 2.84) | 0.29 (0.06, 1.44) | -1.46 (-3.22, 0.30) |
| Week | 0.71 (0.49, 1.04) | 0.70 (0.48, 1.04) | -0.37 (-0.75, -0.001) |
| Treatment×Week | 0.82 (0.50, 1.32) | 0.97 (0.59, 1.60) | 0.09 (-0.38, 0.55) |
Note:
p<0.05,
p<0.01,
p<0.001; the urine test results and weekly primary drug use results were modeled by GEE; the number of days of using primary drugs each week was modeled by growth curve modeling; all three regression models were adjusting for the baseline ASI drug composite score, the type of primary drug, and demographic covariates (age, gender, education, marital status, and employment status).
Results of growth curve modeling are shown in Table 3. Compared to the control group, the intervention group on average had 1.5 fewer days (95% CI: -3.22 – 0.30, p=0.10) of using primary drug each week, but such differences were not statistically significant, after controlling for demographic characteristics. During the study period, participants from both group on average had 0.4 fewer days (95% CI: -0.75 – -0.001, p=0.05) of using primary drug after one additional week, and such differences were not statistically significant. The coefficient for the treatment-time product term was not statistically significant.
3.3. The feasibility and usability of S-Health
Results of the usability items are shown in Table 4. In the intervention group, most participants strongly agreed or agreed that these survey questions are easy to understand (55.3%); they were comfortable answering these questions (68.1%); they were able to remember the number of days or frequency using alcohol, tobacco, or drugs in the past week (53.2%); and smartphone screens easy to use (72.3%). Participants in the intervention group also preferred answering questions on the cellphone (46.8%) relative to in-person interviews (36.2%). A higher proportion of participants in the control group strongly agreed or agreed that these survey questions are easy to understand (75.0%), and they were able to remember their alcohol, tobacco, and drug use status (70.8%) than in the intervention group. However, participants in the intervention group did not differ significantly from the control group in their ratings.
Table 4.
Results of the usability items
| N (%) of participants reporting “strongly agree” or “agree” | Intervention group (N=47) | Control group (N=24) |
|---|---|---|
| These questions were easy to understand. | 26 (55.3) | 18 (75.0) |
| I was comfortable answering these questions about my alcohol, tobacco, and drug use. | 32 (68.1) | 17 (70.8) |
| I was able to remember the number of days or frequency I used alcohol, tobacco, or drugs in the past week. | 25 (53.2) | 17 (70.8) |
| I would be willing to answer questions like these in the annual survey. | 34 (72.3) | 17 (70.8) |
| The smartphone screen was easy to use | 31 (66.0) | 20 (83.3) |
| I would prefer that a person ask me these questions instead of answering them myself on the cellphone. | 17 (36.2) | 10 (41.7) |
| I would prefer to answer these questions myself on a cellphone instead of having a person ask me. | 22 (46.8) | 16 (66.7) |
Note:
p<0.05,
p<0.01,
p<0.001 when comparing the intervention group to the control group. Participants were asked to rate these statements on a scale from 1 to 5 (1=strongly agree, 5= strongly disagree) at the end of the 4-week trial.
4. Discussion
This pilot study examined the feasibility and drug use outcome of a smartphone application (S-Health) to support self-management of participants with SUD in an environment where behavioral treatment options were usually not accessible. This pilot study overcame challenges of logistics and cultural barriers and showed the feasibility to deliver mobile health intervention among individuals with SUD in China.
While the two groups did not differ in slopes (i.e., rates of change in outcomes measured weekly) based on the mixed effects model, the number of days using drug in the past week was significantly lower among participants in the intervention group (Mean= 0.71, SD=1.87) relative to the control group (Mean=2.20, SD=3.06) (p<.05) without regression adjustment. As a pilot study, the relatively small sample size means that this study is underpowered to detect the efficacy of the intervention. For instance, the power to compare urine test results between the intervention group and the control group ranged from 0.15 to 0.53 from week 1 to week 4. We report these findings nevertheless so that the effect sizes can be used to inform future determination of needed sample sizes.
Results of the usability items showed that participants might understand questions better and remember their alcohol, tobacco, and drug use status better with in-person interviews, but they still preferred answering these questions on a cellphone. Compared to participants in the intervention group, a higher proportion of participants in the control group strongly agreed or agreed that these survey questions are easy to understand, and they were able to remember their alcohol, tobacco, and drug use status. Notably, as differences between the intervention group and the control group were not statistically significant, these differences might be due to random errors. Also, both groups preferred answering questions on the cellphone relative to in-person interviews. A previous study using similar usability items found that participants preferred to answer questions about their alcohol, tobacco, and drug use themselves on iPad instead of having a person asking them (Kelly et al., 2014).
