Abstract
Differentiation therapy with all-trans retinoic acid has been a very successful therapeutic strategy in acute promyelocytic leukemia (APL), but the value of differentiation therapy in acute myeloid leukemia (AML) remains to be determined. A number of current treatments, such as tyrosine kinase inhibitors, cytokines, and epigenetic agents, induce differentiation of leukemic cells to some extent, but differentiation is not the main goal of these treatments. Forcing expression of certain transcription factors, such as C/EBP, has also been useful in inducing differentiation in cell lines and in murine models, but an effective way to force expression of these genes in humans is yet to be discovered.
Keywords: acute myeloid leukemia, AML, differentiation, retinoids, PPAR ligands, cytokines, kinase inhibitors, histone deacetylase, methylation, CEBP
Introduction
In 1971, Charlotte Friend developed a cell line eponymously called the Friend cell line that contained a leukemia virus [1]. Friend used dimethyl sulfoxide (DMSO) to transform the Friend cells into red blood cells. This opened the field for a number of researchers to use this cell line to study hemoglobin synthesis and switching of hemoglobin genes. Then, in the myeloid era, three myeloid cell lines were produced that could be induced to differentiate [2–5]. And later, human leukocyte (HL)-60 cell lines were developed that differentiated with exposure to retinoic acid [6,7]. Many other agents can induce differentiation of HL-60 cells, with each agent requiring different molar concentrations for induction (Fig. 1).
Fig. 1.
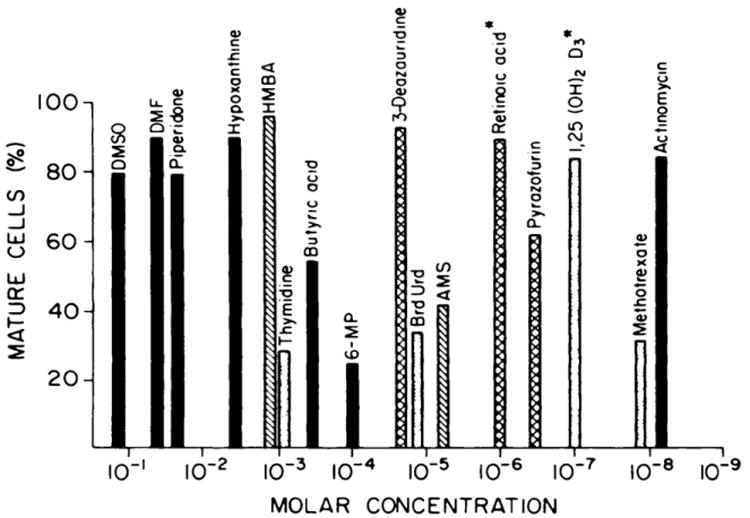
Induction of differentiation of HL-60 cell lines [35]. Compounds with solid bars trigger maturation of HL-60 and both murine M-1 myeloid and Friend cells; compounds with striped bars trigger differentiation of HL-60 and Friend cells; compounds with white bars induce differentiation of HL-60 and M-1 cells; and compounds with hash-marked bars only trigger maturation of human HL-60 cells. Abbreviations: DMSO, dimethyl sulfoxide; DMF, dimethyl formamide; HMBA, hexamethylene bisacetamide; 6-MP, 6-mercaptopurine; BrdUrd, bromodeoxyuridine; 1,25(OH)2D3, 1,25(OH)2 vitamin D3. This research was originally published in Blood. Koeffler HP. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983;62:709-21. ©The American Society of Hematology.
Differentiation agents in acute leukemia
Retinoids
The above studies helped to lead to the discovery of the efficacy of retinoids in acute promyelocytic leukemia (APL). APL cells respond to retinoic acid due to the genomic change common to APL (translocation [15;17]), which results in the PML-RAR α fusion protein that prevents differentiation. PML-RAR α is targeted by retinoic acid, allowing differentiation of leukemic cells. Retinoids are also used for other differentiation defects, such as some skin problems, but in a randomized study of 13-cis-retinoic acid in myelodysplastic syndromes (MDS) [8], no significant difference occurred between the treatment arm and placebo.
