Abstract
Objective
Hypophosphatemia occurs with inadequate dietary intake, malabsorption, increased renal excretion, or shifts between intracellular and extracellular compartments. We noticed the common finding of amino-acid based elemental formula [EF] use in an unexpected number of cases of idiopathic hypophosphatemia occurring in infants and children evaluated for skeletal disease. We aimed to fully characterize the clinical profiles in these cases.
Methods
A retrospective chart review of children with unexplained hypophosphatemia was performed as cases accumulated from various centres in North America and Ireland. Data were analyzed to explore any relationships between feeding and biochemical or clinical features, effects of treatment, and to identify a potential mechanism.
Results
Fifty-one children were identified at 17 institutions with EF-associated hypophosphatemia. Most children had complex illnesses and had been solely fed Neocate® formula products for variable periods of time prior to presentation. Feeding methods varied. Hypophosphatemia was detected during evaluation of fractures or rickets. Increased alkaline phosphatase activity and appropriate renal conservation of phosphate were documented in nearly all cases. Skeletal radiographs demonstrated fractures, undermineralization, or rickets in 94% of the cases. Although the skeletal disease had often been attributed to underlying disease, most all improved with addition of supplemental phosphate or change to a different formula product.
Conclusion
The observed biochemical profiles indicated a deficient dietary supply or severe malabsorption of phosphate, despite adequate formula composition. When transition to an alternate formula was possible, biochemical status improved shortly after introduction to the alternate formula, with eventual improvement of skeletal abnormalities. These observations strongly implicate that bioavailability of formula phosphorus may be impaired in certain clinical settings. The widespread nature of the findings lead us to strongly recommend careful monitoring of mineral metabolism in children fed EF. Transition to alternative formula use or implementation of phosphate supplementation should be performed cautiously with as severe hypocalcemia may develop.
1. Introduction
Hypophosphatemia is uncommon in childhood and can occur either with or without depletion of whole-body phosphate [P] stores [1]. In the acute setting, or upon refeeding after prolonged depletion, phosphate may shift from extracellular to intracellular compartments resulting in transient hypophosphatemia without phosphate depletion. In the more chronic setting, long-term phosphate depletion may occur after prolonged inadequate intake, malabsorption, or excessive urinary losses due to renal tubular disease. As phosphate is abundant in Western diets, inadequate phosphate supply is rarely encountered in the United States [US] and Canada, and observed in very specific situations, such as the breastfed premature infant (owing to the low P content of breast milk). Decreased intestinal absorption of phosphate may occur with generalized malabsorption, vitamin D deficiency or due to the use of phosphate binding agents. One such example has been often reported in individuals abusing antacids, whereby resultant bound phosphate is less available for intestinal absorption [2]. Alternatively, abnormal renal losses of phosphate may occur in the setting of hyperparathyroidism, excess fibroblast growth factor 23 [FGF23], or primary disorders of the renal tubule [1]. Generally, hypophosphatemia encountered in childhood is accompanied by characteristic historic and/or clinical features that suggest the most likely etiology.
The composition of nutritional formulas designed for infants and children follows nationally- and globally-established guidelines to maximize safety and nutritional adequacy across a variety of intakes [3]. When formula is used as the sole source of nutrition, intake is determined by energy requirements and micronutrient intake varies accordingly. Despite this variability in intake, micronutrient intake from formula is usually adequate to meet requirements for most nutrients until intakes fall far below expected for age. As such, micronutrient deficiencies in children consuming infant and pediatric formulas across a wide range of intakes are rare. Furthermore, when low formula volumes are consumed due to low energy requirements, intake of all micronutrients is reduced, resulting in potential deficiencies of multiple micronutrients. Thus isolated micronutrient deficiency in a formula-fed population is not commonly encountered.
In this manuscript, we describe a series of children who had unexpected and unexplained hypophosphatemia, and who had in common the consumption of single brand of amino-acid based nutritional formula.
