Abstract
The oriental armyworm, Mythimna separata, is a major insect pest in China and other Asian countries. Unfortunately, suitable reference genes for quantitative real-time PCR (qRT-PCR) have not been previously identified in M. separata for normalizing target gene expression. In this study, we evaluated the expression stability of eight candidate genes (18S, ACT, EF1-α, GAPDH, RPS7, RPS13, RPL32 and TUB) in M. separata using the comparative ΔCt method, BestKeeper, Normfinder geNorm and ReFinder, a comprehensive software platform. The results indicated that the appropriate reference gene varied depending on the experimental conditions. We found that ACTIN, EF1-α and TUB were optimal for different developmental stages; TUB, RPS13 and EF1-α showed the most stable expresssion in different tissues; RPS13 and 18S were the best reference genes for monitoring expression under high temperature conditions; TUB, RPS13 and RPS7 exhibited the most stable expression under larval-crowding conditions; RPS7, EF1-α, RPL32 and GAPDH were the best for pesticide exposure experiments. This study provides tools for reliable normalization of qRT-PCR data and forms a foundation for functional studies of target gene expression in M. separata.
Introduction
Choosing the appropriate reference gene(s) is critical for assessing the accuracy of target gene expression using quantitative real-time PCR (qRT-PCR) analysis [1]. Ideally, a reference gene should be expressed constantly in samples subjected to selected experimental conditions [2,3]. Recent studies have shown that reference genes have variable expression levels under different biotic (e.g. diverse insect tissues and developmental stages) and abiotic conditions (e.g. temperatures, pesticides, different photoperiods) [4–9]. Yang et al. identified V-ATPase A as the most stable reference gene for different developmental stages in Coleomegilla maculata; however, this gene was the least stable among different C. maculata sexes and dsRNA treatments [10]. Pan et al. identified GAPDH as the most suitable reference gene for tissues and insect sex in Hippodamia convergens, but it was least suitable for temperature stress [11]. Numerous studies now suggest that there is no single ‘universal’ reference gene that can be used for diverse experimental conditions, even within a single species [12–15]. Therefore, it is essential to evaluate the expression stability of reference genes under different experimental treatments before normalizing target gene expression.
The oriental armyworm, Mythimna separata (Walker) (Lepidoptera: Noctuidae) is a migratory insect pest in China and other Asian countries. Currently, M. separata is a serious threat for corn production in China [16–18]. Due to its economic importance, the migratory behavior and regulation of M. separata has been documented [19, 20]. More recently, the development of next-generation sequencing technologies and transcriptome analysis has provided a huge amount of genetic information for expression studies. For example, Liu et al. used transcriptome analysis to identify a large number of genes involved in pesticide resistance, insect development and the stress response in M. separata [21]. Li et al. identified many differentially expressed genes involved in circadian rhythm, hormone regulation, energy metabolism and neurotransmitter receptor pathways by comparing the transcriptomes of migrant and resident M. separata [22]. Furthermore, Chang et al. [23] and Liu et al. [24] identified numerous genes with putative roles in olfactory, sensory and taste transduction by transcriptional analysis of gene expression in the heads and antennae of M. separata. Quantitative examination of gene expression can further our understanding of the molecular mechanisms underlying insect development, the stress response, and behavioral regulation in M. separata and may identify novel targets for controlling this pest.
Actin has been used as a reference gene to normalize the expression of target genes involved in the development, stress response and behavior of M. separata [21, 25–26]. However, these studies did not evaluate the suitability of this gene under different experimental treatments, which may lead to incorrect conclusions regarding the biological functions of these target genes in M. separata. In this study, we utilized the comparative ΔCt method [14], BestKeeper [27, 28], NormFinder [29], and geNorm [13] and a comprehensive software platform-RefFinder [30] to evaluate the stability of eight commonly-used reference genes (18S ribosomal RNA (18S), ACTIN (ACT), elongation factor 1 alpha (EF-1α), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ribosomal protein S7 (RPS7), ribosomal protein S13 (RPS13), ribosomal protein L32 (RPL32) and tubulin (TUB)) during M. separata development, in different M. separata tissues and in response to abiotic (high temperatures and pesticide exposure) and biotic stress (larval crowding). Heat-shock protein 90 (Hsp90) is an abundant and highly conserved molecular chaperone that is induced by multiple environmental factors. We then evaluated the different reference genes using M. separata heat shock protein 90 (Mshsp90) as a target gene to validate our findings. Our results provide a more precise approach to normalize qRT-PCR data in M. separata, which will improve our understanding of target gene functions in this important pest.
Materials and methods
Ethics statement
The M. separata larvae were collected from corn stalks cultivated in Qianxi county, Guizhou province (27°01′39.72″N, 106°20′2.92″E), in 2015. In present study, there were no specific permits being required for the insect collection. No endangered or protected species were involved in the field studies. The ‘‘List of Protected Animals in China” does not contain the M. separata which are common insect.
Insects
The collected larvae (n ≥ 100) were maintained in a climate-controlled chamber at 25 ± 1°C with a 14 L: 10 D photoperiod and 70–80% relative humidity. The larvae were fed with fresh corn leaves until satiated, and then transferred into a plastic box containing wet paper for pupation. Forty newly-emerged pairs were transferred to nylons cage for mating, provided with rice stems for oviposition, and supplied with a 10% (w/v) honey solution.
Sample treatment and collection
Developmental stages and tissues
Developmental stages of M. separata included 100 eggs, 20 first instar larvae, 10 second instar larvae, and three individuals of the remaining stages (3rd-6th instar larvae, pupae and adults). All samples were collected in a microcentrifuge tube (1.5 mL), immediately frozen in liquid nitrogen, and stored at -80°C. Each treatment contained three replications.
Insect tissues (head, epidermis, foregut, midgut, hindgut, and Malpighian tubes) were collected from 6th instar larvae. Tissue collected from three individuals were mixed and constituted one replication, and each treatment was replicated three times.
Stress conditions
M. separata 3rd instar larvae were exposed to elevated temperatures ranging from 31–39°C at 2° increments. Larvae exposed to 24°C constituted the control group. Each temperature consisted of three replications.
Larval crowding trials included five levels: 1, 5, 10, 20, and 30 larvae per plastic bottle (12 x 9 cm, height x diameter). The hatched 1st instar under each densiy were raised as above conditions. When larvae reached the second day of 5th instar (about 15 days), three larvae from same density were collected and considered to be one replication. Each density had three replications.
For insecticide exposure experiments, fresh corn leaves were dipped into five concentrations (0, 1, 2, 4 and 8 μg mL-1) of chlorpyrifos for 10 s. Treated leaves were air-dried and placed in glass petri dishes (9 cm diameter). Twelve 3rd-instar larvae were transferred into the petri dishes and allowed to feed on treated leaves for 24 h. Survival rates were approximately 100, 95, 70, 40, and 15% for 0, 1, 2, 4 and 8 μg mL-1, respectively. Surviving larvae were collected for RNA extraction, and each concentration was replicated three times.