Several studies have utilized all-trans retinoic acid (ATRA) in AML and high-risk MDS. Studies in the United States and the United Kingdom (UK) have not shown an effect of ATRA [9–11], while studies in Germany have shown a benefit to ATRA [12]. In the German study HD98B [12], researchers determined that response to retinoids can be predicted by mutant NPM1 and wild-type FLT3 status, but the Medical Research Council AML12 trial in the UK found no effect even with analysis of samples having a NPM1 mutation [11].
Vitamin D3 is another analog of retinoic acid. Vitamin D3 heterodimerizes with the retinoid X receptor (RXR) and turns on a variety of genes, many of which have antiproliferative and prodifferentiation effects. When vitamin D3 is added to HL-60 cells, the cells differentiate to macrophage-like cells [13], and in vivo activity is good. However, vitamin D causes hypercalcemia [14], so vitamin D analogs that can induce differentiation without hypercalcemia were sought by researchers. Around 100 MDS patients have been treated with vitamin D compounds, but responses have been minor.
PPARγ ligands
Peroxisome proliferator-activated receptor (PPAR)γ ligands bind to the PPAR receptors, which are nuclear hormone receptors, and heterodimerize with RXR. PPARγ ligands have differentiation effects and have been shown to be effective in liposarcomas [15,16], though subsequent studies did not see the same activity. PC3 prostate cancer cell lines can also differentiate in the presence of PPARγ ligands [17], and a large study in prostate cancer showed modestly positive effects [18]. PPARγ ligands also induced differentiation in vivo in myeloid leukemic cells [19], but clinical trials in MDS and leukemias have not shown an effect.
G-CSF
Cloned granulocyte colony-stimulating factor (G-CSF) can also induce differentiation in leukemia cells in vivo [20]. However, evidence suggesting that G-CSF can induce differentiation in fresh leukemia cells is meager. It is more often used to enhance defenses in leukemia rather than cause differentiation. Other cytokines, such as interleukins (IL) 4 and 6, can also induce human myeloid leukemic cell differentiation (Table 1). These cytokines enhance proliferation, are antiapoptotic, and cause differentiation as a natural effect, but are not effective in the in vivo setting.
Table 1.
Cytokines can induce differentiation of human myeloid leukemic cells.
| Cytokine | Leukemia lines/Primary leukemia cells | Differentiation lineage |
|---|---|---|
| EPO | K562 | Erythrocytic |
| G-CSF | U937 | Monocytic |
| GM-CSF | U937, ML-1 | Monocytic |
| IL-4 | U937 | Monocytic |
| IL-6 | K562 | Megakaryocytic |
| SCF | AML blasts | Monocytic |
| SCF or IL-3 | AML blasts | Granulocytic |
| TGF-β | K562 | Erythrocytic |
| TGF-β | HL-60, ML-1, THP-1, U937 | Monocytic |
| TNF-α | HL-60, ML3, U937, AML blasts | Monocytic |
| IFN-α + GM-CSF | CML mononuclear cells | Dendritic |
| TGF-β + TNF-α | U937 | Monocytic |
| IL-3 + SCF + TPO | AML blasts | Megakaryocytic |
| GM-CSF + TNF-α + IL-4 | CS-1, KG-1, MUTZ-3, THP-1, AML blasts, CML blasts | Monocytic |
Abbreviations: AML, acute myeloid leukemia; EPO, erythropoietin; CML, chronic myeloid leukemia; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; SCF, stem cell factor; TGF, transforming growth factor; TNF, tissue necrosis factor; TPO, thrombopoietin.
Congenital neutropenia is a condition associated with a block in differentiation. A proportion of patients with congenital neutropenia have mutation of the G-CSF receptor. When these patients are treated with G-CSF, they experience an increase in mature neutrophils in the peripheral blood. This led to in vitro studies that identified a tyrosine in the distal part of the receptor that was important for induction of differentiation. Elastase is also often mutated in this disease, and apoptosis can account for the lack of differentiation in the peripheral blood [21]. In congenital neutropenia patients, G-CSF enhances survival and decreases apoptosis but has been associated with a higher incidence of leukemia.