2. Patients and methods
This was a retrospective chart review conducted in 17 centres in North America and Ireland between 2014 and 2016. Patients were referred to the various authors because of unexplained mechanism of marked hypophosphatemia in infants and young children, sometimes accompanied by significant bone disease. This case series was compiled when it became evident that several common clinical and biochemical features suggested a limitation in dietary phosphate intake or its intestinal absorption. After the development of a data collection form, centres/ clinicians who had contacted the senior authors regarding affected children provided descriptive details to facilitate comparison of cases. All biochemical measurements were performed at the individual treating centres' clinical laboratories. All data was collected and shared in compliance with the ethics requirements of each centre. After receipt of the supporting information for each case, data was collectively examined to identify similarities and differences, with the objective of describing clinical features, identifying potential risk factors and generating hypotheses regarding etiology of the problem. No cases were excluded because of the use of any particular formula products. Selected clinical case vignettes are provided as examples. (Further details enumerating all cases are found in the Supplementary Table S1).
3. Results
3.1. Cases
3.1.1. Case vignette 1
An 18 month-old boy had a history of esophageal atresia/tracheoesophageal fistula repair and necrotizing enterocolitis. He was orally fed, receiving diuretics and glucocorticoids, and was relatively immobile. After 1 year of Neocate® feeding, he demonstrated irritability associated with knee and skeletal manipulation. A radiographic skeletal survey showed numerous fractures including those of the femur, fibulae, ulna, metatarsals, a metacarpal, and 3 ribs. Upon identification of a low blood P level (1.3 mg/dL; 0.40 mM) and elevated alkaline phosphatase (1137 IU/L), he was treated with calcitriol and oral phosphate. Subsequently the formula was changed to Elecare®, and phosphate supplementation was discontinued, with continue maintenance of a normal serum phosphorus level without added phosphate supplementation. Correction of serum alkaline phosphatase ensued and after 3 months, radiographs improved.
3.1.2. Case vignette 2
A 4-year old girl with a history of hypoxic ischemic encephalopathy, gastric dysmotility, developmental delay and seizures had received Neocate Jr® for 2.6 years. Prior to presentation, she had decreased mobility, and had stopped all crawling and weight-bearing. Healing rib fractures were observed on radiographic examination of the chest; subsequent biochemical evaluation revealed low blood P (1.7 mg/dL; 0.53 mM) and elevated alkaline phosphatase levels (1014 IU/L). She was then treated with oral phosphate supplements, and the formula was changed to Pediasure®. Phosphate supplementation was then discontinued with continued maintenance of a normal serum phosphorus level, and correction of alkaline phosphatase.
3.1.3. Case vignette 3
A 3.5-month old girl (born after a 28 week gestation) with bronchopulmonary dysplasia had a history of feeding intolerance with abdominal distention, which led to feeding with various formulas until milk protein allergy was diagnosed at 2 months of age, when nasogastric Neocate® feeds were begun. Incidental biochemical testing performed 6 weeks later revealed low serum P (2.9 mg/dL; 0.90 mM) and elevated alkaline phosphatase levels (796 IU/L). She was briefly provided with oral phosphate, then changed to Alimentum® formula. Phosphate supplementation was discontinued with maintenance of normal serum P level, and correction of alkaline phosphatase.
