Abstract
Objective
During inflammation, macrophages secrete vesicles carrying RNA, protein and lipids as a form of extracellular communication. In the vessel wall, extracellular vesicles (EVs) have been shown to be transferred between vascular cells during atherosclerosis; however, the role of macrophage-derived EVs in atherogenesis is not known. Here, we hypothesize that atherogenic macrophages secrete miRNAs in EVs to mediate cell-cell communication and promote pro-inflammatory and pro-atherogenic phenotypes in recipient cells.
Approach and Results
We isolated EVs from mouse and human macrophages treated with an atherogenic stimulus (oxidized LDL) and characterized the EV miRNA expression profile. We confirmed the enrichment of miR-146a, miR-128, miR-185, miR-365 and miR-503 in atherogenic EVs compared to controls and demonstrate that these EVs are taken up and transfer exogenous miRNA to naive recipient macrophages. Bioinformatic pathway analysis suggests that atherogenic EV miRNAs are predicted to target genes involved in cell migration and adhesion pathways, and indeed delivery of EVs to naive macrophages reduced macrophage migration both in vitro and in vivo. Inhibition of miR-146a, the most enriched miRNA in atherogenic EVs, reduced the inhibitory effect of EVs on macrophage migratory capacity. EV-mediated delivery of miR-146a repressed the expression of target genes IGF2BP1 and HuR in recipient cells, and knockdown of IGF2BP1 and HuR using siRNA greatly reduced macrophage migration, highlighting the importance of these EV-miRNA targets in regulating macrophage motility.
Conclusion
EV-derived miRNAs from atherogenic macrophages, in particular miR-146a, may accelerate the development of atherosclerosis by decreasing cell migration and promoting macrophage entrapment in the vessel wall.
Introduction
Cell-to-cell communication facilitates the functional coordination of different cell types within a tissue and is a hallmark of multicellular organisms. In addition to cell junctions, adhesion contacts and soluble messengers, cells communicate via the release and transfer of extracellular vesicles (EVs), which have recently emerged as multifaceted regulators of intercellular communication [1–4], [5]. Depending on their size and mechanism of generation, EVs can be classified as exosomes, microvesicles or apoptotic bodies. Exosomes are membrane vesicles ranging from 30 to 150nm in size that are generated by inward budding of the limiting membrane of multivesicular bodies (MVBs). MVBs release exosomes into the extracellular environment by directly fusing with the plasma membrane [6, 7]. Microvesicles, on the other hand, are larger vesicles (with a diameter of 200nm to 1µm) that are produced by direct budding from the cell plasma membrane as shedding vesicles [8, 9]. Secreted EVs can be taken up by target cells through endocytosis, phagocytosis or direct fusion with the plasma membrane, leading to the delivery of EV proteins and RNAs to modulate specific cellular signaling pathways in recipient cells [10].
Like other cell types, macrophages utilize EVs to communicate inflammatory signals. During infection, macrophages secrete exosomes containing pathogen-associated molecular patterns that promote cytokine production and recruitment of other immune cells to inflammatory sites [11]. Moreover, there is increasing evidence that inflammatory macrophages transfer microRNAs (miRNAs) packaged into EVs which alter gene expression in recipient cells [12]. Macrophage-derived microvesicles are enriched in miR-223 and are thought to regulate macrophage differentiation [13]. Although being extensively characterized in infectious inflammation, the role of macrophage-derived EVs, especially the unique EV loading of miRNAs in sterile inflammatory diseases, has not been well studied.
Atherosclerosis is a lipid-driven chronic inflammatory disease where excess cholesterol accumulation in the vessel wall drives a non-resolving immune response [14]. Although many cell types contribute to the disease, macrophages are fundamental to atherosclerotic progression and are one of the most abundant components in atherosclerotic plaques. In the early stages of atherosclerosis, the key inflammatory response to lipid accumulation and endothelial activation is recruitment of circulating monocytes into the vascular intima. Recruited monocytes differentiate into macrophages that scavenge retained modified lipoproteins, transforming into cholesterol-laden foam cells [15, 16]. Excessive free cholesterol accumulation in macrophage-derived foam cells results in activation of downstream cascades including NLRP3 inflammasome, Toll-like receptor (TLR) signaling, and endoplasmic reticulum (ER) stress responses. These inflammatory signals exacerbate not only oxidative stress in the plaques but also the transmigration of additional inflammatory cells including monocytes into the intima [16–18]. Lesion regression requires the resolution of inflammation and macrophage emigration out of the plaques through reverse transmigration. However, during atherosclerosis progression, the expression of retention factors render the macrophage immobile and prevent their emigration out of the artery wall [19], [20, 21]. A better understanding of mechanisms that regulate macrophage motility during atherosclerosis will provide insights into pathways that may have therapeutic value.
It is known that secreted factors such as guidance cues and chemotactic proteins are crucial for controlling monocyte recruitment and macrophage emigration, but whether or not macrophage-derived EVs also play a key role in modulating macrophage migration is unknown. We hypothesized that macrophages can promote pro-inflammatory and pro-atherogenic phenotypes in recipient cells through secretion of EVs containing miRNAs. In the current study, we find that EVs derived from cholesterol-loaded macrophages can inhibit macrophage migration in vitro and in vivo. This effect appears to be mediated by the transfer of several miRNAs, including miR-146a, to recipient macrophages where they repress the expression of specific pro-migratory target genes IGF2BP1 (insulin-like growth factor 2 mRNA-binding protein 1) and HuR (human antigen R or ELAV-like RNA-binding protein 1). Our studies suggest that EV-derived miRNAs secreted from atherogenic macrophages may accelerate the development of atherosclerosis lesions by decreasing cell migration and promoting macrophage entrapment in the vessel wall.
Materials & Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Characterization of macrophage-derived EVs and EV-derived RNA
In order to characterize EVs from atherogenic macrophages, we isolated EVs from primary mouse macrophages treated with or without oxidized LDL (oxLDL). Nanoparticle tracking analysis showed that isolated EVs from both control and oxLDL-treated macrophages ranged in size from 40 to 300nm with a mean particle size of 111 and 125nm, respectively, which is the typical size range of exosomes or small microvesicles [8] (Figure 1A). Quantification of the size of EVs across multiple experiments revealed that there was a small but significant increase in the size of EVs secreted from oxLDL-loaded macrophages (Figure 1B). To further confirm the purity of macrophage-derived EVs, Western Blot analysis was performed and showed that EV samples isolated from control and oxLDL-treated macrophages were positive for CD9 and Tsg101, well-known exosomal markers [6], and were negative for Calnexin, an integral ER membrane protein that was used as a marker for cell contamination (Figure 1C). We then characterized total RNA recovered from macrophage-derived EVs using Bioanalyzer analysis, and found that total EV-derived RNA mainly contained small RNAs and did not contain the prominent peaks of 18S and 28S rRNA (Figure 1D). Bioanalyzer analysis was also used to analyze total RNA isolated from oxLDL-containing media alone (incubated in cell-free wells) which revealed that there was little or no RNA in oxLDL (Supplemental Figure 1A). In addition, we examined the secretion of EVs from atherogenic macrophages using transmission electron microscopy. Results showed that some vesicles were visible as intraluminal vesicles located inside MVBs, which is typical for exosomes (Figure 1E). A different type of vesicle was also observed and characterized as clusters of endomembrane vesicles enclosed by the plasma membrane (Figure 1F). These MVB-like structures are similar to the vesicles released by smooth muscle cells implicated in vascular calcification [22]. Together, these data indicate that primary mouse macrophages secrete EVs that share many characteristics with exosomes including size, marker expression and ultrastructural secretion patterns.
