Organic fertilizer from recycled biowaste was identified as a vehicle for entry of microplastic particles into the environment.
Abstract
The contamination of the environment with microplastic, defined as particles smaller than 5 mm, has emerged as a global challenge because it may pose risks to biota and public health. Current research focuses predominantly on aquatic systems, whereas comparatively little is known regarding the sources, pathways, and possible accumulation of plastic particles in terrestrial ecosystems. We investigated the potential of organic fertilizers from biowaste fermentation and composting as an entry path for microplastic particles into the environment. Particles were classified by size and identified by attenuated total reflection-Fourier transform infrared spectroscopy. All fertilizer samples from plants converting biowaste contained plastic particles, but amounts differed significantly with substrate pretreatment, plant, and waste (for example, household versus commerce) type. In contrast, digestates from agricultural energy crop digesters tested for comparison contained only isolated particles, if any. Among the most abundant synthetic polymers observed were those used for common consumer products. Our results indicate that depending on pretreatment, organic fertilizers from biowaste fermentation and composting, as applied in agriculture and gardening worldwide, are a neglected source of microplastic in the environment.
INTRODUCTION
Plastics are an integral part of everyday life. They fulfill a wide variety of functions, primarily packaging (39.9% of the total plastics used in Europe in 2016) (1). Additional applications are in building and construction; the electrical, electronic, automotive, and agriculture sectors; and, to a lesser extent, consumer and household appliances, furniture, sport, health, and safety (1). Despite its varied applications, approximately 80% of the produced plastic falls into six categories: polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polyurethane (PUR), PE terephthalate (PET), and polystyrene (PS). Worldwide plastic production has increased steadily since 1950, reaching an annual production of 322 million metric tons worldwide in 2015, of which approximately 40% was used in one-way products (1). Not surprisingly, because of inadequate end-of-life treatment, plastics are increasingly found as contaminants in the environment (2). Recently, the World Economic Forum estimated that 32% of plastic packaging is leaking into the environment (3), and models suggest that up to 12.7 million metric tons of plastic litter enters the oceans from land-based sources each year (4). Therefore, the G7 has acknowledged that “plastic litter poses a global challenge” (5). Accordingly, scientists have suggested to “classify plastic waste as hazardous” because it may have “significant ecological impacts, causing welfare and conservation concerns” (6). Among the plastic materials found in the aquatic environment, so-called microplastic particles (MPPs; <5 mm)—mainly fragments, fibers, and spheres—have attracted particular attention (7, 8) because harmful effects of MPPs on various aquatic organisms have been proposed (6, 8–11), linked either to the presence of MPPs per se, to toxic additives, or to potentially harmful microorganisms or chemicals enriched onto them. However, theoretical predictions based on models and empirical studies are often contradictory, and it is not known how effects reported for individual organisms may affect ecosystems (12).
Because of their small size, MPPs may presumably also enter the food web (10) and thus potentially end up in human food (13). There, they pose a risk that is not yet predictable, because the interaction of MPPs with tissue and cells is poorly understood. Investigation of the interaction is further complicated by the fact that MPPs are not single compounds but constitute mixtures of different plastic types, each often consisting of a blend of synthetic polymers, residual monomers, and chemical additives. Furthermore, their morphology (for example, fragments, fibers, or spheres) may influence their effects. In this context, a distinction is typically made between industrially manufactured primary MPPs, originating from cosmetics, household cleaners and other products to which they were purposely added, and secondary MPPs that originate from the disintegration of larger plastics caused by ultraviolet (UV) radiation, mechanical abrasion, and biological degradation (14, 15).
MPPs are detected ubiquitously in aquatic environments across the globe (16–19), reaching values of up to 100,000 particles per cubic meter, with predominantly secondary origin (8). Little is known about the exact origin of this significant contamination, although several pathways through which MPPs may enter surface water have been discussed. Most studies assume a transfer from land, including, but not restricted to, improper disposal of plastic waste, wind distribution, and municipal, as well as industrial wastewater and sewage sludge (10, 18, 20). However, detailed studies regarding MPP production and initial entry into terrestrial ecosystems are currently lacking.
