A putative protein O-fucosyltransferase in Arabidopsis facilitates pollen tube penetration through stigmatic tissue, suggesting that protein O-fucosylation events may play a role in plant reproduction.
Abstract
During pollen-pistil interactions in angiosperms, the male gametophyte (pollen) germinates to produce a pollen tube. To fertilize ovules located within the female pistil, the pollen tube must physically penetrate specialized tissues. Whereas the process of pollen tube penetration through the pistil has been anatomically well described, the genetic regulation remains poorly understood. In this study, we identify a novel Arabidopsis (Arabidopsis thaliana) gene, O-FUCOSYLTRANSFERASE1 (AtOFT1), which plays a key role in pollen tube penetration through the stigma-style interface. Semi-in vivo growth assays demonstrate that oft1 mutant pollen tubes have a reduced ability to penetrate the stigma-style interface, leading to a nearly 2,000-fold decrease in oft1 pollen transmission efficiency and a 5- to 10-fold decreased seed set. We also demonstrate that AtOFT1 is localized to the Golgi apparatus, indicating its potential role in cellular glycosylation events. Finally, we demonstrate that AtOFT1 and other similar Arabidopsis genes represent a novel clade of sequences related to metazoan protein O-fucosyltransferases and that mutation of residues that are important for O-fucosyltransferase activity compromises AtOFT1 function in vivo. The results of this study elucidate a physiological function for AtOFT1 in pollen tube penetration through the stigma-style interface and highlight the potential importance of protein O-glycosylation events in pollen-pistil interactions.
Double fertilization in angiosperms precisely orchestrates the controlled interaction between two nonmotile sperm cells, the egg cell, and the central cell, which results in the generation of a new embryo and surrounding endosperm tissues. Initial pollen grain interaction with stigmatic papillae leads to pollen grain hydration and subsequent formation of a specialized structure called the pollen tube, which serves as a vehicle to transport two sperm nuclei to the distant ovule (Palanivelu and Johnson, 2010; Beale and Johnson, 2013). The pollen tube must penetrate through the stigma-style interface (Elleman et al., 1992; Jiang et al., 2005), rapidly elongate within the transmitting tract (TT; Sessions and Zambryski, 1995), and respond to positional guidance cues that lead the pollen tube to the ovule (Palanivelu et al., 2003; Okuda et al., 2009; Takeuchi and Higashiyama, 2012, 2016; Mizukami et al., 2016). At the ovule, the pollen tube must navigate through the micropylar opening, penetrate through the synergid cell (Sandaklie-Nikolova et al., 2007), and finally rupture to release the sperm cells, in which gamete fusion follows (Sandaklie-Nikolova et al., 2007; Leydon et al., 2015).
The pollen tube must circumvent numerous physical barriers to fertilize the female gamete, and the stigma-style interface represents the first barrier encountered during this process. Ultrastructural microscopy studies of Arabidopsis (Arabidopsis thaliana) pollen-pistil interactions indicate that the elongating pollen tube penetrates through the papillar cell cuticle and cell wall and subsequently grows toward the base of this cell along the surface of the plasma membrane (Elleman et al., 1992; Jiang et al., 2005). After exiting the papillar cell, the pollen tube continues to grow through the middle lamella layer between cells in the stylar tissue and eventually enters the TT (Sessions and Zambryski, 1995; Jiang et al., 2005). While these steps are essential for successful fertilization, relatively few genes involved in pollen tube penetration have been identified, despite the fact that this process markedly alters the pollen tube transcriptome (Qin et al., 2009).
Most plant cells adhere to one another throughout the lifetime of the plant due to interactions between adjacent cell walls (Bouton et al., 2002; Durand et al., 2009; Neumetzler et al., 2012; Verger et al., 2016), but the growing pollen tube must modulate cellular adherence as it interacts with multiple cell types during pollen tube penetration. Therefore, pollen-pistil interactions represent a unique opportunity to investigate the understudied process of cell adhesion in plant systems (Chae and Lord, 2011). Very few factors regulating plant cell adhesion have been described. The disruption or structural alteration of pectic cell wall polysaccharides, which form the middle lamella between adherent cells, causes clear defects in cellular adhesion (Bouton et al., 2002; Durand et al., 2009), suggesting that pectins play an important role in plant cell adhesion. Recently, genetic analyses in Arabidopsis implicated FRIABLE1 (FRB1; Neumetzler et al., 2012) and ESMERELDA1 (ESMD1; Verger et al., 2016) as novel regulators of plant cell adhesion, based on the observations that frb1 mutants exhibit cell adhesion-related phenotypes, such as organ dissociation and separation. Furthermore, esmd1 loss-of-function mutants suppressed these phenotypes and the phenotypes of other known cell adhesion mutants (Verger et al., 2016), suggesting that FRB1 and ESMD1 play opposing roles in the regulation of plant cell adhesion. Interestingly, both genes encode predicted protein O-fucosyltransferases, which are known regulators of cell adhesion and cell-cell communication in animal systems.
In metazoan systems, cell adhesion can directly regulate cell-cell communication and cell fate through the Notch signaling cascade (Kovall et al., 2017). Notch family receptors contain a large extracellular domain composed of multiple Epidermal Growth Factor (EGF) repeat domains (Rebay et al., 1991) as well as an intracellular domain that regulates transcription in response to the extracellular domain binding cognate protein ligands, such as Serrate, Delta, and Jagged. The Notch extracellular domain EGF repeats are heavily glycosylated with Fuc-containing glycans attached to Ser/Thr residues (Okajima et al., 2003; Sasamura et al., 2003; Shi and Stanley, 2003; Rampal et al., 2005). EGF fucosylation is catalyzed by protein O-fucosyltransferase1 (POFT1), which uses GDP-Fuc as a sugar nucleotide donor to attach Fuc to protein targets in the consensus amino acid sequence CXXXX(S/T)C (Wang et al., 2001). These posttranslational glycosylation events potentiate the interaction of Notch with its cognate ligands (Okajima et al., 2003; Stahl et al., 2008). Recently, the x-ray structure of a glycosylated Notch subdomain bound to Jagged was determined, and this structure demonstrated that O-fucosylated residues participate in catch bonds that communicate mechanical information between cells (Luca et al., 2017). Protein O-fucosylation also regulates a number of other metazoan proteins that contain EGF or Thrompospondin Repeat (TSR) domains (Harris and Spellman, 1993; Leonhard-Melief and Haltiwanger, 2010). Whereas this modification plays a well-established role in the regulation of metazoan cell adhesion, it has been relatively understudied in plant systems.
In this study, we identify and characterize a novel Arabidopsis gene, At3g05320, which encodes an O-fucosyltransferase family protein that plays a critical role in pollen tube penetration through the pistil. Phylogenetic analysis showed that this gene was more closely related to metazoan POFT1s than previously described plant POFTs. Therefore, we named this gene Arabidopsis O-FUCOSYLTRANSFERASE1 (AtOFT1). Genetic analyses revealed that oft1 mutants exhibit a marked reduction in overall fertility despite the fact that oft1 pollen tubes grow normally under in vitro conditions. Further analysis revealed that oft1 mutant pollen tubes are compromised in their ability to penetrate the stigma-style interface and elongate through the TT, suggesting that AtOFT1 may play a role in modulating cell adhesion during these events.
RESULTS
Phylogenetic Relationship between Plant and Metazoan Protein O-Fucosyltransferases
To examine the phylogenetic relationship between plant and metazoan POFT1s, the full-length amino acid sequences of Mus musculus, Drosophila melanogaster, Danio rerio, Caenorhabditis elegans, and Homo sapiens POFT1 were used as BLAST queries against the Arabidopsis genome. In each case, a previously uncharacterized Arabidopsis gene (At3g05320) exhibited weak (18%–21%) amino acid sequence identity to these metazoan POFT1s. In contrast, the queried metazoan POFT1s did not identify previously characterized putative Arabidopsis POFTs, such as FRB1 (Neumetzler et al., 2012) and ESMD1 (Verger et al., 2016). To further understand the phylogenetic relationships between At3g05320, other putative Arabidopsis POFTs, and established metazoan POFT1s, a phylogenetic tree containing these protein sequences was constructed (Fig. 1). The Arabidopsis genome contains 38 proteins that are annotated as putative POFTs, in agreement with previous analyses (Neumetzler et al., 2012). At3g05320 was most phylogenetically similar to a subgroup of POFTs that included three other unidentified Arabidopsis genes (At1g53770, At1g17270, and At5g50420) and the metazoan POFT1 family. Interestingly, these protein sequences preferentially clustered with metazoan POFT1s and were only distantly related to previously identified Arabidopsis putative POFTs, suggesting that At3g05320 and its homologs are more similar to metazoan POFT1s in amino acid sequence and potentially in enzymatic function. In light of this phylogenetic similarity, the At3g05320 gene was named AtOFT1.
Figure 1.
Phylogenetic analysis of the Arabidopsis putative POFT family. A neighbor-joining phylogenetic tree was constructed based on the amino acid sequences of members of the putative Arabidopsis protein O-fucosyltransferase family as well as various metazoan POFT1 sequences. Labels at nodes represent bootstrap values based on 1,000 bootstrap trials. The clade containing metazoan POFT1 sequences and related Arabidopsis putative POFTs is colored in red.
AtOFT1 T-DNA Mutants Exhibit Altered Silique Morphology and Reduced Seed Set
Based on the observations that the AtOFT1 sequence is closely related to metazoan POFT1s and that the function of AtOFT1 had not been studied previously, a genetic approach was pursued to examine the physiological role of this gene. Three independent AtOFT1 T-DNA insertions were identified (Supplemental Fig. S1A) in the Arabidopsis T-DNA insertional mutant database (Alonso et al., 2003), and homozygous T-DNA insertions in these mutants were verified by PCR genotyping (Supplemental Fig. S1B). These insertional mutant alleles were renamed oft1-1 (SALK_072442), oft1-2 (SALK_151675), and oft1-3 (WiscDsLox489-492M4; Supplemental Fig. S1A). Reverse transcription (RT)-PCR analysis confirmed that the AtOFT1 transcript was not expressed in these mutants, suggesting that each of these T-DNA lines contain null alleles (Supplemental Fig. S1C).