Figure 1. Characterization of macrophage-derived EVs and EV-derived RNA.
EVs secreted from control and oxLDL-loaded (50 µg/ml for 24h) peritoneal macrophages were purified using ExoQuick-TC solution. (A) EVs were visualized on NanoSight LM10. (B) Mean size of EVs secreted from control and oxLDL-loaded macrophages, Analysis from duplicates from n=3 experments **p<0.01. (C) EVs were subjected to immunoblot analysis of Calnexin, Tsg101 and CD9 in non-reducing conditions. (D) Total RNA was extracted from EVs and was analyzed using Bioanalyzer RNA 6000 Pico chip. (E) Electron microscope images of multivesicular bodies where the membrane invaginates inward to form intraluminal vesicles referred as exosomes. (F) Electron microscope images of a different type of EVs secreted from oxLDL-loaded macrophages.
Differential miRNA expression profile in EVs secreted from cholesterol-loaded macrophages
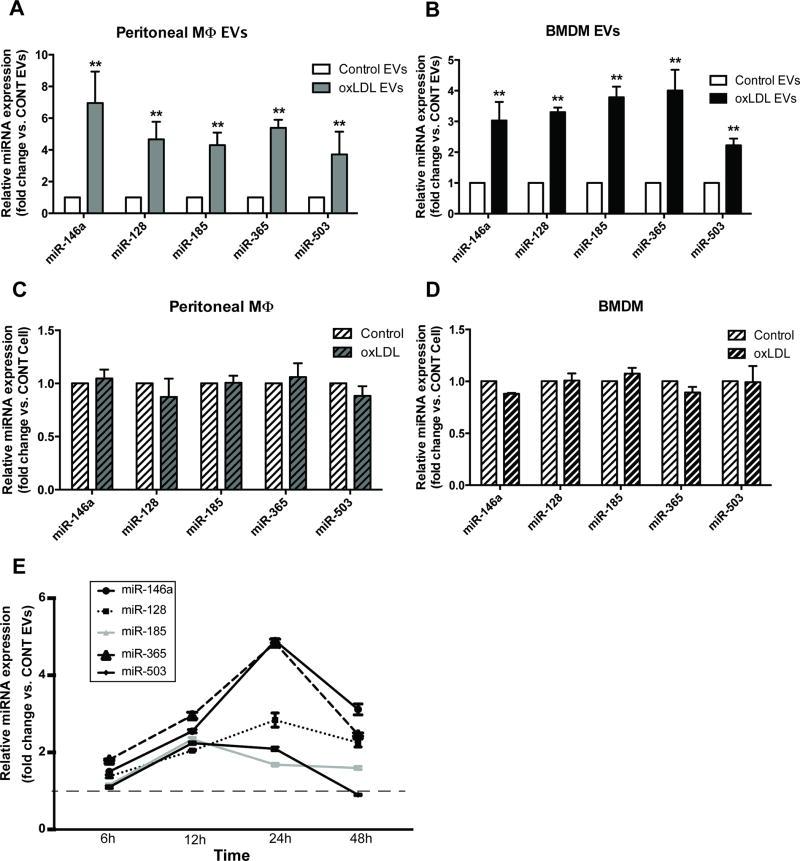
To characterize the EV-derived miRNA signature of atherogenic macrophages, we performed miRNA arrays on atherogenic EVs compared to control EVs and confirmed via qRT-PCR that EVs secreted from cholesterol-loaded macrophages were enriched in miR-146a, miR-128, miR-185, miR-365, and miR-503 (Figure 2A). We also examined the expression of these miRNAs in atherogenic EVs isolated from other macrophages, including mouse primary bone marrow-derived (BMDMs), RAW 264.7, and human THP-1 monocyte-derived macrophages. As expected, the signature of EV-derived miRNAs showed similar patterns of expression in different types of macrophages (Figure 2B and Supplemental Figure 1B-C). Because of the observation that EV-dervied miRNA profiles can be slightly affected by the vesicle isolation method [23, 24], we also isolated EVs from control and cholesterol-loaded macrophages using ultracentrifugation and confirmed the increased expression of top 5 miRNAs in atherogenic EVs (Supplemental Figure 1D). miRNAs are believed to be selectively packaged into EVs and do not necessarily reflect miRNA profiles observed in the parental cells [25]. To determine whether the changes of miRNA expression that occurred in EVs also occurred in the cell of origin, we examined the expression of top candidate miRNAs in the corresponding intracellular RNA samples. Although highly up-regulated in EVs secreted from atherogenic macrophages, the cellular expression levels of miR-146a, miR-128, miR-185, miR-365, and miR-503 in atherogenic macrophages did not significantly change compared to controls (Figure 2C and 2D). We also confirmed that these candidate miRNAs are enriched only in exosomes or small microvesicles secreted from oxLDL-loaded macrophages, not in larger microvesicles isolated from oxLDL-loaded cells nor EVs from LDL-treated macrophages (Supplemental Figure 1E-F). Time course analysis of miRNA expression showed that upregulation of candidate miRNAs in atherogenic EVs occurred early, after 6 hours of oxLDL treatment, with peak expression at 24 hours and persisted until 48-hour post-treatment (Figure 2E). Together, these data indicated that macrophages loaded with oxLDL secrete EVs enriched in miR-146a, miR-128, miR-185, miR-365, and miR-503.
Figure 2. Differential miRNA expression profile in EVs secreted from oxLDL-loaded macrophages.
(A) miRNA expression in EVs isolated from oxLDL-treated peritoneal macrophages (same experimental conditions as Figure 1). (B) miRNA expression in EVs isolated from oxLDL-treated BMDMs. (C) and (D) miRNA expression in corresponding cellular RNA samples. (E) Time course profile of miRNA expression in peritoneal macrophage-derived EVs. Graphs represent the means ± SD from n=3 independent experiments, **p<0.01.
