Abstract
Purpose of Review
The Environmental influences on Child Health Outcomes (ECHO) program’s mission is to enhance the health of children for generations to come. In this manuscript, we describe the structure of the ECHO Coordinating Center (ECHO-CC) and its role in developing the infrastructure for the ECHO program.
Recent Findings
The ECHO-CC supports ECHO’s mission by developing the framework of the ECHO program, coordinating multiple levels of membership in the ECHO community, developing ECHO policies and procedures, and fostering communication and engagement inside and outside of ECHO.
Summary
The ECHO-CC has used a number of innovative methods for organization, communication, and engagement to enable the ECHO program to become greater than the sum of its parts.
Keywords: child health, environmental influences, coordination
Introduction
Coordinating Center Overview
The Environmental influences on Child Health Outcomes (ECHO) program will investigate the longitudinal impact of prenatal, perinatal, and postnatal environmental exposures on child health. This program is uniquely poised to capitalize on existing and future data from ECHO cohort awardees, each of whom are examining different aspects of environmental exposures on child health and development. These 35 longitudinal cohort awardees oversee 83 individual pediatric cohorts; across these 83 individual cohorts, there are 248 sites conducting research at 120 unique institutions. Data from all of these cohorts will be leveraged to answer important research questions related to environmental impacts on pediatric health and development.
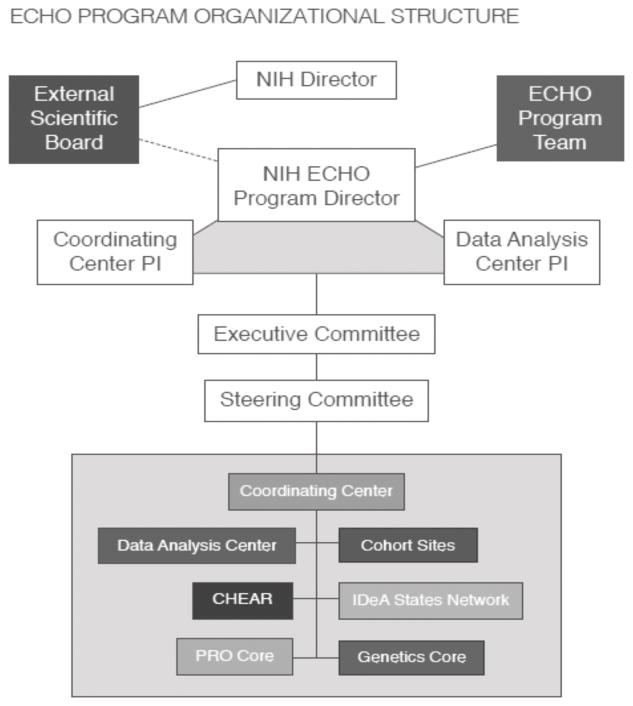
In addition to the cohorts, the ECHO program includes five components: 1) a Coordinating Center; 2) a Data Analysis Center (DAC); 3) a Children’s Health and Exposure Analysis Resource (CHEAR); 4) a Patient-Reported Outcomes (PRO) Core; and 5) the IDeA States Pediatric Clinical Trials Network (ISPCTN) (Figure 1). A standardized, ECHO-wide Cohort Data Collection framework (ECHO-wide Cohort Protocol) will be created to collect and harmonize existing cohort data; this protocol will be expanded to include ongoing data collection. Not only will investigators collect data that is pertinent to a specific area of interest, but they will gather information relative to the focus areas defined by the ECHO Program, which include obesity; positive health; prenatal, perinatal, and postnatal outcomes; upper and lower airway disease; and neurodevelopment. The novelty of the ECHO program lies in its ability to maximize the existing cohort infrastructure to develop one cohesive network that will strive to efficiently address critical research questions about the impact of environmental exposure on key pediatric outcomes with existing and longitudinal data. The ISPCTN is one component of the ECHO program comprised of 17 sites that are designed to provide rural and medically underserved children access to cutting-edge clinical trials; the current research infrastructure of these 17 sites is being expanded to be able to support clinical trials.
