Abstract
Two distinct defense strategies provide a host with survival to infectious diseases: resistance and tolerance. Resistance is dependent on the ability of the host to kill pathogens. Tolerance promotes host health while having a neutral to positive impact of pathogen fitness. Immune responses are almost inevitably defined in terms of pathogen resistance. Recent evidence has shown, however, that several effects attributed to activation of innate and adaptive immune mechanisms, cannot be readily explained with the paradigm of immunity as effectors of microbial destruction. This review focuses on integrating the concept of disease tolerance into recent studies of immune system function related to the regulation and resolution of tissue damage, T cell exhaustion, and tolerance to innocuous antigen.
Introduction
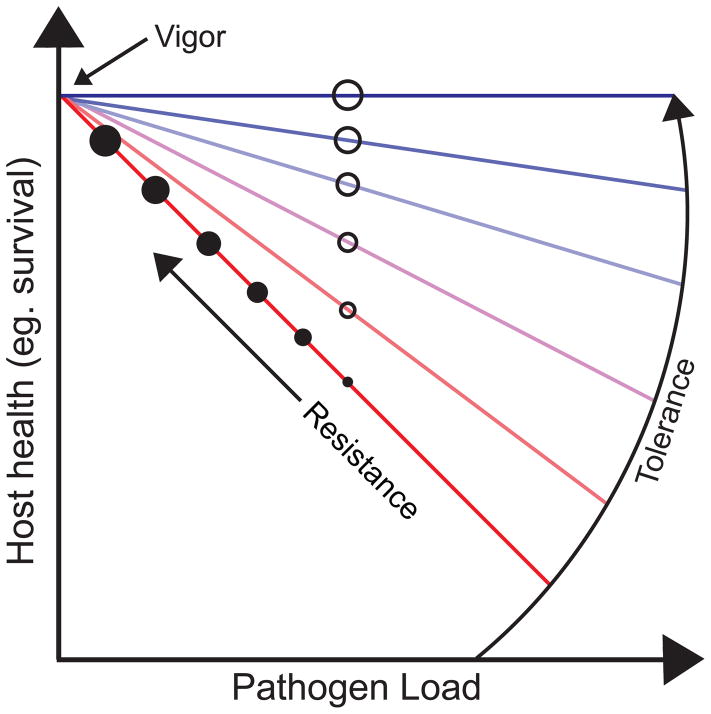
Survival of infectious diseases has traditionally been thought to be entirely dependent on the ability of the host to kill an invading pathogen through the execution of resistance mechanisms [1–3]. In the past decade, a distinct defense strategy called disease tolerance has been recognized as an essential component of the host defense response to infections [1–4]. Disease tolerance is distinct from resistance because it protects the host by promoting host health while having a neutral to positive effect on pathogen fitness. Disease tolerance is not to be confused with immunological tolerance, which involves the elimination of self reactive T cells, although in certain disease contexts immunological tolerance can be considered a disease tolerance mechanism. Experimentally, the contribution of resistance and disease tolerance to any host-microbe system can be distinguished by measuring a parameter of host health (eg. Survival, tissue damage) and examining its relationship to pathogen burden in the relevant tissues (Figure 1) [5]. The past fifty years of immunology have done an excellent job of dissecting the mechanism of resistance. An understanding of the basic mechanisms and contributions of disease tolerance to host defense is a necessary facet for current and future immunological research.
Figure 1.
Overview of host tolerance and resistance measurement. A dose dependent curve can be generated from the relationship between host health (eg. survival) and pathogen burden, where vigor represents uninfected hosts. Host resistance (closed circle) is defined as a decrease in pathogen burden as host health (eg. survival) increases (linear relationship). Increases in host resistance are represented by increasing circle size. In comparison, host tolerance (open circle) is defined as an increase in host health (eg. survival) independently of pathogen burden. Increases in host tolerance are represented by increasing circle size and slope color (red= low tolerance, blue= high tolerance). Adapted from [1,4].
Mechanisms that mediate resistance are well described and involve the response of the immune system and downstream events leading to the execution of microbial killing. The underlying mechanisms that mediate disease tolerance are just beginning to be discovered. While a common assumption is that the mediators of tolerance will be distinct from those that control resistance, recent evidence suggests that functions of the immune system cannot be ascribed to only microbial killing mechanisms and rather also contribute to disease tolerance defenses. In this review, we will discuss recent work suggesting that molecular and cellular components of both the innate and adaptive immune systems are critical regulators of disease tolerance defenses and discuss how these recent advances may shape future research.