Several study limitations need to be considered for interpreting these findings. The present study was based on participants with SUD in Shanghai, which may limit the study’s generalizability considering regional variations in China. The urine test results were recorded as positive if any drug was tested positive, which limited the comparability of urine test results and self-reported TLFB results. In addition, it was not possible to blind patients and clinicians or social workers who assessed patients each week. This may result in potential bias away from the null.
The follow-up rate for this one-month study was as high as 95%. In addition, feasibility issues need to be considered by future researchers when conducting mHealth studies in similar settings. In this study, data security and privacy issues were of top priority. In addition to the normal security procedures (e.g., password protected), S-Health used “*” to refer to drugs in the survey to avoid mentioning “drugs” or the name of any specific drugs (see Appendix). As participants input their responses, S-Health encrypted the data immediately. After participants submitted their responses, all responses were removed from their smartphones and transmitted to the secured server at UCLA. Though individualized feedback was considered important by participants (Schulte et al., 2016), any information suggesting the participant’s drug use status on their cellphone might be a threat to their safety. Thus, participants could not review their own responses over time based on their submitted surveys,, which was a compromise between functionality and data security for participant protection. In addition, participants might not report their behaviors and experiences in real time and natural environments. This was because participants were purposely allowed to complete surveys at any time of their choosing, and participants might not be able to report their drug use immediately due to logistical reasons (e.g., power supply, data plan, working schedules).
Despite these study limitations and relevant challenges, the pilot trial had a high retention rate (95%) and supported the preliminary feasibility and acceptance of the intervention. Further, the pilot outcomes might suggest that the test of the effectiveness of S-Health with larger samples over a longer period of time is promising to provide evidence in support of mHealth as an effective technique for patients with SUD in Asian countries. Future research in this field needs to consider a number of issues, including language and literacy barriers, power supply considerations, data security, and privacy issues (Brian & Ben-Zeev, 2014) to ensure a successful development and implementation of a Smartphone intervention such as S-Health.
Figure 3.
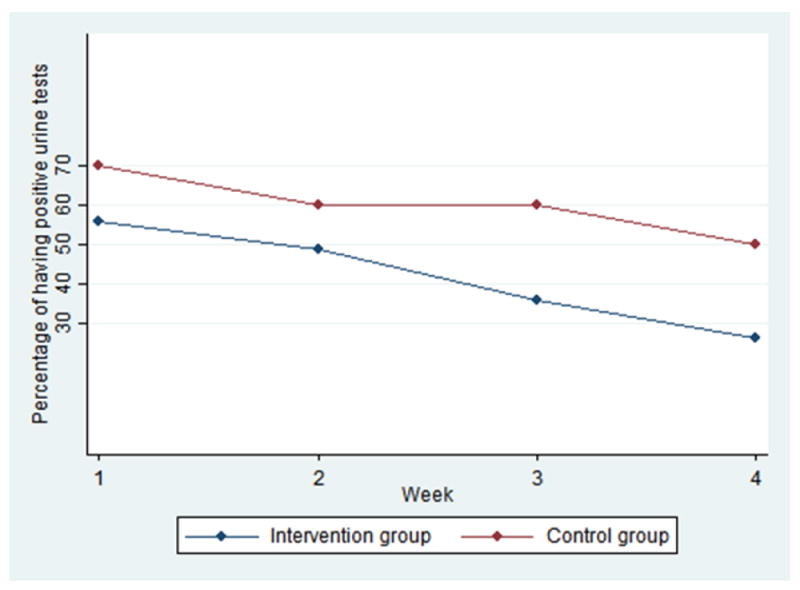
Participants use status over time
Highlights.
S-Health is developed as a bilingual smartphone application based on cognitive behavioral principles to support recovery from drug addiction.
75 participants were recruited in Shanghai, China, for this 1-month pilot randomized controlled study.
This pilot demonstrated the feasibility and potential benefits to deliver mobile health intervention among participants with SUD.
Acknowledgments
This study is funded by National Institute on Drug Abuse (NIDA 5R21 DA25252-2).
Appendix
Daily Survey
Q1: Today I…
Consumed 1-2 drinks.
Consumed 3 or more drinks.
Did not consume alcohol.
Q2: Today I…
Did not smoke any cigarettes.
Smoked less than 10 cigarettes.
