PERPETUAL FLOWERING1 has a dual role regulating meristem fate after cold; it prevents flowering of new axillary branches and antagonizes inflorescence development.
Abstract
The alpine perennial Arabis alpina initiates flower buds during prolonged exposure to cold. In the accession Pajares, we demonstrate that the length of vernalization influences flowering time and inflorescence fate but does not affect the axillary branches that maintain vegetative growth. The expression of floral organ identity genes gradually increases in the main shoot apex during vernalization, correlating with an increase in floral commitment. In northern Arabidopsis (Arabidopsis thaliana) accessions, the length of vernalization modulates the stable silencing of the floral repressor FLOWERING LOCUS C (FLC). We demonstrate that expression of PERPETUAL FLOWERING1 (PEP1), the ortholog of FLC in A. alpina, is similarly influenced by the duration of the exposure to cold. Extended vernalization results in stable silencing of PEP1 in the inflorescence. In contrast, insufficient vernalization leads to PEP1 reactivation after cold treatment, which correlates with delayed flowering and the appearance of floral reversion phenotypes such as bracts and vegetative inflorescence branches. Floral reversion phenotypes are reduced in the pep1-1 mutant, suggesting that PEP1 regulates the fate of the inflorescence after vernalization. The effect of vernalization duration on stable silencing of PEP1 is specific to meristems that initiate flowering during cold treatment. Extended vernalization fails to silence PEP1 in young seedlings and axillary branches that arise from buds initiated during cold treatment, which remain vegetative. We conclude that the duration of vernalization in A. alpina differentially regulates PEP1 in the inflorescence and axillary branches. PEP1 has a dual role regulating meristem fate; it prevents meristems from flowering and antagonizes inflorescence development after vernalization.
Perennials live for many years, and most of them are capable of undergoing several reproductive events during their lifetime. In contrast to annual species, which complete their life cycle after seed set, perennials keep some meristems in a vegetative state to maintain growth from one year to the next. Therefore, the orchestration of developmental and seasonal cues that promote or repress flowering contributes to the perennial growth habit. Many perennials initiate flowering several months or years before anthesis. This growth pattern provides developmental flexibility, which is essential for the perennial life cycle and advantageous in arctic and alpine environments where spring and summer seasons are short (Diggle, 1997; Meloche and Diggle, 2001; Battey and Tooke, 2002). Preformation of flower buds can facilitate rapid anthesis after snowmelt and influences plant responses to environmental cues (Billings and Mooney, 1968; Aydelotte and Diggle, 1997).
We used the alpine perennial Brassicaceae species Arabis alpina as a model to study the effect of prolonged exposure to winter chilling temperatures on flowering and perennial traits. In nature A. alpina flowers soon after snowmelt (Toräng et al., 2015), suggesting that, similar to other alpine species, the induction of flowering and anthesis might be uncoupled. A. alpina accessions such as Pajares have an obligate vernalization requirement for flowering, although other accessions do not require vernalization (Wang et al., 2009; Albani et al., 2012). Pajares plants flower in response to vernalization only if they are grown for at least 5 weeks in long days before cold treatment, suggesting that A. alpina has a juvenile phase, during which it is not competent to flower (Wang et al., 2011; Bergonzi et al., 2013). Under controlled environmental conditions, adult Pajares plants initiate flowering during vernalization, and this transition is marked by an increase in the expression of the floral meristem identity gene AaLEAFY (AaLFY), which becomes detectable after 5 weeks of cold treatment (Wang et al., 2009). However, 7 additional weeks of vernalization are required for the formation of flower buds in the main shoot apex, suggesting that vernalization in A. alpina might also regulate other processes downstream of flowering induction (Wang et al., 2009). Vernalization fails to trigger flowering in axillary branches that arise from buds initiated during cold treatment (Wang et al., 2009; Park et al., 2017). This asynchronous development of different meristems within the same plant contributes to the perennial growth habit (Albani and Coupland, 2010; Bergonzi and Albani, 2011; Wang et al., 2011; Bergonzi et al., 2013; Park et al., 2017).
In A. alpina, flowering in response to vernalization is regulated by PERPETUAL FLOWERING1 (PEP1), the ortholog of FLOWERING LOCUS C (FLC) in the annual model species Arabidopsis (Arabidopsis thaliana; Michaels and Amasino, 1999; Sheldon et al., 2000; Wang et al., 2009). The pep1 mutants and accessions with nonfunctional alleles of PEP1 flower without vernalization (Wang et al., 2009; Albani et al., 2012; Nordström et al., 2013). Furthermore, as shown for FLC, A. alpina PEP1 expression is downregulated during vernalization. However, one key difference between the two species is the timing of flower initiation in relation to FLC/PEP1 temporal expression patterns. In Arabidopsis, flowering is initiated after vernalization; therefore, stable repression of FLC is essential for flowering to occur. In A. alpina, flowering is initiated during vernalization, when PEP1 mRNA levels are low. After vernalization, high PEP1 expression levels ensure the continuation of vegetative growth by repressing flowering in the axillary branches that arise from buds initiated during cold treatment (Wang et al., 2009). Thus, PEP1 provides a second layer of regulation to define the fate of these axillary branches after returning to warm temperatures, in addition to the age-dependent flowering pathway. This role of PEP1 in maintaining vegetative growth is also supported by the phenotype of pep1-1, in which all axillary branches flower (Wang et al., 2009). PEP1 expression pattern resembles that of FLC in extreme northern Arabidopsis accessions, in which insufficient vernalization fails to stably silence FLC (Shindo et al., 2006; Li et al., 2014; Duncan et al., 2015). Interestingly, both in A. alpina and in Arabidopsis, PEP1 and FLC expression are substantially reduced after 6 weeks of vernalization (Shindo et al., 2006; Wang et al., 2009). However, the A. alpina accession Pajares and northern Arabidopsis accessions require longer cold exposure periods to accelerate flowering. In Arabidopsis, this requirement is related to the initial accumulation of the H3K27me3 repressive mark on the FLC locus, which occurs in autumn (Coustham et al., 2012; Duncan et al., 2015). In A. alpina, H3K27me3 levels on PEP1 correlate negatively with PEP1 expression in both flowering and vegetative branches. Interestingly, plants still flower, although PEP1 expression is reactivated in flower buds after 12 weeks of vernalization (Wang et al., 2009; Castaings et al., 2014; Kiefer et al., 2017).
In Arabidopsis, the floral integrators FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1) are directly repressed by FLC in the leaves and shoot apex, respectively (Helliwell et al., 2006; Searle et al., 2006; Deng et al., 2011). SOC1 is the earliest molecular marker upregulated in the shoot apical meristem at floral transition and, together with FRUITFUL (FUL), it plays a redundant role in the maintenance of the inflorescence meristem (Borner et al., 2000; Lee et al., 2000; Samach et al., 2000; Melzer et al., 2008). soc1 mutants, combined with mutations in FUL, fail to maintain the determinacy of the inflorescence, which reverts to vegetative development and produces aerial rosettes (Melzer et al., 2008). Interestingly, soc1 ful double mutants show perennial traits and an extended life cycle, suggesting that lack of inflorescence determinacy might contribute to perennial growth (Melzer et al., 2008). After floral transition, FT is expressed in the inflorescence in an FLC-dependent manner and is required to maintain floral commitment in Arabidopsis (Liu et al., 2014; Müller-Xing et al., 2014). Under short photoperiods, ft mutants produce inflorescences with reverted phenotypes containing vegetative branches with rosette-like cauline leaves (Melzer et al., 2008; Liu et al., 2014). These results support the hypothesis that flowering time and floral commitment might be regulated by the same genes.
In Arabidopsis, inflorescences show two clear zones (I1 and I2) marked by the development of different organs. The I1 zone consists of flowering inflorescence branches, and the I2 zone consists of solitary flowers. The branches in the inflorescence are produced by the basipetal activation of buds on the axils of leaf primordia initiated before floral induction (Hempel and Feldman, 1994). At the same time, flowers in the I2 zone differentiate acropetally, marking floral commitment (Hempel and Feldman, 1994). LFY and the MADS-box transcription factor APETALA1 (AP1) play an instrumental role in this process. Mutations in these meristem identity genes result in the extension of the I1 zone over the I2 zone as flowers are transformed into inflorescence branches (Irish and Sussex, 1990; Mandel et al., 1992; Weigel et al., 1992; Bowman et al., 1993; Weigel and Nilsson, 1995).
In contrast to Arabidopsis, where flower primordia acquire a floral fate very rapidly, in perennials, continuous exposure to flower-inducing stimuli is required to prevent floral reversion, where the flower itself loses floral identity and has vegetative or inflorescence features (Battey and Lyndon, 1990; Hempel et al., 1997; Battey and Tooke, 2002). Here, we show that insufficient vernalization results in floral reversion phenotypes and late flowering in A. alpina, probably because floral commitment was not achieved during cold. Floral reversion phenotypes increase with PEP1 reactivation after the return to warm temperatures, whereas extended vernalization silences PEP1 in the shoot apex and enhances the reproductive potential of the inflorescence. The achievement of a floral fate during vernalization is important for meristem behavior after the plants return to warm temperatures and to maintain stable silencing of PEP1. In contrast, in young plants and axillary vegetative branches, which do not initiate flowering during the cold period, the duration of vernalization has no influence on PEP1 expression after vernalization. Overall, our results show that PEP1 expression is regulated by the duration of vernalization, depending on whether meristems acquire floral or vegetative identity during the cold treatment.