Here, we investigated organic fertilizers (composts, digestates, and percolate-leachates from digestion, which is used as liquid fertilizer) from recycled biowaste as possible vehicles for the entry of MPPs >1 mm into the environment. According to best current practice, after separate collection, organic waste from households and industry is either directly composted or partially digested for biogas/energy production in an anaerobic biogas fermenter, typically followed by composting of the remaining digestates. The recycling of organic waste through composting or fermentation and subsequent application on agricultural land is, in principle, an environmentally sound practice to return nutrients, trace elements, and humus to the soil. However, most household and municipal biowaste is contaminated by plastic material. Sieving and sifting procedures can significantly reduce, but never completely remove, these contaminants. Moreover, most countries allow a certain amount of foreign matter such as plastics in fertilizers; for example, Germany, which has one of the strictest regulations on fertilizer quality worldwide, allows up to 0.1 weight % (wt %) of plastics. In this regulation, particles smaller than 2 mm are not even considered (21). Thus, organic fertilizers may be a source of environmental MPPs that should not be overlooked. Our study is a first attempt to estimate the significance of this entry pathway to the terrestrial environment.
RESULTS
In this investigation, one biowaste composting plant (plant A; aerobic treatment) and one biowaste digester (plant B, “biogas plant”; anaerobic treatment) were studied in detail. The biowaste composting plant (plant A) processes the biowaste from households with a nearly equal amount of green clippings from the area. The plant removes potential nonbiodegradable material, including plastics, as thoroughly as possible by a series of sieving (80 mm), metal separation, and manual sorting steps. The remaining material is subsequently transferred into a box composter for rotting. The plant offers two types of commercial, quality-controlled, certified compost [composting plant (CP) 8 and CP 15 mm], sieved through 8- and 15-mm meshes, respectively. Both composts were sampled. The batch biowaste digester (parallel boxes, plant B) mainly processed biowaste from households with the addition of some green clippings and occasionally energy crops. The mixture is introduced directly into the digester without pretreatment. Instead, the operators remove contaminating materials from the final compost using one or two sieving steps (see Materials and Methods). From plant B, two mature composts (“Digest A” and “Digest B”), a nonmatured fertilizer (“Digest C”), and the pooled percolate (“Digest D”) from the parallel boxes were analyzed.
An agricultural energy crop digester (plant C) processing only energy crops and no biowaste served as a reference. In plant C, the sample (“Energycrop”) was taken from the postdigester outlet, corresponding to an end-of-process sample. This agricultural biogas plant processes energy crops such as corn/grass silage and, to a lesser extent, ground wheat. Ground wheat and silage arrive in plastic encasings, but these are removed before the substrate is passed through the shredder and entered into the fermenter. In addition, a commercially available fertilizer from a second biowaste digester (plant D, processing solely waste from commerce) located in the same area, as well as end-of-process digestate samples from 10 additional agricultural biogas plants (plants E to N), processing feeds such as dung/manure, sunflowers, or waste from fruit processing, together with the regular energy crops, were screened for MPPs.
Quantity of MPPs
With only 20 (CP 8 mm) and 24 (CP 15 mm) particles per kilogram dry weight (Table 1), the MPP load of the certified composts from the biowaste composting plant (plant A) was almost an order of magnitude lower than that determined in the samples from the biowaste digester (plant B), where up to 146 particles per kilogram dry weight were found in the fresh digestate-fertilizer (Digest C). Mature compost from the same biowaste digester (Digest A and Digest B) contained similar amounts of MPPs (70 and 122 particles per kilogram dry weight, respectively), whereas the pooled percolate sample (Digest D) was somewhat less contaminated, containing only 14 particles per kilogram dry weight. In the agricultural energy crop digester (plant C), which served as a “blank” fermenter, no plastic particles were found in the end-of-process digestate (sample Energycrop) (Table 1). The end-of-process samples of digestates from the additional 10 agricultural biogas plants (plants E to N) included in the screening contained only negligible numbers of particles: The samples from eight plants contained no particle, whereas the samples from the other two plants contained one particle each, resembling a maximum of 11 MPPs per kilogram dry weight. In contrast, with 895 MPPs per kilogram dry weight, the sample from the second biowaste digester (plant D) included in the screening contained even higher numbers of MPPs than found in composts (Digests A to C) from the biowaste digester (plant B), despite the fact that plant B processed biowaste collected from households, whereas plant D processed biowaste directly supplied by commerce.
Table 1. Overview of plants and compartments.