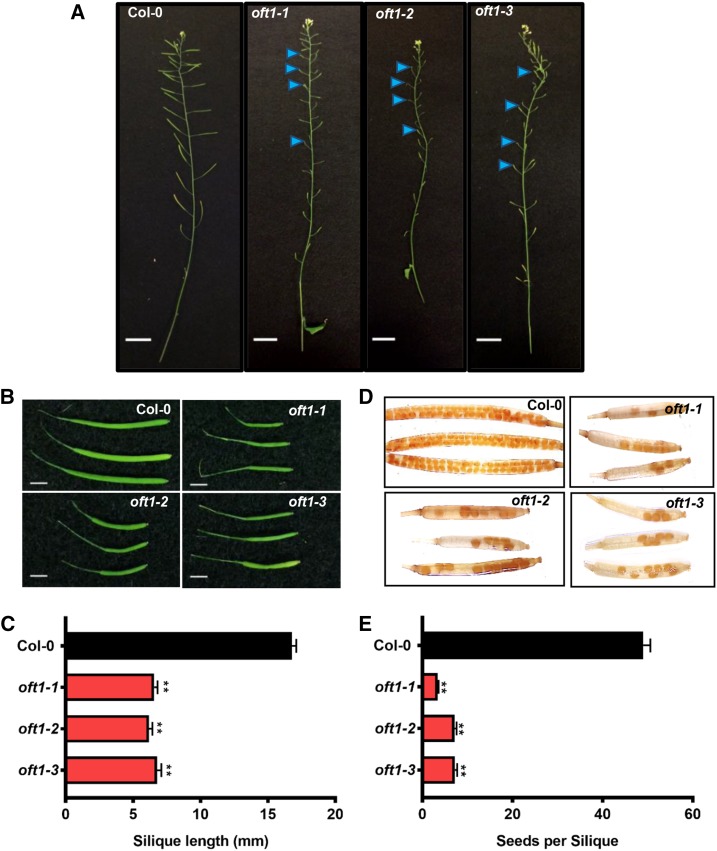
The oft1 mutant lines were phenotypically evaluated at various stages of Arabidopsis development. At the reproductive stage, oft1 mutants flowered normally but developed 65% shorter siliques compared with those of wild-type Columbia-0 (Col-0) control plants (Fig. 2, A–C). The seed set of the oft1 mutants was examined by clearing fully developed siliques and quantifying the number of seeds produced. In contrast to wild-type Col-0 control plants, which produced 45 to 50 seeds per silique, oft1 mutants produced 5- to 10-fold fewer seeds (Fig. 2, D and E), indicating that these mutants are reproductively compromised.
Figure 2.
Phenotypic characterization of oft1 mutant lines. A, Inflorescence morphology of 6-week-old oft1 mutants and wild-type Col-0 controls. Blue arrowheads indicate improperly developed siliques. Bars = 1 cm. B, Representative images of fully developed siliques harvested from 6-week-old oft1 mutant or wild-type Col-0 control plants. The genotypes of siliques are indicated in the top right corner of each image. Bars = 2 mm. C, Quantification of 6-week-old Col-0 (black bar) and oft1 mutant (red bars) silique lengths. Data are means ± se (n = 23). One-way ANOVA indicated a significant difference between Col-0 and oft1 mutants (**, P < 0.01 by Tukey’s posthoc analysis). D, Fully developed siliques of the indicated genotypes were harvested from 6-week-old plants and cleared in 70% (v/v) ethanol as described in “Materials and Methods.” Representative siliques from each genotype are shown. E, Quantification of Col-0 (black bar) and oft1 mutants (red bars) seed set. Data are means ± se (n = 12–22 siliques). One-way ANOVA indicated a significant difference between Col-0 and oft1 mutants (**, P < 0.01 by Tukey’s posthoc analysis).
The Male oft1 Gamete Is Responsible for Reduced Reproductive Success
The observed reproductive defects in oft1 mutants could result from impaired male or female gametophytes. Therefore, the transmission of oft1 mutant alleles in self-fertilization and outcross events was examined. The oft1-1 and oft1-3 alleles respectively contained functional kanamycin and BASTA resistance markers associated with their T-DNA insertions. Transmission of the oft1-2 allele was assessed by PCR genotyping. Selfing oft1+/− mutants would be expected to produce 75% progeny containing the oft1 T-DNA insertion, but oft1 mutant alleles were transmitted to only 51% (oft1-1), 47% (oft1-2), and 52% (oft1-3) of progeny (Table I). The frequency of homozygotes recovered in these selfing events also was estimated by propagating a population of the progeny and visually phenotyping the plants at the reproductive stage. Homozygous mutants were recovered at much lower frequencies than the expected 33% progeny (oft1-1, 3.7% homozygotes [n = 108]; oft1-2, 0.9% homozygotes [n = 108]; and oft1-3, 1.4% homozygotes [n = 72]), indicating that while homozygous mutants can be obtained from self-fertilization events, they are much less common than expected by Mendelian inheritance.
Table I. Segregation distortion analysis of oft1 mutant and complemented lines.
| Parents (♂ × ♀)a | #Rb | #Sb | Total | %R | TEc | χ2d | P |
|---|---|---|---|---|---|---|---|
| oft1-1+/− self | 258 | 247 | 505 | 51.1 | 1.04 | 38.5 | <0.00001 |
| oft1-2+/− self | 51 | 57 | 108 | 47.2 | 0.89 | 11.1 | 0.0009 |
| oft1-3+/− self | 369 | 328 | 697 | 52.9 | 1.13 | 45.2 | <0.00001 |
| Col-0 × oft1-1+/− | 57 | 52 | 109 | 52.3 | 1.10 | 0.11 | 0.74 |
| oft1-1+/− × Col-0 | 0 | 116 | 116 | 0 | 0 | 58 | <0.00001 |
| Col-0 × oft1-3+/− | 364 | 340 | 704 | 51.7 | 1.07 | 0.41 | 0.52 |
| oft1-3+/− × Col-0 | 1 | 1,871 | 1,872 | 0.05 | 5.3 × 10−4 | 934 | <0.00001 |
| Col-0 × oft1-3−/−; 11p::OFT1-GFP+/−e | 84 | 102 | 186 | 45.2 | 0.82 | 0.87 | 0.35 |
| oft1-3−/−; 11p::OFT1-GFP+/− × oft1-3−/−e | 494 | 0 | 494 | 0 | ND | 247 | <0.00001 |
| Col-0 × oft1-3+/−; 11p::OFT1-GFP+/−e | 161 | 158 | 319 | 50.5 | 1.02 | 0.014 | 0.91 |
| oft1-3+/−; 11p::OFT1-GFP+/− × Col-0e | 771 | 1,688 | 2,459 | 31.4 | 0.46 | 171.0 | <0.00001 |
The parent lines of each cross are indicated. Transgenic lines containing the 11p::OFT1-GFP construct are abbreviated as 11p::OFT1-GFP with the indicated genotype.
The total number of resistant (#R) and sensitive (#S) individuals in each cross F1 progeny are indicated. Transmission of alleles was scored as described in “Materials and Methods”: oft1-1 (kanamycin resistance), oft1-2 (PCR genotyping), oft1-3 (BASTA resistance), and 11p::OFT1-GFP (hygromycin resistance).
Transmission efficiency (TE) was calculated as #R/#S. dχ2 was calculated based on the expectation of a 1:1 segregation of transgenes in the resulting F1 populations. eThe aggregate numbers of F1 progeny from at least three independent complemented lines are represented.
To further investigate whether this observed segregation distortion was due to defects in the male or female gametophyte, reciprocal crosses were performed using oft1+/− parents, and the transmission of mutant alleles was evaluated in the F1 progeny (Table I). The resulting F1 progeny would be expected to inherit the oft1 allele at a frequency of 50%, and when oft1+/− pistils accepted Col-0 pollen, 52% (oft1-1) and 51% (oft1-3) of the resulting progeny exhibited herbicide resistance (Table I). In contrast, when oft1+/− pollen was used to fertilize Col-0 pistils, only one transmission event was detected out of 1,872 oft1-3 F1 progeny and no transmission events were observed for oft1-1 F1 progeny, indicating a nearly 2,000-fold reduction in oft1 pollen transmission efficiency (Table I). These results suggest that oft1 mutants exhibit segregation distortion specifically attributed to a defective male gamete.
To confirm that the near-sterile phenotype of oft1 alleles was due to a loss-of-function mutation, a complementation construct was generated consisting of the AtOFT1 genomic DNA sequence fused to the N terminus of GFP under the control of the pollen tube-specific ARABINOGALACTAN PROTEIN11 (AGP11) promoter (Levitin et al., 2008). T1 oft1-3−/−; 11p::OFT1-GFP+/− and oft1-3+/−; 11p::OFT1-GFP+/− seedlings were selected on hygromcyin as described in “Materials and Methods” and propagated to the flowering stage. This construct, which is referred to as 11p::OFT1-GFP, was introduced into the oft1-3−/− and oft1-3+/− genetic backgrounds and fully or partially complemented the seed set phenotype of oft1-3−/− mutants in multiple independent transgenic lines (Supplemental Fig. S2). These plants were utilized in a variety of outcrossing experiments described below, and the resulting F1 progeny of these crosses were assayed for 11p::OFT1-GFP transmission via hygromycin resistance.
Reciprocal crosses were performed with three independent lines harboring an 11p::OFT1-GFP+/− transgene in the oft1-3−/− background (Table I). In this assay, all of the pollen were mutant, and the transmission test evaluated how well the hemizygous pollen harboring the transgene competed with pollen that do not contain the transgene. While an expected 50% transmission was observed through the female gametophyte, a nearly 100% transmission was observed through the pollen (Table I). These results indicate that oft1-3− pollen tubes containing the 11p::OFT1-GFP transgene significantly outcompete oft1-3− pollen tubes in a direct competition assay.