EVs from atherogenic macrophages are taken up by naïve macrophages and transfer microRNA
In order for EV-derived miRNAs to exert paracrine effects, EVs need to be taken up and contents delivered to recipient cells. Following the identification of the most highly dysregulated miRNAs in atherogenic EVs, we proceeded to test whether control and atherogenic EVs could be taken up by naïve macrophages. We established a co-culture system where fluorescently-labeled macrophages were cultured together with naïve (unlabeled) macrophages, separated by a 0.4µm transwell filter to avoid direct cell contact or transfer of larger vesicles. Confocal microscopy analysis showed that naïve cells co-cultured with membrane labeled macrophages took up the label, indicating transfer of small (less than 0.4µm) membranous vesicles between these cells (Figure 3A). We next isolated labeled macrophage-derived EVs from control and oxLDL-treated cells and applied them directly to naïve macrophages. As expected, we observed a rapid uptake of fluorescently-labeled EVs from both control and atherogenic EVs into naïve cells (Figure 3B). Notably, when we inhibited exosome generation using GW4869 [26], the levels of fluorescent signals in naïve cells significantly decreased as compared to control (Figure 3C-D).
Figure 3. EVs from atherogenic macrophages are taken up and deliver exogenous miRNAs to naïve macrophages.
(A-C) Peritoneal macrophages (donor cells) were stained with Vybrant DiO cell labeling solution, and visualization of EV uptake was performed using confocal microscopy. (A) Labeled macrophages were co-cultured with naïve macrophages in the absence/presence of oxLDL (50 µg/ml) for 24h. (B) Labeled macrophages were treated without/with oxLDL (50 µg/ml) for 24h. EVs were then purified using ExoQuick-TC solution and applied to naïve macrophages. (C) Labeled macrophages were pre-treated with GW4869 (10µM) for 24h. Culture media was collected and applied to naïve macrophages. (D) The area of fluorescent signal from (C) was quantified using Image J software using 5 fields for each condition. Graph represents the mean ± SD from n=3 independent experiments. Images are representative of experiments done in triplicate (n=3). (E) Macrophages (donor cells, BMDMs) were transfected with C.elegans miR-54 mimics and then co-cultured with naïve macrophages in the absence/presence of oxLDL (50 µg/ml) for 48h. Total RNA was isolated from naïve macrophages and cel-miR-54 levels were measured by qPCR. The experiment was performed in triplicate (n=3), **p <0.01.
To determine if macrophage-derived EVs can transfer miRNAs to naïve macrophages, we once again employed a co-culture system of macrophages transfected with cel-miR-54 (naturally present only in Caenorhabditis elegans) with naïve macrophages separated by a 0.4µm filter. The expression levels of cel-miR-54 in naïve macrophages were analyzed after 24-hour and 48-hour co-culture and demonstrated that cel-miR-54 is transferred to naïve macrophages (Figure 3E). Overall, this shows that EVs secreted by donor macrophages can be taken up and transfer their miRNA contents to recipient macrophages and these EVs are likely derived from the exosomal pathway.
Atherogenic EVs inhibit macrophage migration in vitro
To determine the potential pathways regulated by the signature of EV-derived miRNA, we performed bioinformatic analysis on the most dysregulated miRNAs in atherogenic EVs (including up- and down-regulated miRNAs) using miRSystem [27]. The rationale for this approach is that although the magnitude of gene repression exerted by a single miRNA is relatively small, distinct miRNAs can simultaneously target many genes in a given pathway and collectively alter cell function. Pathway analysis demonstrated that EV-derived miRNAs secreted from atherogenic macrophages putatively target cell migration pathways, with at least 7 out of 10 dysregulated miRNAs predicted to target genes that control leukocyte transendothelial migration, cell adhesion molecules, regulation of actin cytoskeleton and axon guidance (Table 1); many of which have been shown to play a role in atherosclerosis [28, 29], [30].
Table 1. Bioinformatic pathway analysis of the most significantly up-regulated miRNAs from atherogenic EVs.
miRSystem (http://mirsystem.cgm.ntu.edu.tw/) was used and listed here are the most enriched pathways, including how many miRNAs (out of 10) are predicted to target the pathway. A gene is considered as a potential target of a miRNA using at least 3 prediction algorithms.
| Predicted pathways | Target Genes of Interest | # miRNAs targeting pathway (/10) |
|
|---|---|---|---|
| Pathways Predicted to be targeted by dysregulated miRNAs | Leukocyte Transendothelial Migration | PI3 kinase receptor 1, RhoA, ROCK1, ROCK2, PECAM, Cadherin 5, Cdc42, Protein kinase C-β | 7 |
| Cell Adhesion Molecules | PTEN, PI3 kinase R1, Cell adhesion molecule 1 (CADM1), Cadherin 5, VEGFA, Collagen 1α2, Collagen 4α6, CD34 | 6 | |
| Regulation of Actin Cytoskeleton | Fibronectin, FGF1, FGFR1, FGF12, Actinin alpha 1 (ACTN1), Actinin α4 (ACTN4), Integrin α3 | 7 | |
| Axon Guidance | DCC, UNC5C, Eph receptor A7 (EPHA7), Netrin G1, Semaphorin 3A, Semaphorin 6A, Eph receptor B2 (EPHB2), Eph receptor B1, ROBO1 | 7 | |
| Chemokine Signaling Pathway | CX3CL1, CXCL12, CXCL2, CXCR4, LYN, STAT5B, ELMO1 | 7 |
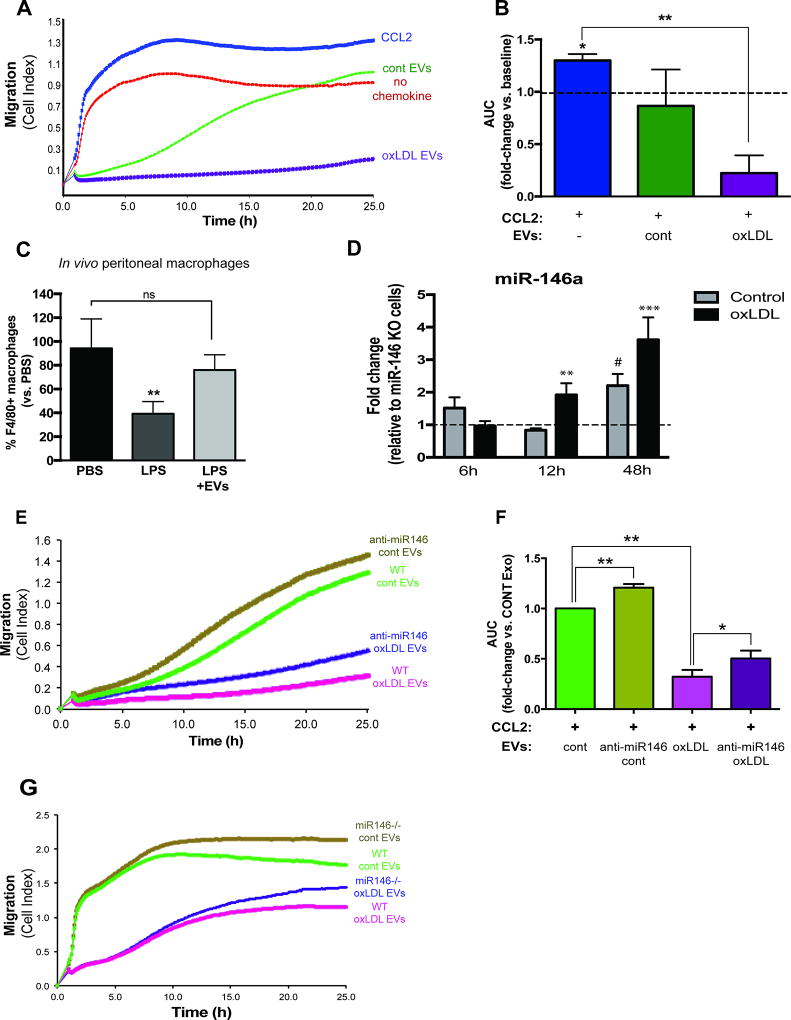