RESULTS
The Duration of Vernalization Determines Inflorescence Fate But Has a Marginal Effect on Axillary Branches Initiated during Vernalization
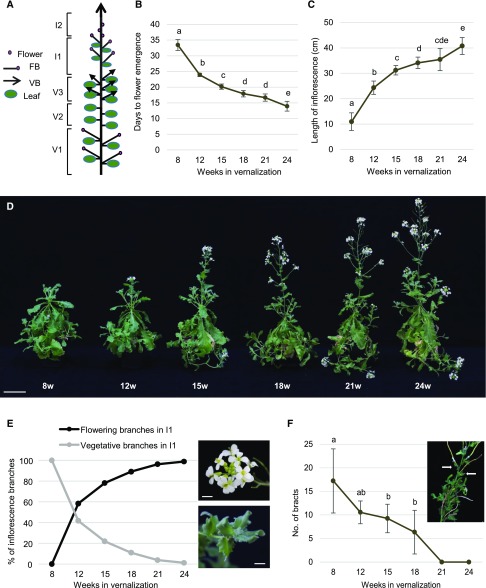
A. alpina flower buds are initiated during vernalization. After vernalization, the main shoot apex gives rise to an inflorescence stem, whereas axillary branches follow different fates depending on whether they initiated before or during cold treatment (Fig. 1A; Wang et al., 2009; Park et al., 2017). The axillary branches initiated before vernalization flower after the return to warm temperatures, similar to the main shoot apex (V1 in Fig. 1A). In contrast, the axillary branches located right below the inflorescence arise from buds initiated during vernalization and remain vegetative afterward (V3 in Fig. 1A; Wang et al., 2009; Park et al., 2017). To test the effect of the vernalization duration on flowering and bud fate (inflorescence and axillary), we subjected plants to different vernalization periods. Plants were grown for 8 weeks in long-day greenhouse conditions and vernalized for 8, 12, 15, 18, 21, or 24 weeks. Treatments were synchronized so that all plants returned to greenhouse conditions on the same day. A longer vernalization period accelerated flowering after the return to warm temperatures (Fig. 1, B and D). Plants vernalized for 24 weeks flowered within 2 weeks after being transferred to the greenhouse, whereas plants vernalized for 8 weeks required an additional 3 weeks in long days to flower. Similarly, plants exposed to longer vernalization periods bolted earlier than plants vernalized for shorter periods (Fig. 1, C and D). The length of the inflorescence was also influenced by the duration of vernalization, with prolonged vernalization resulting in longer inflorescence stems (Fig. 1C). These results suggest that extended vernalization accelerates bolting and flowering after the return to warm temperatures and that the duration of vernalization determines the final length of the inflorescence.
Figure 1.
The length of vernalization determines inflorescence outgrowth and floral reversion in A. alpina. A, Schematic representation of a flowering A. alpina plant after vernalization. Axillary branches have different fates according to their position on the plant: (V1) axillary branches that flower and partially senesce, (V2) dormant axillary buds, and (V3) axillary vegetative branches. The inflorescence consists of the I1 zone with inflorescence branches and the I2 zone with solitary flowers. The I1 branches can be distinguished from the V3 branches by the bolting of the inflorescence stem. B, Flowering time of Pajares plants vernalized for 8, 12, 15, 18, 21, or 24 weeks. C, Length of the inflorescence in Pajares plants vernalized for 8, 12, 15, 18, 21, or 24 weeks measured 8 weeks after vernalization. D, Picture of A. alpina Pajares exposed to 8, 12, 15, 18, 21, or 24 weeks of vernalization (8w, 12w, 15w, 18w, 21w, or 24w) and subsequently grown for 3 weeks in an LD greenhouse. Bar, 10 cm. E, Percentages of flowering and vegetative inflorescence branches in the I1 zone after 8, 12, 15, 18, 21, and 24 weeks of vernalization followed by 8 weeks in a LD greenhouse. Close-up pictures of flowering (top) and vegetative (bottom) I1 inflorescence branches. Bar, 0.5 cm. F, Number of bracts in the I2 zone of plants exposed to 8, 12, 15, 18, 21, and 24 weeks of vernalization followed by 14 weeks in a LD greenhouse. Close-up picture of the bracts in the inflorescence of plants exposed to 12 weeks of vernalization followed by 14 weeks in a LD greenhouse. Bar, 3 cm. Arrows indicate two bracts as an example. The error bars represent the SD; n = 9–12. Letters indicate significant differences determined by omnibus Kruskal-Wallis test followed by pairwise multiple comparison using Mann-Whitney U test (α-value of 0.05). No letters indicate no significant differences.
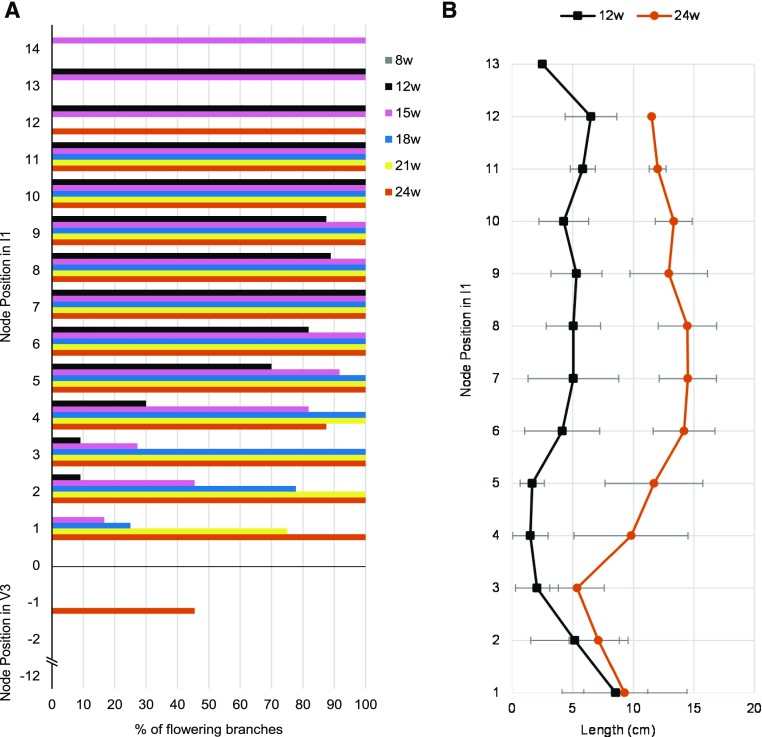
Plants vernalized for 12 weeks or longer produced an inflorescence showing the typical I1 and I2 zonation described in Arabidopsis. Interestingly, I1 inflorescence branches either flowered or stayed vegetative (Fig. 1D). The total number of inflorescence branches in the I1 zone varied according to the duration of vernalization (Supplemental Fig. S1). In addition, the ratio between flowering and vegetative I1 inflorescence branches depended on the duration of vernalization (Fig. 1E). Extended vernalization resulted in an increased frequency of I1 branches that produced flowers and, therefore, a reduction in the number of I1 inflorescence branches that remained vegetative (Fig. 1E). Nevertheless, the commitment of I1 inflorescence branches to flowering showed a similar pattern of growth as in Arabidopsis (Hempel and Feldman, 1994). Inflorescence branches committed basipetally to floral identity according to the duration of vernalization (Fig. 2A; Supplemental Fig. S2). Similarly, the length of the I1 branches increased with vernalization duration and showed a basipetal pattern of growth with higher branches being longer than lower branches (Fig. 2B). In plants vernalized for 8 weeks, inflorescences were abnormal, and occasionally single flowers reverted to inflorescence branches (Supplemental Fig. S3; Wang et al., 2009). Inflorescences remained vegetative even after plants were grown under long days (LDs) for 5 months and showed delayed senescence (Supplemental Fig. S3). A prominent feature of short vernalization periods was the presence of bracts in the axils of solitary flowers in the I2 zone, which is also a phenotype associated with floral reversion (Müller-Xing et al., 2014). Plants vernalized for 21 weeks or longer did not develop bracts, suggesting that prolonged vernalization reduced the vegetativeness of the inflorescence (Fig. 1F). Consequently, the number of siliques produced in the inflorescences increased according to the vernalization period. In general, plants vernalized for less than 15 weeks produced a reduced number of siliques compared to plants exposed to longer vernalization (Supplemental Fig. S4). Overall, our results suggest that at least 15 weeks of vernalization are required for inflorescences to reach their reproductive potential.
Figure 2.
The duration of vernalization determines the commitment to floral identity of inflorescence and V3 branches in a basipetal pattern. A, Ratio of flowering branches after different periods of vernalization (8, 12, 15, 18, 21, and 24 weeks). Nodes are numbered from the bottom of the inflorescence. Positive values represent the I1 inflorescence zone. Negative values represent the V3 zone. The basis of the inflorescence bolted stem is indicated by zero (0). B, Mean length of inflorescence branches at consecutive nodes along the inflorescence in plants vernalized for 12 or 24 weeks followed by 8 weeks in a LD greenhouse. The error bars represent the SD; n = 11–12.