To measure the simultaneous transmission of wild-type, mutant, and complemented AtOFT1 pollen tubes, the transmission of oft1-3− and 11p::OFT1-GFP alleles in outcrosses between Col-0 and oft1-3+/−; 11p::OFT1-GFP+/− was evaluated. For these experiments, the BASTA resistance cassette associated with the oft1-3 T-DNA allele was utilized to evaluate transmission. When oft1-3+/−; 11p::OFT1-GFP+/− females were used with Col-0 pollen, 50% of the resulting F1 progeny inherited the oft1-3 mutant allele, consistent with the expected value of 50% (Table I). However, when oft1-3+/−; 11p::OFT1-GFP+/− pollen was used to pollinate Col-0 pistils, only 31% of the F1 progeny inherited the oft1-3 T-DNA allele, which was significantly less than the expected 50% inheritance (Table I). This result was consistent with the hypothesis that only oft1-3−; 11p::OFT1-GFP+ pollen are able to successfully compete with wild-type pollen (expected value, 33%; χ2 = 2.79, P = 0.09; Table I), suggesting that oft1-3 mutant pollen tubes could only successfully fertilize ovules if they also contained a functional complement copy of the 11p::OFT1-GFP transgene. Overall, these results provide compelling evidence to support the hypothesis that AtOFT1 plays a significant role in pollen tube physiology and that the male gametophyte is solely responsible for the reduction in oft1 fertility.
AtOFT1 Is Expressed in Pollen Tubes
To investigate whether AtOFT1 was expressed in developing pollen or other reproductive tissues, public microarray data were examined using the Pollen RCN Heat Tree expression viewer (www.arabidopsis-heat-tree.org). This analysis revealed that AtOFT1 expression was not observed in various female tissues (Supplemental Fig. S3A). However, AtOFT1 expression was observed in dry pollen, and transcript abundance increased after pollen tube germination. Interestingly, the highest AtOFT1 transcript abundance was observed in 4-h semi-in vivo germinated pollen tubes that had penetrated through dissected stigmatic tissue (Qin et al., 2009), suggesting that AtOFT1 expression increases as the pollen tube interacts with the female tissues. This analysis also revealed that At1g53770 and At5g50420, two other genes that are closely phylogenetically related to AtOFT1, exhibited very different expression profiles in reproductive tissues. At1g53770 was strongly expressed in sperm cells, with lower but significant expression in ovule tissue. At5g50420 was expressed throughout the pistil tissue but was not expressed in the male gametophyte tissues after pollen maturity, suggesting that AtOFT1-like OFTs may play distinct roles in Arabidopsis reproductive tissues due to their nonoverlapping expression patterns. To additionally confirm AtOFT1 pollen tube expression, 1,000 bp of the putative AtOFT1 promoter was fused to a GFP reporter (Supplemental Fig. S3B), and this construct was transformed into wild-type Col-0 plants. Subsequent fluorescence imaging of pollen derived from T1 transformants revealed substantial GFP signal in in vitro germinated pollen tubes (Supplemental Fig. S3, C–F), confirming that AtOFT1 is expressed in growing pollen tubes.
Subcellular Localization of AtOFT1
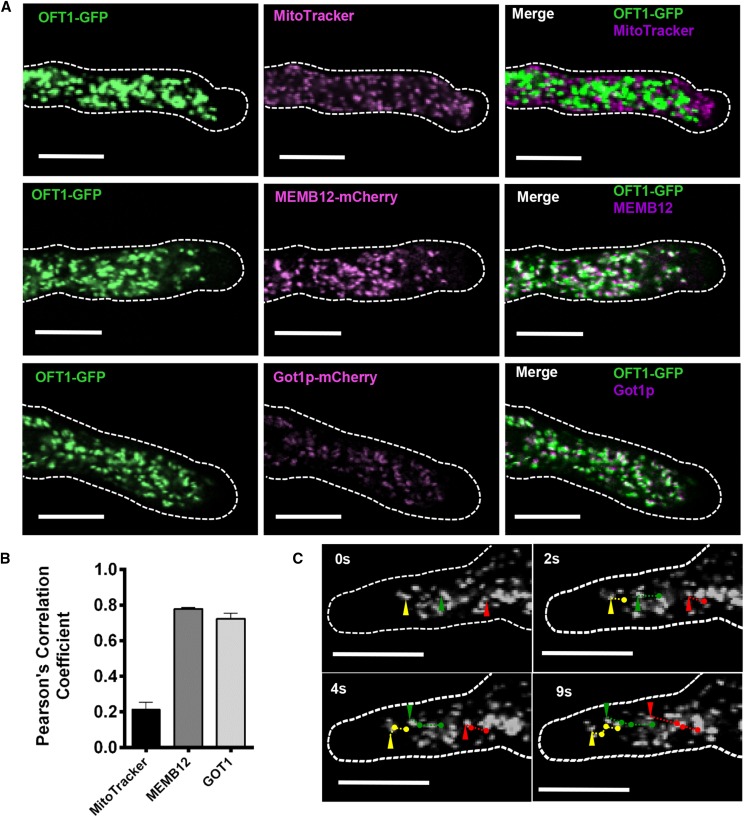
Metazoan POFTs are localized to the endoplasmic reticulum, where they modify target substrates before being further glycosylated in the Golgi apparatus (Luo and Haltiwanger, 2005). To further understand the function of AtOFT1, the previously described 11p::OFT1-GFP transgenic lines were used to investigate the subcellular localization of this protein. Pollen tubes were germinated under in vitro conditions and examined by confocal microscopy 1.5 h after germination. AtOFT1-associated GFP signal was localized to punctate motile intracellular organelles (Fig. 3C; Supplemental Movie S1).
Figure 3.
Subcellular localization of AtOFT1. A, Pollen tubes from 11p::OFT1-GFP-expressing plants stained with 500 nm MitoTracker Orange for 15 min (top row) and 11p::OFT1-GFP and MEMB12- or GOT1-mCherry- (Geldner et al., 2009) coexpressing plants (middle and bottom rows, respectively). Pollen tubes were germinated as described above and visualized by confocal microscopy. The outline of each pollen tube is shown as a dashed white line. OFT1-GFP (left column; green signal), the colocalization marker (middle column; magenta signal), and the merge of each image set (right column; white signal) are shown. Bars = 10 µm. B, Quantitative colocalization analysis of each image set was performed using JACoP (Bolte and Cordelières, 2006), and the Pearson correlation coefficient between OFT1-GFP and MitoTracker (black bar), MEMB12-mCherry (charcoal bar), or Got1p-mCherry (gray bar) was calculated. Data are means ± se (n = 6–15 independent images per colocalization marker). C, Live-cell confocal imaging of OFT1-GFP subcellular localization in a growing pollen tube over time. The outline of the pollen tube is indicated with a dashed white line. Image time points are indicated in the top left corner of each image. The trajectories of three representative particles are indicated individually at their current position for each indicated time point by green, red, and yellow arrowheads, and their corresponding trajectories in successive images are indicated with green, red, and yellow lines, respectively. Scale bars represent 10 μm.
To further investigate this subcellular localization pattern, the predicted subcellular localization of AtOFT1 was examined using the SUBcellular localization database of Arabidopsis proteins (SUBA; Tanz et al., 2013). AtOFT1 was predicted to be mitochondrially localized by SUBA, so 11p::OFT1-GFP-expressing pollen tubes were germinated in vitro and stained with 500 nm MitoTracker Orange. As shown in Figure 3A, AtOFT1-GFP signal did not overlap with MitoTracker Orange-stained mitochondria, and quantitative colocalization analysis using JACoP (Bolte and Cordelières, 2006) revealed a Pearson’s correlation coefficient of only 0.21 between the AtOFT1-GFP and MitoTracker Orange signals (Fig. 3B), suggesting that AtOFT1 is not localized to pollen tube mitochondria as predicted.
Subcellular localization studies previously determined that FRB1 and ESMD1 were localized to the Golgi apparatus in interphase cells (Neumetzler et al., 2012; Verger et al., 2016), suggesting that AtOFT1 also may localize to this organelle. To test this hypothesis, the 11p::OFT1-GFP transgenic construct was introgressed into transgenic plants expressing the established Golgi marker MEMBRIN12 (MEMB12) or GOLGI TRANSPORT1 (Got1p) fused to mCherry (Geldner et al., 2009). Pollen harvested from 6-week-old F1 progeny was germinated in vitro and examined by confocal fluorescence microscopy. These experiments revealed significant localization overlap between AtOFT1-GFP and Golgi marker fluorescent signals (Fig. 3A). Quantitative colocalization analyses revealed that both the MEMB12- and Got1p-mCherry markers colocalized with AtOFT1-GFP signal with Pearson’s correlation coefficients of 0.79 and 0.72, respectively (Fig. 3B), suggesting a high degree of colocalization between AtOFT1-GFP and known Golgi-resident proteins. These results suggest that AtOFT1 localizes to the Golgi apparatus and potentially participates in cellular glycosylation events in this organelle.