Quantitative real-time PCR analysis
Total RNA was extracted using the SV Total RNA isolation system (Promega, WI, USA) introduction and treated with Dnase I to eliminate DNA contamination. The integrity and purity of RNA in all samples were verified by agarose gel electrophoresis and spectrophotometric measurements, respectively. Total RNA was reverse-transcribed to cDNA in 20 μL reaction volumes using the iScript gDNA Clear cDNA Synthesis Kit (Bio-Rad, CA, USA), and the cDNA was used as a template for qRT-PCR analysis. PCR was performed in 20 μL reaction volumes containing 10 μL SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), 1 μL of each gene specific primer (Table 1), 1 μL of cDNA template, and 7 μL of ddH2O. Reactions were conducted in a CFX96 real-time PCR system (Bio-Rad). The PCR parameters were as follows: 95°C for 3 min, 35 cycles of 95°C for 5 s, and 30 s at the Tm of each primer pair (Table 1), followed by melting curve analysis to investigate the specificity of PCR products. Every treatment included three replicates, and each reaction was run in triplicate.
Table 1. Primer sequences used in qRT-PCR.
| Gene | Accession No. | Primer Sequence (5’-3’) | Size (bp) | Tm (°C) | Efficiency (%) | R2 |
|---|---|---|---|---|---|---|
| RPS7 | AB669190 | F: CGCCAACAAACAGAAGAGGC | 219 | 55.5 | 93.2 | 0.997 |
| R: CGCCCCGTAAGCTTCTTGTA | ||||||
| RPS13 | GQ222274 | F: ACTGACTGCTGATGATGTGAAGG | 77 | 57.5 | 106.3 | 0.996 |
| R: TGACACCGATTTGGGAGGG | ||||||
| 18S | FJ875999. | F: CGGCGACGCATCTTTCAA | 85 | 56.8 | 105.7 | 0.997 |
| R: TTCCCCGTTACCCGTGACA | ||||||
| GAPDH | HM055756 | F: AAAATCTCCGTCCTCTCCGA | 140 | 55.8 | 109.9 | 0.990 |
| R: ACCTTCTTGGCACCACCCT | ||||||
| ACT | GQ856238 | F: AACTTCCCGACGGTCAAGTCAT | 168 | 60 | 104.6 | 0.996 |
| R: TGTTGGCGTACAAGTCCTTACG | ||||||
| TUB | EU100016 | F: CGGTAATGCCTGCTGGGAA | 118 | 54.3 | 94.0 | 0.998 |
| R: CTCGCTGAAGAAGGTGTTGAA | ||||||
| RPL32 | AB669190 | F: GTGAAAAAGCGGACGAAAAGA | 187 | 53 | 95.2 | 0.987 |
| R: GGAAACCATTGGGCAGCATA | ||||||
| EF-1α | KR869785 | F: CTCCACTGAGCCCCCATACA | 128 | 59.4 | 108.4 | 0.994 |
| R: CTCCGTGCCAGCCAGAAAT |
Statistical analysis
The raw Ct values were obtained using CFX Manager 3.1 (Bio-RAD). The comparative ΔCt method [14], BestKeeper [27–28], NormFinder [29], and geNorm [13] were used to evaluate the stability of the eight candidate reference genes in all treatments. RefFinder (http://150.216.56.64/referencegene.php) was used to estimate and screen the most suitable reference genes by combining the results of the four methods [30]. To obtain the optimal number of reference genes for normalization, geNorm utilizes the V-value (0.15) to decide whether additional reference genes are necessary. If the V-value exceeds 0.15, three reference genes are required for normalization [13]. The analysis procedures followed the instructions provided with the algorithms. For transcriptional analysis, fold-changes in mRNA expression profiles of Mshsp90 were evaluated using the 2-ΔΔct method [31]. One-way ANOVA was used to detect significances in Mshsp90 expression levels between treatments, followed by a Duncan's multiple range with P ≤ 0.05. All procedures were performed using Data Processing System (DPS) software [32].
Results
Primer specificity, efficiency and Ct values
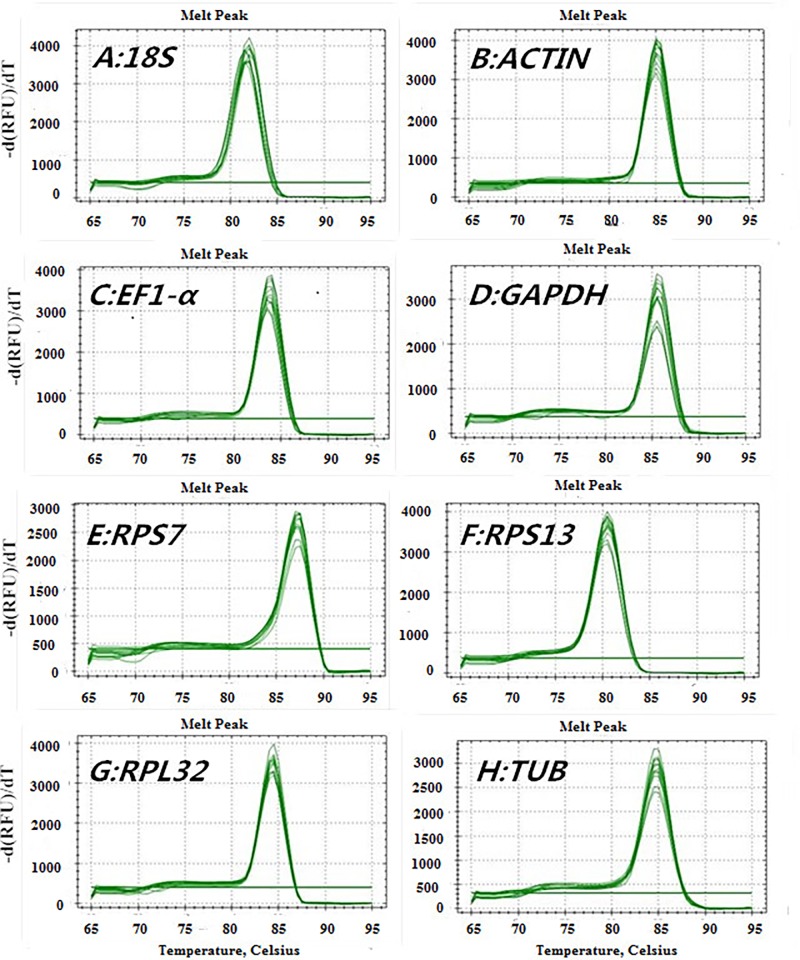
The eight candidate reference genes were amplified as single bands of the predicted size in 1.5% agarose gels (data not shown). Gene-specific amplification was confirmed by a single peak in melting-curve analysis (Fig 1). Furthermore, the amplification efficiency of all eight genes ranged from 93.2% to 109.9% with correlation coefficients (R2) of 98.7% and above (Table 1).
