Abstract
We detected Corynebacterium spp. in raw milk samples of three farms by means of a selective, tellurite-containing medium. The isolated strains were identified based on full 16S rRNA gene sequences and partial rpoB gene sequences as C. xerosis, C. variabile, C. lactis, C. callunae, C. confusum, C. glutamicum and C. crudilactis. The identification based on 16S rRNA and rpoB sequences was not reliable for isolates of C. xerosis. Chemotaxonomic markers of the isolates, fatty acids, acyl type of peptidoglycan, presence and length of mycolic acids, quinone patterns, and polar lipids, were in accord with the known characteristics of these species. Biochemical profiles, analyzed with the API Coryne system, were able to differentiate all groups, but were unable to identify the strains due to an inappropriate database for raw-milk associated corynebacteria. Most of the tested isolates showed a single-substance resistance against oxacillin, but three single isolates were classified as multidrug resistant.
Introduction
Species of the genus Corynebacterium were found ubiquitously in the environment, although often their natural habitat—especially the habitat of nonmedical Corynebacterium species—remains unknown [1]. In various studies, pathogenic corynebacteria were detected in raw milk samples and Corynebacterium spp. are known to cause subclinical mastitis in dairy cows [2]. Corynebacterium bovis is a common agent of bovine subclinical mastitis [3,4] and other species, e.g., C. amycolatum, C. minutissimum, C. ulcerans and C. pseudotuberculosis, were associated with clinical or subclinical bovine mastitis as well [5,6].
Non-pathogenic Corynebacterium species were also frequently isolated from raw milk or raw milk products [7,8]. Among them were also some species with beneficial functions in food processing. For example, the species C. glutamicum and C. variabile are well-known amino-acid producers [9] and the species C. casei, C. mooreparkense, C. ammoniagenes and C. stationis, have been detected on the surface of smear ripened cheese and are supposed to contribute to the flavor of the cheese [10,11].
The knowledge about corynebacterial diversity in raw milk is still fragmentary because of inappropriate routine test systems and high numbers of misidentifications [6,12]. For example, C. xerosis has been considered a serious and frequent human pathogen, until findings [12] indicated that most of the clinical isolates were misidentified strains of C. amycolatum. The identification to species level by analysis of their 16S rRNA gene sequences is sometimes not reliable because the 16S rRNA genes of some species show sequence differences below 2% [13,14]. For these species, the identification by additional genes has been proposed, such as rpoB gene sequencing [15]. Additionally, Corynebacterium species often grow weak on standard laboratory media. Most species show enhanced growth in sheep blood broth or brain-heart infusion, with 0.1–1.0% Tween 80 for the growth of lipophilic species [1,16]. Selective agars for Corynebacterium species are based on tellurite and have been described for C. diphteriae and C. ulcerans [17,18]. They are based on the ability of Corynebacterium species, among other Gram-positive species, to grow in the presence of tellurite, in contrast to most Gram-negative species [19].
The aim of our study was to isolate and characterize Corynebacterium spp. from bulk tank raw cow´s milk of different dairy farms and to illuminate potential pitfalls in the identification process. Corynebacterium species represent only a minor part of the raw milk microbiota [20]. Therefore, we evaluated the use of a selective medium for Corynebacterium species based on brain-heart infusion agar supplemented with tellurite and Tween 80 to detect also slow growing strains with minor abundance.
Material and methods
Evaluation of a selective medium for Corynebacterium spp.
The selective medium used in this study was based on brain-heart infusion (Oxoid Ltd., Hampshire, United Kingdom) solidified with 1.5% (w/v) agar (Oxoid Ltd., Hampshire, United Kingdom). Tween 80 (Merck KGaA, Darmstadt, Germany) was added in concentrations of 0.1% or 1.0% (w/v) [16]. Potassium tellurite trihydrate (Merck KGaA, Darmstadt, Germany) was dissolved in distilled water and added filter-sterilized to the autoclaved medium in concentrations of 0.15 g/L [21], 0.25 g/L [22] or 0.36 g/L [16]. Selectivity of this medium was tested with type strains and isolates of the genus Corynebacterium and with isolates of other non-target genera: C. frankenforstense ST18T, C. lactis RW2-5T, C. glutamicum DSM 20300T, C. amycolatum DSM 6922T, C. camporealensis NS1-11, C. flavescens TS21, C. xerosis M3_I15, C. confusum M3_I13, C. casei M3_I10, Lactococcus lactis subsp. lactis JZ RK-40, Bacillus subtilis M3_I11, Staphylococcus chromogenes M3_I12, Escherichia coli M3_I20, Acinetobacter guillouiae M3_I21 and Pseudomonas gessardii M3_I22. All isolates were identified based on their fatty acid profiles and 16S rRNA gene sequences [8] and except for C. glutamicum DSM 20300T and C. amycolatum DSM 6922T, all strains were recovered from raw milk [8,23].
Cultivation and isolation
Nine raw milk samples were collected from the bulk tank of seven dairy farms (farm B, K, M, N, P1, P2 and F) operated by private farmers, who gave their permission to conduct the study on their site. The dairy farms are located in the greater Bonn area in North Rhine-Westphalia, Germany. Ethical approval of the local authorities was not required because the raw milk samples were taken directly from the bulk tank, in accordance with the owners of the farms. Animals were not affected by the sampling procedure. The milk samples were cultivated on brain-heart infusion agar with 0.25 g/L potassium tellurite and 1.0% Tween 80 (BHT-agar) at 30°C for 48 h. Total bacterial counts were determined on Trypton soy agar (TSA; Merck KGaA, Darmstadt, Germany). Colonies from BHT-agar with bacterial cells of rod-shaped morphology were subcultivated on TSA as presumptive Corynebacterium spp.
16S rRNA and rpoB gene sequencing
Extraction of genomic DNA, amplification and sequencing of 16S rRNA genes was performed as described previously [23,24]. 16S rRNA genes were amplified with the universal bacterial primers 8F (5´-AGAGTTTGATCMTGGC-3´) and 1492R (5´-TACCTTGTTACGACTT-3´) and as sequencing primers we used 787R (5´-GGACTACCAGGGTATCTAAT-3´) and 518F (5´-CCAGCAGCCGCGGTAAT-3´) [24]. Amplification of partial rpoB genes was performed with the Corynebacterium specific primers C2700F (5´-CGWATGAACATYGGBCAGGT-3´) and C3130R (5´-TCCATYTCRCCRAARCGCTG-3´) [15]. These primers were also used as sequencing primers. The obtained sequences were manually edited with Chromas Lite 2.1.1. (Technelysium Pty Ltd, South Brisbane, AU) and assembled with BioEdit 7.2.5. [25] to obtain either almost complete 16S rRNA sequences of 1,400–1,500 base pairs (bp) or partial rpoB gene sequences (300–400 bp). The sequences were compared to the sequences of type strains by using the Basic local alignment search tool (BLAST) [26]. Phylogenetic trees of isolates, closest related type strains and other raw milk associated Corynebacterium species were obtained by maximum-likelihood algorithm with MEGA 6.06. [27] and the alignment of the sequences was performed by ClustalW [28]. Model parameters were estimated using the “find best DNA” option of MEGA and models were chosen according to lowest Bayesian information criterion (BIC) and Akaike information criterion (AIC) values [27]. As best fit substitution model for 16S rRNA gene sequences, the Tamura-3-parameter model was chosen with discrete gamma distribution and presence for invariant sites. The rpoB gene sequences were analyzed based on the Tamura-Nei model with discrete gamma distribution and presence for invariant sites. The nearest-neighbor-interchange search method was used for both trees.