In contrast to what was observed in the main stem, the duration of vernalization had a minor influence on the fate of the axillary branches initiated during vernalization. In some plants, only after 24 weeks of vernalization did the V3 upper branch commit to reproductive development (Fig. 2A; Supplemental Fig. S2). Taken together, these results show that the duration of vernalization influences the architecture and the fate of the inflorescence but has a marginal effect on those axillary buds initiated during cold treatment.
Floral Commitment after Extended Vernalization Correlates with Changes in the Expression of Meristem Identity Genes
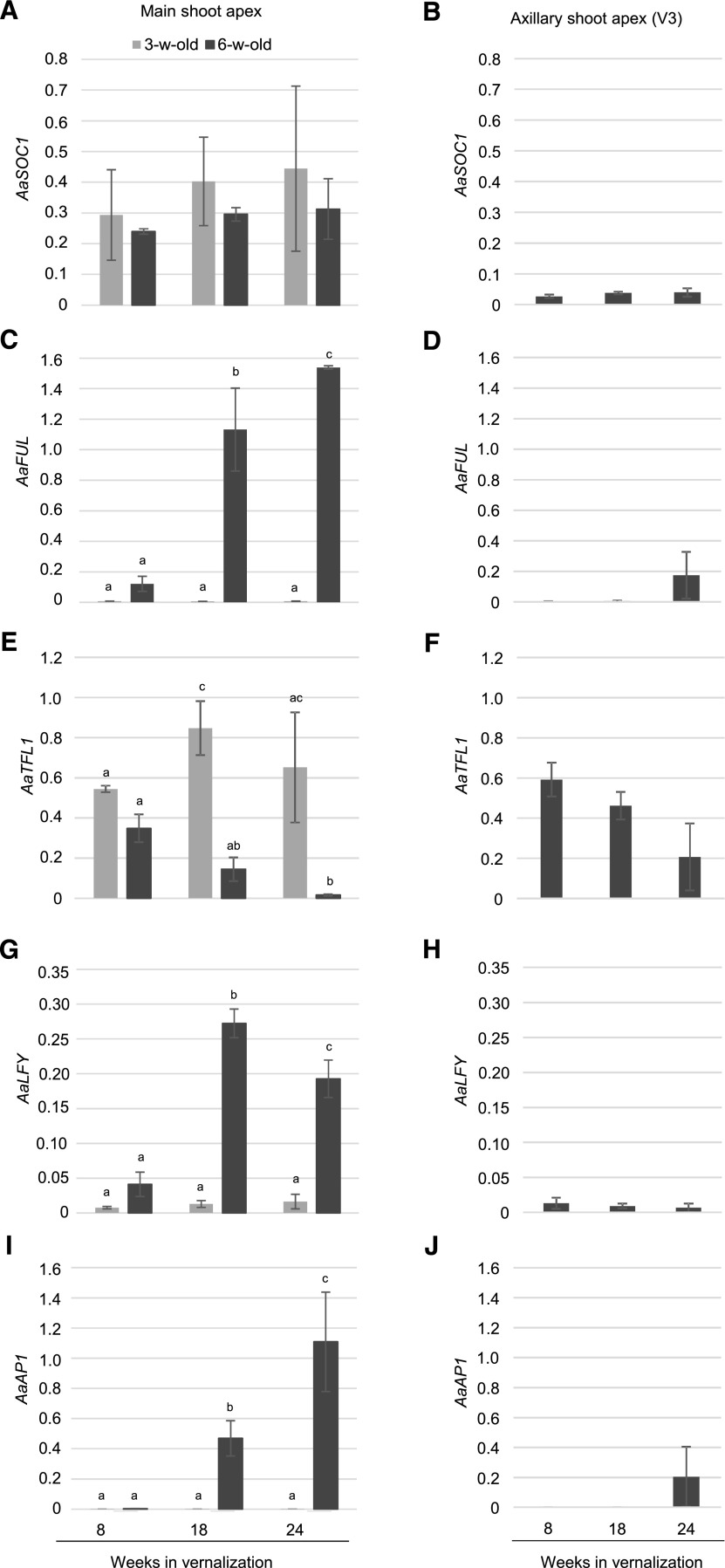
The floral reverting phenotypes observed after short vernalization indicated that floral commitment had not been achieved during cold treatment. We measured the expression of SOC1, FUL, LFY, AP1, and TFL1 orthologs in apices of 3- and 6-week-old plants after different vernalization periods. Three-week-old plants of the A. alpina accession Pajares are not competent to flower in response to cold and remain vegetative during and after vernalization, whereas 6-week-old plants initiate flowering during vernalization (Wang et al., 2011; Bergonzi et al., 2013; Park et al., 2017). Apices of 3-week-old plants are composed of the vegetative shoot apical meristem and leaf primordia. Apices of 6-week-old plants are composed of I1, I2, and V3 initials as well as leaf primordia. AaSOC1 mRNA levels increased similarly in all apices regardless of whether they were reproductive or not at the end of the vernalization treatment (Fig. 3A; Wang et al., 2011). In contrast, AaFUL, AaLFY, AaAP1, and AaTFL1 were differentially expressed between flowering and vegetative apices. AaFUL, AaLFY, and AaAP1 showed higher expression in flowering apices after prolonged vernalization, whereas AaTFL1 mRNA levels were gradually reduced specifically in flowering apices (Fig. 3, C, E, G, and I). Interestingly, AaAP1 expression was barely detectable after 8 weeks of cold treatment, suggesting that floral commitment was not achieved.
Figure 3.
The transcripts of floral meristem identity genes accumulate in the apices of 6-week-old plants that initiate flowering during cold treatment. A, C, E, G, and I, Expression level of AaSOC1, AaFUL, AaTFL1, AaLFY, and AaAP1 in the shoot apical meristem of 3- and 6-week-old Pajares plants at the end of different periods of vernalization. Three- or six-week-old plants were vernalized for 8, 18, or 24 weeks. Apices were harvested at the end of the vernalization treatments. B, D, F, H, and J, Expression level of AaSOC1, AaFUL, AaTFL1, AaLFY, and AaAP1 in V3 axillary branches. Six-week-old plants were vernalized for 8, 18, or 24 weeks and transferred to a LD greenhouse for 5 weeks. The data are the means of two or three biological replicates, and error bars represent the SD. Letters above the columns indicate significant differences determined by multiple pairwise comparisons using Benjamini-Hochberg-corrected P values (α-value of 0.05). Graphs with no letters indicate no significant differences.
In V3 axillary branches the expression of AaSOC1, AaFUL, AaLFY, and AaAP1 was very low, while AaTFL1 expression was high after all cold treatment periods (Fig. 3, B, D, F, H, and J). Our results suggest that floral commitment in A. alpina is marked by the accumulation of AaFUL, AaLFY, and AaAP1 mRNA in the shoot apical meristem at the end of vernalization.