Smoked 10-20 cigarettes (up to 1 pack).
Smoked 21-40 cigarettes (up to 2 packs).
Smoked more than 40 cigarettes (more than 2 packs).
Q3: In the last 24 hours (or, since last survey) what was your highest level of craving for *?
0 – No craving
1 – Minimal craving
2 – Less than moderate craving
3 – Moderate craving
4 – High craving
5 – Extremely high craving
Q4: Have you used * in the last 24 hours (or since last survey)?
Yes
No
If Q4 = 1, ask Q5; otherwise skip to Q8.
Q5: Were you planning to use * today?
Yes
No
Q6: (In the last 24 hours) What time of day did you start using *?
Enter time:
Q7: I was tempted (triggered) to use * today due to…
The way I was feeling.
Who I was with.
Where I was.
Craving.
Was offered/ Saw *.
A situation that had just happened.
If Q7 = 1, ask Q7-1a and Q7-1b; if Q7 = 2, ask Q7-2; if Q7 = 3, ask Q7-3; if Q7 = 4, ask Q7-4; if Q7 = 5, ask Q7-5; if Q7 = 6, ask Q7-6; otherwise skip to Q10a.
Q7-1a: How I was feeling…
Frustrated
Anxious
Depressed
Nervous
Afraid
Upset
Attentive
Inspired
Alert
Hostile
Happy
Bored
Active
Celebratory
Ashamed
Determined
Q7-1b: On a scale of 1=mild to 5=very strong, how strongly were you feeling [EMOTION] at the time you were tempted to use?
Q7-2: Who I was with…
I was with old friends with whom I’d used with before.
My sexual partner was using.
I felt awkward about saying “no” to this person.
Q7-3: Where I was…
I was in a place where I had bought or used in the past.
I went somewhere I thought people might be using but wasn’t planning to use myself.
Q7-4: Craving
I was thinking about how good a high had felt.
I had a strong craving.
I was thinking about using.
Q7-5: Was offered/ Saw substance
At home
At a party
At a friend’s
On the street
Add new choice_____
Q7-6: A situation that had just happened
I had a disagreement with someone
I felt rejected by a sexual partner
I had received a poor review at work or a poor grade at school
Add new choice____
Q8: Were you triggered to take * today?
Yes
No
If Q8 = 1, ask Q9 and repeat Q7; otherwise skip to Q10a.
Q9: How did you cope with that trigger experience?
Relaxation
Meditation/ Breathing
Exercise
Social support (friends, family)
Smoked cigarettes or used other drugs
Add new choice____
Q10a: How are you feeling right now?
Frustrated
Anxious
Depressed
Nervous
Afraid
Upset
Attentive
Inspired
Alert
Hostile
Happy
Bored
Active
Celebratory
Ashamed
Determined
Q10b: On a scale of 1=mild to 5=very strong, how strongly were you feeling [EMOTION] at the time you were tempted to use?
Q10: My main goal area is in…
Substance use
Health/ Mental health
Lifestyle/ Personal responsibility
Community
If Q10 = 1, ask Q10-1; if Q10 = 2, ask Q10-2; if Q10 = 3, ask Q10-3; if Q10 = 4, ask Q10-4. Q10-1: How did you do on a scale of 1-5 meeting your substance use goals today? (1=poor, 5=great)
Q10-2: How did you do on a scale of 1-5 meeting your health/ mental health goals today? (1=poor, 5=great)
Q10-3: How did you do on a scale of 1-5 meeting your lifestyle/ personal responsibility goals in this area today? (1=poor, 5=great)
Q10-4: How did you do on a scale of 1-5 meeting your community-focused goals today? (1=poor, 5=great)
Footnotes
Among the 50 participants in the intervention group, 38 were recruited from MMT clinics, and 12 were recruited from the Shanghai Zi-Qiang consortium. Among the 25 participants in the control group, 20 were from MMT clinics, and 5 were from the Shanghai Zi-Qiang consortium. The proportion of participants recruited from social workers did not differ significantly between the intervention group and the control group.