Chemotaxonomic and biochemical properties
Chemotaxonomic characteristics
Fatty acid patterns, presence and length of mycolic acids, polar lipid patterns, quinones and the acyl type of peptidoglycan were determined as described previously [23].
API Coryne test system
Biochemical tests were performed with the API Coryne test system (BioMèrieux, F) for identifying coryneform bacteria according to the manufacturer’s specifications. Microorganisms were cultured on TSA for 24 h at 30°C and the inoculated test strips were incubated for 24–48 h at 30°C. Reaction profiles were analyzed with the apiwebTM software. The database version was V3.0.
Proteolytic and lipolytic activity
Proteolytic activity was determined on skim milk agar (TSA with 5.0% w/v skim milk powder) and lipolytic activity on tributyrin agar (TSA with 1.0% v/v tributyrin) [29]. Both tests were performed at 30°C for 48 h and at 10°C for 7 d. Strains were considered positive for proteolysis or lipolysis by formation of a transparent halo around the colonies.
Susceptibility against antimicrobial agents
Susceptibility patterns against 16 antimicrobial agents of different classes were determined on Mueller Hinton agar (Oxoid Ltd., Hampshire, United Kingdom) by the agar disk diffusion method. All antimicrobial susceptibility disks were purchased from Oxoid (Hampshire, United Kingdom) or bestbion (Cologne, Germany). The antimicrobial agents used in this study were: penicillin G (6μg, Oxoid), oxacillin (1 μg, Oxoid), ampicillin (10 μg, Oxoid), tetracyclin (30 μg, bestbion), gentamicin (10 μg, bestbion), erythromycin (15 μg, bestbion), trimethoprim/sulfonamide (1.25/23.75 μg, bestbion), ceftiofur (30 μg, bestbion), cefazolin (30 μg, bestbion), cephalothin (30 μg, bestbion), pirlimycin (2 μg, bestbion), amoxicillin/clavulanic acid (20/10 μg, bestbion), kanamycin (30 μg, bestbion), streptomycin (10 μg, bestbion), tobramycin (30 μg, bestbion) and amikacin (30 μg, bestbion). Strains were considered resistant, intermediate or susceptible according to zone diameters of the CLSI document VET01-A4 [30].
Results and discussion
Evaluation of different selective media
All reference strains showed intense growth on brain-heart infusion agar without supplements. The addition of potassium tellurite inhibited the growth of the Gammaproteobacteria strains Escherichia coli M3_I20, Acinetobacter guillouiae M3_I21 and Pseudomonas gessardii M3_I22 at all concentrations. Colonies of the Corynebacterium, Staphylococcus, Bacillus and Lactococcus strains were visible after two days of incubation at 30°C. Earlier studies showed that Gram-negative species are especially sensitive against tellurite and its strongly oxidizing potential [31], whereas Gram-positive genera like Staphylococcus, Enterococcus and Corynebacterium are able to grow in the presence of tellurite [19,31]. The content of Gammaproteobacteria increases during the storage of raw milk in the bulk tank at approximately 4°C [20]. These organisms usually grow fast on standard media and may overgrow Corynebacterium strains, but are inhibited by tellurite.
Strains grown on tellurite formed black and small colonies. Growth of the Corynebacterium, Staphylococcus, Bacillus and Lactococcus reference strains was weaker at 0.36 g/L potassium tellurite than at 0.25 g/L or 0.15 g/L. Therefore, 0.25 g/L tellurite was used as additive for the selective cultivation of Corynebacterium spp. Neither 0.1% nor 1.0% Tween 80 had an enhancing or inhibiting effect on the growth of the non-lipophilic Corynebacterium reference strains. Because the supplementation of Tween 80 is essential for cultivation of lipophilic species, it was added at a concentration of 1.0% to the selective agar.
Bacterial counts and isolation procedure
On BHT-agar, the mean number of bacterial counts in nine raw milk samples was 5.0 x 103 cfu/mL (colony-forming unit) with a range from 1.0 x 102 to 2.4 x 104 cfu/mL. The mean number of bacterial counts on TSA was 8.1 x 104 cfu/mL (range from 2.0 x 103 to 2.7 x 105 cfu/mL). The average ratio of bacterial counts on BHT-agar to total bacterial counts on TSA was 6.1% with a range from 1.2% to 165.0%. Colonies on BHT-agar were screened microscopically for a rod-shaped cell morphology and rods without endospores were subcultivated as presumptive Corynebacterium spp. These isolates (n = 68) were obtained from five raw milk samples taken from three dairy farms.
Identification of raw milk isolates
Raw milk associated Corynebacterium species
The isolates were identified based on their partial or full 16S rRNA gene sequences and partial rpoB gene sequences and out of 68 isolates, 28 were identified as Corynebacterium spp. The other isolates were members of the genera Brevibacterium, Microbacterium, Arthrobacter, Dietzia or Psychrobacillus. The Corynebacterium isolates were assigned to six different species: C. callunae, C. xerosis, C. variabile, C. confusum, C. glutamicum and C. lactis. One isolate, JZ16T, could not be assigned to any of the known Corynebacterium species. For this strain, the new species C. crudilactis was proposed recently [32]. Corynebacterium isolates were recovered from raw milk of three different dairy farms; none were detected in the raw milk samples of the other four dairy farms. This confirms a report [33], in which Corynebacterium species were only detected in 25% of the analyzed raw milk samples. All of the identified Corynebacterium species in this study were recovered from raw milk before, except for C. callunae, and may be part of the natural raw milk microbiota [8,9,23]. C. xerosis was the only species isolated from raw milk of three different dairy farms and isolates of this species have frequently been recovered from raw cow´s milk [6,8]. As it is considered a commensal of the mammalian and bovine mucous membrane [34,35], it may contaminate raw milk as part of the cow’s natural udder microbiota. The isolated Corynebacterium species are considered as non-pathogenic and an impact on human health is unlikely. Except for C. confusum, which is a rare human pathogen and was rarely isolated from clinical material [36,37], but no data is available considering the pathogenicity of C. confusum in animals or the potential of a human infection via zoonotic transmission.