Regulation and resolution of tissue damage
An inevitable consequence of host responses to inflammatory stimuli such as pathogens or physical injury is immunopathology of host tissues. Host encoded mechanisms preventing the onset or supporting resolution of immunopathology in host tissues are components of the disease tolerance defense system [1]. Recent studies have demonstrated that cellular components of the immune system are essential for mediating these health promoting effects. Amphiregulin is an epidermal growth factor receptor ligand that is important for regulating tissue damage induced by viral infections. Earlier work demonstrated that amphiregulin prevented viral-induced lung pathology in mice and suggested that amphiregulin is produced largely by innate lymphoid cells (ILCs) 2 in an IL-33-dependent manner [6]. More recent work has identified an alternative source for amphiregulin in the lung. Arpaira et al. (2015) demonstrated that amphiregulin from T regulatory (Tregs) cells prevents lung immunopathology induced by influenza infection. Interestingly, amphiregulin was efficacious independent of influencing the suppressive capacity of Tregs (T cell receptor (TCR)-independent) [7,8]. Further, they confirmed that IL-33 is critical for amphiregulin induction, along with another “innate type” cytokine, IL-18 [7]. In immunocompromised hosts (Tlr2−/−Tlr4−/−) administration of amphiregulin to animals co-infected with Influenza (LP02ΔdotAΔflaA) and Legionella pneumophila alleviated lung damage and promoted host survival without influencing viral or bacterial load [9]. Interestingly, disease tolerance mediated by amphiregulin may extend beyond protection from respiratory challenges. Administration of dextran sulfate sodium to mice induces mucosal erosion and intestinal injury resulting in a “colitis” like disease. Monticelli et al. (2015) showed that amphiregulin can also alleviate DSS-induced colitis. The intestinal microbiome contributes to the pathogenesis of DSS induced colitis and with without measurements of the microbiome composition and levels, it remains unclear if amphiregulin is mediating resistance or disease tolerance in this context [10].
The mechanisms that regulate amphiregulin induction remain unclear but recent work suggests that proresolving lipid mediators, molecules that possess both resistance and disease tolerance traits, may be one such mechanism [11]. One way in which proresolving lipid mediators may support tolerance mechanisms is through the de novo generation of forkhead box P3 (FoxP3)-expressing Tregs [12,13]. The proresolving lipid mediator maresin-1 promoted the generation of Tregs and regulated cytokine production from ILC2s including increased amphiregulin production in the murine lung [13]. Although amphiregulin contributes to disease tolerance and survival of infections, it remains to be formally tested if the proresolving lipid mediators themselves mediate disease tolerance through their regulation of amphiregulin.
Pattern recognition receptors of the innate immune system are traditionally appreciated for their role in executing antimicrobial responses during challenge. However, several studies over the past decade have extended the function of these sensors to resolving immunopathology through tissue repair functions, suggesting that they are important for mediating disease tolerance. Beneficial microbes, including those of the intestinal microbiome, encode microbial associated molecular patterns (MAMPS) that activate innate immune receptors. In a DSS colitis model, recognition of commensal microbiome derived ligands by Toll Like Receptors (TLRs) was critical for mediating tissue repair in the colon [14]. More recent studies have shown that the various inflammasomes can also promote tissue repair. Inflammasomes are cytosolic multiprotein complexes that are required for the activation of Caspase 1 protease and subsequent maturation of inflammatory responses. Casp1−/−, Asc−/− and Il18−/− mice exhibited greater disease severity, barrier permeability, microbial translocation and defective cell proliferation when treated with DSS. Administration of recombinant IL-18 to Casp1−/− mice reduced disease severity, supporting a role for IL-18 in promoting repair of the gut epithelium. Thus, activation of the inflammasome and other innate immune receptors in the intestine may promote disease tolerance through tissue repair, alleviating dehydration, electrolyte imbalances and anemia that could be caused by gut damage. An important caveat of these studies is that the levels and composition of the microbiomes were not appropriately evaluated and thus it remains to be formally tested whether the effects of these receptors on tissue repair promote disease tolerance or resistance against the microbiome [14–19]. In addition to the role of these receptors at mucosal surfaces, the role these sensors play in tolerance and resistance to disease beyond the gut are being explored [20,21].