When HL-60 cells were treated with the phorbol diester TPA (12-O-tetradecanoylphorbol 13-acetate), these cells differentiated to macrophage-like cells [22]. TPA causes differentiation within 2 days in almost any leukemic cells from any patient, also becoming macrophage-like in appearance and histochemical staining. Some analogs of TPA have been used in clinical trials, but they have not been very effective in general.
Tyrosine kinase inhibitors
Gefitinib is an epidermal growth factor receptor (EGFR) inhibitor that can induce myeloid differentiation in AML cell lines [23], even though EGFR is not expressed on those cells. This suggests that gefitinib has off-target effects. A microarray expression analysis was performed to identify the signature of myeloid differentiation. An RNA interference (RNAi) library screen was also performed to identify the target of gefitinib that was inducing differentiation. These tests identified a different tyrosine kinase, SYK, as gefitinib's target on AML cells. When AML cells are exposed to gefitinib or the analog R406, the level of phosphorylated SYK decreases, and when SYK is inhibited with small hairpin RNA (shRNA), HL-60 and U937 cells were able to differentiate. The SYK inhibitor also worked in vitro to slow growth of human AML in immunodeficient mice.
A number of tyrosine kinase inhibitors might cause some differentiation. Western blot analysis showed that another EGFR inhibitor, erlotinib, also inhibits phosphorylation of JAK2 and STAT5 [24]. The SYK inhibitor R406 can also induce differentiation in acute lymphoblastic leukemia (ALL) B lymphocytes [25], and the BCR/ABL tyrosine kinase inhibitor imatinib mesylate can cause B-cell differentiation in BCR/ABL-positive B-cell ALL [26].
Epigenetic agents
The discovery by Charlotte Friend that DMSO, a polar planar compound, caused differentiation led to the investigation of other polar compounds, such as hexamethylamine bisacetamide (HMBA) [27]. Suberoylanilide hydroxamic acid (SAHA) is a second-generation polar compound that also functions as a histone deacetylase (HDAC) inhibitor and, in addition, is an inducer of cell differentiation [28]. Chromatin remodeling agents, such as DNA methylation inhibitors (5-azacytidine [azacitidine] and 2-deoxy-5-azacytidine [decitabine]) and HDAC inhibitors, have epigenetic control of gene expression. DNA methylation leads to transcriptional silencing, and inhibitors of DNA methylation lead to demethylation and activation of transcription. HDAC inhibitors acetylate histones and other proteins. Both epigenetically modifying families of drugs can also cause cell-cycle arrest, angiogenesis, immune modulation, and apoptosis. These agents also have some antileukemic and anti-MDS effects, and both azacitidine and decitabine have been approved by the FDA for treatment of advanced MDS.
CEBP
The CCAAT/enhancer binding protein (C/EBP) transcription factors are associated with the differentiation process of a variety of mammalian cells, including hematopoietic cells. In AML, C/EBPα function is abrogated by mutations on either the amino or carboxyl ends of C/EBP. Furthermore, common gene fusion products in AML, such as BCR/ABL and AML1-ETO, can downregulate CEBPα, which impedes differentiation to mature myeloid cells. High levels of FLT3 ITD mutation can also downregulate CEBPα. In lymphoid leukemias, the PAX5 gene, a counterpart to C/EBP, is necessary for cells to differentiate from prolymphocytes to mature lymphocytes, and studies have found that 25%–30% of ALL patients have alterations of PAX5 [29,30]. If C/EBPα or C/EBPε expression is genetically induced in myeloid leukemia cells, these cells will differentiate both morphologically and functionally [31,32]. In a murine APL model, forced expression of either C/EBPα or C/EBPε induced differentiation, and overexpression enhanced survival in combination with all-trans retinoic acid [33]. However, ways to force gene expression in humans are unknown, and small molecules to modulate mutant C/EBPα are yet to be discovered.