Extended Vernalization Prevents Reactivation of PEP1 mRNA in Meristems That Achieved Flowering Identity during Vernalization
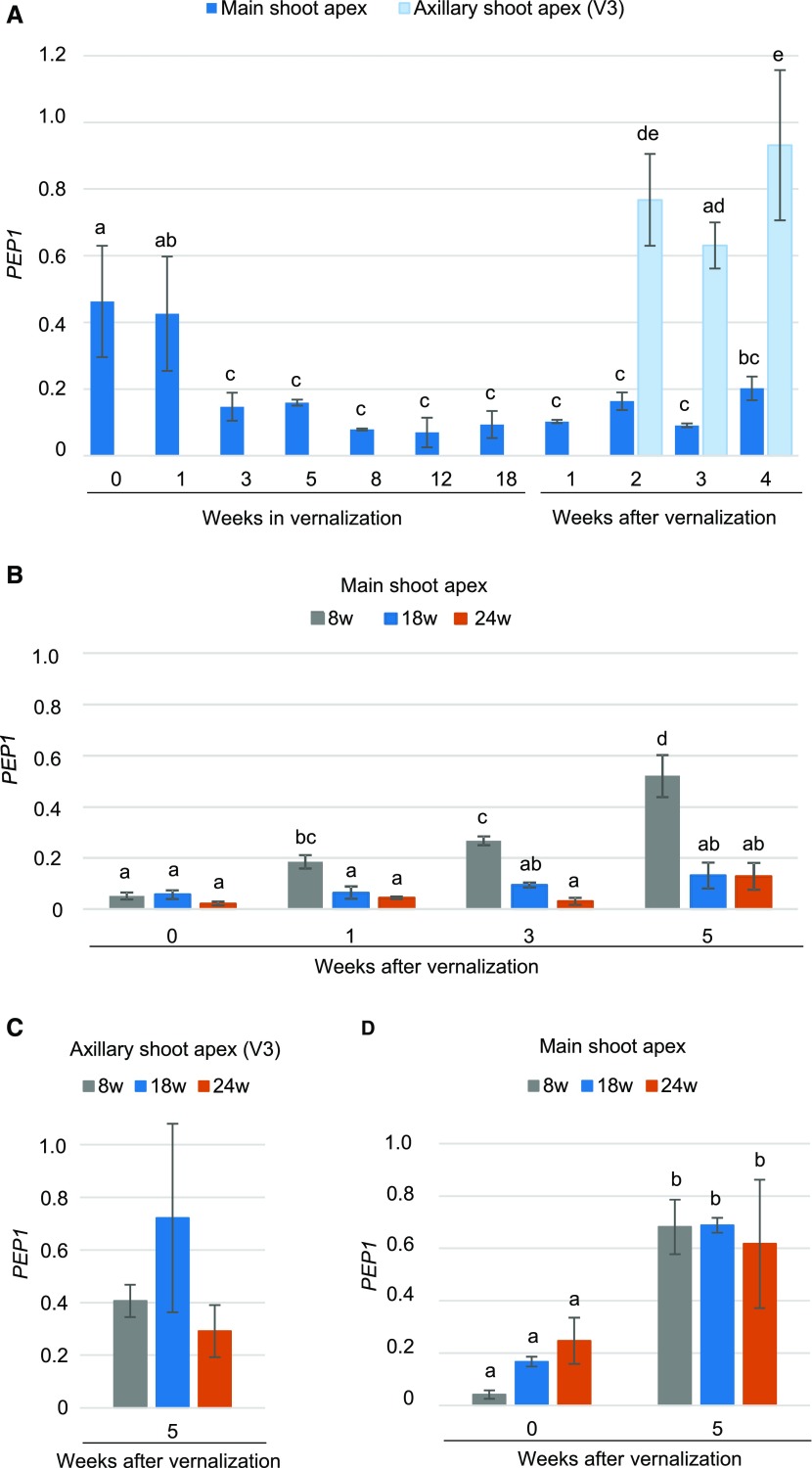
The effect of different vernalization periods on flowering in A. alpina resembled those of northern Arabidopsis accessions such as Lov-1, in which longer vernalization is required to stably silence FLC (Shindo et al., 2006; Finnegan and Dennis, 2007; Angel et al., 2011; Li et al., 2014; Duncan et al., 2015). To check whether the effect of extended vernalization on flowering may be related to the stable silencing of PEP1, we measured PEP1 mRNA levels in apices of plants exposed to 18 weeks of cold. In the main shoot apex, PEP1 expression was downregulated during vernalization and remained low after plants returned to warm temperatures (Fig. 4A). This result is in contrast to previous studies, in which A. alpina plants vernalized for 12 weeks, showed a reactivation of PEP1 mRNA in the main shoot apex after the return to warm temperatures (Wang et al., 2009; Castaings et al., 2014; Kiefer et al., 2017).
Figure 4.
Extended vernalization silences PEP1 in the inflorescence. A, PEP1 expression in the main shoot apex of 6-week-old plants before (0), during (1, 3, 5, 8, 12, and 18 weeks), and after 18 weeks of vernalization. After vernalization, main shoot apices were harvested 1, 2, 3, and 4 weeks after the plants were transferred back to a LD greenhouse. PEP1 expression was also measured in the apices of axillary V3 branches after vernalization. B, PEP1 expression at the end of vernalization in the apices of plants vernalized for 8, 18, and 24 weeks (0 indicates expression at the end of vernalization) and after vernalization (1, 3, and 5 weeks in a LD greenhouse). C, PEP1 expression in the apices of axillary V3 branches vernalized for 8, 18, or 24 weeks and transferred back to a LD greenhouse for 5 weeks. D, PEP1 expression in the main shoot apex of 3-week-old seedlings at the end of 8, 18, or 24 weeks of vernalization (0 indicates expression at the end of vernalization) and after 5 weeks in a LD greenhouse. Letters above the columns indicate significant differences determined by multiple pairwise comparisons using Benjamini-Hochberg-corrected P values (α-value of 0.05) and Hochberg-GT2. Graphs with no letters indicate no significant differences. All data are the means of two or three biological replicates, and error bars represent the SD.
To determine whether differences in growth conditions may explain this discrepancy, we compared PEP1 mRNA levels after short (8 weeks) and extended (18 and 24 weeks) vernalization. As expected, 8 weeks of chilling temperatures were sufficient to ensure full repression of PEP1, such that at the end of all vernalization treatments tested, PEP1 mRNA levels in the main shoot apex were low (Fig. 4, A and B; Wang et al., 2009). In agreement with our previous observations, the duration of the vernalization affected the silencing of PEP1 (Fig. 4, A and B). Vernalization treatments of 18 and 24 weeks achieved stable silencing of PEP1, whereas after 8 weeks of vernalization, PEP1 mRNA levels increased 10-fold in the shoot apex (Fig. 4, A and B). These data are in agreement with previous results showing the influence of vernalization length on the expression of FLC in Arabidopsis and FLC orthologs in other Brassicaceae species (Shindo et al., 2006; D’Aloia et al., 2008; Irwin et al., 2016). However, in A. alpina the up-regulation of PEP1 after 8 weeks of vernalization correlated with the appearance of a vegetative-like inflorescence, suggesting that differential PEP1 silencing after different vernalization periods might contribute to floral reversion (Supplemental Fig. S3).
To check whether extended vernalization also silences PEP1 in the axillary branches that continue vegetative growth (V3 in Fig. 1A), we measured PEP1 mRNA levels in the apices of the V3 branches after vernalization. All plants showed high levels of PEP1 transcript 5 weeks after they were returned to warm temperatures, indicating that the duration of vernalization did not affect PEP1 mRNA levels in V3 axillary branches (Fig. 4, A and C). These results suggest that the role of PEP1 in maintaining vegetative development after flowering is not affected by the length of vernalization, although 24 weeks of vernalization seem to slightly reduce the percentage of V3 branches that remain vegetative (Fig. 2B; Supplemental Fig. S2). The differential silencing of PEP1 in the main shoot apex compared to axillary branches might be due to their identity (flowering or vegetative) at the end of vernalization. To check this hypothesis, we measured PEP1 mRNA levels in main shoot apices of 3-week-old seedlings after different cold treatment periods. PEP1 expression was reactivated after vernalization in the shoot apical meristem of 3-week-old plants regardless of the duration of the cold treatment (Fig. 4D). These results suggest that PEP1 silencing after vernalization is regulated differently in meristems that either achieved floral identity or remained vegetative at the end of vernalization.
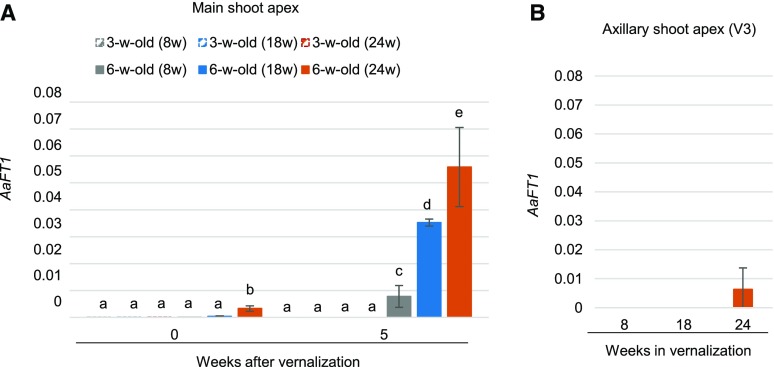
Previous studies in Arabidopsis suggested that FT, which is a target of FLC, is involved in memory of floral commitment (Melzer et al., 2008; Liu et al., 2014; Müller-Xing et al., 2014). Therefore, we compared the expression of the A. alpina FT homolog, AaFT1 (Adrian et al., 2010), in shoot apices after different periods of vernalization. At the end of vernalization, AaFT1 expression was low in all treatments tested (Fig. 5A). When plants were transferred to long days, AaFT1 mRNA was detectable after different cold treatments in flowering plants, but not in apices of 3-week-old vernalized plants or V3 branches (Fig. 5). These results suggest that AaFT1 is upregulated specifically in the apices of flowering plants after vernalization and follows an opposite expression pattern to PEP1.
Figure 5.
AaFT1 transcript gradually accumulates in the apices of 6-week-old plants after different cold treatment periods. A, AaFT1 expression level in the shoot apical meristem of 3- and 6-week-old Pajares plants. Plants were vernalized for 8, 18, or 24 weeks (8w, 18w, and 24w), and apices were harvested at the end of each vernalization treatment (0 indicates expression at the end of vernalization) and 5 weeks after vernalization. B, AaFT1 expression level in the apices of V3 axillary branches vernalized for 8, 18, or 24 weeks and transferred back to a LD greenhouse for 5 weeks. Letters above the columns indicate significant differences determined by multiple pairwise comparisons using Benjamini-Hochberg-corrected P values (α-value of 0.05). Graphs with no letters indicate no significant differences. The data are the means of two or three biological replicates, and error bars represent the SD.
PEP1 Antagonizes the Commitment of the Inflorescence to Reproductive Development after Vernalization
The fact that floral reversion phenotypes after short vernalization correlated with the reactivation of PEP1 mRNA in the shoot apex hinted that PEP1 might regulate the fate of the inflorescence after vernalization. We further explored this by scoring flowering and inflorescence traits in the pep1-1 mutant after different cold treatment periods. To ensure that pep1-1 plants were not induced to flower before prolonged exposure to cold, we vernalized younger plants that were still competent to flower. Five-week-old Pajares and pep1-1 mutant plants were subjected to different vernalization periods (8, 12, 15, 18, or 21 weeks). Vernalization treatments were synchronized so that all plants returned to greenhouse conditions on the same day. pep1-1 plants flowered earlier than the wild-type irrespective of the length of vernalization but responded to different cold treatment periods (Fig. 6A; Supplemental Fig. S5). Moreover, the inflorescence stem was shorter in pep1-1 than in wild-type plants, except in those subjected to eight weeks of vernalization (Supplemental Fig. S5). Taken together, these data show that PEP1 represses flowering and inflorescence outgrowth in A. alpina.