Organismal metabolism and immune crosstalk
Infections lead to profound changes in host metabolism that affect the outcome of the host. For example, many types of infections lead to the sickness induced anorexia that is mediated by the actions of the innate immune system on the central nervous system and brain. In 1979, Murray and Murray showed that blocking the anorexic response was detrimental for host survival of a systemic bacterial infection [22]. In a fruit fly model, Ayres and Schneider showed that any benefits of the anorexic response for host outcome were context dependent [23]. In a systemic Salmonella model, anorexia was beneficial to the host by mediating disease tolerance [23]. However, when challenged with a systemic Listeria infection, the anorexic response rendered the flies less resistant to the infection [23]. Similar context dependent effects of anorexia on host defenses have been demonstrated in the mouse and involve complex interactions between the host metabolism and the immune response to challenge. During systemic bacterial infection, reactive oxygen species (ROS) produced as a component of the resistance response, can cause neuronal damage, that can be reversed by a higher abundance of ketone bodies triggered by the anorexic response leading to disease tolerance. However, during viral infection, higher glucose concentrations mediated by force feeding to override the anorexic response, promoted disease tolerance by preventing unfolded protein response (UPR)-induced neuronal dysfunction. This glucose induced tolerance mechanism was dependent on the innate immune system and type I interferon signaling [24].
The metabolic state of the host will likely affect the pathogen and to understand how sickness induced anorexia and other metabolic adaptations of the host will affect host outcome of infection, this physiological response needs to be studied using systems that allows examination of both host and pathogen behavior. Using a naturally occurring host-pathogen system in which the host and pathogen co-evolved together, Rao et al. (2017) demonstrated that the anorexic response was detrimental to mice orally infected with Salmonella Typhimurium. The anorexic response triggered extraintestinal dissemination of the pathogen leading to accelerated death kinetics. This increased virulence triggered by the anorexic response came at a cost to pathogen transmission to new hosts. The authors found that Salmonella Typhimurium evolved a mechanism to inhibit the anorexic response by preventing inflammasome activation in the small intestine, dampening the inflammatory signal to the brain via the vagus nerve, preventing the induction of the anorexic response [25]. By doing so, the pathogen prevented its extraintestinal invasion, promoting host survival and pathogen transmission. Other mechanisms behind fasting-induced disease tolerance have also been implicated, including a role for autophagy [26,27]. It is been well characterized that fasting can induce autophagy in several host cell types allowing survival by self-protein degradation [28,29]. Interestingly, a study revealed that autophagy induced host tolerance to Staphylococcus aureus lung infection, which depended on its ability to produce alpha-toxin [26]. In these examples, immune-triggered metabolic adaptations of the host during infection regulated behavioral changes in the pathogens that affected their virulence rather than inducing a disease tolerance mechanism. Understanding how microbial virulence is regulated by host physiological responses during infection and how these behavioral changes relate to disease tolerance will be important for future work.
Metabolic perturbations during infection can result from direct effects of the host immune response to infection on metabolic stores. For example, muscle wasting is a common metabolic response that occurs in invertebrates and vertebrates in response to diverse infectious and non-infectious insults. In a Drosophila model, muscle wasting induced by the pathogen Mycobacterium marinum, decreased host survival without affecting pathogen burdens, suggesting that regulation of muscle wasting may contribute to disease tolerance [30]. A more recent study by Schieber et al. (2015) demonstrated that activation of the inflammasome by the intestinal microbiome blocks muscle wasting induced by infectious diseases or chemically induced colitis, and in doing so, promotes disease tolerance. This study showed that during Burkholderia, Salmonella or DSS-induced colitis, a commensal E. coli strain, translocates from the intestine to white adipose tissue deposits. In an NLRC4 inflammasome dependent manner, this commensal induces WAT production of insulin-like growth factor-1 (IGF-1). This is released into circulation and signals to skeletal muscles, preventing induction of muscle specific E3 ubiquitin ligase that induce muscle atrophy [21]. This protection occurs without negatively influencing Burkholderia or Salmonella levels, indicating this E. coli commensal can promote disease tolerance by antagonizing muscle wasting. This study not only attributes innate sensing and immunity to disease tolerance but also suggest a role for microbial composition in the contribution and maintenance of immunity that contributes to disease tolerance [20]. More research is required to detangle the relationship between metabolism and immunity in the context of disease tolerance. For instance, studies are beginning to elucidate the decline of the immune system with aging [31]. Of interest may be the link between dysregulated metabolism and increasing immunity during aging, and the effects on disease tolerance.