AtOFT1 Facilitates Pollen Tube Penetration through the Stigma-Style Interface
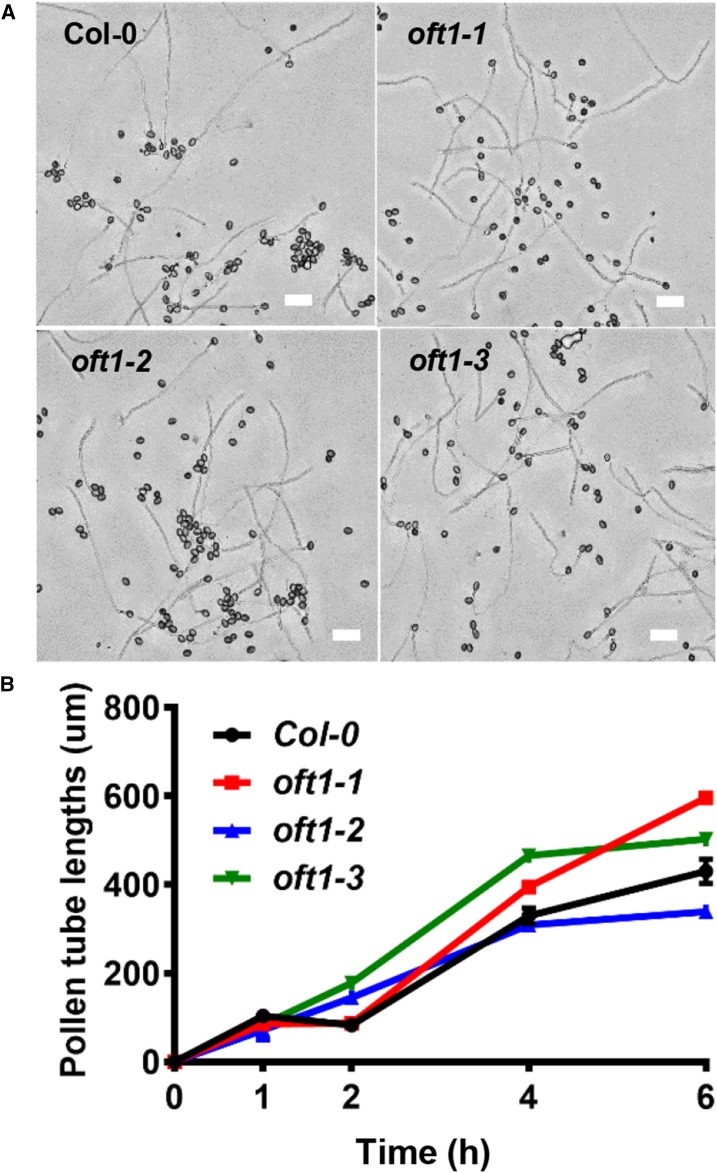
The reciprocal outcross results suggested that oft1 mutants were specifically compromised in some aspect of pollen tube physiology. Mutations that compromise pollen tube elongation often exhibit reduced fertility, so oft1 mutant pollen was germinated in vitro to compare their relative elongation rates and morphology with those of wild-type Col-0 control pollen. As illustrated in Figure 4A, in vitro germinated pollen tubes from all three oft1 mutant lines appeared phenotypically indistinguishable from wild-type Col-0 pollen tubes in terms of overall length and morphology. Pollen tube growth rates from the oft1 mutant lines and wild-type Col-0 control pollen tubes were evaluated further (Fig. 4B). This analysis revealed that, under in vitro conditions, oft1 mutant pollen tubes grew at similar rates to control pollen tubes. To further compare pollen tube elongation rates, pollen harvested from oft1-3−/−; 11p::OFT1-GFP+/− was germinated and visualized using both epifluorescence and bright-field microscopy, facilitating the simultaneous examination of rescued and nonrescued pollen tube elongation rates. Pollen germination rates of complemented and oft1 mutant pollen tubes were indistinguishable (Supplemental Fig. S4A). As shown in Supplemental Figure S4B, pollen tube growth rates of oft1-3−; 11p::OFT1-GFP+ and oft1-3−; 11p::OFT1-GFP− also were essentially identical, further indicating that pollen tube elongation is not compromised in the oft1 mutant. Overall, these observations indicate that oft1 mutant pollen tubes germinate and develop normally, suggesting that these factors cannot explain the reduced transmission of oft1 pollen.
Figure 4.
In vitro pollen tube growth behavior of oft1 mutants. A, Representative images of wild-type Col-0 or oft1 mutant pollen after 6 h of growth. The pollen tube genotype is indicated at the top left corner of each image. Bars = 100 µm. B, Lengths of Col-0 (black line), oft1-1 (red line), oft1-2 (blue line), and oft1-3 (green line) pollen tubes were quantified at the indicated time points. Data are means ± se (n = 30–240).
To further investigate the mechanistic basis of the compromised oft1 reproductive phenotype, pollen tube elongation in the pistil was initially examined. Col-0 or oft1-1−/− mutant pollen was applied to intact, emasculated male sterile1 (ms1; Wilson et al., 2001) mutant pistils. These pistils were harvested after 24 h and stained with Analine Blue as described in “Materials and Methods.” Subsequent fluorescence microscopy imaging revealed that Col-0 pollen tubes had largely traversed the stigma-style interface and extended into the ovary cavity (Fig. 5A, top). In contrast, oft1-1 pollen tubes were largely retained in the stigma-style interface at this time point (Fig. 5A, bottom).
Figure 5.
Pollen tube penetration behavior of oft1 mutants. A, Analine Blue staining of ms1 pistils pollinated with either Col-0 (top) or oft1-1 (bottom) pollen. White arrowheads indicate pollen tube trajectories through the pistil. Bars = 300 µm. B, Representative images of pollen tube emergence from the TT in ms1 pistils dissected at the indicated time points following pollination with either wild-type Col-0 (top row) or oft1-1 (bottom row) pollen. Bars = 0.5 mm. C, Quantification of ms1 stigmas exhibiting pollen tubes emanating from the TT following pollination with Col-0 (black bars), oft1-1 (red bars), oft1-2 (blue bars), or oft1-3 (green bars) homozygous pollen. Data are means ± se (n = 4 independent trials with four to eight stigmas per experimental group per trial). D, Quantification of pollen tube number emerging from ms1 stigmas at the indicated time points following pollination with Col-0 (black line), oft1-1 (red line), oft1-2 (blue line), or oft1-3 (green line). Data are means ± se (n = 4 biological replicates).
To more closely examine this phenomenon, a semi-in vivo assay was utilized to examine the initial stages of pollen tube growth at the stigma-style interface. Emasculated ms1 mutant pistils were pollinated with Col-0 control or homozygous oft1 mutant pollen, dissected from the remainder of the pistil 20 min after pollination, and maintained on a medium surface as described in “Materials and Methods.” Pollinated semi-in vivo (SIV) stigmas were examined over time to determine the rate of pollen tube emergence from the TT and the total number of pollen tubes exiting the TT. In four independent trials, the percentage of stigmas that exhibited at least one pollen tube exiting the TT over the course of the assay was quantified. Wild-type Col-0 pollen tubes exited the TT of 50% of the stigmas by 3 h after pollination (HAP), and 100% of stigmas pollinated with Col-0 pollen exhibited pollen tubes exiting the TT by 3.5 to 4 HAP (Fig. 5, B and C). In contrast, oft1 mutant pollen tubes had only exited the TT of 5% of stigmas by 3 HAP, and only 50% to 75% of oft1 pollinated stigmas exhibited pollen tube exit 4.5 HAP (Fig. 5, B and C). The number of pollen tubes exiting the TT over time also was quantified. Wild-type Col-0 pollen tubes were first observed exiting the TT at 3 HAP, and a linear increase in pollen tube number was observed over the course of the experiment to a maximum of approximately 40 pollen tubes at 8 HAP (Fig. 5D). In contrast, oft1 mutant pollen tubes exhibited a substantial temporal delay in exiting the TT. Approximately five pollen tubes were observed exiting the TT at 4 HAP, and a maximum of 12 pollen tubes were observed at 8 HAP (Fig. 5D). The growth rate of pollen tubes exiting the stigma-style interface also was quantified (Supplemental Fig. S5). While Col-0 control pollen tubes exhibited a rapid growth rate that eventually plateaued at 4 HAP, oft1 mutant pollen tubes exhibited a much slower elongation rate that remained linear over the course of the assay. These mutant pollen tubes also achieved a much shorter final length. These observations suggest that oft1 mutant pollen tubes are compromised in their ability to penetrate the stigma-style interface, despite their ability to elongate normally under in vitro conditions.
To further test the hypothesis that oft1 mutants are unable to efficiently penetrate the stigma-style interface, a semi-in vivo competitive pollen tube penetration assay also was developed. Pollen was harvested from 6-week-old oft1-3−/−;11p::OFT1-GFP+/− T1 transformants and used to pollinate emasculated ms1 pistils following the aforementioned SIV setup. At 4 HAP, the dissected stigmas were observed by fluorescence microscopy. Fluorescent pollen tubes containing the 11p::OFT1-GFP transgene and nonfluorescent oft1-3− mutant pollen tubes would be expected to exit the TT at equal frequencies, but fluorescent pollen tubes were observed to exit the TT at a much higher frequency (85%–90%; Fig. 6A). The proportion of fluorescent and nonfluorescent pollen tubes that did not penetrate the stigma-style interface and elongated away from the stigmatic papillae also was quantified, and 50% of these pollen tubes contained the 11p::OFT1-GFP transgene, indicating that the complementation construct was inserted as a single copy in these transgenic lines. As a control, similar semi-in vivo penetration competition assays were performed with pollen expressing a hemizygous copy of Yellow Fluorescent Protein (YFP) under the control of the pollen tube-specific AUTOINHIBITED CALCIUM ATPASE9 promoter, which we refer to as 9p::YFP (Schiøtt et al., 2004). The pollen tubes emerging from the TT were quantified, and 45% of emerging pollen tubes exhibited 9p::YFP-associated fluorescence (Fig. 6B), suggesting that this control transgene had little influence on pollen tube penetration. Overall, these results suggest that oft1-3− pollen tubes containing the 11p::OFT1-GFP+ construct outcompete oft1-3− mutant pollen tubes for penetration through the stigma-style interface and exit into the TT. Due to the fact that oft1 mutant pollen tubes are capable of normal elongation under in vitro conditions, these results strongly support the hypothesis that oft1 mutant pollen tubes are compromised in their ability to physically penetrate through the stigma-style interface.
Figure 6.