Figure 6.
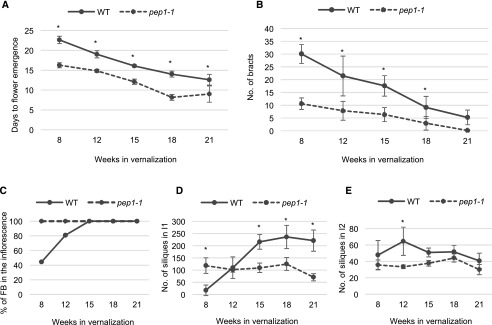
Floral reversion phenotypes are reduced in the pep1 mutant. Phenotypes were measured in wild-type Pajares (WT) and pep1-1 plants exposed to 8, 12, 15, 18, and 21 weeks of vernalization. A, Time to flower emergence; B, number of bracts; C, percentage of flowering I1 inflorescence branches (FB); D, number of siliques in the I1 branches; E, number of siliques in the I2 inflorescence zone. All measurements in B to E were performed at the end of flowering. The error bars represent the SD; n = 9–12. Asterisks indicate significant differences between wild-type and the pep1-1 mutant at each time point determined by multiple pairwise Bonferroni tests (α-value of 0.05).
Interestingly, floral reversion phenotypes were greatly reduced in pep1-1, especially after 8 weeks of vernalization, while wild-type plants showed more enhanced floral reversion (Fig. 6, B and C). The number of bracts subtending solitary flowers in the I2 zone was reduced in pep1-1 compared to the wild type for almost all vernalization lengths applied (Fig. 6B). However, the number of siliques in the I2 zone was similar between Pajares and pep1-1, suggesting that PEP1 does not influence the reproductive potential of the inflorescence in the I2 (Fig. 6E). The most significant phenotype in pep1-1 compared to the wild type was observed in the I1 zone. None of the inflorescence branches in pep1-1 remained vegetative regardless of the duration of vernalization (Fig. 6C). In addition, the number of siliques in these inflorescence branches was very similar for all vernalization durations, suggesting that PEP1 influences the silique number in the I1 zone (Fig. 6D). Nevertheless, after extended vernalization wild-type plants developed more siliques in the I1 zone than pep1-1 plants, suggesting that additional genes contribute to the reproductive potential of the inflorescence in the I1 zone.
PEP1 plays a role in defining the reproductive potential of I1 zone; therefore, we tested whether this is correlated with differential expression of PEP1 among I1 branches. We compared PEP1 expression levels in I1 branches in wild-type plants vernalized for 12 weeks, in which the lower branches in the inflorescence maintained vegetative development while the higher branches flowered (Fig. 2A; Supplemental Fig. S6A). As expected, PEP1 mRNA levels were high in vegetative branches compared to flowering I1 branches (Supplemental Fig. S6B). Similar to PEP1, AaTFL1 mRNA levels were high in vegetative branches compared to flowering I1 branches (Supplemental Fig. S6D). AaFUL and AaAP1 showed the opposite pattern, being less expressed in the vegetative branches compared to flowering branches (Supplemental Fig. S6, C and E). These results indicate that PEP1, AaTFL1, AaFUL, and AaAP1 expression correlates with inflorescence branch fate in the I1 zone. Overall, our data suggest that PEP1 antagonizes the commitment of the inflorescence to reproductive development after vernalization specifically by influencing the fate of the I1 branches.
DISCUSSION
Extended Vernalization Ensures Floral Commitment by Stably Silencing PEP1
Understanding the role of winter chilling temperatures in flowering is important for most species, regardless of whether they follow an annual, biennial, or perennial life strategy. However, low temperatures can regulate different processes depending on the plant’s life strategy. In winter annual and biennial species, cold winters are required to initiate flowering in the spring. Perennial species in temperate or alpine environments initiate flower buds several months or years before flowering; thus, cold winters are important to enhance bud break and achieve uniform flower emergence in the spring (Atkinson et al., 2013).
The quantitative effect of vernalization on flowering has been reported in several Brassicaceae species such as Sinapis alba and Arabidopsis accessions from northern latitudes, in which extended cold exposure is required to accelerate flowering (Shindo et al., 2006; D’Aloia et al., 2008; Li et al., 2014; Duncan et al., 2015). In this study, we investigated whether the duration of vernalization has an effect on flowering in the alpine perennial species A. alpina. Similar to Arabidopsis, longer vernalization gradually accelerates flowering in Pajares plants (Fig. 1). The length of vernalization determines whether PEP1 is successfully silenced after the return to warm temperatures, an effect also described for FLC in Arabidopsis (Fig. 7; Shindo et al., 2006). Thus, A. alpina Pajares plants accelerate flowering in response to vernalization in a similar way to northern Arabidopsis accessions. In both species, after insufficient vernalization, FLC and PEP1 are not stably silenced, and flowering is delayed (Fig. 7; Shindo et al., 2006; Duncan et al., 2015). pep1-1 mutant plants still respond to different cold treatment periods, suggesting that additional genes might regulate flowering in response to vernalization (Fig. 6). FLC-independent mechanisms in the vernalization pathway have also been reported in Arabidopsis as flc-null mutants also respond to vernalization (Michaels and Amasino, 2001; Schönrock et al., 2006). The ortholog of TERMINAL FLOWER1 (AaTFL1) in A. alpina was also previously shown to regulate the duration of vernalization required for flowering (Wang et al., 2011). TFL1 regulates flowering time and inflorescence architecture in Arabidopsis, as tfl1 mutants flower early and develop inflorescences, which terminate prematurely after producing a few flower buds (Ratcliffe et al., 1998). Silencing of AaTFL1 in A. alpina does not abolish the vernalization requirement to flower but allows the plants to respond to shorter cold treatment periods, such that they flower if exposed to only 5 weeks of vernalization (Wang et al., 2011). These data suggest that genes regulating the duration of vernalization required for flowering also play a role in inflorescence determinacy in A. alpina.
Figure 7.
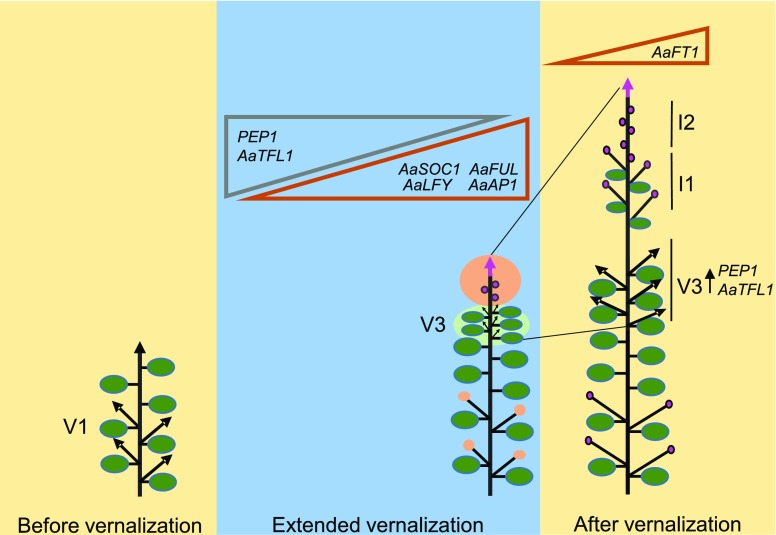
Schematic representation of a model for the genetic regulation of flowering and vegetative branches in a fully vernalized A. alpina plant. Flowering is initiated during vernalization in the apical meristem of the main shoot and in the V1 axillary branches (orange circle). V3 vegetative branches arise from buds below the inflorescence initiated during cold treatment (green circle). The expression of PEP1 and AaTFL1 is downregulated in the main shoot apical meristem, whereas the expression of AaSOC1, AaFUL, AaLFY, and AaAP1 is upregulated (triangles). After the return to LD greenhouse conditions, the I1 and I2 zones of the inflorescence develop and the V1 branches flower. PEP1 remains stably silenced in the shoot apex, and AaFT1 expression is upregulated (triangle). The length of vernalization influences the expression levels of meristem identity genes during vernalization and of PEP1 and AaFT1 after vernalization. In contrast, the V3 branches show high PEP1 and AaTFL1 expression levels irrespective of the duration of vernalization.