Because of the importance of macrophage migration during all stages of atherosclerosis [15, 16], we investigated whether or not EVs secreted from atherogenic macrophages can indeed control migration in recipient cells. We treated naïve macrophages with EVs either from control or oxLDL-treated cells for 48 hours and measured their migration towards CCL2 – a chemokine known to promote macrophage migration [20, 21]. Treatment of naïve macrophages with EVs derived from atherogenic macrophages inhibited the migration of naïve cells towards CCL2 to a greater extent than EVs from control macrophages (80% decrease in migration relative to control, p≤0.01) (Figure 4A-B). Similar results were observed when we examined the effects of atherogenic EVs on the migration ability of RAW 264.7 macrophages (Supplemental Figure 2A-B). To confirm that the inhibitory effect on macrophage migration is specific for EVs, we again inhibited exosome generation using GW4869 and showed that blockade of exosome generation partially relieved the effect of EVs on macrophage migration (Supplemental Figure 2C). Thus, EVs isolated from atherogenic macrophages can be transferred to naïve macrophages and inhibit their migration in vitro.
Figure 4. EVs secreted from atherogenic macrophages inhibit macrophage migration and is partly mediated by miR-146a.
(A-B) BMDMs were cultured without/with oxLDL (50 µg/ml) for 24h and EVs were purified using ExoQuick-TC solution. Isolated EVs were applied to naïve macrophages for 48h. The migration of naïve macrophages towards CCL2 (150 ng/ml) was measured using xCELLigence System. Area under the curve (AUC) was measured for each condition and is shown as mean ± SD of n=3 experiments of quadruplicates, **p<0.01. (C) In vivo migration assay: C57BL6 mice (n=4/group) were injected intraperitoneally (I.P.) with thioglycollate and 4 days later peritoneal macrophages were labeled using 1µm Fluoresbrite microspheres. Mice were pre-treated with EVs (100µg, from RAW macrophages, injected I.P.) or PBS for 24h before treatment with LPS. Three hours following LPS injection, peritoneal cells were collected and the percentage of macrophages in the lavage was quantified by flow cytometry, **p<0.01. (D) Transfer of miR-146a from wild-type macrophages to miR-146a−/− macrophages. Wild-type macrophages (donor cells, BMDMs) were co-cultured with miR-146a−/− macrophages (recipient cells) in the absence/presence of oxLDL (50 µg/ml). Total RNA was isolated from recipient macrophages and miR-146a levels were measured by qPCR. Graphs represent the means ± SD from pooled BMDM of n=5 mice performed in triplicate. **p<0.01, ***p<0.001 vs control and background; # p<0.001 vs background. (E-F) EVs isolated from BMDMs transfected with control anti-miR or anti-miR146a were applied to naïve BMDMs and migration was measured using xCELLigence System as described above. Graphs represent the means ± SD from n=3 independent experiments, *p<0.05 **p<0.01. (G) Control/Atherogenic EVs were isolated from wild-type or miR-146a−/− BMDMs and applied to naïve macrophages and migration was examined using xCELLigence System as described above. Shown here is the representative experiment done in triplicate (n=3).
Macrophage-derived EVs reduce macrophage emigration in vivo
During atherosclerosis regression, plaque macrophages emigrate from the lesion via nearby lymphatics upon cholesterol lowering and removal via efflux pathways [31]. This is similar to mechanisms used by inflammatory macrophages as they rapidly emigrate from the inflamed peritoneum to the draining lymph nodes during resolving peritonitis [32]. Therefore, we used a well-characterized peritonitis model to test the effect of EVs on macrophage emigration in vivo. Mice were first injected with thioglycollate to trigger macrophage accumulation in the peritoneal cavity, after which LPS was delivered to induce the rapid efflux of macrophages from the peritoneum. As previously observed [33], injection of LPS reduced the number of macrophages in the peritoneum by 60% compared to control PBS-injected mice. In contrast, mice pretreated with macrophage-derived EVs prior to LPS stimulation had equivalent macrophages remaining in the peritoneum as PBS treated mice, indicating that macrophage emigration out of the peritoneal cavity was not efficient (Figure 4C). Taken together, these data indicate that macrophage-derived EVs can inhibit macrophage migration both in vitro and in vivo.
Role of miR-146a in macrophage migration
As miR-146a was among the most significantly altered miRNA in atherogenic EVs, we postulated that miR-146a might partially mediate the effects exerted by these EVs. Using a co-culture system, we find that endogenous miR-146a from wild-type macrophages could be transferred to miR-146−/− naive cells, and this process was accelerated with oxLDL (Figure 4D). To test the role of EV miR-146a in macrophage migration, we knocked down miR-146a using anti-miR oligonucleotides and then isolated normal and atherogenic EVs from these cells. Macrophage-derived EVs deficient in miR-146a showed an increased macrophage migration index compared to EVs from control anti-miR macrophages. This increase in migration in the absence of miR-146a was observed regardless of whether the EVs were isolated from control or atherogenic conditions (Figure 4E-F). Additionally, we isolated EVs from miR-146a−/− macrophages, and examined the effects of these EVs on macrophage migration. Naïve macrophages treated with control or atherogenic EVs from miR-146a−/− macrophages exhibited increased migration to CCL2 compared to EVs from WT mice (Figure 4G). Therefore, miR-146a delivered via EVs dampens macrophage migration towards a chemokine stimulus, which is relieved by inhibition of miR-146a.