Figure 1. ECHO Organizational Structure.
This figure displays organizational structure of ECHO.
CHEAR = Children’s Health and Exposure Analysis Resource; ECHO = Environmental influences on Child Health Outcomes; PRO = Patient-Reported Outcomes
The Duke Clinical Research Institute (DCRI) serves as the ECHO Coordinating Center (ECHO-CC), which supports and empowers the broader children’s health research community to catalyze collaboration networks within the ECHO program. ECHO-CC team members formed partnerships across all ECHO program components and cohorts (Figure 1) as a first step towards building an infrastructure that can support multiple levels of membership in the ECHO community. The ECHO-CC’s goal is to make scientific efforts faster and more efficient while protecting human subjects. More specifically, as the ECHO-CC, the DCRI serves as the administrative hub for ECHO, working to coordinate and provide logistical support for all activities of the ECHO Steering Committee (ECHO-SC) and Executive Committee (ECHO-EC), External Scientific Board, National Institutes of Health (NIH) ECHO Team, ECHO pediatric cohorts, and ECHO components. Under the guidance of the ECHO-SC, the ECHO-CC develops and implements ECHO operating policies, creates and maintains effective communications between ECHO and the public, and facilitates information exchange by creating and maintaining the ECHO web site (http://echochildren.org/).
Using a transdisciplinary approach, the ECHO-CC brings together the right people and appropriate tools to enable collaboration and insight, allowing the ECHO Program to be greater than the sum of its parts (1–3). The ECHO-CC encourages this type of collaboration through the facilitation and leadership of working groups, committees, policies, procedures, communications, engagement, and ongoing program evaluations.
Support of ECHO Committees and Working Groups
During the first year of the ECHO program, the ECHO-CC prioritized establishing committees and working groups focused on accelerating engagement, alignment, and decision-making. The ECHO-CC provided guidance, as well as scheduled and hosted meetings for ECHO leadership committees including the ECHO-SC and the ECHO-EC (Figure 1). The ECHO-SC is the main decision-making body within the ECHO program and includes one member from each of the 35 pediatric cohort awardees, representatives from each of the components, and the NIH. ECHO-EC membership is smaller, with two representatives elected as members from the 35 ECHO cohort awardees, as well as one voting member from each component and the NIH. The smaller ECHO-EC allows for more discussions and faster decisions to take place, which are then recommended to the ECHO-SC for approval, thereby increasing ECHO-SC efficiency.
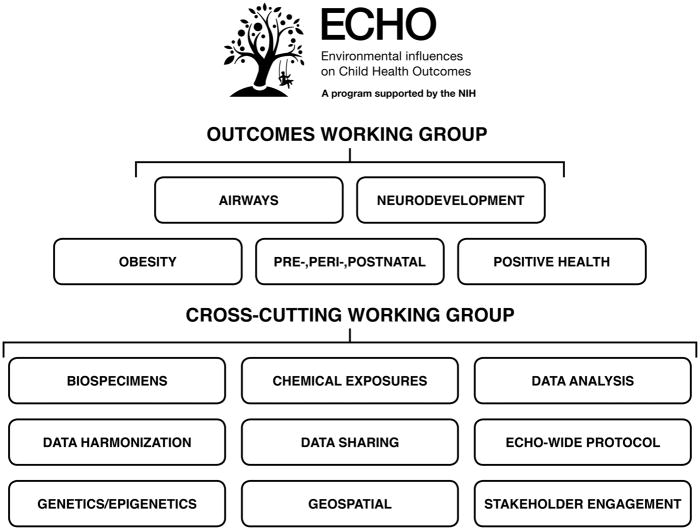
ECHO-CC facilitated the creation and conduct of 14 working groups that contain an average of 84 members (Figure 2). The working groups are chaired by ECHO investigators, and form the backbone of the ECHO program, providing a framework for active, continual engagement to help ECHO constituents meet aggressive project timelines and deliverables. These working groups support the scientific priorities of the ECHO program, performing work independently and collectively as part of an interrelated team. Five Outcomes Working Groups (Airways, Obesity, Neurodevelopment, Prenatal/Perinatal/Postnatal, and Positive Health) gathered at the initial ECHO-SC meeting, developed 18 proposals that leveraged existing data collected by the ECHO cohorts, and solicited 275 key research questions from across the ECHO program. These questions formed the foundation for the ECHO-wide Cohort Protocol. In addition to developing these key research questions, the Outcomes Working Groups partnered with the Protocol Development Working Group to identify which data elements and appropriate measures. Six Cross-Cutting Working Groups (Publications, Biospecimens, Stakeholder Engagement, Data Sharing, Innovations in Data Analysis, and Data Harmonization) developed policies and procedures to support program-wide publication development, as well as data and specimen utilization. Subsequently, ECHO established three additional working groups: the ECHO-wide Cohort Protocol, Chemical Exposures, and Geospatial Working Groups. Non–outcome-focused working groups are defined as Cross-Cutting Working Groups in the ECHO Program.