3.1.4. Case vignette 4
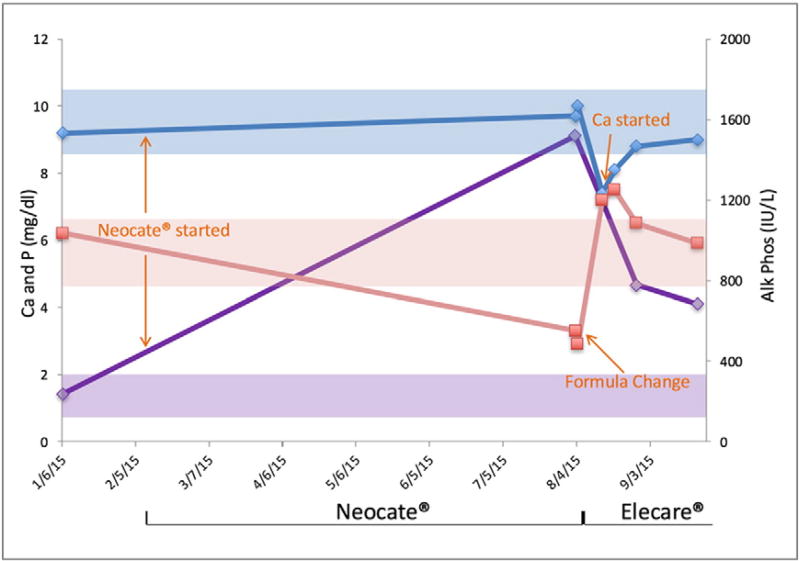
An 8 month-old girl, born after a 31 week gestation, had been fed Neocate® since age 2 months due to a diagnosis of milk protein allergy. She had a history of retinopathy of prematurity, microcephaly, reflux, and hypertonia, and presented after 6 months of elemental formula exposure with a left tibia fracture in the absence of any history of recent trauma. Investigation revealed hypophosphatemia (2.9 mg/dL; 0.90 mM) and elevated alkaline phosphatase levels (1510 IU/L). The formula was changed to Elecare®, with correction of the serum phosphorus level, and alkaline phosphatase activity (Fig. 1). No supplemental phosphate was administered.
Fig. 1.
Changes in serum calcium (blue), phosphorus (pink) and alkaline phosphatase (purple) in an 8 month-old girl fed Neocate® since age 2 months due to milk protein allergy, presenting with a tibial fracture. After the formula was changed to Elecare®, serum phosphorus and alkaline phosphatase levels corrected. Supplemental phosphate was not administered.
3.1.5. Case vignette 5
A 6.5 year-old boy presented to the emergency room with respiratory distress and decreased movement of his right arm for 2 weeks. Severe hypophosphatemia was identified incidentally (serumP, 0.6 mg/dL). He had a history of epidermolysis bullosa and profound global developmental delay. He had been fed Neocate® formula via gastrostomy tube since 3 years of age. Rickets and healing fractures were noted radiographically and phosphate supplementation was begun. One year later, Neocate® formula was changed to Elecare® with subsequent normalization of the serum P level without phosphate supplementation (see Table 1), however persistent diarrhea prompted a change back to Neocate® formula feeds. Two weeks after the resumption of Neocate® feedings, the serum P level decreased to 1.2 mg/dL, requiring the resumption of phosphate supplementation. A second attempt to use Elecare® two months later was well-tolerated, and coincident correction of the serum P level occurred. Eventually radiographic features of rickets healed.
Table 1.
Serum P relationship to formula and phosphate supplementation.
| Date | Serum P (mg/dL) | Formula | Formula change | Phosphate supplementation |
|---|---|---|---|---|
| 11/30/14 | 0.6 | Neocate® | ||
| 12/2014 | Neocate® | Supplementation added | ||
| 11/2015 | 2.5–3.5 | Neocate® to Elecare® | Began tapering | |
| 12/1/15 | 4.5 | Elecare® | Off | |
| 2/25/2016 | Elecare® to Neocate® | |||
| 3/10/16 | 1.2 | Neocate® | Supplementation resumed | |
| 3/16/16 | 3.7 | Neocate® | On | |
| 5/2016 | Neocate® to Elecare® | Began tapering | ||
| 8/24/16 | 4.1 | Elecare® | ||
| 10/26/16 | 4.7 | Elecare® |
3.2. Chart review
Fifty-one children were identified from17 centres in the US, Canada and Europe. Demographic features, and selected clinical characteristics are listed in Table 2. Age at the time of identification ranged from 0.2 to 15.5 years (median, 3.0). Fifty-seven percent of these children were female and 43% were male. Most children (96%) were investigated because of rickets or unexplained single or multiple fractures. The remaining 4% were detected upon biochemical laboratory screening. All identified children were receiving Neocate® formula products as their sole source of nutrition. The formula was administered either via oral or intragastric routes (53%), via the transpyloric route (37%) or using both gastric and transpyloric routes (10%). Median duration of Neocate® feeding was 1.3 years (range 1 week to 8 years). All affected children had complex but highly variable medical histories (see Supplementary Table S1), without a single unifying explanatory feature. Documentation of medication use was available for 44 children (86%), and of these, 91% were exposed to medications that modify gastric acidity, usually proton pump inhibitors [PPIs], with one case of histamine receptor 2-blocker use. Height or length was at the third centile or less for age in 49% of children, and at tenth centile or less in 61%. Weight was at the third centile or less for age in 41% of children, and at tenth centile or less in 49%.