The total number of particles is shown as particles >1 mm per kilogram of dry weight.
| Plant A | Plant B | Plant C | Plant D | Plants E to N | |||||
| Type | Biowaste composting | Biowaste digestion | Energycrop digestion | Biowaste digestion | Agricultural digestion | ||||
| Sampled | CP 8 mm | CP 15 mm | Digest A | Digest B | Digest C | Digest D | End-of-process | Commercial binding | End-of-process |
| Particles per kilogram | 20 | 24 | 70 | 122 | 146 | 14 | 0 | 895 | 0 to 11 |
Polymer size, type, and morphology
Before further analysis, samples were gently fractionated using sieves with mesh sizes of 5, 2, 1, and exceptionally also 0.5 mm. Analysis of MPP size showed that most of the particles collected from the various samples were between 2 and 5 mm (Fig. 1). Only the pooled percolate sample (Digest D) from the biowaste digester (plant B) contained MPPs mostly from 1 to 2 mm. In some samples, we also found MPPs as small as 250 μm. However, because these data are not fully quantitative, we only present data in the size range of 1 to 5 mm. All MPPs were categorized by shape into three subgroups: fragments, fibers, and spheres. Examples are shown in Fig. 2. Most of the MPPs (75 to 100%) were fragments, followed by fibers (0 to 8%) and spheres (0 to 8%).
Fig. 1. Size fractions of MPPs in different fertilizers.
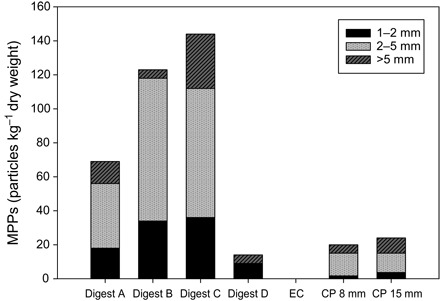
Digests A/B/C/D, biowaste digester; EC, energy crop digester; CP 8 mm/15 mm, biowaste composting plant.
Fig. 2. Examples of MPPs of various shapes found in samples.
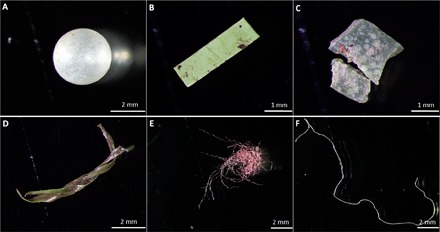
(A) PE sphere. (B) PVC fragment. (C and D) PE fragments. (E) PES fiber. (F) PP fiber.
Attenuated total reflection (ATR)–Fourier transform infrared (FTIR) spectroscopy analysis identified 11 polymer types in the samples: styrene-based polymers (PS, acrylonitrile butadiene styrene, and styrene acrylonitrile), polyester (PES), PE, PP, PET, PVC, PUR, polyvinylidene chloride (PVDC), polyamide (PA), and latex- and cellulose-based polymers (Table 2). Most of the particles found in the high-quality composts from the biowaste composting plant (plant A) were styrene-based polymers (60%; 42%), followed by PE (30%; 33%) for the CP 8- and CP 15-mm samples, respectively. The most abundant polymer types in Digest A (73%) and Digest B (80%) from the biowaste digester (plant B) were also styrene-based polymers, whereas most of the MPPs found in the Digest C from this digester were PES with 38% and PE with 21%. The few polymers found in the additional energy crop digesters (plant E to N) were PP and PVC.
Table 2. MPP abundances in different samples.
Digests A/B/C/D, biowaste digester; EC, energy crop digester, CP 8 mm/15 mm, biowaste composting plant; MPP per kilogram of dry weight; A, proportion of polymer type in specific sample.