Participant compensation was approved by UCLA IRB. Participants received 50 Yuan for the intake interview and 20 Yuan for the intake urine sample, 30 Yuan for each of the three weekly check-ins and urine sample, and 50 Yuan for the 1-month follow-up interview and 20 Yuan for the 1-month follow-up urine sample. For participants in the intervention group, they received an additional 168 Yuan in total for completing daily surveys. After completing all surveys and urine tests, participants in the control group received 230 yuan (about 36 USD), and participants in the intervention group received 398 yuan (about 63 USD).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Kelly SM, Gryczynski J, Mitchell SG, Kirk A, O’Grady KE, Schwartz RP. Validity of Brief Screening Instrument for Adolescent Tobacco, Alcohol, and Drug Use. Pediatrics. 2014;133(5):819–826. doi: 10.1542/peds.2013-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Jama. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002a;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002b;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- Brian RM, Ben-Zeev D. Mobile health (mHealth) for mental health in Asia: objectives, strategies, and limitations. Asian Journal of Psychiatry. 2014;10:96–100. doi: 10.1016/j.ajp.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Chen H, Phillips MR, Cheng H, Chen Q, Chen X, Fralick D, Bueber M, et al. Mental health law of the People’s Republic of China. Shanghai Archives of Psychiatry. 2012;24(6):305–322. doi: 10.3969/j.issn.1002-0829.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, DiClemente R, Prietula M. Taking mHealth Forward: Examining the Core Characteristics. JMIR Mhealth Uhealth. 2016;4(3):e97. doi: 10.2196/mhealth.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Vos T, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- Firth J, Torous J, Yung AR. Ecological momentary assessment and beyond: The rising interest in e-mental health research. Journal of Psychiatric Research. 2016;80:3–4. doi: 10.1016/j.jpsychires.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Gao X, Jackson T, Chen H, Liu Y, Wang R, Qian M, Huang X. There is a long way to go: a nationwide survey of professional training for mental health practitioners in China. Health Policy. 2010;95(1):74–81. doi: 10.1016/j.healthpol.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Gibbons MC, Fleisher L, Slamon RE, Bass S, Kandadai V, Beck JR. Exploring the potential of Web 2.0 to address health disparities. Journal of Health Communication. 2011;16(Suppl 1):77–89. doi: 10.1080/10810730.2011.596916. [DOI] [PubMed] [Google Scholar]
- Gustafson DH, McTavish FM, Chih MY, Atwood AK, Johnson RA, Boyle MG, Isham A. A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA psychiatry. 2014;71(5):566–572. doi: 10.1001/jamapsychiatry.2013.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RC, Chen KY, Broekman B, Mak A. Buprenorphine prescription, misuse and service provision: a global perspective. Advances in psychiatric treatment. 2009;15(5):354–363. doi: 10.1192/apt.bp.108.005975. [DOI] [Google Scholar]
- Ho RC, Zhang MW. Ketamine as a rapid antidepressant: the debate and implications. BJPsych Advances. 2016;22(4):222–233. doi: 10.1192/apt.bp.114.014274. [DOI] [Google Scholar]
- Hser YI, Liang D, Lan YC, Vicknasingam BK, Chakrabarti A. Drug Abuse, HIV, and HCV in Asian Countries. Journal of NeuroImmune Pharmacology. 2016;11(3):383–393. doi: 10.1007/s11481-016-9665-x. [DOI] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA. Relapse prevention - An overview of Marlatt’s cognitive-behavioral model. Alcohol Research & Health. 1999;23(2):151–160. [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang T, Chi H, Chen Y, Li Y, Wang J. Mobile health in China: current status and future development. Asian Journal of Psychiatry. 2014;10:101–104. doi: 10.1016/j.ajp.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Linas BS, Latkin C, Genz A, Westergaard RP, Chang LW, Bollinger RC, Kirk GD. Utilizing mHealth Methods to Identify Patterns of High Risk Illicit Drug Use. Drug Alcohol Depend. 2015;151:250–257. doi: 10.1016/j.drugalcdep.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, George WH. Relapse prevention: Introduction and overview of the model. Addiction. 1984;79(3):261–273. doi: 10.1111/j.1360-0443.1984.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Donovan DM. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. Guilford Press; 2005. [Google Scholar]
- McGahan PL, Griffith JA, Parente R, McLellann AT. Composite Scores Manual. 1986 Retrieved from https://www.tresearch.org/images_specific/CompositeManual.pdf.