Figure 2. ECHO Working Groups.
This figure displays the current ECHO Working Groups: five Outcomes Working Groups (Airways, Obesity, Neurodevelopment, Prenatal/Perinatal/Postnatal, and Positive Health) and the Cross-Cutting Working Groups (Publications, Biospecimens, Stakeholder Engagement, Data Sharing, Innovations in Data Analysis, and Data Harmonization).
ECHO = Environmental influences on Child Health Outcomes
The ECHO-CC provides the central point of contact for each of the working groups through project leaders who are responsible for planning and facilitating all meetings and resulting activities. Project leaders proactively identify working group challenges and needs, and then develop and implement solutions in collaboration with the working group chairs. All working groups, with self-selected membership from across ECHO cohorts and components, meet at least monthly. Working group-specific planning teams set the strategy and direction for each group. Planning teams consist of the working group co-chairs, NIH science officer(s), an ECHO-CC faculty member, and DAC representative(s). Planning teams typically meet two to three times per month.
To promote consistency across the working groups, the ECHO-CC team meets frequently to plan for upcoming deliverables and communication needs across the program. Facilitators often use similar methods and materials to increase efficiency, thereby maximizing impact and results across the teams. The ECHO-CC uses real-time polling during the working group meetings to collect feedback and member votes on key working group decisions. Several working groups have formed subgroups to focus on unique patient populations, research topics, or chapters of policies under development. The ECHO-CC coordinated >500 working group meetings in the first year of the ECHO program.
In the second year of the ECHO program, several working groups will complete their initial purpose as they finalize policies and process documents such as data sharing and biospecimen collecting and handling. Working groups may cease over time, and new working groups may be formed (e.g., Genetics/Epigenetics and Microbiome).
Development of ECHO Policies
The ECHO-CC provided leadership and support for investigators developing ECHO program policies. This support included access to example policies, as well as phone and face-to-face meetings. The ECHO-SC approved the ECHO Publications Policy in May 2017. ECHO Policies for Data Sharing and Biospecimen Utilization are anticipated to be approved in November 2017. The ECHO Publication Policy was developed by the ECHO Publication Working Group. The goals of the policy are to provide structure and transparent processes that will facilitate high-impact science, support consortium priorities, and balance quality and efficiency with a collaborative culture that fosters career development among junior faculty. The ECHO Data Sharing Policy, which is being developed by the Data Sharing Working Group, covers submission of data from several sources including: 1) cohort metadata; 2) ECHO eaRly dAta subMission Protocol (RAMP) data; and 3) ECHO-wide Cohort Protocol data.