In this study, we show that the duration of vernalization determines inflorescence fate by regulating PEP1 stable silencing (Fig. 7 and Supplemental Fig. S6). This role of vernalization through FLC has not been demonstrated in Arabidopsis. The most interesting difference in the role and regulation of vernalization in A. alpina is the fact that flower buds are initiated during prolonged exposure to cold and not after cold as described in Arabidopsis (Wang et al., 2009; Wang et al., 2011). During vernalization, new axillary buds are also initiated in the axils of the leaves below the inflorescence meristem. Branches that arise from these axillary buds (V3 in Fig. 1A) maintain vegetative growth the following season and, therefore, sustain the perennial growth habit of A. alpina. We have previously shown that PEP1 represses flowering in these V3 axillary branches (Wang et al., 2009). Here, we tested the effect of the duration of vernalization on PEP1 expression in the apices of V3 branches. Extended vernalization has a marginal effect on the fate of V3 branches, which show high PEP1 mRNA levels irrespective of the duration of vernalization (Fig. 7). These results suggest that the length of vernalization affects PEP1 silencing specifically in the main shoot apical meristem, but not in the axillary branches that arise from buds initiated during the cold treatment. In V3 branches, it is likely that the high expression of miR156, a microRNA that promotes juvenility and represses flowering, ensures that these shoots remain vegetative (Park et al., 2017). The down-regulation of miR156 accumulation is slowed down by vernalization, but after extended vernalization, miR156 levels are reduced (Bergonzi et al., 2013), which may result in the marginal commitment of V3 branches. The duration of vernalization also increases the reproductive potential of the inflorescence, since inflorescence branches commit basipetally to flowering. After insufficient vernalization, the lower inflorescence branches remain vegetative.
Extended Vernalization Ensures Floral Commitment during Cold Treatment
Flower bud preformation is common among alpine species, as it facilitates acceleration of anthesis and successful seed set in arctic-alpine environments. We demonstrate here that flower initiation during vernalization is associated with the up-regulation of AaSOC1, AaFUL, AaLFY, and AaAP1 and the down-regulation of AaTFL1 in the shoot apex (Fig. 7). Among these genes, AP1 is considered a marker of floral commitment in Arabidopsis (Mandel and Yanofsky, 1995; Hempel et al., 1997; Liljegren et al., 1999; Wagner et al., 1999). Thus, the up-regulation of AaAP1 in the shoot apical meristem probably indicates an increase in the number of floral meristems formed in Pajares during vernalization. The extreme floral reversion phenotypes observed and the absence of detectable AaAP1 expression in the shoot apex after 8 weeks of vernalization highlight that, in A. alpina, floral commitment has to be achieved in the cold. In Arabidopsis, the MADS-box transcription factors SOC1 and FUL act redundantly to regulate the maintenance of the inflorescence meristem (Melzer et al., 2008). Our results show that AaFUL levels in the shoot apex at the end of vernalization correlate with flowering initiation. In contrast, AaSOC1 expression increases irrespective of whether the shoot meristem initiates flowering or remains vegetative during vernalization (Fig. 7). Acceleration of flowering after extended vernalization also correlates with the repression of AaTFL1 in adult plants that will flower after the cold treatment. These results suggest that the mechanisms regulating commitment to flowering in Arabidopsis are partially conserved in A. alpina.
Our data may also give insights into the effects of global warming in arctic-alpine species at the molecular level. Similar to the insufficiently vernalized Pajares plants, which have reduced seed set, ecological studies in the alpine shrub Salix herbacea have demonstrated that early spring snowmelt results in a decrease in plant reproductive output (Supplemental Fig. S3; Wheeler et al., 2016). Plants vernalized for longer than 12 weeks show a typical inflorescence architecture divided into the I1 zone with inflorescence branches and the I2 zone with solitary flowers. However, plants vernalized for 12 or 15 weeks show subtle reverting phenotypes, such as bracts in the I2 zone (Fig. 1). In Arabidopsis, the floral primordium emerges as a cryptic bract, but bracts do not outgrow due to the development of the associated floral meristem (Chandler, 2012). Thus, bracts in Arabidopsis only appear in mutants with compromised floral meristem identity (Mandel et al., 1992; Weigel et al., 1992; Chandler, 2012; Müller-Xing et al., 2014). In A. alpina, the number of bracts in the I2 zone was reduced after longer vernalization periods, following an opposite pattern to the expression of floral identity genes in the inflorescence meristem during cold treatment. These results indicate that extended vernalization in A. alpina ensures floral development during cold treatment and favors the development of the floral meristem over its subtending bract after the return to warm temperatures. In addition, longer vernalization leads to a higher percentage of flowering I1 inflorescence branches (Fig. 1). In plants vernalized for 12 weeks, the higher branches in the inflorescence commit to reproductive development, whereas the lower inflorescence branches remain vegetative. Differences in floral identity within the same inflorescence also correlate with higher expression of AaAP1 and AaFUL in flowering I1 branches compared to vegetative I1 branches (Supplemental Fig. S6). This spatial pattern of commitment to flowering within the inflorescence can also give insights into the sequence of developmental events in the inflorescence meristem. Similarly to Arabidopsis, inflorescence branches in A. alpina commit basipetally to floral identity (Fig. 2; Hempel and Feldman, 1994). The length of inflorescence branches also shows a basipetal pattern, with higher branches being longer than lower branches. These results suggest that flowering in A. alpina is a continuous process, and after flowering induction, prolonged periods of cold temperatures are required for the development of the floral meristem. Longer vernalization leads to the formation of more flower buds during cold treatment and higher expression of floral organ identity markers such as AaAP1 (Fig. 7). Thus, preformation of flower buds during cold treatment facilitates the acceleration of flowering after the return to warm temperatures. A similar requirement for saturating floral-promoting inputs before the return to warm temperatures has been reported in the cold-induced perennials Boronia megastigma and Hypocalymma angustifolium (Day et al., 1994).
Reactivation of PEP1 after Insufficient Vernalization Antagonizes Inflorescence Fate, Likely by Repressing AaFT1
Reverted inflorescences have previously been observed in other perennial Brassicaceae species, such as Arabidopsis halleri growing in nature (Aikawa et al., 2010). Interestingly, the presence of floral reversion phenotypes in A. halleri population coincided with a seasonal up-regulation of AhgFLC (Aikawa et al., 2010). In northern Arabidopsis accessions, despite the up-regulation of FLC after insufficient vernalization, floral reversion has not been demonstrated. However, constitutive expression of FLC in Columbia results in plants that flower late and have aerial rosettes in the inflorescence (Wang et al., 2007). In addition, the Arabidopsis accession Sy-0 has been reported to produce aerial rosettes, which are partially caused by high FLC expression and are abolished with vernalization (Grbić and Bleecker, 1996; Poduska et al., 2003; Wang et al., 2007). In A. alpina, we show that the up-regulation of PEP1 in the inflorescence after vernalization might counteract the commitment to flowering. Besides, AaFT1 mRNA levels in flowering apices gradually increase after vernalization (Fig. 7; Müller-Xing et al., 2014). FT is a direct target of FLC and antagonizes floral reversion in Arabidopsis (Helliwell et al., 2006; Searle et al., 2006; Liu et al., 2014; Müller-Xing et al., 2014). In A. alpina, PEP1 binds directly to AaFT1 chromatin (Mateos et al., 2017), in accordance with our results demonstrating that AaFT1 follows the opposite expression pattern to PEP1 in the shoot apex after vernalization (Fig. 7). In plants that initiate flowering during cold treatment, extended vernalization stably silences PEP1, and AaFT1 is upregulated. However, in young seedlings that do not initiate flowering during cold treatment, extended vernalization fails to silence PEP1, and AaFT1 expression is low. These results lead us to speculate that PEP1 might antagonize inflorescence fate by repressing AaFT1 after vernalization.
Feedback mechanisms that prevent PEP1 reactivation in meristems that achieved floral identity at the end of vernalization might also exist. FLC and PEP1 share targets with AP1 and another MADS-box protein, SEPALLATA3, which also promotes floral organ identity (Kaufmann et al., 2009, 2010; Deng et al., 2011; Mateos et al., 2017). Our results also contribute to the understanding of how flower initiation and maintenance is achieved in perennials, as the expression of FLC- and FT-like genes has been associated with bud break in aspen, apple, and pear trees (Böhlenius et al., 2006; Ito et al., 2016; Kumar et al., 2017). All together, our results highlight the importance of floral repressors such as PEP1/FLC, which are expressed both in vegetative and inflorescence meristems, in modulating the commitment to flowering in different types of shoots.
CONCLUSION
Our study demonstrates that extended vernalization in A. alpina enhances flowering and reduces floral reversion by ensuring flower bud development before exposure to warm temperatures. Seasonal changes in floral promoters or repressors might regulate the outgrowth of the initiated flower buds. We also show that the floral repressor PEP1 regulates the final stages of flowering by antagonizing floral commitment. Extended vernalization silences PEP1 in the inflorescence to accelerate flowering but does not affect PEP1 up-regulation in the vegetative branches ensuring the return to vegetative development after flowering.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Phenotyping
The Arabis alpina Pajares accession and the pep1-1 mutant were used for physiological analysis and expression studies. The accession Pajares was collected in the Cordillera Cantábrica mountains in Spain at 1,400 m altitude (42°59′32′′ N, 5°45′32′′ W), and pep1-1 is an Ethyl methanesulfonate-derived mutant in the Pajares background (Wang et al., 2009). To score flowering and inflorescence traits, plants were grown in an greenhouse under LD conditions (16 h light and 8 h dark) under temperatures ranging from 20°C during the day to 18°C during the night prior to vernalization. All vernalization treatments were performed at 4°C in short day conditions (8 h light and 16 h dark) and experiments were synchronized in such a way that plants vernalized for different periods were moved back to LD greenhouse (16 h light and 8 h dark) on the same day.