Fig 1. Melting curve analysis of eight candidate reference genes.
Genes included: (A) 18S, (B) ACT, (C) EF1-α, (D) GAPDH, (E) RPS7, (F) RPS13, (G) RPL32 and (H) TUB.
To analyze the expression profiles of the eight candidate reference genes, Ct values were calculated for each treatment and all samples, respectively. As shown in Fig 2, Ct values of eight candidate reference genes exhibited relatively different variation for each treatment. Totally, the mean Ct values of the eight genes was less than 25; the lowest and highest mean Ct values were 13.20 for 18S and 24.59 for GAPDH, respectively (Fig 2F).
Fig 2. The cycle threshold (Ct) values of eight candidate reference genes.
The filled circles indicate the mean Ct values for a given reference gene, and vertical bars represent the standard deviation (SD) of the mean.
Expression in different developmental stages and tissues
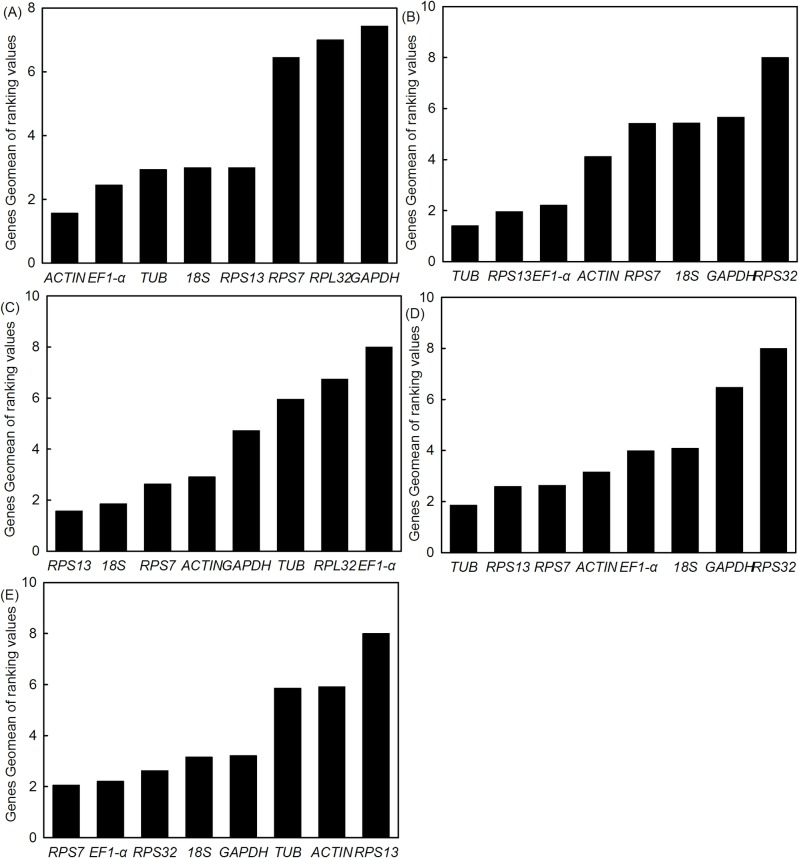
The stability ranking of the eight different reference genes varied widely among the four methods. The ΔCt and geNorm tools identified ACT as the most stable gene, but BestKeeper and NormFinder identified TUB and 18S as most stable, respectively (Table 2). Three methods (ΔCt, NormFinder and geNorm) identified GAPDH as the least stable reference gene, while BestKeeper identified RPS7 as the least stable. When analyzed by RefFinder, the stability order of the reference genes among the different developmental stages was ACT>EF1-α>TUB>18S>RPS13>RPS7>RPL32>GAPDH (Fig 3A).
Table 2. Stability of reference genes in different developmental stages and insect tissues.
| Condition | Rank | Δ Ct | BestKeeper | NormFinder | geNorm | ||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | stability value | Gene | stability value | Gene | Stability value | Gene | Stability value | ||
| Dev. stage | 1.00 | ACT | 1.29 | TUB | 0.43 | 18S | 0.46 | ACT | 0.53 |
| 2.00 | RPS13 | 1.30 | ACT | 0.73 | RPS13 | 0.46 | EF-1α | 0.53 | |
| 3.00 | EF1-α | 1.31 | RPS13 | 0.82 | ACT | 0.48 | TUB | 0.75 | |
| 4.00 | 18S | 1.31 | GAPDH | 0.83 | EF1-α | 0.53 | RPS13 | 0.98 | |
| 5.00 | TUB | 1.42 | 18S | 0.84 | TUB | 0.67 | 18S | 1.07 | |
| 6.00 | RPS7 | 1.73 | EF-1α | 1.10 | RPL32 | 1.11 | RPS7 | 1.28 | |
| 7.00 | RPL32 | 1.80 | RPL32 | 1.38 | GAPDH | 1.19 | RPL32 | 1.38 | |
| 8.00 | GAPDH | 1.91 | RPS7 | 1.67 | GAPDH | 1.18 | GAPDH | 1.51 | |
| Tissues | 1.00 | TUB | 1.21 | TUB | 0.17 | RPS13 | 0.25 | TUB | 0.22 |
| 2.00 | RPS13 | 1.21 | EF1-α | 0.22 | TUB | 0.58 | EF1-α | 0.22 | |
| 3.00 | EF1-α | 1.26 | GAPDH | 0.54 | ACT | 0.66 | RPS13 | 0.81 | |
| 4.00 | ACT | 1.31 | RPS7 | 0.75 | EF1-α | 0.72 | ACT | 0.90 | |
| 5.00 | 18S | 1.45 | RPS13 | 0.80 | 18S | 0.97 | 18S | 0.97 | |
| 6.00 | RPS7 | 1.63 | ACT | 1.04 | RPS7 | 1.32 | RPS7 | 1.14 | |
| 7.00 | GAPDH | 1.73 | 18S | 1.06 | GAPDH | 1.54 | GAPDH | 1.23 | |
| 8.00 | RPS32 | 2.41 | RPL32 | 2.11 | RPS32 | 2.31 | RPS32 | 1.53 | |
Fig 3. Stability ranking of candidate reference genes as determined by RefFinder.
Lower Geomean values indicate more stable expression using RefFinder. Panels represent (A) developmental stages, (B) tissues, (C) high temperature stress, (D) larval crowding, and (E) pesticide exposure.