Semi-in vivo penetration competition assay of oft1-3−/−; 11p::OFT1-GFP+/− pollen tubes. A, GFP and bright-field overlaid images depicting the bottom of an ms1 stigma displaying penetrating pollen tubes. Red arrowheads indicate nonfluorescent pollen tubes, and yellow arrowheads indicate fluorescent pollen tubes. B, Quantification of the percentage of fluorescent pollen tubes emerging from the TT at 4 HAP. Three independent oft1-3−/−;11p:OFT1-GFP+/− transgenic lines (red bars) were assessed. 9p::YFP+/− pollen tubes (black bar) served as a negative control. Data are means ± sd (n ≥ 7).
The Stigma-Style Interface Is a Critical Barrier for oft1 Pollen Tubes
While the stigma-style interface is often considered essential for plant sexual reproduction, fertilization events have been observed in the absence of this interface in tobacco (Nicotiana tabacum), lily (Lilium longiflorum), and eucalyptus (Eucalyptus globulus; van Tuyl et al., 1991; Goldman et al., 1994; Trindade et al., 2001). Our results suggest that AtOFT1 plays a significant role in pollen tube penetration through the stigma-style interface, and to test this hypothesis in more detail, we adapted these decapitation fertilization assays for use in Arabidopsis. In this assay, mature ms1 flowers were emasculated, and the stigma-style interface was excised for the remainder of the pistil. Pollen derived from oft1-3+/− parent plants was applied to the dissected pistil (see experimental design in Supplemental Fig. S6). The pollen germinated and successfully fertilized ovules, resulting in the production of seed. While this process was inefficient compared with normal outcrosses in the presence of a stigma, sufficient seed (19 ± 7 seeds per silique) was produced in these assays to examine oft1-3 mutant allele transmission in the absence of a stigma. Interestingly, transmission of the oft1-3 mutant allele increased dramatically under these conditions, with 29.1% of the resulting progeny inheriting the oft1-3 T-DNA allele (n = 206; transmission efficiency = 0.41). While this result did not reach the 50% progeny that would be expected from Mendelian inheritance, removal of the stigma and style increased transmission efficiency by 773-fold compared with the less than 0.1% transmission that was observed when oft1-3+/− pollen was utilized to pollinate intact pistils. We additionally postulated that this assay format represented a limited pollination scenario that could yield different transmission rates compared with the full pollination experiments carried out above. To examine this possibility, intact ms1 stigmas were pollinated with limiting amounts of oft1-3+/− pollen, and oft1-3 transmission was assayed in the resulting F1 progeny. Under limiting pollination conditions, 2% of the resulting progeny inherited the oft1-3 mutant allele (n = 86), suggesting that oft1 mutant pollen are more competitive under limiting pollination conditions but still ineffective compared with full pollination conditions or pollination in the absence of an intact stigma-style interface. These results suggest that oft1-3 mutant pollen tubes are capable of fertilizing ovules but that the stigma-style interface represents a critical barrier that slows their penetration through these female tissues.
Catalytically Important POFT1 Amino Acids Impact AtOFT1 Function
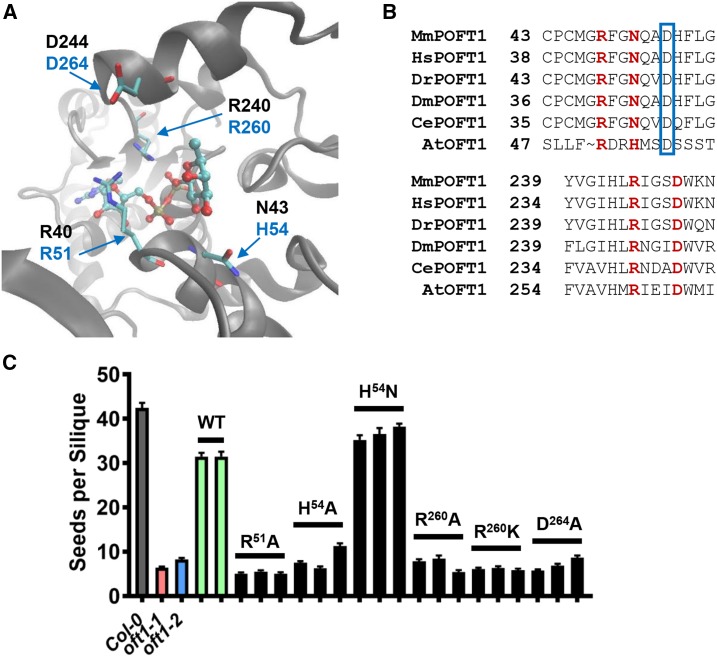
To further examine the amino acid sequence similarities between previously characterized metazoan POFT1s and AtOFT1, we compared the conservation of residues that participate in POFT catalysis between these protein sequences. The x-ray crystal structure of C. elegans POFT1 (CePOFT1) was determined previously in the presence of GDP-Fuc, and this crystal structure illustrates the network of amino acid residues that participate in CePOFT1 substrate selectivity as well as catalysis (Fig. 7A; Lira-Navarrete et al., 2011). The crystal structure of CePOFT1 revealed that the β-phosphate and Fuc moieties of GDP-Fuc are coordinated by a critical Arg residue, CePOFT1R240. CePOFT1N43 also was shown to position the hydroxyl group of the incoming protein substrate in proximity to the fucosyl moiety of GDP-Fuc, hydrogen bond to the Fuc ring, and interact with the α-phosphate of GDP-Fuc. CePOFT1R40 interacts with the ribose ring of GDP-Fuc and also hydrogen bonds to the Fuc ring. Finally, CePOFT1D244 was implicated as a residue that potentially stabilizes the GDP-Fuc-bound conformation of the enzyme. Importantly, in vitro assays indicated that all of these residues are critical for POFT activity and GDP-Fuc binding (Lira-Navarrete et al., 2011). Amino acid sequence alignments between AtOFT1 and metazoan POFT1s revealed that many of these critical residues are conserved in AtOFT1 (Fig. 7B). AtOFT1R260 aligned with CePOFT1R240, the critical Arg residue required for catalysis. Similarly, conserved residues corresponding to CePOFT1R40 and Asp-244 were identified (AtOFT1R51 and Asp-264). Interestingly, CePOFT1N43 was not conserved in AtOFT1, and this residue position contained a His substitution. Based on the hydrogen-bonding function of this residue in the CePOFT1 mechanism (Lira-Navarrete et al., 2011), we postulated that AtOFT1H54 could serve a similar function.
Figure 7.
Structure-function analysis of AtOFT1 putative catalytic residues. A, The active site of C. elegans POFT1 bound to GDP-Fuc (Protein Data Bank identifier 3ZY5) is shown, with critical catalytic residue indices in black font and the corresponding AtOFT1 residues shown in blue font. B, Sequence alignments of the AtOFT1 N-terminal (top) and C-terminal (bottom) regions compared with multiple metazoan POFT1s. Critical catalytic residues are highlighted in red, and the beginning residues for each alignment segment are indicated for reference. An Asp residue used to propose an alternative alignment of the N-terminal domain is boxed in blue. C, Seed set in oft1-1 plants expressing the indicated site-directed mutant constructs of AtOFT1 (black bars). The seed set of Col-0 (gray bar), oft1-1 (red bar), oft1-2 (blue bar), and oft1-1 lines expressing wild-type AtOFT1 (WT; green bars) is shown for reference. Data are means ± se (n = 29–71).
To functionally assess the importance of these conserved catalytic residues (Arg-51, His-54, Arg-260, and Asp-264), site-directed mutagenesis was performed to individually alter these residues in the vector pUBQ10::OFT1-GFP (for a list of mutant constructs, see “Materials and Methods”). The expression of each site-directed mutant was verified by RT-PCR and by confocal microscopy examination of in vitro germinated pollen tubes to confirm proper subcellular localization (Supplemental Fig. S7). The functional importance of each residue was assessed by evaluating the ability of each mutant construct to complement the oft1 seed set phenotype (Fig. 7C). When residues Arg-51, His-54, Arg-260, and Asp-264 were individually mutated to encode Ala, seed set was not restored significantly compared with the homozygous mutant background, which suggested that these residues contribute important structural or catalytic roles in AtOFT1. Similar to CePOFT1, the AtOFT1R260K mutant also was unable to complement the oft1−/− seed set phenotype, which indicated that this residue was fundamentally important in AtOFT1 catalysis due to its stringent requirement for an Arg residue at this position. Interestingly, when AtOFT1H54 was mutated to Asn, the aligned and catalytically relevant residue in CePOFT1, full complementation of the mutant seed set phenotype was observed. Overall, these observations demonstrate the functional importance of catalytically conserved residues between CePOFT1 and AtOFT1, which additionally suggests that these enzymes may share a conserved biochemical mechanism.
DISCUSSION
In this study, we describe the novel role of a previously uncharacterized putative protein O-fucosyltransferase from Arabidopsis (AtOFT1). We demonstrate that AtOFT1 loss-of-function mutants display a near-sterile phenotype that causes as much as a 10-fold reduction in seed set. In addition, pollen transmission efficiency is reduced by nearly 2,000-fold when measured through pollen outcrosses where mutant pollen competes with wild-type pollen for fertilization of ovules (Table I). Interestingly, in vitro germination of oft1 pollen indicates that mutant pollen tubes have similar overall morphology, pollen germination, and pollen tube elongation rates compared with controls (Fig. 4; Supplemental Fig. S4), suggesting that these reproductive defects are not caused by impaired pollen tube growth or abnormal development. However, oft1 mutant pollen tubes exhibited significant delays when penetrating through the stigma-style interface both in intact pistils and during semi-in vivo pollination experiments (Figs. 5 and 6), suggesting that AtOFT1 is required for efficient pollen tube penetration through the stigma and style tissues during the early events underlying double fertilization. Additionally, outcross transmission assays utilizing oft1+/− pollen to fertilize pistils with the stigma-style interface removed indicated that, in the absence of this interface, oft1 mutant pollen tubes are over 700-fold more successful at fertilizing ovules, suggesting that the stigma-style interface represents a critical barrier to fertilization for oft1 mutants. Overall, these results provide a strong argument that AtOFT1 plays a critical role in pollen tube penetration through the stigma-style interface.