Table 2.
Clinical characteristics.
| Clinical characteristics | Total/percentage | |
|---|---|---|
| Age | Range: 0.2–15.5 years | Median: 3.0 |
| Gender | Female | 29/57% |
| Male | 22/43% | |
| Growth | Height/length ≤ 3rd centile | 25/49% |
| Weight ≤ 3rd centile | 21/41% | |
| Formula | Infant | 15/29% |
| Junior | 35/69% | |
| Advance | 1/2% | |
| Feeding route | Oral/gastric | 27/53% |
| Jejunal | 19/37% | |
| Gastric and jejunal | 5/10% | |
| PPI use | Data available in 44 patients | 40/91% |
| Primary treatment | Phosphate supplements | 38/75% |
| Formula change | 13/25% |
Abbreviation: PPIs, proton pump inhibitors.
The results of laboratory investigations were remarkably consistent in all identified children (Table 3). The most striking features were markedly decreased serum phosphate, elevated alkaline phosphatase and normal 25 hydroxyvitamin D [25-OHD] levels. In most cases, serum phosphate and alkaline phosphatase values were far outside of reference ranges. Calcium (ionized and/or total) and PTH levels were usually in the normal range. Urinary phosphate was undetectable or extremely low in all cases, and tubular reabsorption of phosphate (TRP) [4] approached 100% when it could be calculated from the available data. When 1,25 dihydroxyvitamin D [1,25(OH)2D] had been measured (n=37), it was consistently and significantly elevated, and thus may be a highly sensitive biomarker for this phenomenon. Circulating FGF23 levels (measured in only two cases) were low.
Table 3.
Biochemical profile.
| Biochemical profile | |||
|---|---|---|---|
|
| |||
| Mean (SD) | Reference values | ||
| Serum | Ca (mg/dL) | 9.6 (0.8)a | 8.8–10.2 |
| P (mg/dL) | 2.1 (0.7) | 1–12 months: 3.0–7.5 | |
| 1–11 years: 3.5–6.5 | |||
| 11–15 years: 3.5–5.3 | |||
| Alk Phos (IU/L) | 1123 (586) | 1 month–6 years: 80–380 | |
| 6–12 years: 60–480 | |||
| 12–15 years: 90–570 (male) | |||
| 60–280 (female) | |||
| PTH (pg/mL) | 39 (22, median) | 10–69 | |
| 25-OHD (ng/mL) | 37 (14) | 20–50 | |
| 1,25(OH)2D (pg/mL) | 254 (124) | <30 days: <10–72 | |
| 1 month–17 years: 15–90 | |||
| Urine | TRP (%)b | 98 (3) | <0.25 years: 61–86 |
| 0.3–0.5 years: 68–93 | |||
| 0.6–1 year: 80–89 | |||
| 1–2 years: 78–93 | |||
| 3–10 years: 81–98 | |||
| 11–15 years: 86–99 | |||
.
Abbreviations: Ca, calcium; P, phosphate; Alk Phos, alkaline phosphatase; PTH, parathyroid hormone; 25-OHD, 25 hydroxyvitamin D; 1,25(OH)2 D, 1,25 dihydroxyvitamin D; SD, standard deviation; TRP, tubular reabsorption of phosphate. [Reference range for 1,25(OH)2D is that in use at ESOTERIX/LabCore; reference ranges for all other serum values are those in use at Yale-New Haven Hospital. Values for TRP are those reviewed by Ardeshirpour [4].
Values are in Mean (SD, unless otherwise indicated).
n= 9 where calculable, 25 had undetectable urinary P.