In different insect tissues, the ΔCt, BestKeeper and geNorm methods identified TUB as the most stable reference gene, whereas RPS13 was optimal using NormFinder (Table 2). With respect to the least stable gene, the four methods were in agreement and indicated that RPL32 had the lowest expression stability in different tissues (Table 2). According to RefFinder, the stability ranking of the eight reference genes in different insect tissues was TUB>RPS13>EF1-α>ACT>RPS7>18S>GAPDH>RPL32 (Fig 3B).
Stability of reference genes during abiotic and biotic stress
The ΔCt and NormFinder methods recommended RPS13 as the most stable reference gene in experiments involving high temperature stress; however, analysis by BestKeeper and geNorm indicated that RPS7 and 18S were most stable, respectively (Table 3). All four methods identified EF1-α as the least stable reference gene (Table 3). RefFinder analysis indicated the following stability ranking of reference genes for high temperature stress: RPS13>18S>RPS7>ACT>GAPDH>TUB>RPL32>EF1-α (Fig 3C).
Table 3. Stability of reference genes during abiotic and biotic stress.
| Conditions | Rank | Δ Ct | BestKeeper | NormFinder | geNorm | ||||
|---|---|---|---|---|---|---|---|---|---|
| Gene |
stability value | Gene |
stability value | Gene |
Stability value |
Gene |
Stability value |
||
| High temperature | 1.00 | RPS13 | 0.47 | RPS7 | 0.47 | RPS13 | 0.05 | 18S | 0.23 |
| 2.00 | 18S | 0.47 | RPS13 | 0.57 | 18S | 0.08 | ACT | 0.23 | |
| 3.00 | RPS7 | 0.49 | 18S | 0.57 | RPS7 | 0.10 | RPS13 | 0.24 | |
| 4.00 | ACT | 0.49 | EF1-α | 0.59 | ACT | 0.14 | RPS7 | 0.26 | |
| 5.00 | GAPDH | 0.55 | TUB | 0.64 | GAPDH | 0.22 | GAPDH | 0.28 | |
| 6.00 | TUB | 0.71 | ACT | 0.71 | TUB | 0.36 | RPL32 | 0.36 | |
| 7.00 | RPL32 | 0.73 | RPL32 | 0.81 | RPL32 | 0.44 | TUB | 0.45 | |
| 8.00 | EF1-α | 1.24 | GAPDH | 0.89 | EF1-α | 0.83 | EF-1α | 0.64 | |
| Larval crowding | 1.00 | TUB | 0.75 | RPS13 | 0.14 | TUB | 0.14 | EF1-α | 0.46 |
| 2.00 | RPS7 | 0.76 | 18S | 0.36 | RPS7 | 0.21 | ACT | 0.46 | |
| 3.00 | RPS13 | 0.82 | RPS7 | 0.37 | RPS13 | 0.28 | TUB | 0.53 | |
| 4.00 | ACT | 0.89 | TUB | 0.49 | 18S | 0.38 | RPS7 | 0.63 | |
| 5.00 | 18S | 0.90 | ACT | 0.66 | ACT | 0.45 | RPS13 | 0.68 | |
| 6.00 | EF1-α | 0.96 | EF-1α | 0.68 | EF1-α | 0.51 | GAPDH | 0.72 | |
| 7.00 | GAPDH | 1.02 | GAPDH | 0.73 | GAPDH | 0.59 | 18S | 0.78 | |
| 8.00 | RPS32 | 1.45 | RPL32 | 0.90 | RPL32 | 0.96 | RPL32 | 0.95 | |
| Pesticide exposure | 1.00 | EF1-α | 1.33 | GAPDH | 0.30 | EF1-α | 0.22 | RPS7 | 0.21 |
| 2.00 | RPS7 | 1.36 | RPS7 | 0.53 | RPS7 | 0.56 | RPL32 | 0.21 | |
| 3.00 | RPS32 | 1.39 | RPL32 | 0.56 | RPL32 | 0.56 | 18S | 0.65 | |
| 4.00 | 18S | 1.60 | TUB | 1.01 | 18S | 0.79 | ACT | 0.80 | |
| 5.00 | GAPDH | 1.67 | 18S | 1.20 | GAPDH | 0.86 | EF1-α | 0.94 | |
| 6.00 | TUB | 1.88 | EF1-α | 1.24 | TUB | 1.03 | GAPDH | 1.05 | |
| 7.00 | ACT | 1.93 | RPS13 | 1.32 | ACT | 1.11 | TUB | 1.20 | |
| 8.00 | RPS13 | 1.94 | ACT | 2.08 | RPS13 | 1.74 | RPS13 | 1.60 | |
In larval crowding trials, ΔCt and NormFinder recommended TUB as the most stable reference gene, whereas BestKeeper and geNorm selected RPS13 and EF1-α as most stable, respectively. All four methods identified RPL32 as the least stable reference gene (Table 3). According to RefFinder, the stability ranking of reference genes for larval crowding was TUB>RPS13>RPS7>ACT>EF1-α>18S>GAPDH>RPL32 (Fig 3D).
In the pesticide exposure experiments, ΔCt and NormFinder indicated that EF1-α was the most stable gene, but GAPDH and RPS7 showed optimal stability using BestKeeper and geNorm analysis, respectively. The ΔCt, NormFinder and geNorm methods identified RPS13 as the least stable gene, whereas BestKeeper identified ACT as least stable (Table 3). RefFinder indicated the following stability ranking in pesticide exposure trials: RPS7>EF1-α>RPL32>GAPDH>18S>TUB>RPS13>ACT (Fig 3E).
Optimal number of reference genes based on geNorm
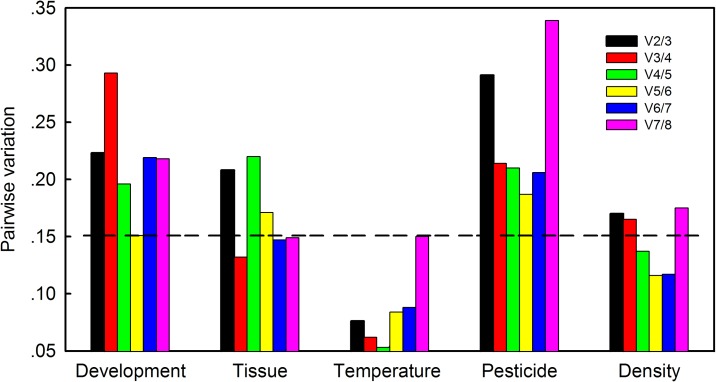
The geNorm algorithm uses mean expression stability values to determine the optimal number of reference genes for a given experimental condition. geNorm analysis of M. separata developmental stages and exposure to pesticides resulted in V-values that all exceeded 0.15 (Fig 4), indicating that three reference genes were required for reliable normalization (Table 4). With respect to different tissues of M. separata, the first value below 0.15 emerged at V3/4, suggesting that three reference genes were required for reliable normalization (Fig 4; Table 4). In high temperature experiments, all stability values were lower than 0.15, thus suggesting that two reference genes were sufficient for normalization (Fig 4; Table 4). In the larval crowding trial, the first value below 0.15 was observed at V4/5, suggesting that four reference genes were required for reliable normalization in larval density experiments (Fig 4; Table 4).