For the characterization of flowering and inflorescence traits in Pajares after different periods of vernalization (8, 12, 15, 18, 21, and 24 weeks), plants were grown for 8 weeks before they were vernalized. For the characterization of flowering and inflorescence traits in Pajares versus pep1-1, plants were grown for 5 weeks in LD greenhouse conditions to ensure that pep1-1 did not initiate flowering before vernalization. Flowering time was measured by recording the date in which the first flower opened. Length of the Pajares inflorescence stem was measured at eight weeks after vernalization or when the last flower in the inflorescence opened. In Pajares, the inflorescence is well defined and is always above a zone of vegetative branches that senesce after flowering. In pep1-1, the inflorescence is not well defined as the vegetative zone is missing. To indicate the beginning of the inflorescence in pep1-1, the last leaf was marked before plants entered vernalization. Inflorescence traits were measured at 8 and 14 weeks after vernalization or when the last flower in the inflorescence opened. The number of siliques was scored 8 weeks after vernalization to avoid loss of siliques or when the last flower in the inflorescence opened. The number of siliques was measured as a proxy for yield in I1 and I2 zones separately. All experiments were performed with at least 12 plants.
Gene Expression Analysis
To follow the expression patterns of PEP1 in the main shoot apex, Pajares plants were grown for 3 or 6 weeks in long days before being vernalized for different periods (8, 18, and 24 weeks). All treatments were synchronized so that the plants were transferred to the greenhouse on the same day. Main shoot apices of 3- and 6-week-old vernalized plants were harvested at the end of the different periods of vernalization, and 1, 3, and 5 weeks after vernalization. From 6-week-old vernalized plants, axillary vegetative apices (V3 in Fig. 1A) were harvested 5 weeks after vernalization. Apices of inflorescence branches of plants vernalized for 12 weeks, which had either flowering (Node 7 in I1) or vegetative (Node 1 in I1) identity were harvested 8 weeks after vernalization. An average of 15 apices were pooled in each sample. Apices from the main shoot apex were harvested at the same time from 15 different plants. Five seedlings per pot were grown in the case of 3-week-old plants, and individual plants were grown in each pot in the case of 6-week-old plants. Samples from apices of V3 axillary branches were harvested from 10 6-week-old plants, two V3 axillary branches per plant. Samples from I1 inflorescence branches were harvested from 10 6-week-old plants grown in individual pots. Two pools were harvested simultaneously, a pool of apices in node 1 of the inflorescence and a pool of apices in node 7.
Total plant RNA was extracted using the RNeasy Plant Mini Kit (Qiagen), and a DNase treatment was performed with Ambion DNAfree-kit DNase treatment and removal (Invitrogen) to reduce any DNA contamination. Total RNA (1.5 µg) was used to synthesize cDNA through reverse transcription with SuperScript II Reverse Transcriptase (Invitrogen) and oligo dT (18) as a primer. Two microliters of a cDNA dilution (1:5) was used as the template for each quantitative PCR. The RT-qPCR was performed using a CFX96 Real-Time System (Bio-Rad) and the iQ SYBR Green Supermix detection system. Each data point was derived from two independent replicates in 3-week-old plants or three independent replicates in 6-week-old plants and is shown as mean ± s.d.m. AaRAN3 and AaPP2A were used for expression data normalization.
Primers used for RT-qPCR for PEP1, AaSOC1, AaLFY, AaTFL1, AaRAN3, and AaPP2A were described previously (Wang et al., 2009, 2011; Bergonzi et al., 2013). Primers for AaAP1 are as follows: AaAP1_F, ATGAGAGGTACTCTTACGCCGA, and AaAP1_R, GTCATCTCCAAGATAATGCCTC. Primers for AaFUL are as follows: AaFUL_F, GGATACTTGAACGCTATGATCG, and AaFUL_R, TCAACGAATCAAGATCTTCCCC. Primers for AaFT1 are as follows: AaFT1_F. GATCTAAGGCCTTCTCAAGTCCAA, and AaFT1_R, CTGTCGGAACAATATCAGCACGATA (Wang, 2007).
Statistical Analysis
Statistical analyses were performed using the R software. Data distribution for time to flower emergence, number of inflorescence branches, total number of siliques and number of siliques in I1 and I2 were checked with a Shapiro-Wilk test of normality. The Kruskal-Wallis test was employed as an omnibus test to detect significant differences as data were not normally distributed. This was followed by a post-hoc test for pairwise multiple comparisons using the Mann-Whitney U test. The type I error rate (α) was set at 0.05, and the Bonferroni P value adjustments method was used. For the pep1-1 physiological analysis, we conducted multiple pairwise Bonferroni tests (α = 0.05) to detect significant differences between Pajares and pep1-1. Here, a nonparametric test could not be conducted due to ties created during rank assignment.
To detect significant differences in gene expression, we controlled for a false discovery rate of 0.05 when conducting multiple pairwise comparisons by using Benjamini-Hochberg-corrected P values or Hochberg-GT2.
Treatments with significant differences are represented with letters or asterisks.
Accession Numbers
Sequence data used in this article can be found in the GenBank/EMBL databases under the following accession numbers: cDNA of PEP1 (FJ755930), coding sequence of AaLFY (JF436956), coding sequence of AaSOC1 (JF436957), AaAP1 (KFK41337.1), coding sequence of AaTFL1 (JF436953), AaFUL (KFK27856.1), AaFT1 (KFK41391.1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The duration of vernalization influences the number of branches in the inflorescence.
Supplemental Figure S2. Inflorescence branches in A. alpina commit basipetally to floral identity.
Supplemental Figure S3. The length of vernalization determines inflorescence reversion and senescence.
Supplemental Figure S4. Extended vernalization increases the number of siliques in the inflorescence.
Supplemental Figure S5. The pep1 mutant flowers earlier than Pajares but also responds to different periods of vernalization.
Supplemental Figure S6. PEP1 antagonizes the commitment of the inflorescence to flowering.
Supplemental Table 1. Statistical differences in Figure 4B determined by multiple pairwise comparisons using Benjamini-Hochberg corrected P values.
Acknowledgments
We thank William Hughes for helpful assistance with the statistical analysis of the data, Yan Zeng for the excellent technical assistance, Alice Vayssières and Yanhao Zhou for useful discussions and Nick Battey, Wim Soppe, and Ute Höcker for critical reading of the manuscript. We thank Eva Madrid-Herrero and George Coupland for providing the AaFUL RT-qPCR primers.
Footnotes
This work was supported by the DFG Priority Programme 1530 (Flowering time control: from natural variation to crop improvement) and the Excellence Cluster EXC1028.
Articles can be viewed without a subscription.