Various therapeutic approaches were employed across the multiple patients and centres, and evolved with increasing experience with the management of the cases. Initially, phosphorus supplementation was employed with continued use of Neocate® formula in most cases (75%). Calcium, vitamin D, and/or calcitriol were occasionally required, to treat severe hypocalcemia, which often occurred rapidly following the addition of phosphate supplements (Fig. 1). In other cases (25%) the formula product was substituted with breast milk, alternate formula products, or parenteral feeds. As with supplemental phosphate administration, hypocalcemia was also often precipitated when the formula product was substituted. Overall, 47% of the children developed hypocalcemia following intervention, and was occasionally severe. In one case, severe hypocalcemia may have contributed to a seizure episode and a subsequent cardiac arrest. After observing the correction of hypophosphatemia with formula product changes, several patients originally treated with phosphate also underwent changes in formula as to simplify their medication regimens. Nevertheless, four cases were intolerant of formula change (diarrhea or anaphylaxis) and required resumption of Neocate® with added phosphate supplements. A representative example of the biochemical and radiographic changes occurring at presentation and with application of treatment is shown in Figs. 1 and 2. In other cases, hypophosphatemia was evident as early as one week after the introduction of Neocate®, but was not recognized until several months later.
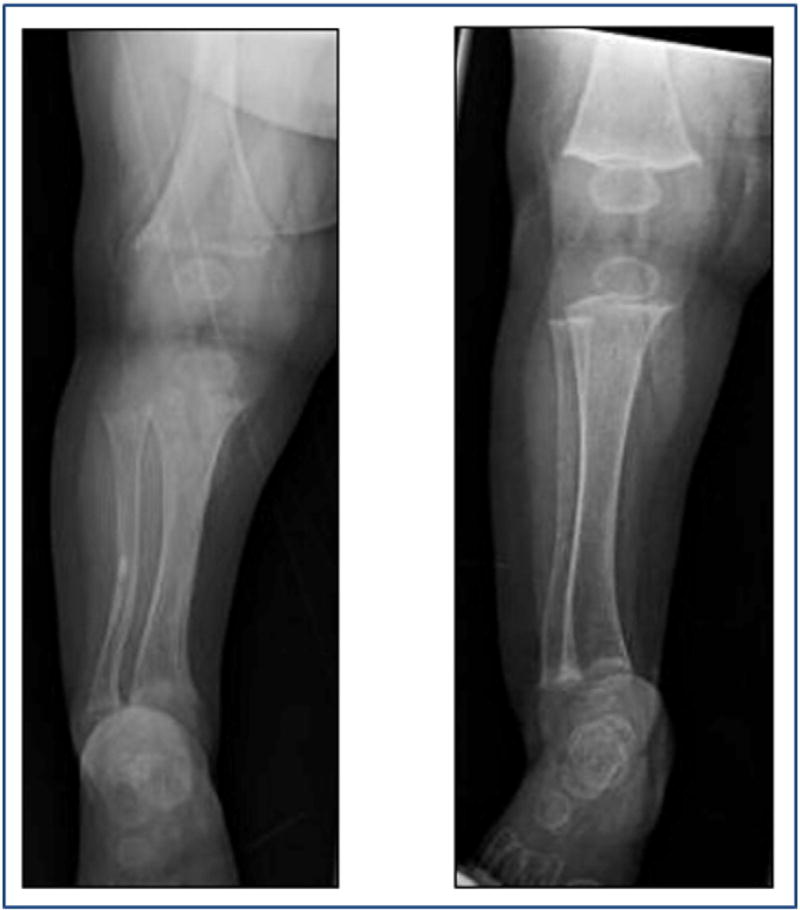
Fig. 2.
Radiographs showing severe rickets at presentation (left) and improvement 5 weeks after formula change (right).
Overall, biochemical abnormalities resolved when supplements were introduced or formula was changed, and bone disease improved with continued therapy. In all cases where frequent follow up data is available, correction of the serum P occurred within 2–7 days after intervention, however no systematic determination of the rapidity of this correction was possible. Decrements in the elevated serum alkaline phosphatase values occurred within weeks, but the correction to the normal range was quite variable and may reflect the chronicity of precedent hypophosphatemia and severity of the bone disease. Radiographic improvement of rachitic disease was often evident by 4–8 weeks when X-ray images were available in this time frame.