16S rRNA and rpoB gene sequence analyses
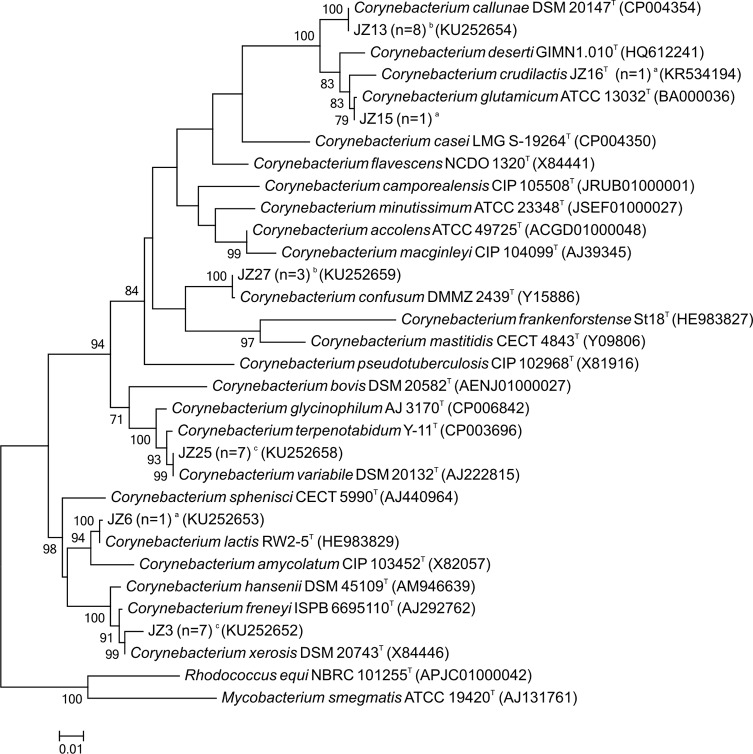
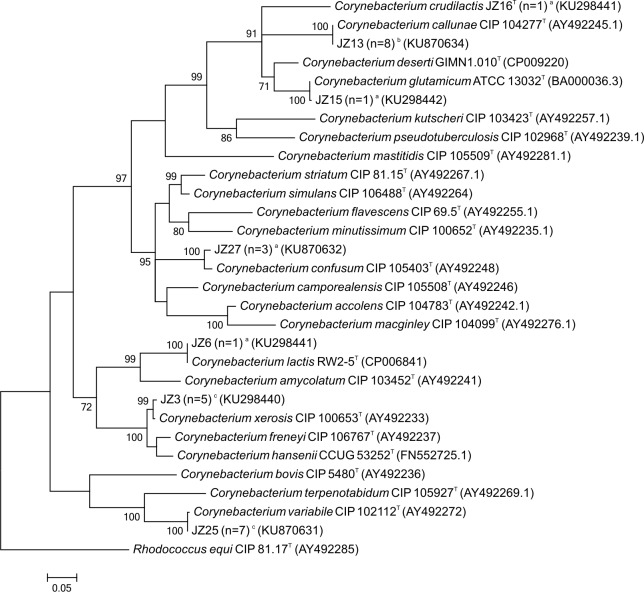
Results of 16S and rpoB gene sequencing are given in Table 1 and GenBank Accession numbers of the gene sequences in S1 Table. Phylogenetic relationships of the isolates and type strains based on maximum-likelihood analysis of their 16S rRNA or rpoB gene sequences are shown in Figs 1 and 2. The identification of C. lactis, C. callunae, and C. confusum was reliably based on a pairwise similarity of at least 99.7% to the 16S rRNA gene sequences of the type strains. Additionally, the pairwise similarity to the gene sequences of the next related type strain did not exceed the proposed threshold for species delineation of 97.8% [38]. The isolates identified as C. variabile, C. xerosis, C. glutamicum and C. crudilactis were not reliably differentiated from their next relatives by 16S rRNA gene sequencing and the deviation of their16S rRNA gene sequences ranged from 0.1–2.1%. Partial rpoB gene sequence analyses were needed to reliably identify the isolates of C. variabile, C. glutamicum and C. crudilactis. Here, the pairwise similarity to the next related type strain was below 90.2% and complied with the proposed cutoff for species delineation of 95.0% [39]. However, C. xerosis and the most closely related species C. freneyi were hardly distinguishable by rpoB gene sequencing as well. The deviation of their rpoB gene sequences ranged between 2.5 and 4.5% and the average pairwise similarity was 95.4% (94.9–95.8%). This supports findings [15] that the rpoB gene sequence similarity of C. xerosis and C. freneyi is the highest among all Corynebacterium species, which are closely related. For C. xerosis and C. freneyi, restriction length polymorphism analysis of the 16S-23S spacer region has been proposed to clearly differentiate the two species [40]. Additionally, multilocus sequence analyses of several housekeeping genes, e.g. atpA, dnaA, fusA, odhA, or whole genome sequencing can be applied to improve the resolution of phylogenetic relationships between these closely related Corynebacterium species [41].
Table 1. Pairwise similarity of 16S rRNA and rpoB gene sequences of the isolates and type strains.
| Strains | Next-related type strain | % Similarity |
||
|---|---|---|---|---|
| 16S rRNA | rpoB | |||
| Partial | full | |||
| JZ2 | Corynebacterium xerosis DSM 20743T | 100.0 | 99.5 | 100.0 |
| JZ3 | Corynebacterium xerosis DSM 20743T | 100.0 | 99.4 | 98.6 |
| JZ1 | Corynebacterium xerosis DSM 20743T | 100.0 | - | 99.5 |
| JZ4 | Corynebacterium xerosis DSM 20743T | 100.0 | - | 99.8 |
| JZ5 | Corynebacterium xerosis DSM 20743T | 100.0 | - | - |
| JZ19 | Corynebacterium xerosis DSM 20743T | 100.0 | - | - |
| N1 | Corynebacterium xerosis DSM20743T | 100.0 | 99.2 | 97.6 |
| JZ6 | Corynebacterium lactis RW2-5T | 99.4 | 99.7 | 99.8 |
| JZ20 | Corynebacterium varabile DSM 20132T | 100.0 | 100.0 | 99.5 |
| JZ25 | Corynebacterium varabile DSM 20132T | 100.0 | 100.0 | 99.5 |
| JZ10 | Corynebacterium varabile DSM 20132T | 100.0 | - | 99.5 |
| JZ11 | Corynebacterium varabile DSM 20132T | 100.0 | - | 99.5 |
| JZ21 | Corynebacterium varabile DSM 20132T | 100.0 | - | 99.5 |
| JZ28 | Corynebacterium varabile DSM 20132T | 100.0 | - | 99.5 |
| JZ29 | Corynebacterium varabile DSM 20132T | 100.0 | - | 99.5 |
| JZ13 | Corynebacterium callunae DSM 20147T | 99.9 | 99.9 | 100.0 |
| JZ14 | Corynebacterium callunae DSM 20147T | 99.9 | 99.9 | 100.0 |
| JZ22 | Corynebacterium callunae DSM 20147T | 99.9 | - | 100.0 |
| JZ23 | Corynebacterium callunae DSM 20147T | 99.9 | - | 100.0 |
| JZ26 | Corynebacterium callunae DSM 20147T | 99.9 | - | 100.0 |
| JZ32 | Corynebacterium callunae DSM 20147T | 99.7 | - | 100.0 |
| JZ34 | Corynebacterium callunae DSM 20147T | 99.6 | - | 100.0 |
| JZ36 | Corynebacterium callunae DSM 20147T | 99.6 | - | 100.0 |
| JZ15 | Corynebacterium glutamicum ATCC 13032T | 99.8 | 99,9 | 99.5 |
| JZ16 | Corynebacterium crudilactis JZ16T | 100.0 | 100.0 | 100.0 |
| JZ27 | Corynebacterium confusum DMMZ 2439T | 100.0 | 99.9 | 98.1 |
| FF1 | Corynebacterium confusum DMMZ 2439T | 99.9 | 99.9 | 98.1 |
| FF3 | Corynebacterium confusum DMMZ 2439T | 99.9 | 99.9 | 98.4 |
Fig 1. Phylogenetic relationships of representative isolates and related type strains from their 16S rRNA gene sequences.
Bootstrap values greater than 70% based on 1,000 replicates are indicated at each node. Bar, 0.01 substitutions per nucleotide position. aIsolated species detected in one sample of one dairy farm. bIsolated species detected in more than one sample of one dairy farm. cIsolated species detected in samples of more than one dairy farm.
Fig 2. Phylogenetic relationships of representative isolates and related type strains from their rpoB gene sequences.
Bootstrap values greater than 70% based on 1,000 replicates are indicated at each node. Bar, 0.05 substitutions per nucleotide position. aIsolated species detected in one sample of one dairy farm. bIsolated species detected in more than one sample of one dairy farm. cIsolated species detected in samples of more than one dairy farm.