EV-derived miR-146a may inhibit macrophage migration via targeting IGF2BP1 and HuR
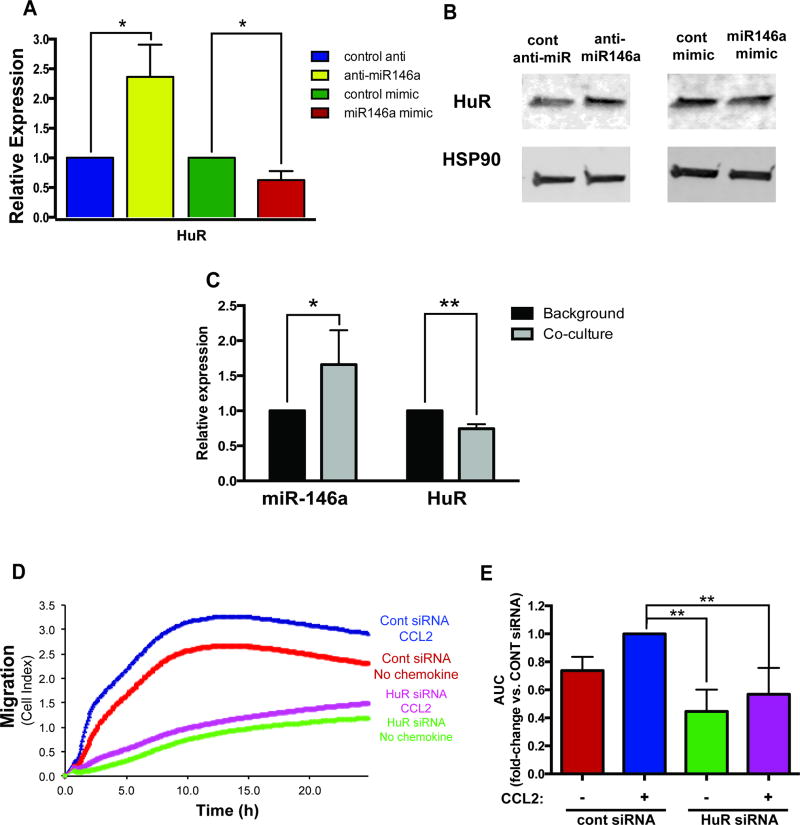
We next sought to understand the mechanisms underlying the inhibitory effects of EV-derived miR-146a on macrophage migration. Based on bioinformatics analysis, a number of potential genes predicted to be targeted by miR-146a are also implicated in cell migration pathways, including IGF2BP1 and HuR. Both IGF2BP1 and HuR are RNA binding proteins that modulate the expression of β-actin and have well-characterized roles in promoting cell migration [34, 35]. Over-expression of miR-146a suppressed and inhibition of miR-146a elevated the expression of IGF2BP1 at both the mRNA and protein level (Figure 5A-B). Luciferase reporter constructs containing the 3’UTR IGF2BP1 showed that miR-146a directly represses the UTR activity of IGF2BP1 (Figure 5C), confirming that it is a direct target of miR-146a. To test whether EV-derived miR-146a targets recipient cell IGF2BP1, we co-cultured miR-146a enriched macrophages with naïve macrophages and demonstrated that miR-146a was transferred to naïve cells where the expression levels of IGF2BP1 were concomitantly decreased (Figure 5D). To examine the function of IGF2BP1 in regulating macrophage migration, we measured the migration of macrophages in which IGF2BP1 was knocked down by short interfering RNA (siRNA). Inhibition of IGF2BP1 reduced macrophage migration towards CCL2 (Figure 5E-F) confirming its role as a modulator of migration in immune cells.
Figure 5. IGF2BP1 is a target of EV-derived miR-146a.
(A-B) IGF2BP1 mRNA (A) and protein (B) expression in BMDMs transfected with control anti-miR/anti-miR146a and control mimic/miR-146a mimic. TRAF6, a validated target of miR-146a, was used as a control for transfection efficiency. (C) Reporter 3’UTR assay for the human IGF2BP1 UTR. Graph depicts luciferase activity relative to control miR after 24h, **p<0.01. (D) Co-culture of miR-146a enriched macrophages and naïve macrophages resulted in an increased expression of miR-146a and a decreased expression of IGF2BP1 in naïve cells in the absence/presence of oxLDL for 72h. (E-F) Knockdown of IGF2BP1 using siRNA reduces macrophage migration. BMDMs were transfected with control or IGF2BP1 siRNA for 72h. The migration of transfected cells towards CCL2 (150 ng/ml) was measured using the xCELLigence System. All graphs in this figure represent the means ± SD from n=3 independent experiments, *p<0.05 **p<0.01. Western blot is representative of a single experiment done in triplicate (n=3).
In agreement with the previous study that identified HuR as a direct gene target of miR-146a [36], qRT-PCR and Western blot analysis of macrophages showed that levels of HuR mRNA and protein increased or decreased when miR-146a were knocked down or over-expressed, respectively (Figure 6A-B). Similar to what was observed with IGF2BP1, transfer of miR-146a from transfected macrophages to naïve macrophages increased the levels of miR-146a and reduced the expression levels of HuR in recipient cells (Figure 6C). Knock down of HuR using siRNA also reduced migration of macrophages towards CCL2 (FIGURE 6D-E). Together these data confirm that IGF2BP1 and HuR functionally promote macrophage migration, and both IGF2BP1 and HuR are targets of EV-derived miR-146a, which may contribute to the reduced chemotaxis in EV-treated cells.
Figure 6. HuR is a target of EV-derived miR-146a.
(A-B) HuR mRNA (A) and protein (B) expression in BMDMs transfected with control anti-miR/anti-miR146a and control mimic/miR-146a mimic. (C) Co-culture of miR-146a transfected macrophages in the absence/presence of oxLDL for 72hs with naïve macrophages resulted in an increased expression of miR-146a and a decreased expression of HuR in naïve cells. (D-E) Knockdown of HuR using siRNA reduces macrophage migration. BMDMs were transfected with control or HuR siRNA for 72h. The migration of transfected cells towards CCL2 (150 ng/ml) was measured using the xCELLigence System. All graphs in this figure represent the means ± SD from n=3 independent experiments, *p<0.05, **p <0.01. Western blot is representative of a single experiment done in triplicate (n=3).
miR-146a is elevated in atherosclerotic plaques and its targets IGF2BP1 and HuR are de-repressed in lesions from miR-146−/− mice
Because a dysregulation of miR-146a might alter macrophages in the vessel wall during atherogenesis, we examined miR-146a expression in lesions from Apoe−/− mice fed a chow or high-cholesterol diet. There was a significant upregulation in the expression of miR-146a in atherosclerotic lesions from Apoe−/− fed a high-cholesterol diet compared to mice fed a chow diet (Figure 7A). To extend these findings to humans, we analyzed miRNA expression in carotid plaques from patients with atherosclerosis, which revealed a significant increase of miR-146a expression compared to disease-free control arteries (Figure 7B). Because we find that miR-146a in EVs dampens macrophage migration, high levels of plaque miR-146a may adversely impact the ability of macrophages to emigrate from the plaque, and contribute to lesion development. To understand how miR-146a targets may be regulated in the atherosclerotic plaque, we examined the aortic expression of IGF2BP1 and HuR in lethally irradiated Ldlr−/− mice transplanted with wild-type or miR146a−/− bone marrow and fed a high cholesterol diet for 12 weeks. We found that IGF2BP1 and HuR expression was increased in lesions from Ldlr−/− mice receiving miR146a−/− bone marrow compared to mice receiving wild-type bone marrow transplant (Figure 7C-D). Together, these data suggest that atherosclerotic lesions contain high levels of miR-146a compared to normal arteries, and, in the absence of miR-146, target genes IGF2BP1 and HuR are upregulated and may play a role in lesion development.