4. Discussion
We describe a cohort of infants and children with previously unrecognized hypophosphatemia accompanied in most cases by significant bone disease. All had in common the use of amino-acid based infant or pediatric formulas. The formulas were prepared by a single manufacturer (Nutricia: Neocate Infant®, Neocate Junior®, and Neocate Advance®), although the possibility that hypophosphatemia may occur with other amino-acid based formulas cannot be excluded. Review of records from several patients in the series documented hypophosphatemia early in the course of formula use, but was not initially interpreted as related to the feedings. As some features had been evident several years prior to this analysis, it is unlikely that alterations in formulation or manufacturing processes account for the findings. It is presently unclear why some children experience the complication while others do not. Preliminary findings from a recent prospective study found no significant mineral deficiencies when Neocate® was used in children with isolated milk protein allergy and who were otherwise healthy [5].
The patients that we describe have in common complicated and diverse clinical histories without obvious unifying features. Many, but not all, were born prematurely, though hypophosphatemia was often observed far beyond the neonatal period. The majority were affected with gastro-intestinal diagnoses. Most were tube-fed via gastric or post-pyloric routes. Thus the hypophosphatemia may reflect an as yet unidentified interaction between patient factors and formula. The formula products used would be expected to meet or exceed recommended intakes of all minerals, including phosphorus. For instance Neocate Infant® formula contains 82.2 mg of phosphorus per 100 kcal [6], yet hypophosphatemia occurred. This is comparable to the phosphorus content of alternate amino acid formulas (Elecare® contains 84.2 mg of phosphorus/100 kcal).
The biochemical profile in all infants strongly suggests limited intestinal absorption of phosphate. The maximal renal conservation of phosphate documented in most cases at the time of diagnosis is a normal physiologic response to phosphate depletion, and excludes renal phosphate losses as causal. The marked elevation in circulating 1,25(OH)2D levels is a classic endocrine response to phosphate deprivation [7]. In two cases where available, circulating FGF23 levels were in the low range, as would be expected to occur with inadequate dietary phosphate, and allowing for the increase in 1,25(OH)2D.
Although these findings implicate that the hypophosphatemia resulted from reduced mineral bioavailability from the formula, an underlying mechanism remains unclear. Furthermore, the marked elevation in serum phosphorus following phosphate supplementation or formula change suggests a robust capacity of the native intestine to absorb phosphate when an alternate form of the mineral is provided. The hyperphosphatemia in this acute setting is likely explained by upregulation of Na-Pi2b sodium-phosphate cotransporters in the intestine, as would occur during phosphate starvation [8]. The accompanying low urinary phosphate excretion is best explained by upregulation of renal tubular Na-Pi2a and Na-Pi2c cotransporters, a well-known adaptation to phosphate starvation [9]. As it takes some time to downregulate these adaptive responses, the marked increase in serum phosphorus acutely after provision of a bioavailable form of the mineral would be predicted to occur, as the upregulated transporters would allow for robust intestinal absorption and renal tubular reabsorption of phosphate, compromising the capacity to excrete the newly acquired phosphate load.