Chemotaxonomic and biochemical features of Corynebacterium isolates
Fatty acid pattern
All isolated Corynebacterium strains showed long-chain saturated and unsaturated fatty acids, as described for the genus Corynebacterium [1]. In contrast to other genera of the Corynebacteriales, the species of this genus contain no or low amounts of tuberculostearic acid [1]. Species-specific fatty acid patterns were detected for the seven identified Corynebacterium species (Table 2). The species showed quantitative and qualitative differences among one another, especially in the presence of minor compounds. For example, C. lactis JZ6 was clearly separated from the other strains because it contained minor amounts of the fatty acids C17:1 cis 9 (9.9%) and C17:0 (17.1%) and the strains of C. variabile contained little to moderate amounts (2.3–15.3%) of the diagnostic compound tuberculostearic acid (TBSA; C18:0 10-methyl). Only few Corynebacterium species contain moderate amounts of TBSA, e.g. C. variabile, C. ammoniagenes and C. bovis [1]. Traces of TBSA were detected for C. confusum as well [36], but this could not be confirmed in this study. Fatty acid analyses may allow differentiation between C. xerosis and the closely related C. freneyi. According to Funke and Frodl [40], strains of C. freneyi contain little amounts of the unsaturated fatty acid C17:1 cis 8, which was not detected for C. xerosis in this study (Table 2). Additionally, C. freneyi strains contain lower levels of C18:1 cis 9 (21%) [40], compared to C. xerosis, where C18:1 cis 9 was the main fatty acid (66.8–85.3%; Table 2). The strains of C. confusum contained large amounts (18.6–46.5%) of an unidentified component (ECL 16,697) that even had, in one case, a higher percentage than the main fatty acid C18:1 cis 9. Mass spectra revealed these compounds as saturated and unsaturated aldehydes, which were presumptive pyrolysis products of the corynemycolic acids [42]. The fatty acid composition proved to be a useful feature for a differentiation of Corynebacterium species because each one of the seven different species showed a unique fatty acid profile. Additionally, the presence of diagnostic fatty acids, e.g. TBSA, which are only present in a few Corynebacterium species, enables a quick distinction between species.
Table 2. Fatty acid composition (with standard deviation in parentheses) of isolated strains.
| Item |
C. xerosis |
C. lactis | C. variabile | C. callunae | C. glutamicum | C. crudilactis | C. confusum |
|---|---|---|---|---|---|---|---|
| No. of isolates | 7 | 1 | 7 | 8 | 1 | 1 | 3 |
| FA (%) | |||||||
| C14: 0 | 0.2 (0.2) | ||||||
| ECL 14.926 a | 8.6 (4.7) | 3.7 | 0.7 (0.4) | ||||
| C15:0 | 0.2 | 0.6 | |||||
| C16:1 cis 7 | 0.1 (0.1) | 0.1 (0.0) | |||||
| C16:0 | 3.0 (2.4) | 8.4 | 39.9 (9.0) | 36.9 (1.5) | 39.4 | 26.8 | 16.3 (2.9) |
| ECL 16.697 a | 3.9 (2.9) | 21.0 (7.5) | 8.5 | 3.4 | 29.8 (14.7) | ||
| C17:1 cis 9 | 9.9 | ||||||
| ECL 16.938 a | 0.4 (0.5) | 1.8 (0.6) | 1.2 | 1.1 | 1.2 (0.7) | ||
| C17:0 | 0.1 (0.2) | 17.1 | 1.6 | ||||
| ECL 17.373 a | 0.2 (0.5) | ||||||
| C18:1 cis 9 | 78.0 (6.5) | 46.4 | 48.7 (13.3) | 56.4 (6.5) | 46.8 | 65.7 | 48.7 (11.5) |
| C18:0 | 18.8 (5.1) | 18.2 | 8.6 (4.7) | 0.1 | 0.8 | 3.2 (1.6) | |
| C18:0 10-methyl | 6.8 (4.7) |
aUnknown compound with a specific equivalent chain length (ECL).
Mycolic acids, acyl type of peptidoglycan, quinones, polar lipid pattern
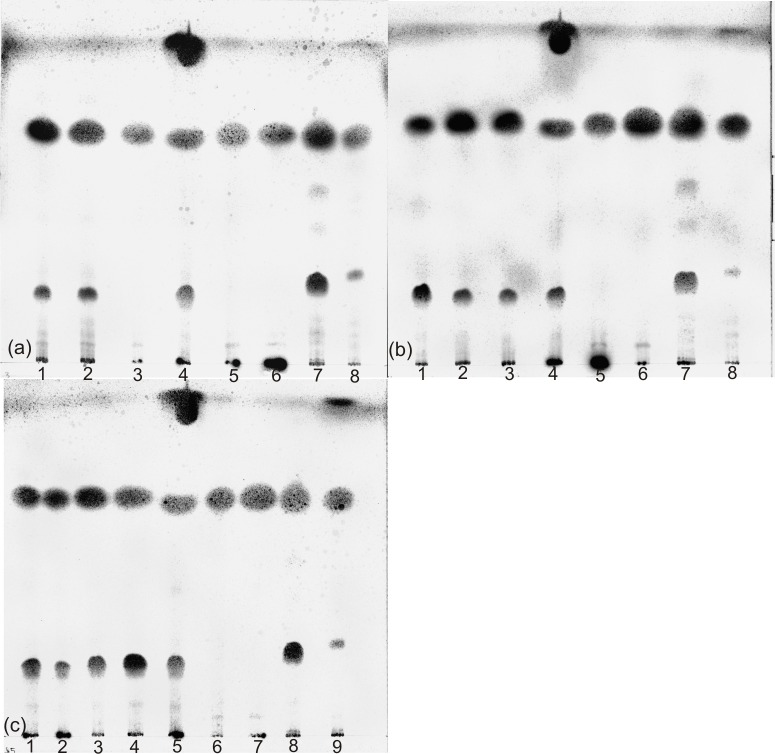
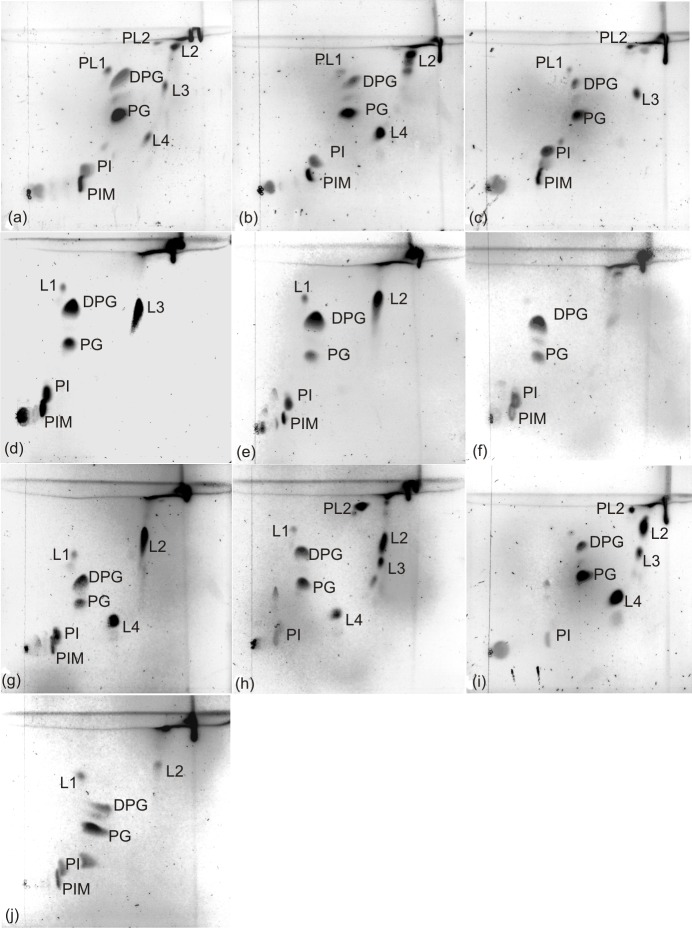
A summary of the chemotaxonomic characteristics of representative isolates is given in Table 3. The isolated strains contained mycolic acids with a chromatographic mobility comparable to the mycolic acids of C. glutamicum DSM 20300T, as described for members of these species [1], except for strain C. lactis JZ6. C. lactis belongs, together with the species C. amycolatum, C. caspium, C. ciconiae and C. kroppenstedtii, to the small group of corynebacteria without mycolic acids [1,23]. Mycolic acids are long-chained, saturated and unsaturated, β-hydroxy fatty acids with a long α-alkyl branch, characteristically synthesized by members of the order Corynebacteriales [43]. They vary in structure, chain length and in the degree of unsaturation between the different genera of the order Corynebacteriales and allow a differentiation of the genus Corynebacterium that characteristically shows short mycolic acids with 22–38 carbons [1]. All strains showed the acetyl type of peptidoglycan. Dihydrogenated menaquinones with nine isoprene units [MK-9 (H2)] were detected as major menaquinones (> 60%) for all of the strains, except for the strains of C. confusum, which