Figure 7. The expression of miR-146a and its targets, IGF2BP1 and HuR, in atherosclerosis.
(A) Aortic expression of miR-146a in ApoE−/− mice fed a chow or high-cholesterol (0.2%) diet for 6 weeks. Graphs represent the means ± SD from pooled cDNA of n=5 mice per group, **p<0.01. (B) Human miR-146a expression in non-diseased arteries (normal) or carotids from humans with atherosclerosis (plaques) from the Biobank of Karolinska Endarterectomy (BiKE). (C-D) Aortic expression of IGF2BP1 (C) and HuR (D) in Ldlr−/− mice transplanted with wild-type bone marrow or miR146a-KO bone marrow. These mice were fed a high-cholesterol (1.25%) diet for 12 weeks. Graphs represent means ± SEM from n=4 mice per group.
Discussion
miRNAs are important modulators of multiple signaling pathways involved in atherosclerosis [37]. The complexity of miRNA-mediated pathway control has expanded since the discovery that miRNAs can be released and function as paracrine molecules to regulate gene expression in recipient cells [38]. miRNAs are packaged into EVs by a broad range of cell types, and different cell types involved in atherosclerosis progression are known to secrete miRNA-containing EVs [39, 40]. In this study, we demonstrate that atherogenic macrophages secrete EVs containing a specific set of miRNAs, which can be taken up by naïve macrophages and mediate the transfer of miRNAs to naïve cells. Functionally, we show that EVs derived from macrophages can inhibit the migration of naïve cells in vitro and in vivo. The effect of EVs on macrophage migration is partially attributable to the transfer of miR-146a to naïve cells where it represses the expression of specific target genes IGF2BP1 and HuR. Our study is the first to explore communication between macrophages in an atherogenic scenario, and suggest that miRNAs may act as paracrine modulators in the development of atherosclerosis.
EVs are primarily classified based on their size and mechanism of secretion. In atherosclerosis, exosomes, microvesicles and apoptotic bodies can mediate signals between cells in the plaque [40]. Although vascular ECs, SMCs and platelets have been shown to bi-directionally shuttle miRNA between cells via exosomes and apoptotic bodies [41–43], the current study is the first to show that lipid-laden macrophages can functionally alter pro-atherogenic phenotypes in recipient macrophages by directly transferring miRNAs via EVs. Although we cannot entirely exclude the role of microvesicles and/or apoptotic bodies in this macrophage-to-macrophage communication, our results using inhibitors, co-culture and nanoparticle tracking analysis suggests that EVs used in our study are likely exosomes.
Accumulation of lipid-laden macrophages in the artery wall, which results from the imbalance of macrophage recruitment to plaque and macrophage emigration from plaque to regional lymph nodes, contributes to the inflammatory propagation of atherosclerosis and plaque instability [16, 31]. Thus, macrophage migration is central to the development of atherosclerosis. Our study shows that EVs secreted from macrophages can block macrophage migration towards a chemokine stimulus (CCL2), which along with its receptor, CCR2, can promote myeloid trafficking to the lymph nodes during inflammation [44, 45]. Our findings parallel the observation that oxLDL induces the migratory arrest of macrophages in vitro which involves focal adhesion kinase and reorganization of the actin cytoskeleton [33, 46]. We find that EV-derived miR-146a represses the expression of several genes implicated in cell migration pathways including HuR and IGF2BP1. The RNA-binding protein HuR is a validated target of miR-146a [36], and in several cancer cell lines, HuR promotes cell migration by stabilizing β-actin and Snail mRNAs [34, 47]. Here, we confirm that EV-derived miR-146a decreases HuR in macrophages. Knockdown of HuR using RNA interference reduces migration towards CCL2 stimulus, confirming a role of this protein in modulating macrophage migration. We also identify the RNA-binding protein IGF2BP1 as a novel target of both endogenous and EV-derived miR-146a. IGF2BP1 is a potent oncogenic factor that regulates the migration and invasiveness of tumor cells by interfering with the translation of MAPK4 and β-actin [35, 48]. Supporting the role of IGF2BP1 in regulating macrophage migration, IGF2BP1 knockdown strongly reduced macrophage migration, and we noted the lower expression of IGF2BP1 in cholesterol-loaded macrophages which have impaired chemotaxis (data not shown). Thus, foam cells may promote migration arrest in other macrophages via the secretion of EVs containing miR-146a, which along with other factors, may inhibit macrophage migration via downregulation of RNA-binding proteins IGF2BP1 and HuR.
To confirm our in vitro findings, we used a model of thioglycollate-elicited peritonitis to study macrophage emigration in vivo [20, 33, 49]. We show that macrophage-derived EVs reduce macrophage migration to lymph nodes in response to LPS, confirming a role of macrophage-derived EVs in regulating macrophage emigration out of inflamed tissues and into the lymph nodes. Interestingly, EVs from mycobacteria-infected macrophages has also been shown to induce cell migration in vitro and in vivo [50, 51], suggesting that EVs from atherogenic macrophages may target similar pathways but exert opposing effects. Our in vivo and in vitro data suggest that EVs secreted from atherogenic macrophages decrease macrophage migration and may promote macrophage entrapment in the vessel wall, which would accelerate the development of atherosclerosis. However, we cannot exclude the possibility that atherogenic EVs may dampen inflammatory cell migration into the arterial intima. Blocking monocyte recruitment, which is crucial for both plaque progression and regression, may be advantageous or disadvantageous in atherosclerosis depending on the timing of inhibition [52]. Therefore, whether EVs from atherogenic macrophages have beneficial effects on the development of atherosclerosis still needs further investigation.