The Biospecimen Working Group is developing the Biospecimen Policy in two parts: 1) the Collection, Processing, and Storage Policy; and 2) the Biospecimen Utilization Policy. The Collection, Processing, and Storage Subgroup is writing this section of the policy to ensure that samples are collected in the most versatile method possible to facilitate their eventual use for multiple analysis types. The Specimen Utilization Subgroup is addressing biospecimen issues, which include defining access to ECHO biospecimens collected by the 83 ECHO cohorts prior to and during the ECHO-wide Cohort Protocol; this section of the policy seeks to expedite ECHO sample access to efficiently examine a broad range of early environmental exposures on pediatric health.
Communication and Stakeholder Engagement
To raise awareness of the ECHO program and ensure the broadest reach possible, the ECHO-CC focuses substantial effort on engagement and communication (4). We recognize that the success of ECHO is dependent on the consortium’s ability to provide targeted research opportunities, high-quality data, and relevant information to both the research community and the public (5, 6). The ECHO-CC provides regular progress updates to the community of 1,200 ECHO investigators via the biweekly newsletter. The ECHO-CC also develops materials outlining ECHO’s purpose, progress, and results to external stakeholders, as well as provides regular ECHO progress updates on the public web site.
To promote collaboration and transparency, the ECHO-CC strongly supports stakeholder engagement not only within the ECHO community, but across the research and participant advocacy community at large (7–10). The ECHO Stakeholder Engagement Working Group has held webinars for ECHO cohorts and the ISPCTN to inform best practices for participant recruitment, re-contact and re-consent, and community advisory boards and social media in research activities. The ECHO-CC provided specific training for its staff on stakeholder and participant engagement in research. Training also included best practices for productive teamwork, actions and agreements, consensus building, and personality/relationship dynamic management, so that the ECHO-CC project team leaders could use this knowledge in their working group facilitation and other ECHO program tasks. Lastly, we introduced ECHO-CC project leaders to a variety of crowdsourcing tools and opportunities that may be deployed to enhance member engagement in their working group or leadership meetings and activities.
As we move into the second year of ECHO, the ECHO-CC will focus on engaging stakeholders in informed consent (11–15) and policy development work, which will include developing mechanisms to take in feedback from participants and caregivers on the ECHO consent process and forms (16); enhancing the communication toolkit in response to stakeholder needs as work evolves (ECHO overview slides, brochure, branding guidelines, logos); and supporting the Stakeholder Engagement Working Group to develop methods and publications that assess participant recruitment, retention, and study results receipt.
ECHO-CC Output
In the first year, the ECHO Program focused on developing two program-wide protocols: RAMP and the ECHO-wide Cohort Protocol. The ECHO-CC and the DAC proposed RAMP to collect existing data from cohorts as a pilot to: 1) initiate and identify challenges in transferring data to DAC; 2) determine visit structures for child measurements across the ECHO cohorts; 3) describe characteristics of participants in the cohorts; 4) test the single institutional review board (IRB) processes; and 6) initiate data harmonization tasks.
The ECHO-wide Cohort Protocol is a novel, multi-level, longitudinal, data platform that will specify elements and measures for data collection across all 83 ECHO cohorts. The ECHO-wide Cohort Protocol Working Group led program-wide activities in order to identify which data are best positioned to address key scientific questions about environmental influences on child health outcomes. The Outcomes Working Groups provided the initial proposed key research questions and associated data elements. Next, the ECHO-wide Cohort Protocol Working Group established six life-stage subcommittees, organized by pediatric life stage (preconception/prenatal, perinatal, infancy, early childhood, middle childhood, and adolescence). The ECHO-wide Cohort Protocol Working Group met at least twice weekly, including two in-person meetings. The team combined the input from the life-stage subcommittees and Outcomes Working Groups into a comprehensive list of potential data elements and associated measures for data collection.
In the second year, the ECHO-CC will transition support from ECHO-wide Cohort Protocol development to implementation. The ECHO-CC, in collaboration with other ECHO components, will develop study documents (data collection forms, informed consent forms, manual of operations, enrollment materials), processes (IRB approval, data transfer, biospecimen transfer), resources (communication plans, refresher training materials, recurring project meetings), and operational plans (data management plan, data monitoring plan, site monitoring plan). The ECHO-CC will organize and train sites on protocol procedures. Close partnership and communication with enrolling sites will help the ECHO-CC identify barriers and develop targeted solutions for individual sites (17). The ECHO-CC will oversee the establishment of a single IRB for ECHO. The ECHO-CC will manage the single IRB, as well as be responsible for protocol and amendment submission to the single IRB.