Fig 4. Optimal number of reference genes for normalization of gene expression in M. separata.
Pairwise variation (Vn/Vn+1) was determined between normalization factors (NFn and NFn+1) using the geNorm program. Values less than 0.15 indicated that additional genes were not required for normalization of gene expression.
Table 4. Recommended reference genes for different experimental conditions.
| Experimental conditions | No. reference genes | Recommended reference genes |
|---|---|---|
| Developmental stages | 3 | ACT, EF1-α and TUB |
| Tissues | 3 | TUB, RPS13 and EF1-α |
| High temperature | 2 | RPS13 and 18S |
| Larval crowding | 3 | TUB, RPS13 and RPS7 |
| Pesticide exposure | 4 | RPS7, EF1-α, RPL32 and GAPDH |
Validation of selected reference genes using Mshsp90
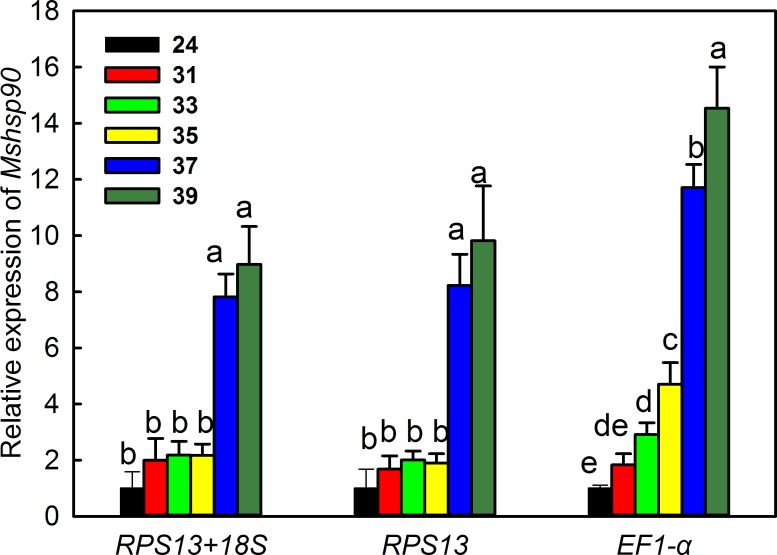
The relative expression of Mshsp90 was used to validate the recommended reference genes (RPS13 and 18S) during exposure of M. separata to high temperature stress. When RPS13 and 18S were used in combination or RPS13 was used alone to normalize the data, there was no significant difference in Mshsp90 expression from 24–35°C; however, expression showed a significant, marked increase at 37 or 39°C (RPS13+18S: F17,5 = 35.254, P = 0.0001; RPS13: F17,5 = 16.112, P = 0.0001) (Fig 5). In contrast, when the least stable reference gene, EF-1α, was used to normalize the data, Mshsp90 expression showed a significant increase in expression at 33°C and higher (F17,5 = 151.511, P = 0.0001) (Fig 5). In general, the expression of Mshsp90 was somewhat lower when the optimal reference genes (RPS13 and 18S) were used for normalizing expression as compared to the least stable gene (EF-1α) (Fig 5).
Fig 5. Relative expression of Mshsp90 during high temperature stress.
Relative expression is shown for Mshsp90 normalized with the most stable reference genes for high temperature stress (RPS13 and 18S) and the least stable gene, EF1-α. Columns represent mean expression at 24, 31, 33, 35, 37 and 39 oC, vertical bars show the SD of three replications, and columns labeled with different letters represent significant difference at P ≤ 0.05.
Discussion
qRT-PCR is a widely-used technique for quantifying transcription of target genes in expression studies, which is a critical factor in characterizing gene function [33]. However, the accuracy of target gene expression largely relies on choosing the appropriate internal control, which is commonly known as a reference or ‘housekeeping’ gene. Consequently, it is critical to validate the expression stability of reference gene(s) under different experimental treatments prior to using them to normalize gene expression.
The M. separata genome has been sequenced, and transcriptomes for insect development, exposure to stressful conditions, and migratory behavior have been described for this species [21–24]. Numerous qRT-PCR studies have been conducted with M. separata using reference genes utilized for other insect species; however, the use of inappropriate reference genes may lead to misinterpretation of data [34–35]. Therefore, in the current study we used five software programs to evaluate the expression stability of eight candidate reference genes in M. separata exposed to five different experimental conditions. The mean Ct values of the eight reference genes varied from 13.20 (18S) to 24.59 (GAPDH), and standard deviations of Ct values ranged from 1.2 (GAPDH) to 2.6 (EF1-α) (Fig 2). Collectively these results indicated that the reference gene transcripts varied in abundance and the overall expression level could varied considerably among experimental treatments. Thus, it is likely that the expression levels of target genes will also show substantial variation depending on the reference gene and experimental conditions.
With the advent of next-generation sequencing techniques, numerous studies have been conducted to identify and select reference genes in human, animal, plant and insect species [36]. In arthropods, at least 45 species have been investigated for reference gene identification and selection under diverse experimental conditions [37]. These include agricultural and urban pests [2, 7, 37–46], beneficial predators [11, 47], pollinators [48–49], and other economically important insects [2, 50]. These studies concur with our study and provide documentation that reference gene expression varies with the experimental conditions.
Our data demonstrated that four computational methods (e.g. ΔCt, BestKeeper, NormFinder, and geNorm) resulted in different stability rankings for the eight reference genes. For example, ΔCt and geNorm recommended ACT as the most stable reference gene for different stages of development, but BestKeeper and NormFinder methods recommended TUB and 18S as the most stable, respectively (Table 2). The ΔCt and NormFinder methods identified RPS13 as the best reference gene for the high temperatures, while BestKeeper and geNorm identified RPS7 and 18S as the most stable genes, respectively (Table 3). These varied results may be attributed to the different algorithms used by the four programs [51]. To address this problem, we used the online software RefFinder [30] to generate the final stability ranking. Analysis using RefFinder indicated that ACT was the most stable gene during M. separata development; TUB was the preferred reference gene in different tissues and in the larval crowding trials; and RPS13 and RPS7 were the most stable reference genes during exposure to high temperatures and pesticides, respectively (Fig 3).