EVs isolated from oxLDL-loaded macrophages showed enrichment of several miRNAs including miR-146a, miR-128, miR-185, miR-365, and miR-503. Notably, oxLDL did not induce changes in the intracellular levels of these miRNAs in donor cells. Previous studies also demonstrated that the expression of RNAs in secreted vesicles does not reflect the intracellular content of RNAs, and selective packaging of miRNAs into vesicles may be crucial for the specificity of the biological functions of secreted miRNAs [53, 54]. The results from our study indicate that oxLDL stimulation of macrophages induces the selective enrichment of miRNAs into exosome-like EVs, and this is not simply a result of packaging of abundant miRNAs into microvesicles or the passive release of miRNAs that is commonly observed during cell death. Moreover, individual cell types appear to secrete a unique assortment of EVs containing a specific set of cargo proteins and RNAs, which allow a precise and regulated signal to be communicated to recipient cells. In our case, several specific miRNAs including miR-146a, miR-128, miR-185, miR-365 and miR-503 were up-regulated in EVs isolated from cholesterol-loaded macrophages. In contrast, EVs derived from LPS-stimulated macrophages showed enrichment of different signature of miRNAs including miR-21, miR-126, miR-146a/b, miR-212, miR-222 [12]. The mechanisms that define cargo selection and packaging into vesicles remain poorly understood. Some evidence suggests that sumoylated heterogenous nuclear ribonucleoproteins (hnRNPs) direct the loading of miRNAs into exosomes through recognition of specific short motifs [55]. MEK-ERK (mitogen-activated protein kinase kinase/extracellular-signal-regulated kinase) signaling downstream of activated KRAS was shown to inhibit sorting of Argonaute (Ago) 2 and Ago2-dependent miRNA let-7a into tumor cell-derived exosomes [56]. In addition, the RNA-binding protein Y-box protein 1 (YBX1) is required for the sorting of miR-223 in exosomes secreted by HEK293T cells [57]. Collectively these studies strongly support the notion that small RNAs are selectively packed into EVs and that different cells may use diverse RNA sorting mechanisms. Additionally, in our study we could only partially EV generation by treating with GW4869, likely because GW4869 inhibits only one pathway of exosome secretion, namely neutral sphingomyelinase, and only reduces EV secretion by 40–50%. Whether macrophages secrete EVs via the ESCRT/Rab machinery from MVBs, and how these mechanisms are unique in certain cell types awaits further insights. And finally, there is the possibility that LDL can co-precipitate in the EV preparations from our oxLDL-treated cells, resulting in some carryover of oxLDL into naïve cells. However, when cells are treated with 50µg/ml as in our study, the majority of oxLDL is taken up by macrophages after 5h [58]. Thus, in cells treated with EVs from oxLDL-treated BMDMs, there is likely only a minor contribution of contaminating oxLDL.
Many of the atherogenic EV-enriched miRNAs found in our study have been shown to play a role in inflammatory and lipid homeostasis pathways. For example, miR-128 acts as a key post-transcriptional regulator controlling networks of lipid and energy metabolism genes such as LDL receptor, ATP-binding cassette transporter A1, sirtuin 1, and insulin receptor substrate 1 [59]. Interestingly, miR-146a inhibits NF-κB and mitogen activated kinase-like protein (MAPK) signaling via targeting TNF-receptor-associated factor 6 (TRAF6), interleukin 1 receptor associated kinase 1 or 2 (IRAK1/2) and HuR, which limits inflammatory responses in a diverse range of cells [36, 60–62]. miR-146a also decreases lipid uptake and cytokine release in oxLDL-activated macrophages through repressing the expression of TLR4 [63]. These observations have classified miR-146a as an anti-inflammatory miRNA, and indeed exogenous delivery of miR-146a to atherosclerosis-prone mice resulted in the accumulation of miR-146a in macrophages and a reduction in plaque size after 6 weeks of treatment [62]. However, more recently, macrophage miR-146a in atherosclerosis lesion progression was evaluated directly in Ldlr−/− mice receiving bone marrow from wild-type or miR-146a−/− mice. After 12 weeks, miR-146−/− bone marrow recipients had significantly less aortic lesion development compared to wild-type recipients [64]. Importantly, we now show that in these same mice, both IGF2BP1 and HuR expression is elevated, in agreement with these genes serving as miR-146a targets. These two studies suggest that macrophage- and endothelial-cell miR-146a play different roles during atherogenesis. We and others find that miR-146a is significantly up-regulated in atherosclerotic plaques [65] as well as in the circulation of coronary artery disease patients and is associated with an increased risk of atherosclerosis [66]. In the atherosclerotic milieu, the reduction in macrophage migration would promote the retention of macrophages and plaque progression, suggesting EV-derived miR-146a may be pro-atherogenic. Collectively these results suggest a potential pro-atherogenic role for miR-146a in the plaque. Given that endogenous miR-146a levels in vitro remain at steady state whereas EV levels increase upon oxLDL treatment, the mechanisms that govern miR-146a targeting are likely dependent on cell of origin and stimulation. Moreover, the individual contributions of the other miRNAs enriched in atherogenic EVs were not tested in this study, therefore we can only conclude that the reduction in macrophage migration observed in EV-treated cells is partially impacted by the enrichment of miR-146a, but may be influenced by other EV-derived miRNAs.
In conclusion, our study provides evidence that macrophages under pro-atherogenic conditions secrete EVs containing a specific signature of miRNAs, which can be transferred to nearby macrophages and functionally alter recipient cell function. As shown in the model in Figure 8, our study has characterized a new intercellular communication during atherogenesis, in which atherogenic macrophages secrete EVs and deliver miRNAs to naïve macrophages where EV-derived miRNAs repress the expression of specific target genes implicated in migration pathways, leading to decreased macrophage migration and potential entrapment in the atherosclerotic plaque.
Figure 8. Model of macrophage-to-macrophage communication during atherogenesis.
Atherogenic macrophages secrete EVs containing specific miRNAs including miR-146a. These EVs mediate the transfer of miRNAs to naïve macrophages where EV-derived miRNAs repress the expression of target genes involved in migration pathways such as IGF2BP1 and HuR, leading to decreased macrophage migration and potential entrapment in the atherosclerotic plaque.
Supplementary Material
Hightlights.
While there has been a significant progress in the understanding of how lipids and inflammatory cells initiate lesion development, less is known about how macrophages become entrapped in the atherosclerotic plaque.
Our study shows that macrophages loaded with cholesterol secrete extracellular vesicles and deliver microRNA to neighboring macrophages.
These pro-atherogenic extracellular vesicles exert an inhibitory effect on migration in recipient cells, which may contribute to the chemotactic arrest seen in plaque progression.
miR-146a secreted from macrophages may surprisingly act as a pro- rather than anti-atherogenic factor.
Combined with the observation that miR-146a is paradoxically increased in human and mouse atherosclerotic lesions, our data suggests that extracellular vesicles secreted from lipid-loaded macrophages may contribute directly to the pro-atherogenic phenotype of foam cells and may partially act through miR-146a.
Acknowledgments
We thank Dr. Arkadiy Reunov for his expertise with the electron microscopy.
Sources of Funding
This work was supported by funding from the Canadian Institutes for Health Research (FND148448 to KJR, PJT148487 and Canada Research Chair to JEF), the Heart and Stroke Foundation of Canada (KJR) and the National Institutes of Health (KJR, R01 HL119047).
Non-standard abbreviations
- EV
extracellular vesicle
- oxLDL
oxidized low-density lipoprotein
- miR, miRNA
microRNA
Footnotes
Disclosures
None.