Mineral solubility is a well-recognized problem in the preparation of parenteral nutrition solutions, affected by concentrations of calcium, phosphorus and amino-acids, as well as temperature and pH [10]. As precipitation issues are not evident with many formulas of similar mineral content, formula mineral concentration is not a likely explanation, however phosphate used in Neocate is listed as dibasic calcium phosphate, whereas calcium phosphate and potassium phosphate are used in Elecare, so differences exist in the species of phosphate salts employed in these formulas. Moreover, pH impacts mineral solubility and may vary from patient to patient, giving rise to variable clinical presentation, and the pH of the environment into which formula is delivered could significantly impact mineral solubility in vivo. Many children in this series were treated with gastric acid modifying agents, mostly PPI medications, likely resulting in greater than physiologic gastric pH. Nevertheless, no identifiable measures of systemic acid/base abnormalities were accompanied by these findings. Furthermore, correction of phosphate status occurred without alteration of acid-modifying (or other) medications. In addition, several children were fed postpylorically where the local pH is expected to be neutral [11]. Increasing pH (particularly in the non-acid range) adversely affects calcium and phosphate solubility and offers an attractive hypothesis for the apparently impaired mineral absorption [10]. A preliminary study describes 4 cases of hypophosphatemia in association with high doses of PPI medications in children receiving gastrostomy tube feedings [12], however, formula use was not reported. In our series formula change and/or phosphate supplementation resulted in correction of the serum phosphorus level while patients continued to receive acid-modifying agents. In case vignette 5, the reappearance of hypophosphatemia when Neocate formula was re-introduced after brief discontinuation emphasizes the peculiar association of the Neocate formula and hypophosphatemia. Another consideration is the possibility that materials in feeding tubes could bind to phosphate products, thereby affecting delivery of phosphate to the child, however this seems unlikely in view of the multiplicity of centres identifying the problem, and the use of various feeding methods (via gastrostomy, as well as oral, nasogastric, and transpyloric feeds). Case vignette 5 describes the occurrence of hypophosphatemia with a specific formula, which was not evident after a formula change using the same feeding method.
As this condition has not previously been described and optimal management is unknown, the treatment of children in this series varied considerably. Biochemical findings and bone radiographs improved with mineral supplementation, or formula change. We have not identified any specific differences among these formulas that would explain a potential difference in the bioavailability of phosphate. Caution is necessary in initiating phosphate supplementation or transition to a new formula and should be done gradually to avoid severe hyperphosphatemia and hypocalcemia. The “hungry bone” syndrome may further compound this phenomenon as rapid skeletal mineralization ensues [13]. Continued monitoring to ensure clinical improvement, normalization of biochemical abnormalities is indicated, and periodic follow up is warranted to avoid recurrent abnormalities.
It appears from an increasing recognition of cases that the problem is not sporadic. After bringing this issue to the attention of the manufacturer, a statement was issued directed to prescribers of the formula recommending periodic monitoring of serum levels of micronutrients, including phosphate, in children with complex diseases [14]. We recommend periodic measurement of serum phosphorus, calcium and alkaline phosphatase; potential benefits of early identification and treatment of the condition would be predicted to offset the small costs of screening, at least until specific risk factors can be delineated. In children with overt clinical findings such as linear growth failure, radiologic osteopenia, rickets or fractures, a more extensive evaluation is indicated, including measurement of serum phosphorus, calcium, creatinine, alkaline phosphatase, 25-OHD and PTH. Assessment of urinary phosphorus and creatinine excretion and calculation of the tubular reabsorption of phosphate provide definition of renal phosphate handling, and measurement of serum 1,25(OH)2D may serve as a corroborative test.
Limitations of this study include its retrospective nature, and the primarily descriptive data available. The lack of uniformity in the data collection makes it difficult to establish a necessary duration of exposure for the development of the condition. These constraints also preclude the identification of a specific mechanism for the finding, however the physiologic markers identified were sufficient to lead clinicians to management directed toward enhancing the nutritional phosphorus supply, which successfully corrected the condition. Finally, we cannot confirm a distinct host factor(s) which may contribute to the presumed impairment in phosphate availability, and can only speculate in this regard.
5. Conclusion
Clinicians should be aware of the potential for significant hypophosphatemia and bone disease in children receiving amino-acid based formula products (especially Neocate®), and particularly those with medically complex conditions receiving formula as their sole source of nutrition. The children in this series were managed with supplemental phosphate or alternative formula products, implicating reduced bioavailability of phosphorus in the formula for certain individuals. Patient-related risk-factors, and an understanding of the mechanism by which the availability of the phosphate provided in the formula is limited or compromised require further investigation.
Supplementary Material
Acknowledgments
We thank Dr. Renee Mobley for her assistance with preparation of the graphic material and Drs. Mobley and Melinda Sharkey for informative and instructive discussion.
Funding source
Dr. Gordon was funded in part by NIH/NIDDK grant T32DK065522. No other funding was secured for this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bone.2017.02.003.