Conclusions
Leukemic cells gain their growth advantage in part by blocking their own differentiation. Many of the current treatments do induce differentiation to some extent. The ability to reprogram somatic cells to a pluripotent state, through nuclear transfer, cell fusion, or forced expression of a cocktail of genes, is promising [34]. This gives hope that researchers will eventually develop ways to force leukemic cells to differentiate into mature cells, such as neutrophils.
Footnotes
Conflict of interest statement: No relevant financial relationships with any commercial interest.
References
- 1.Friend C, Scher W, Holland JG, Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971;68:378–82. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichikawa Y, Pluznik DH, Sachs L. Feedback inhibition of the development of macrophage and granulocyte colonies. I. Inhibition by macrophage. Proc Natl Acad Sci U S A. 1967;58:1480–6. doi: 10.1073/pnas.58.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paran M, Ichikawa Y, Sachs L. Feedback inhibition of the development of macrophage and granulocyte colonies. II. Inhibition by granulocytes. Proc Natl Acad Sci U S A. 1969;62:81–7. doi: 10.1073/pnas.62.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metcalf D. Clonal extinction of myelomonocytic leukemic cells by serum from mice injected with endotoxin. Int J Cancer. 1980;25:225–33. doi: 10.1002/ijc.2910250210. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf D. Clonal analysis of the action of GM-CSF on the proliferation and differentiation of myelomonocytic leukemic cells. Int J Cancer. 1979;24:616–23. doi: 10.1002/ijc.2910240515. [DOI] [PubMed] [Google Scholar]
- 6.Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980;77:2936–40. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978;75:2458–62. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koeffler HP, Heitjan D, Mertelsmann R, Kolitz JE, Schulman P, Itri L, et al. Randomized study of 13-cis retinoic acid v placebo in the myelodysplastic disorders. Blood. 1988;71:703–8. [PubMed] [Google Scholar]
- 9.Estey EH, Thall PF, Pierce S, Cortes J, Beran M, Kantarjian H, et al. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin +/- all-trans retinoic acid +/- granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93:2478–84. [PubMed] [Google Scholar]
- 10.Burnett AK, Milligan DW, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. Modification or dose or treatment duration has no impact on outcome of AML in older patients: Preliminary results of the UK NCRI AML14 trial. Blood. 2006;106:162a. abstr 543. [Google Scholar]
- 11.Burnett AK, Hills RK, Green C, Jenkinson S, Koo K, Patel Y, et al. The impact on outcome of the addition of all-trans retinoic acid to intensive chemotherapy in younger patients with nonacute promyelocytic acute myeloid leukemia: overall results and results in genotypic subgroups defined by mutations in NPM1, FLT3, and CEBPA. Blood. 2010;115:948–56. doi: 10.1182/blood-2009-08-236588. [DOI] [PubMed] [Google Scholar]
- 12.Schlenk RF, Dohner K, Kneba M, Gotze K, Hartmann F, Del VF, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG Trial AML HD98B. Haematologica. 2009;94:54–60. doi: 10.3324/haematol.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes PJ, Marcinkowska E, Gocek E, Studzinski GP, Brown G. Vitamin D3-driven signals for myeloid cell differentiation–implications for differentiation therapy. Leuk Res. 2010;34:553–65. doi: 10.1016/j.leukres.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrich A, Kahl B, Bailey H, Kim K, Turman N, Juckett M. Phase II study of doxercalciferol for the treatment of myelodysplastic syndrome. Leuk Lymphoma. 2008;49:57–61. doi: 10.1080/10428190701713648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A. 1999;96:3951–6. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci U S A. 1997;94:237–41. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shappell SB, Gupta RA, Manning S, Whitehead R, Boeglin WE, Schneider C, et al. 15S-Hydroxyeicosatetraenoic acid activates peroxisome proliferator-activated receptor gamma and inhibits proliferation in PC3 prostate carcinoma cells. Cancer Res. 2001;61:497–503. [PubMed] [Google Scholar]