showed MK-8 (H2) as major menaquinone. The analysis of bacterial isoprenoid quinones and polar lipids is used for the characterization of corynebacteria and related genera [44,45,46]. The phospholipids diphosphatidylglycerol (DPG) and phosphatidylglycerol (PG) were detected in all of the strains. Phosphatidylinositol (PI) and phosphatidylinositol mannoside (PIM) were detected as well, but whereas PI was present in all strains, PIM was not detected in the strains of C. variabile. Aminolipids were not detected. This confirms data from earlier publications, where DPG, PG, PI and PIM were detected for C. xerosis, C. glutamicum and C. lactis [23,47,48]. Phospholipid patterns were not determined so far for C. variabile, C. confusum and C. callunae. PG and phosphatidylethanolamine (PE) are useful markers to differentiate corynebacteria from the related genera Mycobacterium, Nocardia, Gordonia and Rhodococcus [48]. PG was detected in substantial amounts only in Corynebacterium species and not in the genera Mycobacterium, Nocardia or Gordonia [48]. In contrast, PE is absent only in members of the genus Corynebacterium, but not in the other genera of the order Corynebacteriales [1, 48]. While DPG is a common compound in bacteria, PI was only detected in Actinomycetes and Corynebacteriales. Not all of the polar lipids could be identified in this study. Some strains contained molybdenum blue negative lipids with a high mobility in the second dimension. According to literature, this is characteristic for acidic glycolipids [49] and they have been detected for C. xerosis and C. bovis as well [48]. Thin-layer chromatograms of mycolic acids and polar lipids are given in Figs 3 and 4.
Table 3. Chemotaxonomic characteristics of representative isolates of each Corynebacterium species.
| Species a | Strains | FA pattern | Mycolic acids b | Peptido-glycan-type | Menaquinones c | Polar lipids d | |
|---|---|---|---|---|---|---|---|
| +++ | + | ||||||
| Corynebacterium xerosis | JZ2, JZ3, N1 |
C18:1 cis 9, C18:0 | coryne-mycolates | Acetyl | MK-9 (H2) | MK-8 (H2) | DPG, PG, PI, PIM |
| Corynebacterium lactis | JZ6 | C18:1 cis 9, C18:0, 17:0, C17:1 cis 9 | n.d. | Acetyl | MK-9 (H2) | DPG, PG, PI, PIM | |
| Corynebacterium variabile | JZ20, JZ25 | C18:1 cis 9, C18:0, C18:0 10-methyl | coryne-mycolates | Acetyl | MK-9 (H2) | MK-8 (H2) | DPG, PG, PI |
| Corynebacterium callunae | JZ13, JZ14 | C18:1 cis 9, C16:0 | coryne-mycolates | Acetyl | MK-9 (H2) | MK-8 (H2) | DPG, PG, PI, PIM |
| Corynebacterium glutamicum | JZ15 | C18:1 cis 9, C16:0, C15:0 | coryne-mycolates | Acetyl | MK-9 (H2) | MK-8 (H2) | DPG, PG, PI, PIM |
| Corynebacterium crudilactis | JZ16T | C18:1 cis 9, C15:0, C16:0, C17:0 | coryne-mycolates | Acetyl | MK-9 (H2) | MK-8 (H2) | DPG, PG, PI, PIM |
| Corynebacterium confusum | JZ27, FF1, FF3 | C18:1 cis 9, C17:0 | coryne-mycolates | Acetyl | MK-8 (H2) | MK-9 (H2) | DPG, PG, PI, PIM |
aIdentification according to 16S rRNA and rpoB gene sequences.
bMycolic acids: n.d. = no Mycolic acids detected; corynemycolates = Mycolic acids with mobility identical with those of Corynebacterium glutamicum DSM 20300T using thin-layer chromatography.
cMenaquinones: +++ = main component (>60%); + = minor component (<40%).
dPolar lipids: DPG = diphosphatidylglycerol. PG = phosphatidylglycerol; PI = phosphatidylinositol; PIM = phosphatidylinositol mannoside.
Fig 3. Mycolic acid analysis of isolates and reference strains of the suborder Corynebacterineae.
Lanes of chromatogram (a): 1, C. xerosis JZ2; 2, C. xerosis JZ3; 3, C. lactis JZ6; 4, C. glutamicum DSM 20300T; 5, C. amycolatum DSM 6922T; 6, C. lactis RW 2-5T; 7, Rhodococcus rhodochrous DSM 43241T; 8, Gordonia terrae DSM 43249T. Lanes of chromatogram (b): 1, C. confusum JZ27; 2, C. variabile JZ20; 3, C. variabile JZ25; 4, C. glutamicum DSM 20300T; 5, C. amycolatum DSM 6922T; 6, C. lactis RW 2-5T; 7, R. rhodochrous DSM 43241T; 8, G. terrae DSM 43249T. Lanes of chromatogram (c): 1, C. callunae JZ13; 2, C. callunae JZ14; 3, C. glutamicum JZ15; 4, C. crudilactis JZ16T; 5, C. glutamicum DSM 20300T; 6, C. amycolatum DSM 6922T; 7, C. lactis RW 2-5T; 8, R. rhodochrous DSM 43241T; 9, G. terrae DSM 43249T.
Fig 4. Polar lipid patterns of isolates.
C. xerosis JZ2 (a), C. xerosis JZ3 (b), C. lactis JZ6 (c), C. callunae JZ13 (d), C. callunae JZ14 (e), C. glutamicum JZ15 (f), C. crudilactis JZ16T (g), C. variabile JZ20 (h), C. variabile JZ25 (i), C. confusum JZ27 (j). DPG, diphosphatidylglycerol; PG, phosphatidylglycerol; PI, phosphatidylinositol; PIM, phosphatidylinositol mannoside; PL1 –PL2, phospholipids (molybdenum blue positive); L1 –L4, polar lipids (molybdenum blue negative).