T cell exhaustion and disease tolerance
T cell exhaustion is a process in which there is a loss of proliferative, cytotoxic capacity and potential apoptotic death of the T cell [32]. On the one hand, T cell exhaustion can be viewed as a mechanism to promote disease tolerance, for example during graft vs host disease or autoimmunity, because it dampens the responsiveness of T cells. On the other hand it can contribute to disease such as cancer or chronic infection [33]. Therefore, targeting T cell exhaustion, either positively or negatively, affects disease tolerance. Cellular metabolism has emerged as regulator of T cell exhaustion. The cytotoxic efficacy of T cells is positively influenced under hypoxic conditions and mediated by hypoxia-inducible factor 1-α (HIF1-α) [34]. HIF1-α also negatively controlled the development of Type 1 regulatory T cells (Tr1) [35,36]. Recent research has found ways of targeting T cell metabolism, including HIF1-α, pathways for disease treatment. Clever et al. (2016) showed that in T cells, the oxygen-sensing prolyl-hydroxylase (PHD) represses HIF-driven glycolytic metabolism. PHD suppressed pulmonary inflammation driven by innocuous antigen. However, inhibiting PHD improved cancer immunotherapy [37]. In some cases, negatively influencing T cell function through metabolic targeting restores disease tolerance. For example, Yin et al. (2015) were able to reverse a mouse model of autoinflammatory lupus (B6.Sle1.Sle2.Sle3) by treating with the mitochondrial inhibitor metformin and the glucose metabolism inhibitor 2-deoxy-d-glucose (2DG). These inhibitors reduced IFN-γ production from CD4+ T cells that entered an exhausted T cell state [38].
Recently the role of the epigenome has been established in T cell exhaustion. A study by Schietinger et al. (2012) suggested that there is a specific gene expression profile in CD8+ T cells that is characteristic of a tolerant state [39]. It was later established that exhausted T cells have a specific epigenetic fingerprint [40]. Links between the epigenome and T cell exhaustion are further validated by the development of successful targeted treatments. This has been recently emphasized in a study that showed that de novo DNA methylation induced T cell exhaustion and that PD-1 immunotherapy could not overcome this DNA-methylation state. However, the combination of both programmed cell death protein-1 (PD-1) immunotherapy and blocking methylation was efficacious at tumor control. Current studies have also identified a crossover between controlling the epigenome and T cell metabolism [41]. For instance, like HIF1-α, 2-hydroxyglutarate (S-2HG) is induced under hypoxic conditions in CD8+ T cells and is dependent on HIF1-α. S-2HG treatment reversed exhausted states and increased anti-tumor efficacy. Interestingly, the effects of S-2HG were dependent on histone and DNA methylation [42]. A more recent study suggested that S-2HG also controls methylation of the Foxp3, and inhibited Treg differentiation and induced Th17 cells. Inhibition of a pathway [with (aminooxy)acetic acid] upstream of S-2HG resulted in disease tolerance in a model of experimental autoimmune encephalomyelitis [43].
Like in the regulation of tissue damage and immunometabolism and disease tolerance, environmental factors have been implicated in mechanisms of T cell exhaustion. Recent studies showed that the composition of the gut microbiota facilitated the efficacy of cancer immunotherapy [(cytotoxic T-lymphocyte-associated protein 4) CTLA-4 and PD-L1] in mice, making the drugs more efficacious at alleviating T cell exhaustion [44,45].