| CP 8 mm | CP 15 mm | Digest A | Digest B | Digest C | Digest D | EC | ||||||||
|
MPP per kilogram |
A (%) |
MPP per kilogram |
A (%) |
MPP per kilogram |
A (%) |
MPP per kilogram |
A (%) |
MPP per kilogram |
A (%) |
MPP per kilogram |
A (%) |
MPP per kilogram |
A (%) |
|
| Styrene-based polymer |
12 | 60 | 10 | 42 | 51 | 73 | 97 | 80 | 10 | 7 | 0 | 0 | 0 | 0 |
| PES | 1 | 5 | 0 | 0 | 2 | 3 | 2 | 2 | 56 | 38 | 14 | 100 | 0 | 0 |
| PE | 6 | 30 | 8 | 33 | 6 | 9 | 3 | 2 | 31 | 21 | 0 | 0 | 0 | 0 |
| PP | 0 | 0 | 4 | 17 | 3 | 4 | 2 | 2 | 24 | 16 | 0 | 0 | 0 | 0 |
| PET | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 16 | 11 | 0 | 0 | 0 | 0 |
| Cellulose-based polymer |
0 | 0 | 0 | 0 | 6 | 9 | 11 | 9 | 5 | 3 | 0 | 0 | 0 | 0 |
| PVDC | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PVC | 1 | 5 | 1 | 4 | 0 | 0 | 5 | 4 | 2 | 1 | 0 | 0 | 0 | 0 |
| Latex | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| PUR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| PA | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| ∑ MPP | 20 | 24 | 70 | 122 | 146 | 14 | 0 | |||||||
DISCUSSION
Organic waste from private households and industry is increasingly seen as a valuable source of both fertilizer and energy. Processing organic waste by fermentation and/or composting is a sustainable means of producing organic fertilizer for agriculture and private gardening, thereby reducing the need for chemical fertilizers. An initial anaerobic fermentation step (production of biogas) before composting is often proposed because this produces energy in a sustainable manner and helps to economically run a plant (production of electricity and heat) while avoiding the drawbacks of conventional biogas production from energy crops (monocropping, rivalry to esculents). Moreover, an initial fermentation step reduces the amount of methane—a more potent greenhouse gas than carbon dioxide—released into the atmosphere, as compared to composting alone.
Current practice for collecting organic waste fractions from private households calls for separate collecting bins. Theoretically, a pure organic fraction very suitable to composting/biogas fermentation should be obtained. However, in practice, most biowaste contains contaminants, often including plastics. Organic materials from commercial sources, such as the food and drink industries, tend to be less contaminated by plastics, but in particular, unsold food items often arrive in packaged form, some parts of which may then also enter the respective biowaste processing plant. The fact that all the samples from biowaste processing plants investigated in this study contained a certain number of MPPs is therefore not surprising, although a detailed quantitative analysis has been lacking so far. Most of the MPPs were “fragments,” most likely secondary MPPs produced through breakdown of larger plastic materials, such as bags and containers, used for packaging. This hypothesis is corroborated by the fact that styrene-based polymers and PE tended to predominate among the identified materials (that is, materials used mainly for packaging and wrapping). In contrast, none of the samples from the investigated agricultural energy crop digesters contained significant amounts of MPPs, indicating that agricultural crops are only rarely contaminated with plastic items.
However, the relative distribution of MPPs among the different polymer types was not necessarily consistent for all samples from a given plant. For example, although polymer distribution was similar in Digests A and B from the biowaste digester (plant B), a different distribution was found in Digest C from the same plant. The three composts/fertilizers (Digests A to C) from plant B were sampled simultaneously. Because they had matured for differing lengths of time, the observed differences in MPP composition may very well reflect seasonal changes in biowaste composition. However, because no samples of the original feed substrate were available, this aspect could not be further investigated.
The final processing step for compost is typically sieving using 8-, 10-, or even 15-mm mesh sizes. MPPs, defined as particles smaller than 5 mm, will therefore pass through these sieves and enter the compost. Here, samples were gently fractionated using sieves with mesh sizes of 5, 2, 1, and exemplarily 0.5 mm before the analysis. In most samples, MPP sizes ranged between 2 and 5 mm. The only exception was the percolate sample (Digest D) from plant B, which mainly contained particles between 1 and 2 mm. This may be because the percolate is filtrated because it passes through the fermenter content, and retention increases with particle size. This may also explain why the percolate sample contained a comparatively low number of MPPs compared to the other samples from plant B.
Although particles as small as 250 μm were found in some of the fractions, most likely because they had attached to larger fragments and were therefore retained, the smallest MPP size that could be examined with certitude in this study was 1 mm. At present, quantitative evaluation of smaller particles via the existing methodology is very difficult because the removal of the high organic load is extremely challenging and hampers reliable analysis (22). Hence, quantitative results are presented in this study only for the size range of 1 to 5 mm. Studies focusing on aquatic environments have reported that sites contaminated by MPPs in the range of 1 to 5 mm typically also contain an even higher amount of particles <1 mm, presumably created through further fragmentation of larger MPPs (23). However, fragmentation into a size <1 mm is perhaps more likely in the natural environment, where mechanical forces act (for example, wave action at a beach), than in biowaste treatment plants. Nevertheless, it cannot be excluded at present writing that MPPs <1 mm are produced during biowaste treatment as well (for example, due to the mechanical forces present during the various sieving steps), indicating that actual MPP numbers in fertilizer originating from biowaste may be much higher. This needs further study, particularly in view of the intended use of the material as organic fertilizer.