ACT, an important structural protein, is expressed at various levels in lot of cell types. It is considered as the most ideal reference gene in qRT-PCR analysis. Our data indicated that ACT was the most stable gene across differenct developmental stages of M. separata, which is consitent with results found in Apis mellifera [52], Schistocerca gregaria [37], Drosophila melanogaster [53], Plutella xylostella and Chilo suppressalis[2], Chortoicetes terminifera[54], Diuraphis noxia[55] and Liriomyza trifolii [56]. However, ACT has been regarded as an unsuitable reference gene for qRT-PCR analysis of developmental stages of Frankliniella occidentalis [43], Nilaparvata lugens [57].
TUB, a type of cytoskeletal structure protein, is another commonly used reference gene. In this study, TUB was considered as the most appropriate reference gene for tissue and larval crowding studies, which is similar to results found in Locusta migratoria[58]. To the best of our knowledge, TUB has been reported unsuitable to normalize gene expression in the brain of desert locust [37] and in virus-infected planthoppers[59].
Ribosomal protein (RP), a principal component of ribosomes, involves in multiple physiologcial processes of organisms, including intracellular protein biosynthesis, DNA repair, cell differentiation, and so on [60]. Our results suggested that the RPS13 showed the most stable expression under high temperature conditions, whereas the RPS7 exhibited the most stable expression under pesticide exposure conditions. These results were similar with privious studies on different developmental stages and photoperiods conditions for Plutella xylostella[41] and tissues for feline species[61]. Recently, other memembers of RP gene family have been reported to show the most stable expression in Tribolium castaneum (RPS3: [62]), Spodoptera litura (RPS10: [7]), N.lugens (RPS11: [57]), Cimex lectularius (RPL18: [63]), Helicoverpa armigera (RPL28: [64], F.occidentalis (RPL32: [43]) and Schistocerca gregaria (RP49: [37]).
Additionally, in previous studies, a single reference gene was used to normalize expression of target genes under multiple experimental conditions. In our study, RPS7 was ranked as fairly stable under high temperature stress, larval crowding, and pesticide exposure (Fig 3C–3E); therefore, RPS7 could be used as a reference gene for these experimental conditions in M. separata.
Previous studies have demonstrated that a single reference gene is insufficient to normalize expression data and may lead to inappropriate conclusions regarding the biological function of target genes [42, 65]. Therefore, some researchers have advocated the use of multiple reference genes, and this practice has improved the expression stability of target genes [11, 42, 47]. Our results demonstrated that three reference genes were required for normalizing gene expression in M. separata developmental stages, tissue types and pesticide exposure trials; two reference genes were sufficient to normalize expression during high temperature stress; and four reference genes were needed to obtain reliable normalization during larval crowding (Fig 4).
To further validate the reference genes in M. separata, the expression of Mshsp90 was evaluated during high temperature stress. hsp90 functions as a molecular chaperone and plays multiple roles in the stress response of many organisms. Our results suggested that relative expression level of Mshsp90 was relatively constant from 24 to 35°C, but significantly up-regulated at 37 and 39°C when normalized by the recommended reference gene, RPS13 (Fig 5). A similar result was also observed when expression data were normalized using a combination of reference genes, e.g. RPS13 and 18S (Fig 5). However, when Mshsp90 expression data was normalized with the least stable reference gene, EF1-α, expression showed a significant upregulation at lower temperatures (33 and 35°C) (Fig 5). These results showed that the arbitrary selection of a reference gene may lead to misleading results regarding the function of a target gene. Therefore, the choice of optimal reference genes is essential for determining the accuracy of expression results, especially for subtle differences. To improve the accuracy of results, it may be necessary to use a panel of selected reference genes for each experimental condition.
Conclusions
In summary, we used five software programs to evaluate the expression stability of eight reference genes under different biotic and abiotic conditions. Based on our comprehensive analysis, different reference genes and combinations of reference genes should be used to normalize gene expression in M. separata subjected to different experimental conditions. Our results provide reliable normalization of qRT-PCR data and also lay a foundation for future functional studies of target gene expression in M. separata.
Acknowledgments
We would like to thank Li Yin-Shuang for help in conducting experiments and Hu Yang and Bender Carol L for language edition.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was funded by Special Fund for Agro-scientific Research in the Public Interest of China (201403031), National Natural Science Foundation of China (31601633), Natural Science Foundation of Guizhou province (20142121) and Talent Foundation of Guizhou Academy of Agricultural Science (201301).
References
- 1.Ling D, Salvaterra PM. Robust RT-qPCR data normalization: Validation and selection of internal reference genes during post-experimental data analysis. PLoS ONE. 2011; 6: e17762 doi: 10.1371/journal.pone.0017762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teng X, Zhang Z, He G, Yang L, Li F. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in four lepidopteran insects. J Insect Sci. 2012; 12: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Wang Q, Zhang B. Evaluation and selection of reliable reference genes for gene expression under abiotic stress in cotton (Gossypium hirsutum L.). Gene. 2013; 530: 44–50. doi: 10.1016/j.gene.2013.07.084 [DOI] [PubMed] [Google Scholar]
- 4.Selvey S, Thompson EW, Matthaei K, Lea RA, Irving MG, Griffiths LR. β-ACT—an unsuitable internal control for RT-PCR. Mol Cell Probe. 2001; 15: 307–311. [DOI] [PubMed] [Google Scholar]