Innocuous antigens, immunity and disease tolerance
While disease tolerance is conceptually distinct from immunological tolerance, we propose that in certain contexts, immune mechanisms that control the lack of response to innocuous antigens, such as those oral fed or self-antigen will function as disease tolerance mechanisms. The function of Tregs is critical for this process [46–50] and re-establishment of oral tolerance (desensitization) through immunotherapy are targeted towards Tregs [51]. This is achieved through administration of the antigen in increments of increasing doses, which can expand antigen-specific Tregs [52]. Some clinical trials have been successful [53–55], however, it is becoming clear that this process is complicated and issues such as the age of antigen introduction and route of administration are critical to preventing sensitization and inducing desensitization to antigen [56–58]. Further, the role of environmental factors cannot be ignored, such as viral infection or the gut microbiota, as these can influence the sensitization process and inflammatory responses to innocuous dietary antigens [59,60].
Conclusions
Host immune mechanisms can promote both resistance and tolerance to disease. Most research efforts into infectious diseases focus on resistance mechanisms. However, the use of host resistance mechanisms can come at a cost, the emergence of an arms race between host and pathogen. The studies highlighted in this review suggest that tolerance to disease (eg. infection) is not just dependent on cellular and whole host metabolism, and environmental factors but also host immunity. Investigations into the promotion of host disease tolerance through modulation of immunity by medications or modulation of environmental factors will help eliminate the competitive nature of resistance and can be applied to a range of disorders, not just infection. The emerging evidence demonstrating that the immune system is a mediator of disease tolerance raises the interesting question of what which function of the immune system evolved first: to mediate disease tolerance or resistance?
Highlights.
Resistance and tolerance are two separate components of the host immune response
Immune mechanisms of disease tolerance function independently of pathogen killing
Studying tolerance to pathogens could reveal novel immune mechanisms
Tolerance requires collaboration between host metabolism and immunity
Environmental factors (eg. microbiota) influence immunity and tolerance to disease
Acknowledgments
Funding
JSA is supported by NIH grant R01AI114929, The Nomis Foundation, the Searle Scholar Foundation, the Ray Thomas Edward Foundation, the Helmsley Charitable Trust and CCFA Senior Award.
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 2.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc Lond B Biol Sci. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *4.Ayres JS. Cooperative Microbial Tolerance Behaviors in Host-Microbiota Mutualism. Cell. 2016;165:1323–1331. doi: 10.1016/j.cell.2016.05.049. A perspective that reviews the current literature of disease tolerance to pathogens but also expolores the idea that pathogen behaviour may also be employed by beneficial bacteria (eg. commensal microbiota) in order to promote host tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 6.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. Demonstrates that the majority of lung amphiregulin was produced by Tregs which was critical to tolerance to immunopathology against viral lung infection and whose mechanism occurred independly of the TCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carney K, Chang YM, Wilson S, Calnan C, Reddy PS, Chan WY, Gilmartin T, Hernandez G, Schaffer L, Head SR, et al. Regulatory T-cell-intrinsic amphiregulin is dispensable for suppressive function. J Allergy Clin Immunol. 2016;137:1907–1909. doi: 10.1016/j.jaci.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, Medzhitov R. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340:1230–1234. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A. 2015;112:10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8:353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, et al. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. 2015;194:863–867. doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol. 2011;186:7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Schieber AM, Lee YM, Chang MW, Leblanc M, Collins B, Downes M, Evans RM, Ayres JS. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science. 2015;350:558–563. doi: 10.1126/science.aac6468. Evidence that the gut microbiome was critical for supporting disease tolerance to a range of inflammatory insults. Mice harbouring a gut commensal E. coli capable of stimuliting IGF-1 signaling via inflammasome activation in white adipose tissue promoted host tolerance to muscle wasting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray MJ, Murray AB. Anorexia of infection as a mechanism of host defense. Am J Clin Nutr. 1979;32:593–596. doi: 10.1093/ajcn/32.3.593. [DOI] [PubMed] [Google Scholar]