- McLellan AT. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction. 2002;97(3):249–252. doi: 10.1046/j.1360-0443.2002.00127.x. [DOI] [PubMed] [Google Scholar]
- Monney G, Penzenstadler L, Dupraz O, Etter JF, Khazaal Y. mHealth App for Cannabis Users: Satisfaction and Perceived Usefulness. Frontiers in Psychiatry. 2015;6:120. doi: 10.3389/fpsyt.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QLT, Nguyen LH, Tran BX, Phan HTT, Le HT, Nguyen HD, Latkin C, et al. Co-financing for viral load monitoring during the course of antiretroviral therapy among patients with HIV/AIDS in Vietnam: A contingent valuation survey. PloS one. 2017;12(2):e0172050. doi: 10.1371/journal.pone.0172050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poushter J. Smartphone Ownership and Internet Usage Continues to Climb in Emerging Economies. 2016 Retrieved from http://www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climb-in-emerging-economies/
- Runyan JD, Steenbergh TA, Bainbridge C, Daugherty DA, Oke L, Fry BN. A smartphone ecological momentary assessment/intervention “app” for collecting real-time data and promoting self-awareness. PLoS One. 2013;8(8):e71325. doi: 10.1371/journal.pone.0071325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte M, Liang D, Wu F, Lan YC, Tsay W, Du J, Hser YI, et al. A Smartphone Application Supporting Recovery from Heroin Addiction: Perspectives of Patients and Providers in China, Taiwan, and the USA. Journal of NeuroImmune Pharmacology. 2016;11(3):511–522. doi: 10.1007/s11481-016-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21(4):486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SG, Wu Z. Rapid scale up of harm reduction in China. International Journal of Drug Policy. 2007;18(2):118–128. doi: 10.1016/j.drugpo.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Tang YL, Zhao D, Zhao C, Cubells JF. Opiate addiction in China: current situation and treatments. Addiction. 2006;101(5):657–665. doi: 10.1111/j.1360-0443.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- Thompson ER. Development and validation of an internationally reliable short-form of the positive and negative affect schedule (Panas) Journal of Cross-Cultural Psychology. 2007;38(2):227–242. doi: 10.1177/0022022106297301. [DOI] [Google Scholar]
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Affairs. 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- Weaver ER, Horyniak DR, Jenkinson R, Dietze P, Lim MS. “Let’s get Wasted!” and Other Apps: Characteristics, Acceptability, and Use of Alcohol-Related Smartphone Applications. JMIR Mhealth Uhealth. 2013;1(1):e9. doi: 10.2196/mhealth.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZY, Wang Y, Detels R, Bulterys M. Towards ending HIV/AIDS among drug users in China. Addiction. 2015;110:1–3. doi: 10.1111/add.12809. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chow EP, Zhuang X, Liang Y, Wang Y, Tang C, Wilson DP, et al. Methadone maintenance treatment participant retention and behavioural effectiveness in China: a systematic review and meta-analysis. PLoS One. 2013;8(7):e68906. doi: 10.1371/journal.pone.0068906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MW, Ho RC. Enabling psychiatrists to explore the full potential of E-health. Frontiers in psychiatry. 2015;6:177. doi: 10.3389/fpsyt.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MW, Tsang T, Cheow E, Ho CS, Yeong NB, Ho RC. Enabling psychiatrists to be mobile phone app developers: insights into app development methodologies. JMIR mHealth and uHealth. 2014;2(4):e53. doi: 10.2196/mhealth.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MW, Ho RC. Tapping onto the potential of Smartphone applications for psycho-education and early intervention in Addictions. Frontiers in psychiatry. 2016a;7:40. doi: 10.3389/fpsyt.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MW, Fang P, Ho R. Global outreach and user preferences of a smartphone application developed for drinkers. Technology and Health Care. 2016b;24(4):495–501. doi: 10.3233/THC-161143. [DOI] [PubMed] [Google Scholar]
- Zhang MW, Tran BX, Nguyen HLT, Le HT, Long NH, Hinh ND, Tu NH, et al. Using online respondent driven sampling for Vietnamese youths’ alcohol use and associated risk factors. Healthcare informatics research. 2017a;23(2):109–118. doi: 10.4258/hir.2017.23.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MW, Ho R. Smartphone applications for immersive virtual reality therapy for internet addiction and internet gaming disorder. Technology and Health Care, Technology and Health Care. 2017b;25(2):367–372. doi: 10.3233/THC-161282. [DOI] [PubMed] [Google Scholar]
- Zhao M, Li X, Hao W, Wang Z, Zhang M, Xu D. A preliminary study of the reliability and validity of the addiction severity index. Journal of Chinese Medicine Research. 2004;4(8):679–680. [Google Scholar]
- Luo W, Wu Z, Wei X, Jia W, Zhang Q, Li L, Liu Z, et al. Adaptation and evaluation of Chinese version of Addiction Severity Index-V and its usage for assessing addiction of patients in methadone maintenance treatment clinics. Chinese Journal of Drug Dependence. 2007;16(5):373–378. [Google Scholar]