Although all samples from the biowaste treatment plants contained MPPs, significant differences in the level of contamination were observed. High-quality compost (“quality seal” label) from the biowaste composting plant (plant A) contained less than 25 MPPs per kilogram dry weight, whereas the contamination of the composts/digestates from the biowaste digester (plant B) was nearly an order of magnitude higher. Several factors may have contributed to this result. Although aerobic rotting (composting) reduces the dry mass of the material by approximately 50%, anaerobic conversion to biogas, followed by composting, will often achieve a reduction of more than 80%. Nondigested material, such as MPPs, is therefore enriched by a factor of 5 during anaerobic biowaste digestion but by only a factor of 2 during simple composting. Concomitantly, in the biowaste composting plant (plant A), biowaste from private households was mixed with at least equal amounts of green clippings. The latter is typically much less contaminated with plastics and thus dilutes the MPP contamination. In addition, an elaborate substrate preparation protocol is in place at the biowaste composting plant (plant A), which attempts to remove contaminating materials as thoroughly as possible before the substrate enters the composter. Finally, temperatures of up to 75°C are reached during aerobic rotting (composting as in plant A), whereas most anaerobic biowaste digesters, such as plant B, are operated between 45° and 55°C. This will directly influence, for example, the fraction of cellulose-based MPPs found in the final compost, which, in consequence, was nondetectable in the samples from the composting plant (plant A). In addition to the lower temperature, a lack of oxygen and UV radiation will also block potential MPP degradation pathways in the anaerobic biowaste digesters, such as plant B, compared to aerobic composting, as in plant A. A recent study testing the degradability of PE and PET in an active anaerobic environment at 50°C showed no appreciable degradation of polymers over the investigation period of 500 days (24). In particular, PE and PS, which were detected in all samples from the biowaste treatment plants, are known to be highly persistent in the environment. It is therefore likely that these particles, once released, will accumulate in nature over time.
In Germany alone, which has one of the strictest regulations on fertilizer quality worldwide, more than 12 million metric tons of biowaste were either composted or passed through municipal biogas plants in 2013 (25). This quantity of biowaste translates into more than 5 million metric tons of compost from these plants, most of which is used in traditional agriculture and gardening. We recorded particle counts varying from 14 to 895 particles per kilogram dry weight (when conservatively calculated, 1-kg compost contains approximately 50% dry weight content) for MPPs larger than 1 mm, together with a yet unquantified number of smaller particles. Although our data may not be representative of all biowaste treatment plants, an extrapolation based on our results suggests that, in Germany alone, although counting only particles >1 mm, between 35 billion and 2.2 trillion MPPs are potentially introduced via this pathway into the environment each year.
An evaluation of our data is difficult because there is no other quantitative study on MPPs in compost available. However, our data can at least be compared with similar potential sources of MPPs such as sewage sludge, which is also used for fertilization of agricultural land. When considering only MPPs >1 mm, recent studies on the MPP contamination of sewage sludge have found concentrations ranging between 0 and 300 particles per kilogram dry weight in the analyzed samples (26, 27). The highest concentration found for sewage sludge in the latter study is by a factor of 3 lower than the highest concentration found in the compost samples in our study. However, as stated above, sewage sludge may be contaminated with an even higher amount of smaller MPPs (<1 mm) indicated by recent studies, which have found between 1000 and 24,000 particles per kilogram dry weight (26, 27). For various reasons, sewage sludge is in the public opinion increasingly seen as problematic waste inappropriate for redistribution into the environment, probably not least because of the contamination with heavy metals, residual pharmaceuticals, and also artificial fibers. The latter was detectable in agricultural soils up to 15 years after application of sewage sludge (28). This abandonment is not the case for composts and digestates from biowaste processing plants, which, in principle, do constitute valuable organic fertilizers.
However, compared to sewage sludge, which, in Germany, is routinely incinerated, fertilizer contaminated with MPPs from biowaste processing plants inevitably enters the environment. Because Germany has one of the strictest regulations on fertilizer quality worldwide, we here report only on the “best case scenario,” whereas the MPP contamination in countries with less strict regulations may be even higher.