- 23.Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 2009;7:e1000150. doi: 10.1371/journal.pbio.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **24.Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, Medzhitov R. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell. 2016;166:1512–1525. e1512. doi: 10.1016/j.cell.2016.07.026. Revealed an opposing effect of fasting on disease tolerance to bacterial or viral infection. During systemic bacterial infection the anorexic response was protective due to high abundance of keytone bodies, however, during viral infection force feeding was protective due to high glucose concentrations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **25.Rao S, Schieber AM, O’Connor CP, Leblanc M, Michel D, Ayres JS. Pathogen-Mediated Inhibition of Anorexia Promotes Host Survival and Transmission. Cell. 2017;168:503–516. e512. doi: 10.1016/j.cell.2017.01.006. Authors showed that the anorexic response due to oral bacterial infection by Salmonella Typhimurium was detrimental. Authors demonstrated that Salmonella has evolved a mechanism to inhibit the host anorexic response (by gut-brain axis) to increase pathogen transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer K, Reyes-Robles T, Alonzo F, 3rd, Durbin J, Torres VJ, Cadwell K. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe. 2015;17:429–440. doi: 10.1016/j.chom.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Niekerk G, Loos B, Nell T, Engelbrecht AM. Autophagy--A free meal in sickness-associated anorexia. Autophagy. 2016;12:727–734. doi: 10.1080/15548627.2016.1147672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 29.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol. 2006;16:1977–1985. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 31.Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, Ganio EA, Fragiadakis GK, Spitzer MH, Douchet I, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med. 2017;23:174–184. doi: 10.1038/nm.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu SR, Reddy P. Tissue tolerance: a distinct concept to control acute GVHD severity. Blood. 2017;129:1747–1752. doi: 10.1182/blood-2016-09-740431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsiao HW, Hsu TS, Liu WH, Hsieh WC, Chou TF, Wu YJ, Jiang ST, Lai MZ. Deltex1 antagonizes HIF-1alpha and sustains the stability of regulatory T cells in vivo. Nat Commun. 2015;6:6353. doi: 10.1038/ncomms7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, et al. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell. 2016;166:1117–1131. e1114. doi: 10.1016/j.cell.2016.07.032. Expression of an oxygen sensing protein by T-cells was critical for tolerance to innocuous antigens in the lung but endorses tumor cell colonization. These proteins supported the development of Tregs and decreased cytotoxic T cell abundance and inhibition allowed clearance of tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra218. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, Carter R, Awad W, Neale G, Thomas PG, et al. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell. 2017;170:142–157. e119. doi: 10.1016/j.cell.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Velica P, You J, Chia GS, Sim J, Doedens A, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu T, Stewart KM, Wang X, Liu K, Xie M, Kyu Ryu J, Li K, Ma T, Wang H, Ni L, et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature. 2017 doi: 10.1038/nature23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 50.Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamad A, Burks W. Oral tolerance and allergy. Semin Immunol. 2017 doi: 10.1016/j.smim.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O’Riordan G, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caminiti L, Pajno GB, Crisafulli G, Chiera F, Collura M, Panasci G, Ruggeri P, Guglielmo F, Passalacqua G. Oral Immunotherapy for Egg Allergy: A Double-Blind Placebo-Controlled Study, with Postdesensitization Follow-Up. J Allergy Clin Immunol Pract. 2015;3:532–539. doi: 10.1016/j.jaip.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, Wood RA. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135:1275–1282. e1271–1276. doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burks AW, Wood RA, Jones SM, Sicherer SH, Fleischer DM, Scurlock AM, Vickery BP, Liu AH, Henning AK, Lindblad R, et al. Sublingual immunotherapy for peanut allergy: Long-term follow-up of a randomized multicenter trial. J Allergy Clin Immunol. 2015;135:1240–1248. e1241–1243. doi: 10.1016/j.jaci.2014.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, Brough H, Marrs T, Radulovic S, Craven J, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N Engl J Med. 2016;374:1733–1743. doi: 10.1056/NEJMoa1514210. [DOI] [PubMed] [Google Scholar]
- 58.Lionetti E, Castellaneta S, Francavilla R, Pulvirenti A, Tonutti E, Amarri S, Barbato M, Barbera C, Barera G, Bellantoni A, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295–1303. doi: 10.1056/NEJMoa1400697. [DOI] [PubMed] [Google Scholar]
- 59.Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, Meisel M, Kim SM, Discepolo V, Pruijssers AJ, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356:44–50. doi: 10.1126/science.aah5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caminero A, Galipeau HJ, McCarville JL, Johnston CW, Bernier SP, Russell AK, Jury J, Herran AR, Casqueiro J, Tye-Din JA, et al. Duodenal Bacteria From Patients With Celiac Disease and Healthy Subjects Distinctly Affect Gluten Breakdown and Immunogenicity. Gastroenterology. 2016;151:670–683. doi: 10.1053/j.gastro.2016.06.041. [DOI] [PubMed] [Google Scholar]