API Coryne
Identification of Corynebacterium species by the API Coryne test system is a fast and easy method and earlier studies showed that the numbers of misidentifications of clinical isolates are relatively low [50,51,52]. Results of the API Coryne test system for our isolates are shown in Table 4. The numerical code was obtained after an incubation period of 24 h. Test strips of the strains of C. xerosis and C. confusum were incubated 48 h because of their weak growth after 24 h. All of the tested strains were positive for pyrazinamidase and negative for gelatinase, pyrolidonyl arylamidase, N-acetyl-β-glucosaminidase and fermentation of glycogen and xylose. The numerical code was identical for the strains within the species C. variabile, C. callunae and C. confusum (Table 4), the three strains of C. xerosis showed different results in the API Coryne test. Two of the three tested strains of C. xerosis (JZ2 and JZ3) were negative for α-glucosidase activity and two strains (JZ3 and N1) were negative for alkaline phosphatase. This may also differentiate the isolated C. xerosis strains from the closely related C. freneyi. Strains of C. freneyi are described consistently positive for α-glucosidase activity and alkaline phosphatase [40]. None of the isolates were correctly identified by this test system, which is explained by the lack of these raw milk associated species in the present database version. Therefore, correct identification of milk-associated Corynebacterium species is critical with this system.
Table 4. Numerical code of representative isolates generated with the API Coryne test system.
| Species a | Strain | Numerical code | Apiweb-Identification d | |
|---|---|---|---|---|
| Sign. Taxa | % ID (T) | |||
| Corynebacterium xerosis b | JZ2 | 3500365 | Incorrect profile c | |
| JZ3 | 2000325 | Corynebacterium group G | 48.5 (0.72) | |
| N1 | 2410344 | Actinomyces neuii ssp. anitratus | 95.0 (0.46) | |
| Corynebacterium lactis | JZ6 | 2100304 | C. jeikeium | 93.6 (1.0) |
| Corynebacterium callunae | JZ13 | 2000325 | Corynebacterium group G | 48.5 (0.72) |
| JZ14 | 2000325 | Corynebacterium group G | 48.5 (0.72) | |
| Corynebacterium glutamicum | JZ15 | 3201325 | C. glucuronolyticum | 98.3 (0.92) |
| Corynebacterium crudilactis | JZ16T | 3241304 | C. glucuronolyticum | 94.6 (0.72) |
| Corynebacterium variabile | JZ20 | 2011004 | C. urealyticum | 82.4 (0.67) |
| JZ25 | 2011004 | C. urealyticum | 82.4 (0.67) | |
| Corynebacterium confusum b | JZ27 | 3100304 | C. propinquum | 83.4 (1.0) |
| FF1 | 3100304 | C. propinquum | 83.4 (1.0) | |
| FF3 | 3100304 | C. propinquum | 83.4 (1.0) | |
aIdentification according to 16S rRNA and rpoB gene sequences.
bIncubation of test strips for 48 h.
cIncorrect profile indicated by the apiwebTM Software.
d Strains were subcultured on TSA for 24 h at 30°C and results were obtained after 24 h incubation of the test strips at 30°C.
Proteolytic and lipolytic activity
In order to determine the potential as food spoiling organisms, proteolytic and lipolytic activity of the Corynebacterium isolates were tested at 30°C and 10°C. None of the Corynebacterium strains showed proteolytic activity. Lipolytic activity was detected for the strains of C. lactis, C. callunae, C. xerosis and C. variabile at 30°C and the strains of C. variabile were able to perform lipolysis at 10°C as well. Although growth below 20°C is rarely detected within the genus Corynebacterium [1], all raw milk isolates, except strains of the species C. confusum, were able to grow at 10°C on TSA within 48 h.
Antimicrobial susceptibility of Corynebacterium isolates
Nine selected strains were tested for susceptibility against 16 different antimicrobial agents: C. lactis JZ6, C. callunae JZ13, C. glutamicum JZ15, C. crudilactis JZ16T, C. variabile JZ20, C. confusum JZ27 and C. xerosis JZ2, JZ3 and N1. All of the strains were resistant against oxacillin. Additionally, C. callunae JZ13 was resistant against pirlimycin and C. glutamicum JZ15 against streptomycin. The three isolates C. xerosis N1, C. confusum JZ27 and C. crudilactis JZ16T [32] were resistant against antimicrobial agents of three different classes, which qualified them as multidrug resistant (MDR) according to the definition of the CLSI [30]. C. xerosis N1 and C. confusum JZ27 were resistant against oxacillin, erythromycin and pirlimycin and C. crudilactis JZ16T was resistant against oxacillin, ampicillin, thrimethoprim/sulfonamide, kanamycin and streptomycin [32]. Multidrug resistance has been described for clinically relevant Corynebacterium species, e.g. C. resistens, C. striatum or C. amycolatum, but rarely for non-medical corynebacteria [53,54,55]. Susceptibilities of raw milk associated Corynebacterium isolates against antimicrobial agents has not been described so far, expect for C. bovis and C. amycolatum isolates from bovine mammary glands [56]. Here, the Corynebacterium isolates were generally susceptible against the 15 tested antimicrobial agents. This supports findings of this study that antimicrobial resistance of raw milk associated corynebacteria and the potential to distribute antimicrobial resistance is generally low, except for single strains with high levels of antimicrobial resistance.
Conclusion
Results of this study confirm that Corynebacterium species are a minor but regular part of the raw milk microbiome. Additionally, strains of other genera of the phylum Actinobacteria were isolated from raw milk on the selective medium in this study, e.g. Arthrobacter and Dietzia, which are rarely described as raw milk associated bacteria. Abundance and impact on raw milk of these organisms may need further investigation. A delineation of Corynebacterium spp. from other closely related genera of the order Corynebacteriales is possible by chemotaxonomic markers. Some of these markers, especially fatty acid profiles or the presence or absence of mycolic acids could also be used to differentiate between several milk-associated species within this genus. Sequencing of the 16S rRNA gene is appropriate for the identification of most Corynebacterium strains. For some species, a reliable identification needs additional sequence information from a less conserved gene like the rpoB gene, but for some closely related species, like C. xerosis and C. freneyi, additional tests (fatty acid pattern, α-glucosidase activity and alkaline phosphatase) are highly recommended. The high physiological diversity within the genus Corynebacterium, covering amino-acid producers, colonizers of smear-ripened cheese but also animal and human pathogens, gives reasons for further in-depth analyses of raw milk associated Corynebacterium species.
Supporting information
(PDF)
Acknowledgments
This project was conducted in the Center of Integrated Dairy Research (CIDRe), University of Bonn (Bonn Germany). We thank Susanne Plattes (CIDRe) and Iris Gockel-Böhner (Institute of Animal Science, University of Bonn) for their help in collecting raw milk samples.
Data Availability
All gene sequences are available from the GenBank database (accession numbers KU252651, KU252652, KU252653, KU252654, KU252655, KU252656, KU252657, KU252658, KU252659, KU252600, KU252661, KU252662, KU298439, KU298440, KU298441, KU298442, KU298443, KU870629, KU870630, KU870631, KU870632, KU870633, KU870634, KX359581, KX359582, KX359583, KX359584, KX359585, KX359586, KX359587, KX359588, KX359589, KX359590, KX359591, KX359592, KX359593, KX359594, KX359595, KX965686, KX965687, KX965688, KX965689, KX965690, KX965691, KX965692, KX965693, KX965694, KX965695, KX965696, KX965697, KX965698, KY013617, KY013618, KR534194).