- 18.Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, et al. Effects of ligand activation of peroxisome proliferatoractivated receptor gamma in human prostate cancer. Proc Natl Acad Sci U S A. 2000;97:10990–5. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konopleva M, Elstner E, McQueen TJ, Tsao T, Sudarikov A, Hu W, et al. Peroxisome proliferator-activated receptor gamma and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol Cancer Ther. 2004;3:1249–62. [PubMed] [Google Scholar]
- 20.Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, et al. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232:61–5. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- 21.Skokowa J, Germeshausen M, Zeidler C, Welte K. Severe congenital neutropenia: inheritance and pathophysiology. Curr Opin Hematol. 2007;14:22–8. doi: 10.1097/00062752-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Rovera G, Santoli D, Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979;76:2779–83. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stegmaier K, Corsello SM, Ross KN, Wong JS, DeAngelo DJ, Golub TR. Gefitinib induces myeloid differentiation of acute myeloid leukemia. Blood. 2005;106:2841–8. doi: 10.1182/blood-2005-02-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehrer S, Ades L, Braun T, Galluzzi L, Grosjean J, Fabre C, et al. Erlotinib exhibits antineoplastic off-target effects in AML and MDS: a preclinical study. Blood. 2008;111:2170–80. doi: 10.1182/blood-2007-07-100362. [DOI] [PubMed] [Google Scholar]
- 25.Wossning T, Herzog S, Kohler F, Meixlsperger S, Kulathu Y, Mittler G, et al. Deregulated Syk inhibits differentiation and induces growth factor-independent proliferation of pre-B cells. J Exp Med. 2006;203:2829–40. doi: 10.1084/jem.20060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein F, Feldhahn N, Harder L, Wang H, Wartenberg M, Hofmann WK, et al. The BCR-ABL1 kinase bypasses selection for the expression of a pre-B cell receptor in pre-B acute lymphoblastic leukemia cells. J Exp Med. 2004;199:673–85. doi: 10.1084/jem.20031637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuben RC, Wife RL, Breslow R, Rifkind RA, Marks PA. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976;73:862–6. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richon VM, Webb Y, Merger R, Sheppard T, Jursic B, Ngo L, et al. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc Natl Acad Sci U S A. 1996;93:5705–8. doi: 10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller CB, Mullighan CG, Downing JR. Mutations in the B-cell transcription factor PAX5 found in B-progenitor acute lymphoblastic leukemia impair normal PAX5 activity. Blood. 2006;108:185a. abstr 613. [Google Scholar]
- 30.Nowak D, Kawamata N, Niebuhr B, Nowak V, Mossner M, Nahar RR, et al. The Pax5 fusion product Pax5-C20orf112 causes downregulation of pre-B cell receptor genes and induces differential proliferation patterns in B-lymphoblastic cell lines. Blood. 2009;114:526. abstr 1284. [Google Scholar]
- 31.Wagner K, Zhang P, Rosenbauer F, Drescher B, Kobayashi S, Radomska HS, et al. Absence of the transcription factor CCAAT enhancer binding protein alpha results in loss of myeloid identity in bcr/abl-induced malignancy. Proc Natl Acad Sci U S A. 2006;103:6338–43. doi: 10.1073/pnas.0508143103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duprez E, Wagner K, Koch H, Tenen DG. C/EBPbeta: a major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells. EMBO J. 2003;22:5806–16. doi: 10.1093/emboj/cdg556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YJ, Jones LC, Timchenko NA, Perrotti D, Tenen DG, Kogan SC. CCAAT/enhancer binding proteins alpha and epsilon cooperate with all-trans retinoic acid in therapy but differ in their antileukemic activities. Blood. 2006;108:2416–9. doi: 10.1182/blood-2006-02-003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Malley J, Woltjen K, Kaji K. New strategies to generate induced pluripotent stem cells. Curr Opin Biotechnol. 2009;20:516–21. doi: 10.1016/j.copbio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Koeffler HP. Induction of differentiation of human acute myelogenous leukemia cells: therapeutic implications. Blood. 1983;62:709–21. [PubMed] [Google Scholar]