However, advantages and disadvantages of the continuation of using biowaste for fertilizer production need to be carefully balanced, particularly because studies on the impact of MPPs on terrestrial life forms are still inconclusive. It cannot be excluded that, analogous to aquatic systems, MPPs can accumulate in the soil detrital food web (29). At least one study has shown that (pristine) PE particles mixed with litter and offered to earthworms for uptake led to higher mortality and a reduced growth rate (30). Another study showed that polybrominated diphenyl ether, a substance mixed into polymers as a flame retardant, is bioaccessible and can enter soils after volatilization or polymer deterioration. Accumulation in earthworms was shown, and transfer to higher trophic levels is likely (31). However, it is unknown whether these additives are still present in secondary MPPs after fermentation and/or composting. In addition, it cannot be excluded that MPPs in the investigated size range, or smaller, exert a direct influence on active microbiota in biowaste treatment plants or soils, which has not been considered yet in the literature. Hence, further studies on the possible consequences and impacts of MPP contamination of fertilizers originating from biowaste treatment plants for soil quality and soil life forms are necessary before any risk assessment can be undertaken.
MATERIALS AND METHODS
Biowaste composting plant (aerobic treatment), plant A
The biowaste compositing plant (plant A) processes approximately 8000 metric tons per year (t/a) of biowaste solely from households, together with approximately 12,000 t/a of green clippings. The plant commercializes quality-controlled, certified composts of two compost qualities (sieving with 8- and 15-mm sieves), both of which were sampled. Arriving biowaste was initially sieved using an 80-mm mesh. The fraction <80 mm was passed through the metal separator and then directly placed into the rotting containers for fast initial decomposition. The fraction >80 mm was sorted manually to remove stones, metals, plastics, and glass. Afterward, the material was mechanically shredded and again added to the sieving drum. In the rotting container, temperatures >70°C were reached. After initial rotting, the compost was left to mature and stabilize in open piles for several months, followed by a final sieving step to reach the desired final corn sizes of below 8 and 15 mm, respectively.
Biowaste digester (anaerobic treatment), plants B and D
The investigated biowaste digester (plant B) was a nonstirred, discontinuous box fermentation system. The plant comprised several quadrangular box digesters, each with a volume of 945 m3 and a filling capacity of 500 m3, which corresponds to a mass of 350 metric tons of organic material. All boxes were equipped with a floor heating and operated at temperatures between 40° and 45°C. The substrate consisted of a pourable mixture of biowaste (11,000 t/a, solely from households) and green clippings (3000 t/a) with a water content below 15 wt %. The composition of the substrate follows seasonal changes. In the winter, the substrate is occasionally supplemented with energy crops.
To initiate the fermentation in the box, fresh substrate was predigested via aerobic digestion for several days and mixed with two volumes of fermenter content. The mixture was added into a fermenter box using an excavator. Afterward, the box was locked, assuring anaerobic conditions, and inoculated by sprinkling with percolate from other boxes. No mechanical treatment or manual presorting took place. After 28 days of fermentation, the box was emptied, 30 volume percent (volume %) of the digestate was removed, and the rest was mixed with 30 volume % of fresh substrate. Subsequently, the digestate was sieved (20-mm mesh) to remove impurities, such as stones, larger plastics, and metals, before it is processed to fertilizer and potting soil using an aerobic composting process. To produce high-quality compost, digestates were matured for 11 to 13 months and sieved using a 10-mm mesh. Lower-quality fertilizer was matured for only 8 to 9 months, and no additional sieving step was performed at 10 mm.
In addition, to expand the range and verify our findings, 1.5 liters of liquid fertilizer for private and agricultural use produced by another anaerobic plant (plant D) was screened for MPPs. This plant processes 16,000 t/a biowaste from commerce, particularly waste from the local market, as well as waste from food and drink industries.
Energy crop digester, plant C and plants E to N
The agricultural energy crop digester (plant C) serving as a presumably uncontaminated reference in this study was a standard two-stage “wet-digester” tank system consisting of a 30-m3 unit for feeding, a 400-m3 plug-flow fermenter with spool agitators, and a 1000-m3 agitated postdigester. The fermenter and postdigester were equipped with heating aggregates and operated between 42° and 45°C. The plant converts approximately 3200 t/a of corn silage and 200 t/a of ground wheat, together with varying amounts of grass silage, and produces approximately 950,000 Nm3 of biogas per year. Before feeding, the silage was removed from its plastic encasing and passed through a mechanical shredder. Enough water was added to ensure pumpability. In addition, similar end-of-process samples were taken from 10 additional agricultural biogas plants (plants E to N), with feeds ranging from dung/manure, sunflowers, or waste from fruit processing, together with regular energy crops; none of these plants processed any biowaste. Whatever material arrived in plastic encasings was taken from these foils before being either mechanically shredded or directly entered into the digester.