References
- Adrian J, Farrona S, Reimer JJ, Albani MC, Coupland G, Turck F (2010) cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell 22: 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa S, Kobayashi MJ, Satake A, Shimizu KK, Kudoh H (2010) Robust control of the seasonal expression of the Arabidopsis FLC gene in a fluctuating environment. Proc Natl Acad Sci USA 107: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani MC, Castaings L, Wötzel S, Mateos JL, Wunder J, Wang R, Reymond M, Coupland G (2012) PEP1 of Arabis alpina is encoded by two overlapping genes that contribute to natural genetic variation in perennial flowering. PLoS Genet 8: e1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani MC, Coupland G (2010) Comparative analysis of flowering in annual and perennial plants. Curr Top Dev Biol 91: 323–348 [DOI] [PubMed] [Google Scholar]
- Angel A, Song J, Dean C, Howard M (2011) A Polycomb-based switch underlying quantitative epigenetic memory. Nature 476: 105–108 [DOI] [PubMed] [Google Scholar]
- Atkinson CJ, Brennan RM, Jones HG (2013) Declining chilling and its impact on temperate perennial crops. Environ Exp Bot 91: 48–62 [Google Scholar]
- Aydelotte A, Diggle P (1997) Analysis of developmental preformation in the alpine herb Caltha leptosepala (Ranunculaceae). Am J Bot 84: 1646. [PubMed] [Google Scholar]
- Battey NH, Lyndon RF (1990) Reversion of flowering. Bot Rev 56: 162–189 [Google Scholar]
- Battey NH, Tooke F (2002) Molecular control and variation in the floral transition. Curr Opin Plant Biol 5: 62–68 [DOI] [PubMed] [Google Scholar]
- Bergonzi S, Albani MC (2011) Reproductive competence from an annual and a perennial perspective. J Exp Bot 62: 4415–4422 [DOI] [PubMed] [Google Scholar]
- Bergonzi S, Albani MC, Ver Loren van Themaat E, Nordström KJ, Wang R, Schneeberger K, Moerland PD, Coupland G (2013) Mechanisms of age-dependent response to winter temperature in perennial flowering of Arabis alpina. Science 340: 1094–1097 [DOI] [PubMed] [Google Scholar]
- Billings WD, Mooney HA (1968) The ecology of artic and alpine plants. Biol Rev Camb Philos Soc 43: 481–529 [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, Gleissner R, Wisman E, Apel K, Melzer S (2000) A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J 24: 591–599 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Alvarez J, Weigel D, Meyerowitz EM, Smyth DR (1993) Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119: 721–743 [Google Scholar]
- Castaings L, Bergonzi S, Albani MC, Kemi U, Savolainen O, Coupland G (2014) Evolutionary conservation of cold-induced antisense RNAs of FLOWERING LOCUS C in Arabidopsis thaliana perennial relatives. Nat Commun 5: 4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JW. (2012) Floral meristem initiation and emergence in plants. Cell Mol Life Sci 69: 3807–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustham V, Li P, Strange A, Lister C, Song J, Dean C (2012) Quantitative modulation of polycomb silencing underlies natural variation in vernalization. Science 337: 584–587 [DOI] [PubMed] [Google Scholar]
- D’Aloia M, Tocquin P, Périlleux C (2008) Vernalization-induced repression of FLOWERING LOCUS C stimulates flowering in Sinapis alba and enhances plant responsiveness to photoperiod. New Phytol 178: 755–765 [DOI] [PubMed] [Google Scholar]
- Day JS, Loveys BR, Aspinall D (1994) Environmental-control of flowering of Boronia megastigma (Rutaceae) and Hypocalymma angustifolium (Myrtaceae). Aust J Bot 42: 219–229 [Google Scholar]
- Deng W, Ying H, Helliwell CA, Taylor JM, Peacock WJ, Dennis ES (2011) FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc Natl Acad Sci USA 108: 6680–6685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle P. (1997) Extreme preformation in alpine Polygonum viviparum: An architectural and developmental analysis. Am J Bot 84: 154. [PubMed] [Google Scholar]
- Duncan S, Holm S, Questa J, Irwin J, Grant A, Dean C (2015) Seasonal shift in timing of vernalization as an adaptation to extreme winter. eLife 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan EJ, Dennis ES (2007) Vernalization-induced trimethylation of histone H3 lysine 27 at FLC is not maintained in mitotically quiescent cells. Curr Biol 17: 1978–1983 [DOI] [PubMed] [Google Scholar]
- Grbić B, Bleecker AB (1996) An altered body plan is conferred on Arabidopsis plants carrying dominant alleles of two genes. Development 122: 2395–2403 [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES (2006) The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J 46: 183–192 [DOI] [PubMed] [Google Scholar]
- Hempel FD, Feldman LJ (1994) Bidirectional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta 192: 276–286 [Google Scholar]
- Hempel FD, Weigel D, Mandel MA, Ditta G, Zambryski PC, Feldman LJ, Yanofsky MF (1997) Floral determination and expression of floral regulatory genes in Arabidopsis. Development 124: 3845–3853 [DOI] [PubMed] [Google Scholar]
- Irish VF, Sussex IM (1990) Function of the apetala-1 gene during Arabidopsis floral development. Plant Cell 2: 741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JA, Soumpourou E, Lister C, Ligthart JD, Kennedy S, Dean C (2016) Nucleotide polymorphism affecting FLC expression underpins heading date variation in horticultural brassicas. Plant J 87: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Saito T, Sakamoto D, Sugiura T, Bai S, Moriguchi T (2016) Physiological differences between bud breaking and flowering after dormancy completion revealed by DAM and FT/TFL1 expression in Japanese pear (Pyrus pyrifolia). Tree Physiol 36: 109–120 [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Muiño JM, Jauregui R, Airoldi CA, Smaczniak C, Krajewski P, Angenent GC (2009) Target genes of the MADS transcription factor SEPALLATA3: integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol 7: e1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Wellmer F, Muiño JM, Ferrier T, Wuest SE, Kumar V, Serrano-Mislata A, Madueño F, Krajewski P, Meyerowitz EM, et al. (2010) Orchestration of floral initiation by APETALA1. Science 328: 85–89 [DOI] [PubMed] [Google Scholar]
- Kiefer C, Severing E, Karl R, Bergonzi S, Koch M, Tresch A, Coupland G (2017) Divergence of annual and perennial species in the Brassicaceae and the contribution of cis-acting variation at FLC orthologues. Mol Ecol 26: 3437–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Gupta K, Pathania S, Swarnkar MK, Rattan UK, Singh G, Sharma RK, Singh AK (2017) Chilling affects phytohormone and post-embryonic development pathways during bud break and fruit set in apple (Malus domestica Borkh.). Sci Rep 7: 42593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, Lee JS, Kwon YM, Lee I (2000) The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev 14: 2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Filiault D, Box MS, Kerdaffrec E, van Oosterhout C, Wilczek AM, Schmitt J, McMullan M, Bergelson J, Nordborg M, et al. (2014) Multiple FLC haplotypes defined by independent cis-regulatory variation underpin life history diversity in Arabidopsis thaliana. Genes Dev 28: 1635–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta GS, Yanofsky MF (1999) Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 11: 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Farrona S, Klemme S, Turck FK (2014) Post-fertilization expression of FLOWERING LOCUS T suppresses reproductive reversion. Front Plant Sci 5: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360: 273–277 [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF (1995) A gene triggering flower formation in Arabidopsis. Nature 377: 522–524 [DOI] [PubMed] [Google Scholar]
- Mateos JL, Tilmes V, Madrigal P, Severing E, Richter R, Rijkenberg CWM, Krajewski P, Coupland G (2017) Divergence of regulatory networks governed by the orthologous transcription factors FLC and PEP1 in Brassicaceae species. Proc Natl Acad Sci USA 114: E11037–E11046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche CG, Diggle PK (2001) Preformation, architectural complexity, and developmental flexibility in Acomastylis rossii (Rosaceae). Am J Bot 88: 980–991 [PubMed] [Google Scholar]
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T (2008) Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat Genet 40: 1489–1492 [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM (2001) Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13: 935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Xing R, Clarenz O, Pokorny L, Goodrich J, Schubert D (2014) Polycomb-group proteins and FLOWERING LOCUS T maintain commitment to flowering in Arabidopsis thaliana. Plant Cell 26: 2457–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström KJ, Albani MC, James GV, Gutjahr C, Hartwig B, Turck F, Paszkowski U, Coupland G, Schneeberger K (2013) Mutation identification by direct comparison of whole-genome sequencing data from mutant and wild-type individuals using k-mers. Nat Biotechnol 31: 325–330 [DOI] [PubMed] [Google Scholar]
- Park JY, Kim H, Lee I (2017) Comparative analysis of molecular and physiological traits between perennial Arabis alpina Pajares and annual Arabidopsis thaliana Sy-0. Sci Rep 7: 13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduska B, Humphrey T, Redweik A, Grbić V (2003) The synergistic activation of FLOWERING LOCUS C by FRIGIDA and a new flowering gene AERIAL ROSETTE 1 underlies a novel morphology in Arabidopsis. Genetics 163: 1457–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ (1998) A common mechanism controls the life cycle and architecture of plants. Development 125: 1609–1615 [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Schönrock N, Bouveret R, Leroy O, Borghi L, Köhler C, Gruissem W, Hennig L (2006) Polycomb-group proteins repress the floral activator AGL19 in the FLC-independent vernalization pathway. Genes Dev 20: 1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G (2006) The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 20: 898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES (2000) The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC). Proc Natl Acad Sci USA 97: 3753–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo C, Lister C, Crevillen P, Nordborg M, Dean C (2006) Variation in the epigenetic silencing of FLC contributes to natural variation in Arabidopsis vernalization response. Genes Dev 20: 3079–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toräng P, Wunder J, Obeso JR, Herzog M, Coupland G, Ågren J (2015) Large-scale adaptive differentiation in the alpine perennial herb Arabis alpina. New Phytol 206: 459–470 [DOI] [PubMed] [Google Scholar]
- Wagner D, Sablowski RW, Meyerowitz EM (1999) Transcriptional activation of APETALA1 by LEAFY. Science 285: 582–584 [DOI] [PubMed] [Google Scholar]
- Wang R. (2007) Flowering-time control and perennialism in Arabis alpina, a perennial relative of Arabidopsis thaliana. PhD thesis. University of Cologne, Cologne, Germany [Google Scholar]
- Wang R, Albani MC, Vincent C, Bergonzi S, Luan M, Bai Y, Kiefer C, Castillo R, Coupland G (2011) Aa TFL1 confers an age-dependent response to vernalization in perennial Arabis alpina. Plant Cell 23: 1307–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Farrona S, Vincent C, Joecker A, Schoof H, Turck F, Alonso-Blanco C, Coupland G, Albani MC (2009) PEP1 regulates perennial flowering in Arabis alpina. Nature 459: 423–427 [DOI] [PubMed] [Google Scholar]
- Wang Q, Sajja U, Rosloski S, Humphrey T, Kim MC, Bomblies K, Weigel D, Grbic V (2007) HUA2 caused natural variation in shoot morphology of A. thaliana. Curr Biol 17: 1513–1519 [DOI] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69: 843–859 [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O (1995) A developmental switch sufficient for flower initiation in diverse plants. Nature 377: 495–500 [DOI] [PubMed] [Google Scholar]
- Wheeler JA, Cortes AJ, Sedlacek J, Karrenberg S, van Kleunen M, Wipf S, Hoch G, Bossdorf O, Rixen C (2016) The snow and the willows: earlier spring snowmelt reduces performance in the low-lying alpine shrub Salix herbacea. J Ecol 104: 1041–1050 [Google Scholar]