The genetic mechanisms underlying pollen tube penetration through the pistil remain unclear (Elleman et al., 1992; Jiang et al., 2005). In this study, we identify AtOFT1 as a gene encoding a putative protein O-fucosyltransferase that facilitates pollen tube penetration through the stigma and style tissues. We postulate that this behavior could be explained by at least three different physiological mechanisms. First, AtOFT1 may be required for the pollen tube to respond to currently unidentified positional guidance response cues within the style and TT. Although responses to specific guidance cues in these tissues must be explored further, in this scenario, oft1 mutant pollen tubes could fail to perceive such a stimulus that potentiates their ability to efficiently fertilize ovules. In line with this hypothesis, similar reduced pollen tube penetration behavior has been observed in Arabidopsis VACUOLAR PROTEIN SORTING41 mutants (vps41; Hao et al., 2016). The authors of that study suggested that vps41 plants are not able to turn over unidentified guidance cue receptors, leading to reduced pollen tube growth in the style, ultimately causing reduced pollen tube penetration rates. Second, pollen tubes could penetrate female tissues by simply degrading cell wall material that connects cells within the style and TT. In this case, AtOFT1 could enable the secretion of cell wall-degrading enzymes by the pollen tube (Jiang et al., 2005) or posttranslationally glycosylate these enzymes to promote their hydrolytic activity. Finally, a third scheme could be imagined in which AtOFT1 participates in the synthesis of a pollen tube cell wall component that confers mechanical strength during penetration though the female tissues. Indeed, other Arabidopsis putative protein O-fucosyltransferases, such as FRB1, MSR1, and MSR2, display alterations in cell wall architecture and composition (Neumetzler et al., 2012; Wang et al., 2013; Verger et al., 2016). However, oft1 mutant pollen tubes germinated normally and displayed no obvious morphological abnormalities, such as early pollen tube tip swelling or bursting, suggesting that if AtOFT1 does contribute to the penetrative capacitance of the pollen tube cell wall, it must only become necessary in the presence of the female tissues.
Protein O-fucosylation and the enzymes that catalyze this posttranslational modification are relatively understudied in plant systems (Neumetzler et al., 2012; Verger et al., 2016). Metazoan protein O-fucosyltransferases glycosylate numerous substrates, and these fucosylation events often critically regulate protein-protein interactions involved in cell adhesion and cell-cell communication. Supporting the hypothesis that POFTs perform similar functions in plant cells, the few POFT-like genes that have been characterized in plants demonstrate cell adhesion-related phenotypes. For example, FRB1 plays an important role in cell adhesion during Arabidopsis growth and development, as these mutants exhibit cell sloughing and dissociation, suggesting that this putative POFT plays a role in vegetative cell adhesion throughout the plant (Neumetzler et al., 2012). Interestingly, loss-of-function mutations in ESMD1, a second putative POFT, were described recently as suppressor mutations of frb1 mutant cell adhesion-related phenotypes as well as other genes known to play a role in plant cell adhesion (Verger et al., 2016). These observations suggest that POFT-like glycosylation events may antagonize one another or serve as a form of cell-cell communication during cellular adherence.
The substrates of AtOFT1 and all other POFT-like plant genes remain unidentified. Metazoan POFT1s utilize GDP-Fuc to fucosylate specific Ser or Thr residues in CXXXX(S/T)C consensus sequences within EGF repeat or TSR domains (Wang et al., 2001). A previous study identified protein sequences in the Arabidopsis genome that contained EGF repeat-like domains and noted that TSR-like domains were absent from the Arabidopsis genome (Verger et al., 2016). Very few EGF-like domains were identified in this study, and the majority of these proteins were transmembrane receptors, including Wall Associated Kinases, which are known to sense pectic cell wall polysaccharides during development and plant defense (Kohorn, 2016). It is currently unclear whether AtOFT1 utilizes GDP-Fuc or this enzyme fucosylates similar substrates as its metazoan counterparts. However, of the 38 putative POFTs in the Arabidopsis genome, AtOFT1 is most phylogenetically similar to metazoan POFT1 and additionally shares similar residues of critical importance to enzymatic activity. Mutagenesis of these critical residues in AtOFT1 leads to loss of function in vivo (Fig. 7), suggesting that AtOFT1 may perform a similar enzymatic function to metazoan POFT1s. We additionally note that a biochemically verified POFT was described recently in Arabidopsis (Zentella et al., 2017). Arabidopsis SPINDLY was demonstrated to directly fucosylate specific Ser/Thr residues in the DELLA protein, a critical regulator of GA signaling. These fucosylation events activated the GA signaling pathway by modulating protein-protein interactions between DELLA and other known transcriptional activators of light and brassinosteroid signaling responses. SPINDLY is not phylogenetically related to metazoan POFT1s, suggesting that either metazoan-like POFTs have acquired novel functions in plant systems or that protein O-fucosylation enzymes have evolved multiple times throughout evolution.
CONCLUSION
Overall, the results from this study provide strong evidence that AtOFT1 plays a critical role in pollen tube penetration through the stigma and/or style tissues. Additional genetic and cell biological evidence suggests that a Golgi-localized fucosyltransferase system may be required for pollen tube growth through pistil tissues. While the substrates of AtOFT1 are currently unknown, it is possible that these substrates include cell surface receptors or structural proteins that are secreted into the extracellular matrix, and identifying AtOFT1 substrates will be the subject of future studies. The discovery of a nearly sterile oft1 phenotype provides a unique opportunity to use robust genetic and cell biological techniques to investigate how POFTs are utilized in plant systems, from pollen-pistil interactions during sexual reproduction to overall plant growth and maintenance.
MATERIALS AND METHODS
Plant Growth and Maintenance
Arabidopsis (Arabidopsis thaliana) seeds were sterilized in seed-cleaning solution (3% [v/v] sodium hypochlorite and 0.1% [w/v] SDS) for 20 min at 25°C. Seeds were washed five times in sterile water and incubated at 4°C for 48 h before plating. Seeds were germinated on Murashige and Skoog (MS) medium (1/2× MS salts, 10 mm MES-KOH, pH 5.7, 1% [w/v] Suc, and 1% [w/v] phytoagar) and grown vertically for 10 d under long-day conditions (16 h of light/8 h of dark) at 24°C. These seedlings were transferred to soil and propagated in a Percival AR-66L2 growth chamber under long-day conditions until seed set. Arabidopsis outcrosses were performed as described previously (Myers et al., 2009). For seed set imaging, siliques from 6-week-old adult wild-type Col-0 or homozygous oft1 mutant Arabidopsis plants were harvested and incubated for 48 h at 25°C in 70% (v/v) ethanol under fluorescent light. Seeds in the cleared siliques were visualized with a Leica EZ4HD video dissecting microscope at 35× magnification.
For plant transformations, Agrobacterium tumefaciens GV3101 cells harboring various plant expression constructs were grown in 10 mL of Luria-Bertani cultures supplemented with 100 µg mL−1 spectinomycin, 50 µg mL−1 gentamycin, and 25 µg mL−1 rifampicin for 16 h at 30°C. These cultures were used to seed 1 L of Luria-Bertani cultures containing spectinomycin, gentamycin, and rifampicin at the concentrations indicated above and grown for 16 h at 30°C. Cells were harvested by centrifugation at 3,000g for 15 min, and the resulting pellets were resuspended in 450 mL of 5% (w/v) Suc supplemented with 0.05% (v/v) Silwet L-77. Five-week-old flowering Arabidopsis plants were transformed via the floral dip method (Clough and Bent, 1998). T1 transformants were identified on selection medium (1/2× MS salts, 1% [w/v] Suc, 0.8% [w/v] phytoagar, 10 mm MES-KOH, pH 5.7, 100 µg mL−1 cefotaxime, 15 µg mL−1 BASTA, or 25 µg mL−1 hygromycin) after a 10-d incubation at 24°C under long-day conditions. Resistant seedlings were selected and transferred to soil.
Isolation of the AtOFT1 Knockout Line
AtOFT1 (At3g05320) was queried against the Salk Institute T-DNA insertional mutant database (Alonso et al., 2003), and three potential T-DNA lines were identified (oft1-1, SALK_072442; oft1-2, SALK_151675; and oft1-3, WiscDsLox489-492M4). These seed populations were propagated on MS medium as described above, transplanted to soil, and grown under long-day conditions at 24°C. Genomic DNA isolation for PCR genotyping was performed essentially as described previously (Edwards et al., 1991) with slight modifications (Villalobos et al., 2015). Each genomic DNA sample was analyzed by PCR genotyping using locus-specific primers as well as the appropriate T-DNA-specific left border primer (Supplemental Table S1) and ExTaq polymerase (Takara Bio). Reactions were cycled under the following conditions: 95°C initial denaturation for 5 min, 35 cycles of 95°C (30 s), 52°C (30 s), and 72°C (1.5 min), and a final extension at 72°C for 7 min. The resulting PCR products were separated on 1% (w/v) agarose gels and documented with a Bio-Rad Gel Doc XR+ image-analysis workstation. The oft1-3−/− line was backcrossed to Col-0 to remove a second T-DNA insertion and its associated BASTA resistance marker.
Cloning of Transgenic Constructs
An OFT1 promoter fragment containing 1,000 bp upstream of the predicted start codon and 12 bp after the start codon was cloned using genomic DNA isolated as described above, promoter-specific primers (pOFT1-1000 pENTR F and pOFT1 pENTR R; Supplemental Table S1), and Phusion DNA polymerase. PCRs were cycled under the following conditions: 98°C initial denaturation for 5 min, followed by 35 cycles of 98°C (30 s), 52°C (30 s), and 72°C (2 min), and a final elongation for 7 min at 72°C. The resulting DNA fragment was resolved on a 1% (v/v) low-melting-point agarose gel, excised, and gel purified with the QiaQuick gel extraction kit (Qiagen). This extracted DNA fragment was cloned into the pENTR-D-TOPO vector according to the manufacturer’s instructions (ThermoFisher Scientific). The AtOFT1 promoter sequence was transferred into the pIGWB-504 binary vector (Nakagawa et al., 2007) using LR Clonase II according to the manufacturer’s instructions.