- 5.Dhar A, Bowers RM, Licon KS, Veazey G, Read B. Validation of reference genes for quantitative measurement of immune gene expression in shrimp. Mol Immunol. 2009; 46: 1688–1695. doi: 10.1016/j.molimm.2009.02.020 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Han Z, Yan S, Mao A, Wang B, Ren H, et al. Evaluation of suitable reference gene for real-time PCR in human umbilical cord mesenchymal stem cells with long-term in vitro expansion. In Vitro Cell Dev Biol Anim. 2010; 46: 595–599. doi: 10.1007/s11626-010-9318-y [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Yuan M, Gao X, Kang T, Zhan S, Wan H, et al. Identification and validation of reference genes for gene expression analysis using quantitative PCR in Spodoptera litura (Lepidoptera: Noctuidae). PLoS ONE. 2013; 8: e68059 doi: 10.1371/journal.pone.0068059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansal R, Mittapelly P, Cassone BJ, Mamidala P, Redinbaugh MG, Michel A. Recommended reference genes for quantitative PCR analysis in soybean have variable stabilities during diverse biotic stresses. PLoS ONE. 2015; 10: e0134890 doi: 10.1371/journal.pone.0134890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann A, Lehmann R, Beckert A, Vilcinskas A, Franta Z. Selection and evaluation of tissue specific reference genes in Lucilia sericata during an immune challenge. PLoS ONE. 2015; 10: e0135093 doi: 10.1371/journal.pone.0135093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Pan H, Noland J, Zhang D, Zhang Z, Liu Y, et al. Selection of reference genes for RT-qPCR analysis in a predatory biological control agent, Coleomegilla maculata (Coleoptera: Coccinellidae). Sci Rep. 2016; 5: 18201 doi: 10.1038/srep18201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan H, Yang X, Siegfried BD, Zhou X. A comprehensive selection of reference genes for RT-qPCR analysis in a predatory lady beetle, Hippodamia convergens (Coleoptera: Coccinellidae). PLoS ONE. 2015; 10: e0125868 doi: 10.1371/journal.pone.0125868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, et al. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999; 75: 291–295. [DOI] [PubMed] [Google Scholar]
- 13.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004; 64: 5245–5250. doi: 10.1158/0008-5472.CAN-04-0496 [DOI] [PubMed] [Google Scholar]
- 14.Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 2006; 7: 33 doi: 10.1186/1471-2199-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR—a perspective. J Mol Endocrinol. 2005; 34: 597–601. doi: 10.1677/jme.1.01755 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang Z, Jiang Y, Zeng J, Gao Y, Cheng D. Preliminary analysis of the outbreak of the third-generation army worm Mythimna separata in China in 2012. Plant Prot. 2012; 38: 1–8. [Google Scholar]
- 17.Jiang Y, Li C, Zeng J, Liu J. Population dynamics of the armyworm in China: A review of the past 60 years’ research. Chin J Appl Entomol. 2014; 51: 890–898. [Google Scholar]
- 18.Jiang X, Zhang L, Cheng Y, Luo L. Novel features, occurrence trends and economic impact of the oriental armyworm, Mythimna separata (Walker) in China. Chin J Appl. Entomol. 2014; 51: 1444–1449. [Google Scholar]
- 19.Jiang X, Luo L, Zhang L, Sappington T, Hu Y. Regulation of migration in Mythimna separata (Walker) in China: a review integrating environmental, physiological, hormonal, genetic, and molecular factors. Environ Entomol. 2011; 40: 516–533. doi: 10.1603/EN10199 [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Zhang L, Chen Y, Luo L. Current status and trends in research on the oriental armyworm, Mythimna separata (Walker) in China. Chin J Appl Entomol. 2014; 51: 881–889. [Google Scholar]
- 21.Liu Y, Qi M, Chi Y, Wuriyanghan H. De novo assembly of the transcriptome for oriental armyworm Mythimna separata (Lepidoptera: Noctuidae) and analysis on insecticide resistance-related genes. J Insect Sci. 2016; 16: 92; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W. The transcriptome sequencing and gene expression analysis of migrant and resident Mythimna separata (Walker). M. Sc. Thesis, Chinese Academy of Agricultural Sciences. 2017.
- 23.Chang X, Nie X, Zhang Z, Zeng F, Lv L, Zhang S, et al. De novo analysis of the oriental armyworm Mythimna separata antennal transcriptome and expression patterns of odorant binding proteins. Comp Biochem Physiol. 2017; 22: 120–130. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Wang X, Lei C, Zhu F. Sensory genes identification with head transcriptome of the migratory armyworm, Mythimna separata. Sci Rep. 2017; 7: 46033 doi: 10.1038/srep46033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Luo L, Jiang X. Starvation influences allatotropin gene expression and juvenile hormone titer in the female adult oriental armyworm, Mythimna separata. Arch Insect Biochem Physiol. 2008; 68: 63–70. doi: 10.1002/arch.20255 [DOI] [PubMed] [Google Scholar]
- 26.Bao W, Cao B, Zhang Y, Wuriyanghan H. Silencing of Mythimna separata chitinase genes via oral delivery of in planta-expressed RNAi effectors from a recombinant plant virus. Biotechnol Lett. 2016; 38: 1961–1966. doi: 10.1007/s10529-016-2186-0 [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl MW. Quantification strategies in real-time PCR In: Bustin SA, editor. A-Z of quantitative PCR. La Jolla: International University Line; 2004. pp. 89–113. [Google Scholar]
- 28.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004; 26: 509–515. [DOI] [PubMed] [Google Scholar]
- 29.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Panepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002; 3: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol. 2012; 80: 75–84. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001; 29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Q, Zhang C. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013; 20: 254–260. doi: 10.1111/j.1744-7917.2012.01519.x [DOI] [PubMed] [Google Scholar]
- 33.Hong SY, Seo PJ, Yang MS, Xiang F, Park CM. Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant. Biol. 2008; 8: 112 doi: 10.1186/1471-2229-8-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Bioph Meth. 2000; 46: 69–81. [DOI] [PubMed] [Google Scholar]
- 35.Ferguson BS, Nam H, Hopkins RG, Morrison RF. Impact of reference gene selection for target gene normalization on experimental outcome using real-time qRT-PCR in adipocytes. PLoS ONE. 2010; 5: e15208 doi: 10.1371/journal.pone.0015208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman JR, Waldenström J. With reference to reference genes: a systematic review of endogenous controls in gene expression studies. PLoS ONE. 2015; 10: e0141853 doi: 10.1371/journal.pone.0141853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Hiel MB, Van Wielendaele P, Temmerman L, Van Soest S, Vuerinckx K, Huybrechts R, et al. Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC Mol Biol. 2009; 10: 56 doi: 10.1186/1471-2199-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nijhof AM, Balk JA, Postigo M, Jongejan F. Selection of reference genes for quantitative RT-PCR studies in Rhipicephalus (Boophilus) microplus and Rhipicephalus appendiculatus ticks and determination of the expression profile of Bm86. BMC Mol Biol. 2009; 10: 112 doi: 10.1186/1471-2199-10-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majerowicz D, Alves-Bezerra M, Logullo R, Fonseca-de-Souza AL, Meyer-Fernandes JR, Braz GR, et al. Looking for reference genes for real-time quantitative PCR experiments in Rhodnius prolixus (Hemiptera: Reduviidae). Insect Mol Biol. 2011; 20: 713–722. doi: 10.1111/j.1365-2583.2011.01101.x [DOI] [PubMed] [Google Scholar]