Sampling
All samples were stored in glass jars to avoid contamination by plastics. In the case of the energy crop digester (plant C), a 2-liter sample was taken from the outlet pipe of the postdigester after a certain amount of digestate was discharged to avoid clotted residues. The 10 additional agricultural biogas plants (plants E to N) included in the study were sampled in the same way as the agricultural biogas plant (plant C).
Four 0.75-liter subsamples were taken from the biowaste digester (plant B) and pooled from a compost (Digest A) matured for 11 months and a compost (Digest B) matured for 13 months. Both were sieved with a mesh size of 10 mm. In addition, one compost (Digest C) sample, which had not been matured beforehand, was sieved with a mesh size of 20 mm. For each compost sample, four subsamples were taken equidistantly at a constant height per heap according to the heap size (50 cm for heap A, 30 cm for heap B, and 1.5 m for heap C). The first subsample was always taken at a distance of 1 m from the wall, and every subsequent subsample was taken at an interval of 1 m from the previous subsample. Compost heap C was sampled from the rightmost end to the middle to maintain the greatest possible distance from the adjacent heap (which had not yet undergone sieving) to avoid contamination with objects that would not have passed the sieving process. In addition to the compost, 5.5-liter samples were taken from the percolate at the outlet of the pipeline pooling the percolate from all fermenter boxes (Digest D). In the second anaerobic biowaste digestion plant (plant D) in the study, a representative sample was drawn from commercially available 5-liter bindings.
In the case of the biowaste composting plant (plant A), two 40-liter batches of compost were purchased and subsampled to a 3-liter volume. One batch was sieved with a mesh size of 8 mm (“CP 8 mm”), and the other was sieved with a 15-mm mesh (“CP 15 mm”).
Isolation of MPPs
For MPP isolation, samples were wet-sieved through three stacked stainless steel sieves with mesh sizes of 5, 2, and 1 mm and exemplarily 500 μm (see below). Objects >5 mm were thoroughly rinsed over the sieves with filtered water and filtered ethanol (30%) to remove any attached MPPs. The material remaining on the sieves was visually presorted under a Leica M50 stereomicroscope. Potential plastic particles were photographed, sized at a magnification of ×40 with a digital camera for microscopy (Olympus DP26), and stored for further analysis using ATR-FTIR spectroscopy (see below). Additional samples from 10 agricultural biogas plants and one liquid fertilizer were treated equally, with the exception of sieving with mesh sizes of 1 mm and 500 μm.
FTIR spectroscopy
A Bruker Tensor 27 FTIR spectrometer equipped with a germanium crystal for measurements in the ATR mode was used for spectral analysis of the putative MPPs. Following 16 background scans, 16 sample scans were performed with a spectral resolution of 8 cm−1 within a range of 3940 to 800 cm−1. The measured spectra were identified by comparison with reference spectra from a custom-made spectral polymer library. The library includes 131 records and contains not only the most common plastic polymers but also natural materials such as silicate, chitin, cotton, or keratin (32).
Determination of dry weight
For standardization, the dry weight of each pooled sample (n = 5) was determined by weighing before and after drying at 60°C to a constant weight.
Acknowledgments
We want to thank U. Wilczek, H. Martirosyan, and M. Preiss for help with the experiments. Furthermore, we want to thank the editor and the anonymous reviewers for valuable and helpful comments on the manuscript and B. Trotter for linguistic improvements. Institutional Review Board and/or Institutional Animal Care and Use Committee guidelines were followed with human or animal subjects. Funding: The authors acknowledge that they received no funding in support of this research. Author contributions: C.L. and R.F. designed the study. N.W., J.N.M., M.G.J.L., and S.P. performed the experiments. N.W., M.G.J.L., S.P., C.L., and R.F. wrote and revised the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.Plastics - The facts 2016, An Analysis of European Plastics Production, Demand and Waste Data (PlasticsEurope, 2016).
- 2.Green Paper - On a European Strategy on Plastic Waste in the Environment (European Commission, 2013).