The ECHO-CC is also preparing to oversee the ECHO biorepository, which will provide sample collection kitting, shipping instructions, and training in accordance with the ECHO Biospecimens Working Group policies. The Biospecimens Working Group (including members of the NIH, Coordinating Center, and DAC) and the Children’s Health and Exposure Analysis Resource (CHEAR) will work together to develop and operationalize the processes for sample management and analyses.
A unique aspect of the ECHO program is the ECHO Opportunities and Infrastructure Fund (OIF), which will support projects that introduce new research, tools, and technologies. OIF projects should provide benefit to the ECHO program as a whole and the child health community at large. The OIF will encourage collaborations among ECHO awardees to promote transdisciplinary research. The data, tools, and resources generated through OIF will be rapidly shared with ECHO members and subsequently made available to the scientific community and general public. To determine research priorities for each funding cycle, the ECHO-SC will assess ECHO program priorities and determine the targeted area of scientific focus. The ECHO-CC will prepare a tailored request for application for each award cycle. OIF awards will be offered in five different cycles, with approximately 10–12 OIFs awarded per cycle. The ECHO-CC will establish an OIF Review Committee to evaluate applications and rank proposals for funding. Once awardees are selected, the ECHO-CC will contract directly with awardees and oversee progress on their research projects.
ECHO-CC Evaluation and Improvement
The ECHO-CC monitors overall programmatic impact and solicits feedback from ECHO components and cohorts through surveys, direct invitation (via conference calls and face-to-face meetings), and feedback received through cohort representatives on the ECHO-EC. To test the reach and impact of ECHO, the ECHO-CC tracks overall metrics, such as number of manuscripts, timelines for manuscript production, satisfaction surveys following face-to-face Steering Committee meetings, use of the ECHO collaboration web site, and newsletter views. We recognize that not all feedback should result in a programmatic shift; therefore, feedback, suggestions, and metrics will be reviewed on an ongoing basis with the ECHO-EC. Process improvement suggestions will be made based on recurring feedback themes. The ECHO-CC operations team is physically co-located to enable sharing of information, concerns, best practices, and lessons learned in real time. The complete ECHO-CC team (faculty, communications, and operations) meet together regularly and have frequent one-on-one meetings to facilitate effective program coordination.
Conclusion
ECHO’s mission is to enhance the health of children for generations to come. The ECHO-CC supports this mission by developing the infrastructure of the ECHO program, coordinating multiple levels of membership in the ECHO community, developing ECHO policies and procedures, and fostering engagement inside and outside of the ECHO program. By ensuring that the right people and appropriate tools work together, the ECHO-CC enables the ECHO program to be greater than the sum of its parts.
Key Points.
The Environmental influences on Child Health Outcomes (ECHO) program’s mission is to enhance the health of children for generations to come.
ECHO Coordinating Center (ECHO-CC) supports ECHO’s mission by developing the framework of the program, coordinating multiple levels of membership in the community, developing policies and procedures, and fostering communication and engagement inside and outside of ECHO.
By ensuring that the right people and appropriate tools work together, the ECHO-CC enables the ECHO program to be greater than the sum of its parts.
Acknowledgments
Sources of Funding
This work was funded under NIH grant NIH-1U2C-OD02337501.
Footnotes
Conflict of Interest Disclosures
PB Smith: Dr. Smith reports no relevant disclosures.
S Knox: Ms. Knox reports no relevant disclosures.
DK Benjamin, Jr.: Dr. Benjamin, Jr. reports no relevant disclosures.