- 40.Li R, Xie W, Wang S, Wu Q, Yang N, Yang X, et al. Reference gene selection for qRT-PCR analysis in the sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE. 2013; 8: e53006 doi: 10.1371/journal.pone.0053006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu W, Xie W, Zhang Z, Wang S, Wu Q, Liu Y, et al. Exploring valid reference genes for quantitative real-time PCR analysis in Plutella xylostella (Lepidoptera: Plutellidae). Int J Biol Sci. 2013; 9: 792–802. doi: 10.7150/ijbs.5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues TB, Khajuria C, Wang H, Matz N, Cunha Cardoso D, Valicente FH, et al. Validation of reference housekeeping genes for gene expression studies in western corn rootworm (Diabrotica virgifera virgifera). PLoS ONE. 2014; 9: e109825 doi: 10.1371/journal.pone.0109825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Y, Li H, Lu M, Du Y. Evaluation and validation of reference genes for qRT-PCR normalization in Frankliniella occidentalis (Thysanoptera:Thripidae). PLoS ONE. 2014; 9: e111369 doi: 10.1371/journal.pone.0111369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, An S, Li Z, Wu F, Yang Q, Liu Y, et al. Identification and validation of reference genes for normalization of gene expression analysis using qRT-PCR in Helicoverpa armigera (Lepidoptera: Noctuidae). Gene. 2015; 555: 393–402. doi: 10.1016/j.gene.2014.11.038 [DOI] [PubMed] [Google Scholar]
- 45.Sun M, Lu M, Tang X, Du Y. Exploring valid reference genes for quantitative real-time PCR analysis in Sesamia inferens (Lepidoptera: Noctuidae). PLoS ONE. 2015; 10: e0115979 doi: 10.1371/journal.pone.0115979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi C, Yang F, Zhu X, Du E, Yang Y, Wang S, et al. Evaluation of housekeeping genes for quantitative real-time PCR analysis of Bradysia odoriphaga (Diptera: Sciaridae). Int J Mol Sci. 2016; 17: 1034 doi: 10.3390/ijms17071034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales MA, Mendoza BM, Lavine LC, Lavine MD. Walsh DB, Zhu F. Selection of reference genes for expression studies of xenobiotic adaptation in Tetranychus urticae. Int J Biol Sci. 2016; 12: 1129–1139. doi: 10.7150/ijbs.16319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reim T, Thamm M, Rolke D, Blenau W, Scheiner R. Suitability of three common reference genes for quantitative real-time PCR in honey bees. Apidologie. 2013; 44: 342–350. [Google Scholar]
- 49.Hornakova D, Matouskova P, Kindl J, Valterova I, Pichova I. Selection of reference genes for real-time polymerase chain reaction analysis in tissues from Bombus terrestris and Bombus lucorum of different ages. Anal. Biochem. 2010; 397: 118–120. doi: 10.1016/j.ab.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Xia Q, Cheng J, Duan J, Zhao P, Chen J, et al. Reference genes identified in the silkworm Bombyx mori during metamorphism based on oligonucleotide microarray and confirmed by qRT-PCR. Insect Sci. 2008; 15: 405–413. [Google Scholar]
- 51.Coker JS, Davies E. Selection of candidate housekeeping controls in tomato plants using EST data. Biotechniques. 2003; 35: 740–749. [DOI] [PubMed] [Google Scholar]
- 52.Scharlaken B, Graaf DC, Goossens K, Brunain M, Peelman LJ, Jacobs FJ. (2008) Reference gene selection for insect expression studies using quantitative real-time PCR: the head of the honeybee, Apis mellifera, after a bacterial challenge. J Insect Sci. 2008; 8: 33. [Google Scholar]
- 53.Ponton F, Chapuis MP, Pernice M, Sword GA, Simpson SJ. Evaluation of potential reference genes for reverse-transcription-qPCR studies of physiological responses in Drosophila melanogaster. J Insect Physiol. 2011; 57: 840–850. doi: 10.1016/j.jinsphys.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 54.Chapuis MP, Tohidi ED, Dodgson T, Blondin L, Ponton F, et al. Assessment and validation of a suite of reverse transcription-quantitative PCR reference genes for analyses of density-dependent behavioural plasticity in the Australian plague locust. BMC Mol Biol. 2011; 12(1): 7 doi: 10.1186/1471-2199-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha DK, Smith CM. Selection of reference genes for expression analysis in Diuraphis noxia (Hemiptera: Aphididae) fed on resistant and susceptible wheat plants. Sci Rep. 2014; 4 (3): 5059 doi: 10.1038/srep05059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang Y, Chen J, Lu M, Gao Y, Tian Z, Gong W, et al. Selection and validation of reference genes for quantitative real-time PCR analysis under different experimental conditions in the leafminer Liriomyza trifolii (Diptera: Agromyzidae). PLoS ONE. 2017; 12(7): e0181862 doi: 10.1371/journal.pone.0181862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan M, Lu Y, Zhu X, Wan H, Shakeel M, Zhan S, et al. Selection and evaluation of potential reference genes for gene expression analysis in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) using reverse-transcription quantitative PCR. PLoS ONE. 2014; 9: e86503 doi: 10.1371/journal.pone.0086503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Q, Li Z, Cao J, Zhang S, Zhang H, Wu X, et al. Selection and Assessment of Reference Genes for Quantitative PCR Normalization in Migratory Locust Locusta migratoria (Orthoptera: Acrididae). PLoS ONE. 2014; 9(6): e98164 doi: 10.1371/journal.pone.0098164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serazin-Leroy V, Denis-Henriot D, Morot M, De Mazancourt P, Giudicelli Y. Semi-quantitative RT-PCR for comparison of mRNAs in cells with different amounts of housekeeping gene transcripts. Mol Cell Probe. 1998; 12: 283–291. [DOI] [PubMed] [Google Scholar]
- 60.Ladror DT, Frey BL, Scalf M, Levenstein ME, Artymiuk JM, Smith LM. Methylation of yeast ribosomal protein S2 is elevated during stationary phase growth conditions. Biochem Biophys Res Commun. 2014; 445: 535–541. doi: 10.1016/j.bbrc.2014.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kessler Y, Helfer-Hungerbuehler AK, Cattori V, Meli ML, Zellweger B, Ossent P, et al. Quantitative TaqMan real-time PCR assays for gene expression normalisation in feline tissues. BMC Mol Biol. 2009;10: 106 doi: 10.1186/1471-2199-10-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lord JC, Hartzer K, Toutges M, Oppert B. Evaluation of quantitative PCR reference genes for gene expression studies in Tribolium castaneum after fungal challenge. J Microbiol Methods. 2010; 80: 219–221. doi: 10.1016/j.mimet.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 63.Mamidala P, Rajarapu SP, Jones SC, Mittapalli O. Identification and Validation of Reference Genes for Quantitative Real-Time Polymerase Chain Reaction in Cimex lectularius. J Med Entomol. 2011; 48: 947–951. [DOI] [PubMed] [Google Scholar]
- 64.Shakeel M, Zhu X, Kang T, Wan H, Li J. Selection and evaluation of reference genes for quantitative gene expression studies in cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J Asia-Pac Entomol. 2015; 18: 123–130. [Google Scholar]
- 65.de Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, Kamps WA, et al. Evidence based selection of housekeeping genes. PLoS ONE. 2007; 2: e898 doi: 10.1371/journal.pone.0000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.