The rescue construct 11p::OFT1-GFP encodes AtOFT1-GFP-6xHis-TEV protease site-Halo tag-6xHis. The AtOFT1 genomic sequence was amplified by PCR using gene-specific primers (OFT1 cloning F and R) and transferred into a modified pGreenII vector system for plant expression (Hellens et al., 2000), with a kanamycin selection marker for bacteria and a hygromycin marker for plants. The DNA sequence of this construct is provided in Supplemental Figure S8. The 11p promoter corresponds to the upstream regulatory region for AGP11 (At3g01700) with the addition of a 5′ untranslated region containing an intron from proton pump AHA3 (At5g57350; Frietsch et al., 2007). The GFP does not contain an S65T modification for enhanced fluorescence, in order to maintain a better tolerance of acidic pH values that are often found in compartments in the secretory pathway. The HaloTag from Promega provides a tag for purification, along with two 6×His tags.
Site-directed mutagenesis plasmids were fashioned using the vector pUBQ10::OFT1-GFP, which encodes AtOFT1 with GFP fused to the C terminus under the control of the Ubiquitin10 promoter. The AtOFT1 genomic DNA sequence was amplified using gene-specific primers (OFT1-pENTR F and OFT1-pENTR R; Supplemental Table S1). PCRs were cycled under the following conditions: 98°C initial denaturation for 5 min, followed by 30 cycles of 98°C (30 s), 55°C (30 s), and 72°C (1 min), and a final elongation for 5 min at 72°C. The resulting DNA fragment was gel purified and cloned into pENTR-D-TOPO as described above. The AtOFT1 sequence was transferred into the plant-compatible Gateway vector, pUBC-GFP (Grefen et al., 2010), using LR Clonase II according to the manufacturer’s instructions. Individual site-directed mutagenesis PCRs were assembled subsequently using OFT1/pENTR-D-TOPO as a template and nucleotide-specific mutagenic primers (Supplemental Table S1) to create six plasmids containing a single altered amino acid residue. PCRs were cycled under the following conditions: 98°C initial denaturation for 5 min, followed by 35 cycles of 98°C (30 s), 60°C (30 s), and 72°C (4.5 min), and a final elongation for 15 min at 72°C. Following DpnI digestion for 2 h at 37°C, reactions were transformed directly into chemically competent Escherichia coli.
All constructs were verified by Sanger DNA sequencing at the Nevada Genomics Center (http://www.ag.unr.edu/genomics). Following sequence validation, all constructs were transformed into A. tumefaciencs GV3101 and then transformed into the indicated Arabidopsis background as described using the standard floral dip method (Clough and Bent, 1998).
Phylogenetic Analysis of Putative Arabidopsis OFTs
The AtOFT1 amino acid sequence was used as a BLAST query to identify putative POFT homologs in the Arabidopsis genome using the WU-BLAST function on The Arabidopsis Information Resource Web site (www.arabidopsis.org). The amino acid sequences of Mus musculus (NP_536711.3), Drosophila melanogaster (AAF58290.1), Danio rerio (NP_991283.3), Homo sapiens (NP_056167.1), and Caenorhabditis elegans (ABA29469.1) were obtained from the National Center for Biotechnology Information protein sequence database. Protein sequences were aligned using the multiple alignment mode in ClustalX2 (www.clustal.org), and incomplete sequences were removed. This alignment was used to create a neighbor-joining phylogenetic tree consisting of 1,000 independent bootstrap trials in MEGA7 (http://www.megasoftware.net/). The resulting phylogenetic trees were viewed and analyzed in MEGA7.
The molecular model of C. elegans POFT1 was generated using the Visual Molecular Dynamics software version 1.9.3. The crystal structure of this protein was generated in complex with GDP-Fuc by Lira-Navarrete et al. (2011) and accessed through the Protein Data Bank (identifier 3ZY5).
In Vitro Pollen Tube Growth Assays
Pollen was harvested from the opened flowers of 6-week-old plants through gentle blotting action of the floral opening across a 76- × 25-mm glass slide containing 750 μL of solid pollen germination medium (PGM; 5 mm CaCl2, 0.01% [w/v] boric acid, 5 mm KCl, 10% [w/v] Suc, 1 mm MgSO4, and 1.5% [w/v] low-melting-point agarose, pH 7.5; Boavida and McCormick, 2007) cooled to 25°C. Pollen was germinated in the dark at 25°C in a humidified chamber for the indicated time period. Before imaging, liquid PGM (lacking low-melting-point agarose) was applied to the surface of the agar medium, and a coverslip was added. Pollen tube growth and morphology were visualized with a Keyence BZ-X700 microscope under bright-field illumination using a 10× 0.45 numerical aperture (NA) air objective. Pollen tube lengths were quantified using ImageJ (imagej.nih.gov/ij/). For confocal microscopy of AtOFT1 subcellular localization, pollen was harvested as described previously from flowers of 6-week-old oft1-3−/− plants expressing 11p::OFT1-GFP or this transgenic line crossed with either Got1p-mCherry or MEMB12-mCherry Golgi marker lines (Geldner et al., 2009) and germinated for 1.5 h. Before imaging with an Olympus FluoView FV1000 line-scanning confocal microscope equipped with 40× 1.3 NA and 60× 1.4 NA oil objectives, 488- and 543-nm excitation laser lines (GFP emission filter 500–530; Tetramethylrhodamine emission filter 555–615), liquid PGM was applied to the surface of the agar medium pad and a coverslip was added. For colocalization imaging using MitoTracker staining, the 11p::OFT1-GFP pollen tubes were treated with 500 nm MitoTracker Orange for 15 min prior to coverslip addition and imaging. MitoTracker Orange was visualized using the Tetramethylrhodamine filter described above. Images were processed in Fiji (https://fiji.sc/). Colocalization statistics were calculated using JACoP (Bolte and Cordelières, 2006).
Pollen Tube Penetration Assays
Pollen from 6-week old plants was collected from Col-0 or homozygous oft1 mutant lines and used to pollinate mature, emasculated ms1 stigmas. Twenty minutes after pollination, stigmas were dissected from the parent plant using a razor blade and transferred to a PGM-agarose pad on a microscope slide as described above. Samples were incubated at 25°C in a humidified chamber in the dark for 2 HAP. Pollen tube emergence from the TT was observed over time. Dissected stigmas were visualized using a Leica EZ4HD dissecting microscope at 35× magnification over the indicated time intervals starting at 2 HAP. After each set of images was collected for a given time point, the samples were returned to the humidified chamber in the dark until the next set of images was collected.
Semi-in vivo assays utilizing fluorescently labeled pollen tubes were assembled identically. Mature pollen was collected from 11p::OFT1-GFP+/−; oft1-3−/− or 9p::YFP+/− transgenic lines and used to pollinate mature, emasculated ms1 pistils, which were then dissected and incubated as described above. At 4 HAP, pollen tubes emerging from the style were examined using a Keyence BZ-X700 microscope at 20× magnification. Sequential Z-stack images using bright-field illumination, GFP (excitation = 470 nm, emission = 525 nm), or YFP (excitation = 500 nm, emission = 530 nm) were used to construct images. The Z-stacks were assembled and processed in Keyence BZ-X Analyzer software and analyzed subsequently for total pollen tubes emerging from the style as well as the proportion of fluorescent and nonfluorescent tubes.
Analine Blue Staining of Pollinated Pistils
Mature, emasculated ms1 flowers were pollinated with either Col-0 or oft1-3−/− mutant pollen and incubated under normal growth conditions for 24 h. Following incubation, pistils were dissected away from the remainder of the plant and subjected to Analine Blue staining (Mori et al., 2006). Images were acquired at 20× with a Keyence BZ-X710 epifluorescence microscope fitted with a 4′,6-diamino-phenylindole filter cube.
RNA Isolation, cDNA Synthesis, and RT-PCR
RNA from 7-d-old seedlings from each homozygous oft1 mutant line and Col-0 was isolated using the PureLink Plant RNA Kit (ThermoFisher Scientific) following the manufacturer’s instructions. Genomic DNA contamination was eliminated from the extracts using the Turbo DNA-free Kit (Ambion), and the synthesis of first-strand cDNA was carried out using the Invitrogen SuperScript III First-Strand Synthesis System, both according to the manufacturer’s protocol (ThermoFisher Scientific). The resulting cDNA library for each line was probed for AtOFT1 transcript as well as ACTIN7 (At1g33160) for amplification reference by PCR. Reactions were assembled using 24 ng of cDNA as template and the respective gene-specific primers (OFT1-pENTR F and OFT1-pENTR R or ACTIN7 F and ACTIN7 R; Supplemental Table S1). Reactions probing for the AtOFT1 transcript were cycled under the following conditions: 98°C initial denaturation for 5 min, followed by 30 cycles of 98°C (30 s), 55°C (30 s), and 72°C (1 min), and a final elongation for 5 min at 72°C. Reactions probing for the ACTIN7 transcript were cycled under the following conditions: 98°C initial denaturation for 30 s, followed by 30 cycles of 98°C (10 s), 55°C (20 s), and 72°C (30 s), and a final elongation for 5 min at 72°C. The resulting PCR products were separated on 1% (w/v) agarose gels and visualized with a Bio-Rad Gel Doc XR+ image-analysis workstation.