References
- 1•.Horowitz CR, Shameer K, Gabrilove J, et al. Accelerators: sparking innovation and transdisciplinary team science in disparities. Int J Environ Res Public Health. 2017:14. doi: 10.3390/ijerph14030225. pii: E225. This article describes the successful use of team science in the setting of a community-academic research partnership board in order to foster collaboration across sectors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Mielke J, Vermaßen H, Ellenbeck S. Ideals, practices, and future prospects of stakeholder involvement in sustainability science. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1706085114. pii: 201706085. [Epub ahead of print]. This survey highlights some of the tensions felt by investigators as they engage stakeholder in developing research projects. These include time needed to engage stakeholders and trade-offs on some of the scientific goals of the research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid RW, Brouwer CR, Jackson EW, Lila MA. A need for a transdisciplinary environment: the Plant Pathways Elucidation Project. Trends Plant Sci. 2014;19:485–487. doi: 10.1016/j.tplants.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Acosta JD, Whitley MD, May LW, et al. Stakeholder perspectives on a culture of health: key findings. Rand Health Q. 2017;6:6. [PMC free article] [PubMed] [Google Scholar]
- 5.Weinfurt KP, Hernandez AF, Coronado GD, et al. Pragmatic clinical trials embedded in healthcare systems: generalizable lessons from the NIH Collaboratory. BMC Med Res Methodol. 2017;17:144. doi: 10.1186/s12874-017-0420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindson N, Richards-Doran D, Heath L, et al. Setting research priorities in tobacco control: a stakeholder engagement project. Addiction. 2017;112:2257–2271. doi: 10.1111/add.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institutes of Health (NIH) all of Us Research Program. Communications and Engagement. [Accessed December 6, 2017];NIH All of Us web site. https://allofus.nih.gov/about/program-components/communications-and-engagement.
- 8.Pediatric Trials Network. For Health Care Professionals. [Accessed December 6, 2017];Pediatric Trials Network web site. https://www.pediatrictrials.org/for-health-care-professionals.
- 9.Salloum RG, Shenkman EA, Louviere JJ, Chambers DA. Application of discrete choice experiments to enhance stakeholder engagement as a strategy for advancing implementation: a systematic review. Implement Sci. 2017;12:140. doi: 10.1186/s13012-017-0675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lobb R, Colditz GA. Implementation science and its application to population health. Annu Rev Public Health. 2013;34:235–251. doi: 10.1146/annurev-publhealth-031912-114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson EE, Newman SB, Matthews AK. Improving informed consent: stakeholder views. AJOB Empir Bioeth. 2017;8:178–188. doi: 10.1080/23294515.2017.1362488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholls SG, Tessier L, Etchegary H, et al. Stakeholder attitudes towards the role and application of informed consent for newborn bloodspot screening: a study protocol. BMJ Open. 2014;4:e006782. doi: 10.1136/bmjopen-2014-006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platt T, Platt J, Thiel DB, et al. ‘Cool! and creepy’: engaging with college student stakeholders in Michigan’s biobank. J Community Genet. 2014;5:349–362. doi: 10.1007/s12687-014-0190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarini BA, Goldenberg A, Singer D, et al. Not without my permission: parents’ willingness to permit use of newborn screening samples for research. Public Health Genomics. 2010;13:125–130. doi: 10.1159/000228724. [DOI] [PubMed] [Google Scholar]
- 15.Platt J, Bollinger J, Dvoskin R, et al. Public preferences regarding informed consent models for participation in population-based genomic research. Genet Med. 2014;16:11–18. doi: 10.1038/gim.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enzinger AC, Wind JK, Frank E, et al. A stakeholder-driven approach to improve the informed consent process for palliative chemotherapy. Patient Educ Couns. 2017;100:1527–1536. doi: 10.1016/j.pec.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn KE, Hahn CL, Kramer JM, et al. Using central IRBs for multicenter clinical trials in the United States. PLoS One. 2013;8:e54999. doi: 10.1371/journal.pone.0054999. [DOI] [PMC free article] [PubMed] [Google Scholar]