- 3.L. Neufeld, F. Stassen, R. Sheppard, T. Gilman, Eds., The New Plastics Economy: Rethinking the Future of Plastics (World Economic Forum, 2016). [Google Scholar]
- 4.Jambeck J. R., Geyer R., Wilcox C., Siegler T. R., Perryman M., Andrady A., Narayan R., Law K. L., Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015). [DOI] [PubMed] [Google Scholar]
- 5.G7 leaders, Leadersʼ Declaration (2015); www.g7germany.de/Content/EN/_Anlagen/G7/2015-06-08-g7-abschluss-eng_en.pdf?__blob=publicationFile&v=3.
- 6.Rochman C. M., Browne M. A., Halpern B. S., Hentschel B. T., Hoh E., Karapanagioti H. K., Rios-Mendoza L. M., Takada H., Teh S., Thompson R. C., Policy: Classify plastic waste as hazardous. Nature 494, 169–171 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Ivar do Sul J. A., Costa M., The present and future of microplastic pollution in the marine environment. Environ. Pollut. 185, 352–364 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Wright S. L., Thompson R. C., Galloway T. S., The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 178, 483–492 (2013). [DOI] [PubMed] [Google Scholar]
- 9.McCormick A., Hoellein T. J., Mason S. A., Schluep J., Kelly J. J., Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 48, 11863–11871 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Browne M. A., Crump P., Niven S. J., Teuten E., Tonkin A., Galloway T., Thompson R., Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 45, 9175–9179 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Lithner D., Larsson Å., Dave G., Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 409, 3309–3324 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Galloway T. S., Cole M., Lewis C., Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 1, 0116 (2017). [DOI] [PubMed] [Google Scholar]
- 13.van Cauwenberghe L., Janssen C. R., Microplastics in bivalves cultured for human consumption. Environ. Pollut. 193, 65–70 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Andrady A. L., Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Cole M., Lindeque P., Halsband C., Galloway T. S., Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 62, 2588–2597 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Imhof H. K., Ivleva N. P., Schmid J., Niessner R., Laforsch C., Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 23, R867–R868 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Zbyszewski M., Corcoran P., Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water Air Soil Pollut. 220, 365–372 (2011). [Google Scholar]
- 18.Wagner M., Scherer C., Alvarez-Muñoz D., Brennholt N., Bourrain X., Buchinger S., Fries E., Grosbois C., Klasmeier J., Marti T., Rodriguez-Mozaz S., Urbatzka R., Dick Vethaak A., Winther-Nielsen M., Reifferscheid G., Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 26, 12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eerkes-Medrano D., Thompson R. C., Aldridge D. C., Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 75, 63–82 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Duis K., Coors A., Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 28, 2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.B. Kehres, H&K Aktuell, Änderung der Düngemittelordnung (BGK e.V., 2015).
- 22.Löder M. G. J., Imhof H. K., Ladehoff M., Löschel L. A., Lorenz C., Mintenig S., Piehl S., Primpke S., Schrank I., Laforsch C., Gerdts G., Enzymatic purification of microplastics in environmental samples. Environ. Sci. Technol. 51, 14283–14292 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Imhof H. K., Laforsch C., Wiesheu A. C., Schmid J., Anger P. M., Niessner R., Ivleva N. P., Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Res. 98, 64–74 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Selke S., Auras R., Nguyen T. A., Castro Aguirre E., Cheruvathur R., Liu Y., Evaluation of biodegradation-promoting additives for plastics. Environ. Sci. Technol. 49, 3769–3777 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Federal Statistical Office (2017); www.destatis.de/EN/Homepage.html.
- 26.Mintenig S. M., Int-Veen I., Löder M. G. J., Primpke S., Gerdts G., Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 108, 365–372 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Mahon A. M., O’Connell B., Healy M. G., O’Connor I., Officer R., Nash R., Morrison L., Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 51, 810–818 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Zubris K. A. V., Richards B. K., Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 138, 201–211 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Rillig M. C., Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 46, 6453–6454 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Huerta Lwanga E., Gertsen H., Gooren H., Peters P., Salánki T., van der Ploeg M., Besseling E., Koelmans A. A., Geissen V., Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 50, 2685–2691 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Gaylor M. O., Harvey E., Hale R. C., Polybrominated diphenyl ether (PBDE) accumulation by earthworms (Eisenia fetida) exposed to biosolids-, polyurethane foam microparticle-, and Penta-BDE-amended soils. Environ. Sci. Technol. 47, 13831–13839 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Löder M. G. J., Kuczera M., Mintenig S., Lorenz C., Gerdts G., Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ. Chem. 12, 563–581 (2015). [Google Scholar]