Accession Numbers
Accession numbers are as follows: AtOFT1 (At3g05320), FRB1 (At5g01100), ESMD1 (At2g01480), MSR1 (At3g21190), MSR2 (At1g51630), SPINDLY (At3g11540), H. sapiens POFT1 (NP_056167.1), M. musculus POFT1 (NP_536711.3), D. rerio POFT1 (NP_991281.3), C. elegans POFT1 (ABA29469.1), and D. melanogaster POFT1 (AAF58290.1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. PCR genotyping verification of oft1 T-DNA insertions and AtOFT1 transcript abundance.
Supplemental Figure S2. Seed set measurements of 11p::OFT1-GFP complement lines.
Supplemental Figure S3. Tissue expression analysis of AtOFT1.
Supplemental Figure S4. In vitro pollen germination and elongation rates of oft1-3 rescued pollen.
Supplemental Figure S5. Pollen tube behavior following emergence from SIV pistils.
Supplemental Figure S6. Decapitation assay experimental design.
Supplemental Figure S7. Expression verification of AtOFT1 site-directed mutant constructs.
Supplemental Figure S8. Sequence and relevant features of the 11p::OFT1-GFP complementation construct.
Supplemental Table S1. Oligonucleotide primers used in this study.
Supplemental Movie S1. AtOFT1-GFP subcellular dynamics in growing pollen tubes.
Footnotes
D.K.S., D.M.J., J.B.R.L., and I.S.W. were supported by startup funds through the University of Nevada, Reno, Department of Biochemistry and Molecular Biology. D.M.J. and I.S.W. were supported by National Science Foundation IOS award 1449068. D.K.S. was also supported by a National Science Foundation Graduate Research Fellowship and a Nevada Agricultural Experiment Station Award (NEV00382). Additionally, the confocal microscope used in this study is supported by an NIH COBRE award (award number RR024210).
Articles can be viewed without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Beale KM, Johnson MA (2013) Speed dating, rejection, and finding the perfect mate: advice from flowering plants. Curr Opin Plant Biol 16: 590–597 [DOI] [PubMed] [Google Scholar]
- Boavida LC, McCormick S (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52: 570–582 [DOI] [PubMed] [Google Scholar]
- Bolte S, Cordelières FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232 [DOI] [PubMed] [Google Scholar]
- Bouton S, Leboeuf E, Mouille G, Leydecker MT, Talbotec J, Granier F, Lahaye M, Höfte H, Truong HN (2002) QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. Plant Cell 14: 2577–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Lord EM (2011) Pollen tube growth and guidance: roles of small, secreted proteins. Ann Bot 108: 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Durand C, Vicré-Gibouin M, Follet-Gueye ML, Duponchel L, Moreau M, Lerouge P, Driouich A (2009) The organization pattern of root border-like cells of Arabidopsis is dependent on cell wall homogalacturonan. Plant Physiol 150: 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman CJ, Franklin-Tong V, Dickinson HG (1992) Pollination in species with dry stigmas: the nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytol 121: 413–424 [DOI] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Dénervaud-Tendon V, Hyman DL, Mayer U, Stierhof YD, Chory J (2009) Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J 59: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MHS, Goldberg RB, Mariani C (1994) Female sterile tobacco plants are produced by stigma-specific cell ablation. EMBO J 13: 2976–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, Blatt MR (2010) A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J 64: 355–365 [DOI] [PubMed] [Google Scholar]
- Hao L, Liu J, Zhong S, Gu H, Qu LJ (2016) AtVPS41-mediated endocytic pathway is essential for pollen tube-stigma interaction in Arabidopsis. Proc Natl Acad Sci USA 113: 6307–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RJ, Spellman MW (1993) O-Linked fucose and other post-translational modifications unique to EGF modules. Glycobiology 3: 219–224 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D (2005) VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17: 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD. (2016) Cell wall-associated kinases and pectin perception. J Exp Bot 67: 489–494 [DOI] [PubMed] [Google Scholar]
- Kovall RA, Gebelein B, Sprinzak D, Kopan R (2017) The canonical Notch signaling pathway: structural and biochemical insights into shape, sugar, and force. Dev Cell 41: 228–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard-Melief C, Haltiwanger RS (2010) O-Fucosylation of thrombospondin type 1 repeats. Methods Enzymol 480: 401–416 [DOI] [PubMed] [Google Scholar]
- Levitin B, Richter D, Markovich I, Zik M (2008) Arabinogalactan proteins 6 and 11 are required for stamen and pollen function in Arabidopsis. Plant J 56: 351–363 [DOI] [PubMed] [Google Scholar]
- Leydon AR, Tsukamoto T, Dunatunga D, Qin Y, Johnson MA, Palanivelu R (2015) Pollen tube discharge completes the process of synergid degeneration that is initiated by pollen tube-synergid interaction in Arabidopsis. Plant Physiol 169: 485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira-Navarrete E, Valero-González J, Villanueva R, Martínez-Júlvez M, Tejero T, Merino P, Panjikar S, Hurtado-Guerrero R (2011) Structural insights into the mechanism of protein O-fucosylation. PLoS ONE 6: e25365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca VC, Kim BC, Ge C, Kakuda S, Wu D, Roein-Peikar M, Haltiwanger RS, Zhu C, Ha T, Garcia KC (2017) Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science 355: 1320–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Haltiwanger RS (2005) O-Fucosylation of notch occurs in the endoplasmic reticulum. J Biol Chem 280: 11289–11294 [DOI] [PubMed] [Google Scholar]
- Mizukami AG, Inatsugi R, Jiao J, Kotake T, Kuwata K, Ootani K, Okuda S, Sankaranarayanan S, Sato Y, Maruyama D, et al. (2016) The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance. Curr Biol 26: 1091–1097 [DOI] [PubMed] [Google Scholar]
- Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T (2006) GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 8: 64–71 [DOI] [PubMed] [Google Scholar]
- Myers C, Romanowsky SM, Barron YD, Garg S, Azuse CL, Curran A, Davis RM, Hatton J, Harmon AC, Harper JF (2009) Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J 59: 528–539 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, et al. (2007) Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 71: 2095–2100 [DOI] [PubMed] [Google Scholar]
- Neumetzler L, Humphrey T, Lumba S, Snyder S, Yeats TH, Usadel B, Vasilevski A, Patel J, Rose JK, Persson S, et al. (2012) The FRIABLE1 gene product affects cell adhesion in Arabidopsis. PLoS ONE 7: e42914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima T, Xu A, Irvine KD (2003) Modulation of notch-ligand binding by protein O-fucosyltransferase 1 and fringe. J Biol Chem 278: 42340–42345 [DOI] [PubMed] [Google Scholar]
- Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, et al. (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458: 357–361 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114: 47–59 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Johnson MA (2010) Functional genomics of pollen tube-pistil interactions in Arabidopsis. Biochem Soc Trans 38: 593–597 [DOI] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5: e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampal R, Li AS, Moloney DJ, Georgiou SA, Luther KB, Nita-Lazar A, Haltiwanger RS (2005) Lunatic fringe, manic fringe, and radical fringe recognize similar specificity determinants in O-fucosylated epidermal growth factor-like repeats. J Biol Chem 280: 42454–42463 [DOI] [PubMed] [Google Scholar]
- Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S (1991) Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell 67: 687–699 [DOI] [PubMed] [Google Scholar]
- Sandaklie-Nikolova L, Palanivelu R, King EJ, Copenhaver GP, Drews GN (2007) Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol 144: 1753–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamura T, Sasaki N, Miyashita F, Nakao S, Ishikawa HO, Ito M, Kitagawa M, Harigaya K, Spana E, Bilder D, et al. (2003) Neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development 130: 4785–4795 [DOI] [PubMed] [Google Scholar]
- Schiøtt M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101: 9502–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions RA, Zambryski PC (1995) Arabidopsis gynoecium structure in the wild and in ettin mutants. Development 121: 1519–1532 [DOI] [PubMed] [Google Scholar]
- Shi S, Stanley P (2003) Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci USA 100: 5234–5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Uemura K, Ge C, Shi S, Tashima Y, Stanley P (2008) Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem 283: 13638–13651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2012) A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol 10: e1001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531: 245–248 [DOI] [PubMed] [Google Scholar]
- Tanz SK, Castleden I, Hooper CM, Vacher M, Small I, Millar HA (2013) SUBA3: a database for integrating experimentation and prediction to define the SUBcellular location of proteins in Arabidopsis. Nucleic Acids Res 41: D1185–D1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade H, Boavida LC, Borralho N, Fiejo JA (2001) Successful fertilization and seed set from pollination on immature non-dehisced flowers of Eucalyptus globulus. Ann Bot 87: 469–475 [Google Scholar]
- van Tuyl JM, van Dien MP, van Creij MGM, van Kleinwee TCM, Franken J, Bino RJ (1991) Application of in vitro pollination, ovary cultures, ovule culture, and embryo rescue for overcoming incongruity barriers in interspecific Lilium crosses. Plant Sci 74: 115–126 [Google Scholar]
- Verger S, Chabout S, Gineau E, Mouille G (2016) Cell adhesion in plants is under the control of putative O-fucosyltransferases. Development 143: 2536–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos JA, Yi BR, Wallace IS (2015) 2-Fluoro-L-fucose is a metabolically incorporated inhibitor of plant cell wall polysaccharide fucosylation. PLoS ONE 10: e0139091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mortimer JC, Davis J, Dupree P, Keegstra K (2013) Identification of an additional protein involved in mannan biosynthesis. Plant J 73: 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shao L, Shi S, Harris RJ, Spellman MW, Stanley P, Haltiwanger RS (2001) Modification of epidermal growth factor-like repeats with O-fucose: molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J Biol Chem 276: 40338–40345 [DOI] [PubMed] [Google Scholar]
- Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ (2001) The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J 28: 27–39 [DOI] [PubMed] [Google Scholar]
- Zentella R, Sui N, Barnhill B, Hsieh WP, Hu J, Shabanowitz J, Boyce M, Olszewski NE, Zhou P, Hunt DF, et al. (2017) The Arabidopsis O-fucosyltransferase SPINDLY activates nuclear growth repressor DELLA. Nat Chem Biol 13: 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]