Disclosures
Thomas O. Carpenter and Linda Casey have performed consulting services for Nutricia, North America. The other authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of interest
Ruth Faircloth, MD: The views expressed are those of the authors and do not reflect the official policy or position of the US Army, Department of Defense nor the US Government. The other authors have no potential conflicts of interest to disclose.
References
- 1.Imel EA, Econs MJ. Approach to the hypophosphatemic patient. J. Clin. Endocrinol. Metab. 2012;97(3):696–706. doi: 10.1210/jc.2011-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insogna KL, Bordley DR, Caro JF, Lockwood DH. Osteomalacia and weakness from excessive antacid ingestion. JAMA. 1980;244(22):2544–2546. [PubMed] [Google Scholar]
- 3.Food and Agricultural Organization of the United Nations, World Health Organization. Standard for infant formula and formulas for special medical purposes intended for infants; Codex Stan 72, Codex Alimentarius. [Last accessed May 5, 2016];International Food Standards. 1981 www.fao.org/input/download/standards/288/CXS_072e_2015.pdf.
- 4.Ardeshirpour L, Cole DEC, Carpenter TO. Evaluation of bone and mineral disorders. Pediatr. Endocrinol. Rev. 2007;5(Suppl. 1):584–598. [PubMed] [Google Scholar]
- 5.Harvey BM, Harthoorn LF, Burks AW. Mineral status of infants requiring dietary management of cow's milk allergy by using an amino acid-based formula. World Congress of Pediatric Gastroenterology, Hepatology and Nutrition (WCPGHAN), Montreal Canada. J. Pediatr. Gastroenterol. Nutr. 2016;63(Suppl. 2):S399. (1172) [Google Scholar]
- 6.Nutricia products guide/website. http://nutricia.com/our-products/paediatrics-nutrition/neocate/what-is-neocate.
- 7.Prié D, Friedlander G. Reciprocal control of 1,25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clin. J. Am. Soc. Nephrol. 2010;5(9):1717–1722. doi: 10.2215/CJN.02680310. [DOI] [PubMed] [Google Scholar]
- 8.Hattenhauer O, Traebert M, Murer H, Biber J. Regulation of small intestinal Na-Pi type IIb cotransporter by dietary phosphate intake. Am. J. Phys. 1999;277(4):G756–G762. doi: 10.1152/ajpgi.1999.277.4.G756. [DOI] [PubMed] [Google Scholar]
- 9.Blaine J, Weinman EJ, Cunningham R. The regulation of renal phosphate transport. Adv. Chronic Kidney Dis. 2011;1(2):77–84. doi: 10.1053/j.ackd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 10.MacKay M, Jackson D, Eggert L, Fitzgerald K, Cash J. Practice-based validation of calcium and phosphorus solubility limits for pediatric parenteral nutrition solutions. Nutr. Clin. Pract. 2011;26(6):708–713. doi: 10.1177/0884533611426435. [DOI] [PubMed] [Google Scholar]
- 11.Ovesen L, Bendtsen F, Tage-Jensen U, et al. Intraluminal pH in the stomach, duodenum, and proximal jejunum in normal subjects and patients with exocrine pancreatic insufficiency. Gastroenterology. 1986;90(4):958–962. doi: 10.1016/0016-5085(86)90873-5. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe-Wylie M, Mouzaki M, Sochett E, Harrington J. Chronic high dose proton-pump inhibitors as a cause of hypophosphatemic rickets. Abstract at Endocrine Society's 98th Annual Meeting and Expo. 2016 Apr 1–4; (Boston http://press.endo-crine.org/doi/10.1210/endo-meetings.2016.BCHVD.16.FRI-348)
- 13.Witteveen JE, van Thiel S, Romijn JA, Hamdy NA. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur. J. Endocrinol. 2013;168(3):R45–R53. doi: 10.1530/EJE-12-0528. [DOI] [PubMed] [Google Scholar]
- 14.Update on monitoring micronutrients. Nutricia learning center website. http://www.nutricialearningcenter.com/globalassets/pdfs/gi-allergy/update-on-monitoring-micronutrients_mar2016.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.