Funding Statement
The authors recieved no specific funding for this work.
References
- 1.Bernard KA, Funke G. Genus I. Corynebacterium Lehmann and Neumann 1896, 350AL emend. Bernard, Wiebe, Burdz, Reimer, Ng. Singh, Schindle and Pacheo 2010, 877 In: Goodfellow M, Kämpfer P, Busse HJ, Trujillo ME, Suzuki K, Ludwig W, Whitman WB, editors. Bergey’s Manual of Systematic Bacteriology. New York: Springer; 2012. pp. 245–289. [Google Scholar]
- 2.Gonçalves JL, Tomazi T, Barreiro JR, Beuron DC, Arcari MA, Lee SHI, et al. Effects of bovine subclinical mastitis caused by Corynebacterium spp. on somatic cell count, milk yield and composition by comparing contralateral quarters. Vet J. 2016; 209: 87–92. doi: 10.1016/j.tvjl.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 3.Haltia L, Honkanen-Buzalski T, Spiridonova I, Olkonen A, Myllys V. A study of bovine mastitis, milking procedures and management practices on 25 Estonian dairy herds. Acta Vet Scand. 2006; 48: 22 doi: 10.1186/1751-0147-48-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schukken YH, González RN, Tikofsky LL, Schulte HF, Santisteban CG, Welcome FL, et al. CNS mastitis: Nothing to worry about? Vet Microbiol. 2009; 134: 9–14. doi: 10.1016/j.vetmic.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 5.Hommez J, Devriese LA, Vaneechoutte M, Riegel P, Butaye P, Haesebrouck F. Identification of nonlipophilic Corynebacteria isolated from dairy cows with Mastitis. J Clin Microbiol. 1999; 37: 954–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts JL, Lowery DE, Teel JF, Rossbach S. Identification of Corynebacterium bovis and other Coryneforms isolated from bovine mammary glands. J Dairy Sci. 2000; 83: 2373–2379. doi: 10.3168/jds.S0022-0302(00)75126-5 [DOI] [PubMed] [Google Scholar]
- 7.Dolci P, Barmaz A, Zenato S, Pramotton R, Alessandrina V, Cocolin L, et al. Maturing dynamics of surface microflora in Fontina PDO cheese studied by culture-dependent and–independent methods. J Appl Microbiol. 2009; 106: 278–287. doi: 10.1111/j.1365-2672.2008.04001.x [DOI] [PubMed] [Google Scholar]
- 8.Weber M, Geißert J, Kruse M, Lipski A. Comparative analysis of bacterial community composition in bulk tank raw milk by culture-dependent and culture-independent methods using the viability dye propidium monoazide. J Dairy Sci. 2014; 97: 6761–6776. doi: 10.3168/jds.2014-8340 [DOI] [PubMed] [Google Scholar]
- 9.Fricker M, Skanseng B, Rudi K, Stessl B, Ehling-Schulz M. Shift from farm to dairy tank milk microbiota revealed by a polyphasic approach is independent from geographical origin. Int J Food Microbiol. 2011; 145: 24–30. [DOI] [PubMed] [Google Scholar]
- 10.Bockelmann W, Hoppe-Seyler T. The surface flora of bacterial smear-ripened cheeses from cow´s and goat´s milk. Int Dairy J. 2001; 11: 307–314. [Google Scholar]
- 11.Brennan NM, Brown R, Goodfellow M, Ward AC, Beresford BT, Simpson PJ, et al. Corynebacterium mooreparkense sp. nov. and Corynebacterium casei sp. nov., isolated from the surface of a smear-ripened cheese. Int J Syst Evol Microbiol. 2001; 51: 843–852. doi: 10.1099/00207713-51-3-843 [DOI] [PubMed] [Google Scholar]
- 12.Funke G, Lawson PA, Bernard KA, Collins MD. Most Corynebacterium xerosis strains identified in the routine clinical laboratory correspond to Corynebacterium amycolatum. J Clin Microbiol. 1996; 34: 1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renaud FN, Aubel D, Riegel P, Meugnier H, Bollet C. Corynebacterium freneyi sp. nov., α-glucosidase-positive strains related to Corynebacterium xerosis. Int J Syst Evol Microbiol. 2001; 51: 1723–1728. doi: 10.1099/00207713-51-5-1723 [DOI] [PubMed] [Google Scholar]
- 14.Renaud FN, Le Coustumier A, Wilhem N, Aubel D, Riegel P, Bollet C, et al. Corynebacterium hansenii sp. nov., an α-glucosidase-negative bacterium related to Corynebacterium xerosis. Int J Syst Evol Microbiol. 2007; 57: 1113–1116. doi: 10.1099/ijs.0.64665-0 [DOI] [PubMed] [Google Scholar]
- 15.Khamis A, Raoult D, La Scola B. RpoB gene sequencing for identification of Corynebacterium species. J Clin Microbiol. 2004; 42: 3925–3931. doi: 10.1128/JCM.42.9.3925-3931.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riegel P, de Briel D, Prévost G, Jehl F, Monteil H. Genomic diversity among Corynebacterium jeikeium strains and comparison with biochemical characteristics and antimicrobial susceptibilities. J Clin Microbiol. 1994; 32: 1860–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal E, Eylan E. Cystine–Serum–Tellurite: A differential medium for Corynebacterium diphteriae. Med Microbiol Immunol. 1973; 158: 165–169. [DOI] [PubMed] [Google Scholar]
- 18.Tinsdale GFW. A new medium for the isolation and identification of C. diphteriae based on the production of hydrogen sulphide. J Pathol Bacteriol. 1947; 59: 461–466. [Google Scholar]
- 19.Von Graevenitz A, Bernard K. Genus Corynebacterium–Medical In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The Prokaryotes. New York: Springer; 2006. pp. 819–842. [Google Scholar]
- 20.Raats D, Offek M, Minz D, Halpern M. Molecular analysis of bacterial communities in raw cow milk and the impact of refrigeration on its structure and dynamics. Food Microbiol. 2011; 28: 465–471. doi: 10.1016/j.fm.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 21.Baird-Parker AC. An improved diagnostic and selective medium for isolating coagulase-positive Staphylococci. J Appl Bacteriol. 1962; 25: 12–19. [Google Scholar]
- 22.Zebovitz E, Evans JB, Niven CF. Tellurite-Glycine Agar: a selective plating medium for the quantitative detection of coagulase-positive Staphylococci. J Bacteriol. 1955; 70: 686–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiertz R, Schulz SC, Müller U, Kämpfer P, Lipski A. Corynebacterium frankenforstense sp. nov. and Corynebacterium lactis sp. nov., isolated from raw cow milk. Int J Syst Evol Microbiol. 2013; 63: 4495–4501. doi: 10.1099/ijs.0.050757-0 [DOI] [PubMed] [Google Scholar]
- 24.Buchholz-Cleven BEE, Rattunde B, Straub KL. Screening for genetic diversity of isolates of anerobic Fe(II)–oxidizing bacteria using DGGE and whole-cell hybridization. Syst Appl Microbiol. 1997; 20: 301–309. [Google Scholar]
- 25.Hall TA. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999; 41: 95–98. [Google Scholar]
- 26.Altschul SF, Gish W, Miller W, Myer EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215: 403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 27.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28: 2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrigan WF. Laboratory Methods in Food Microbiology. 3rd edn. San Diego: Academic Press; 1998. [Google Scholar]
- 30.CLSI. Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals 4th ed. Wayne: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 31.Walter EG, Taylor DE. Plasmid-mediated resistance to tellurite: expressed and cryptic. Plasmid. 1992; 27: 52–64. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann J, Rückert C, Kalinowski J, Lipski A. Corynebacterium crudilactis sp. nov., a new Corynebacterium species isolated from raw cow´s milk. Int J Syst Evol Microbiol. 2016; 66: 1–6. [DOI] [PubMed] [Google Scholar]
- 33.Vacheyrou M, Normand A-C, Guyot P, Cassagne C, Piarroux R, Bouton Y. Cultivable microbial communities in raw cow milk and potential transfers from stables of sixteen French farms. Int J Food Microbiol. 2011; 146: 253–262. doi: 10.1016/j.ijfoodmicro.2011.02.033 [DOI] [PubMed] [Google Scholar]
- 34.Coyle MB, Lipsky BA. Coryneform bacteria in infectious diseases: clinical and laboratory aspects. Clin Microbiol Rev. 1990; 3: 227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonçalves JL, Tomazi T, Barreiro JR, Braga PA, Ferreira CR, Araújo Junior JP, et al. Identification of Corynebacterium spp. isolated from bovine intramammary infections by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Vet Microbiol. 2014; 173: 147–151. doi: 10.1016/j.vetmic.2014.06.028 [DOI] [PubMed] [Google Scholar]
- 36.Funke G, Osorio CR, Frei R, Riegel P, Collins MD. Corynebacterium confusum sp. nov., isolated from human clinical specimens. Int J Syst Bacteriol. 1998; 48: 1291–1296. doi: 10.1099/00207713-48-4-1291 [DOI] [PubMed] [Google Scholar]
- 37.Bernard KA, Munro C, Wiebe D, Ongsansoy E. Characteristics of rare or recently described Corynebacterium species recovered from human clinical material in Canada. J. Clin. Microbiol. 2002; 40: 4375–4381. doi: 10.1128/JCM.40.11.4375-4381.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M, Oh H-S, Park S-C, Chun J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014; 64: 346–351. doi: 10.1099/ijs.0.059774-0 [DOI] [PubMed] [Google Scholar]
- 39.Khamis A, Raoult D, La Scola B. Comparison between rpoB and 16S rRNA gene sequencing for molecular identification of 168 clinical isolates of Corynebacterium. J Clin Microbiol. 2005; 43: 1934–1936. doi: 10.1128/JCM.43.4.1934-1936.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funke G, Frodl R. Comprehensive study of Corynebacterium freneyi strains and extended and emended description of Corynebacterium freneyi Renaud, Aubel, Riegel, Meugnier, and Bollet 2001. J Clin Microbiol. 2008; 46: 638–643. doi: 10.1128/JCM.01491-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.König C, Meinel DM, Margos D, Konrad R, Sing A. Multilocus sequence typing of Corynebacterium ulcerans provides evidence for zoonotic transmission and for increased prevalence of certain sequence types among toxigenic strains. J. Clin. Microbiol. 2002; 52: 4318–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lechevalier MP, Lechevalier H, Horan AC. Chemical characteristics and classification of nocardiae. Can J Microbiol. 1973; 19: 965–972. [DOI] [PubMed] [Google Scholar]
- 43.Puech V, Chami M, Lemassu A, Lanéelle M-A, Schiffler B, Gounon P, et al. Structure of the cell envelope of Corynebacteria: importance of the non-covalently bound lipids in the formation of the cell wall permeability barrier and fracture plane. Microbiology. 2001; 147: 1365–1382. doi: 10.1099/00221287-147-5-1365 [DOI] [PubMed] [Google Scholar]
- 44.Bendinger B, Kroppenstedt RM, Klatte S, Altendorf K. Chemotaxonomic differentiation of coryneform bacteria isolated from biofilters. Int J Syst Bacteriol. 1992; 42: 474–486. doi: 10.1099/00207713-42-3-474 [DOI] [PubMed] [Google Scholar]
- 45.Brennan PJ, Lehane DP. The Phospholipids of Corynebacteria. Lipids. 1971; 6: 401–409. [DOI] [PubMed] [Google Scholar]
- 46.Yamada Y, Inouye G, Tahara Y, Kondô K. The menaquinone system in the classification of cornyeform and nocardioform bacteria and related organisms. J Gen Appl Microbiol. 1976; 22: 203–214. [Google Scholar]
- 47.Hoischen C, Krämer R. Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J Bacteriol. 1990; 172: 3409–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minnikin DE, Patel PV, Alshamaony L, Goodfellow M. Polar lipid composition in the classification of Nocardia and related bacteria. Int J Syst Bacteriol. 1977; 27: 104–117. [Google Scholar]
- 49.Wilkinson SG. Cell walls of Pseudomonas species sensitive to ethylendiaminetetraacetic acid. J Bacteriol. 1970; 104: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konrad R, Berger A, Huber I, Boschert V, H'rmansdorfer S, Busch U, et al. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry as a tool for rapid diagnosis of potentially toxigenic Corynebacterium species in the laboratory management of diphtheria-associated bacteria. Euro Surveill. 2010; 15: 43. [DOI] [PubMed] [Google Scholar]
- 51.Renaud FN, Dutaur M, Daoud S, Aubel D, Riegel P, Monget D, et al. Differentiation of Corynebacterium amycolatum, C. minutissimum, and C. striatum by Carbon Substrate Assimilation Tests. J Clin Microbiol. 1998; 36: 3698–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soto A, Zapardiel J, Soriano F. Evaluation of API Coryne system for identifying coryneform bacteria. J Clin Pathol. 1994; 47: 756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernard KA. The genus Corynebacterium and other medically relevant Coryneform-like bacteria. J Clin Microbiol. 2012; 50: 3152–3158. doi: 10.1128/JCM.00796-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Miguel-Martinez I, Fernandez-Fuertes F, Ramos-Macias A, Bosch-Benitezand JM, Martin-Sanchez AM. Sepsis due to multiply resistant Corynebacterium amycolatum. Eur J Clin Microbiol. 1996; 15: 617–618. [DOI] [PubMed] [Google Scholar]
- 55.Otsuka Y, Kawamura Y, Koyama T, Iihara H, Ohkusu K, Ezaki T. Corynebacterium resistens sp. nov., a new multidrug-resistant coryneform bacterium isolated from human infections, J Clin Microbiol, 2005; 43: 3713–3717. doi: 10.1128/JCM.43.8.3713-3717.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watts JL, Rossbach S. Susceptibilities of Corynebacterium bovis and Corynebacterium amycolatum isolates from bovine mammary glands to 15 antimicrobial agents. Antimicrob Agents Chemother. 2000; 44: 3476–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All gene sequences are available from the GenBank database (accession numbers KU252651, KU252652, KU252653, KU252654, KU252655, KU252656, KU252657, KU252658, KU252659, KU252600, KU252661, KU252662, KU298439, KU298440, KU298441, KU298442, KU298443, KU870629, KU870630, KU870631, KU870632, KU870633, KU870634, KX359581, KX359582, KX359583, KX359584, KX359585, KX359586, KX359587, KX359588, KX359589, KX359590, KX359591, KX359592, KX359593, KX359594, KX359595, KX965686, KX965687, KX965688, KX965689, KX965690, KX965691, KX965692, KX965693, KX965694, KX965695, KX965696, KX965697, KX965698, KY013617, KY013618, KR534194).