References
- 1.Kjenseth A, Fykerud T, Rivedal E, Leithe E. Regulation of gap junction intercellular communication by the ubiquitin system. Cell. Signal. 2010;22:1267–1273. doi: 10.1016/j.cellsig.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Denef C. Paracrinicity: The story of 30 years of cellular pituitary crosstalk. J. Neuroendocrinol. 2008;20:1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer SJ. Intercellular communication and cell-cell adhesion. Science. 1992;255:1671–1677. doi: 10.1126/science.1313187. [DOI] [PubMed] [Google Scholar]
- 4.Majka M, Janowska-wieczorek A, Ratajczak J, Ehrenman K, Pietrzkowski Z, Kowalska A, Gewirtz AM, Emerson SG, Ratajczak MZ, Kowalska MA. Numerous growth factors, cytokines, and chemokines are secreted by human normal hematopoiesis in an autocrine/paracrine manner. Am. Soc. Hematol. 2014;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 5.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 6.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol. Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Bobrie A, Colombo M, Raposo G, Théry C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 8.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, El Andaloussi S, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2012;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald MK, Tian Y, Qureshi RA, Gormley M, Ertel A, Gao R, Lopez EA, Alexander GM, Sacan A, Fortina P, Ajit SK. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain. 2014;155:1527–1539. doi: 10.1016/j.pain.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, Shah P, Wisler J, Eubank TD, Tridandapani S, Paulaitis ME, Piper MG, Marsh CB. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121:984–995. doi: 10.1182/blood-2011-08-374793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lusis A. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 18.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr. Opin. Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Gils JM, Derby MC, Fernandes LR, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanschel A, Seibert T, Hewing B, Ramkhelawon B, Ray TD, Van Gils JM, Rayner KJ, Feig JE, O’Brien ER, Fisher EA, Moore KJ. Neuroimmune guidance cue semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler. Thromb. Vasc. Biol. 2013;33:886–893. doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krohn JB, Hutcheson JD, Martínez-Martínez E, Aikawa E. Extracellular vesicles in cardiovascular calcification: Expanding current paradigms. J. Physiol. 2016;594:2895–2903. doi: 10.1113/JP271338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82:1024–1032. doi: 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- 24.Rekker K, Saare M, Roost AM, Kubo AL, Zarovni N, Chiesi A, Salumets A, Peters M. Comparison of serum exosome isolation methods for microRNA profiling. Clin. Biochem. 2014;47:135–138. doi: 10.1016/j.clinbiochem.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Hu G, Drescher KM, Chen XM. Exosomal miRNAs: Biological Properties and Therapeutic Potential. Front. Genet. 2012;3:1–9. doi: 10.3389/fgene.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu TP, Lee CY, Tsai MH, Chiu YC, Hsiao CK, Lai LC, Chuang EY. MiRSystem: An integrated system for characterizing enriched functions and pathways of microRNA targets. PLoS One. 2012;7:e42390. doi: 10.1371/journal.pone.0042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008;28:2003–2008. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsoyi K, Jang HJ, Nizamutdinova IT, Park K, Kim YM, Kim HJ, Seo HG, Lee JH, Chang KC. PTEN differentially regulates expressions of ICAM-1 and VCAM-1 through PI3K/Akt/GSK-3β/GATA-6 signaling pathways in TNF-α-activated human endothelial cells. Atherosclerosis. 2010;213:115–121. doi: 10.1016/j.atherosclerosis.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 30.Van Gils JM, Ramkhelawon B, Fernandes L, Stewart MC, Guo L, Seibert T, Menezes GB, Cara DC, Chow C, Kinane TB, Fisher EA, Balcells M, Alvarez-Leite J, Lacy-Hulbert A, Moore KJ. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler. Thromb. Vasc. Biol. 2013;33:911–919. doi: 10.1161/ATVBAHA.112.301155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 33.Young MP, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dormoy-Raclet V, Ménard I, Clair E, Kurban G, Mazroui R, Di Marco S, von Roretz C, Pause A, Gallouzi IE. The RNA-binding protein HuR promotes cell migration and cell invasion by stabilizing the beta-actin mRNA in a U-rich-element-dependent manner. Mol. Cell. Biol. 2007;27:5365–5380. doi: 10.1128/MCB.00113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stöhr N, Hüttelmaier S. IGF2BP1: A post-transcriptional “driver” of tumor cell migration. Cell Adhes. Migr. 2012;6:312–318. doi: 10.4161/cam.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013;5:949–966. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinberg MW, Moore KJ. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016;118:703–720. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc. Res. 2013;100:7–18. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 40.Chistiakov DA, Orekhov AN, Bobryshev YV. Extracellular vesicles and atherosclerotic disease. Cell. Mol. Life Sci. 2015;72:2697–2708. doi: 10.1007/s00018-015-1906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 42.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 43.Ramakrishnan DP, Hajj-Ali RA, Chen Y, Silverstein RL. Extracellular vesicles activate a CD36-dependent signaling pathway to inhibit microvascular endothelial cell migration and tube formation. Arterioscler. Thromb. Vasc. Biol. 2016;36:534–544. doi: 10.1161/ATVBAHA.115.307085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato N, Ahuja SK, Quinones M, Kostecki V, Reddick RL, Melby PC, Kuziel WA, Ahuja SS. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokin. J. Exp. Med. 2000;192:205–218. doi: 10.1084/jem.192.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jimenez F, Quinones MP, Martinez HG, Estrada CA, Clark K, Garavito E, Ibarra J, Melby PC, Ahuja SS. CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-kappa B. J. Immunol. 2010;184:5571–5581. doi: 10.4049/jimmunol.0803494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Kennedy DJ, Ramakrishnan DP, Yang M, Huang W, Li Z, Xie Z, Chadwick AC, Sahoo D, Silverstein RL. Oxidized LDL – bound CD36 recruits an Na+/ K+-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci. Signal. 2015;8:ra91. doi: 10.1126/scisignal.aaa9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong R, Lu JG, Wang Q, He XL, Chu YK, Ma QJ. Stabilization of Snail by HuR in the process of hydrogen peroxide induced cell migration. Biochem. Biophys. Res. Commun. 2007;356:318–321. doi: 10.1016/j.bbrc.2007.02.145. [DOI] [PubMed] [Google Scholar]
- 48.Stöhr N, Köhn M, Lederer M, Glaß M, Reinke C, Singer RH, Höttelmaier S. IGF2BP1 promotes cell migration by regulating MK5 and PTEN signaling. Genes Dev. 2012;26:176–189. doi: 10.1101/gad.177642.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao C, Lawrence DA, Strickland DK, Zhang L. Aspecific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 2005;106:3234–3241. doi: 10.1182/blood-2005-03-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh PP, Smith VL, Karakousis PC, Schorey JS. Exosomes Isolated from Mycobacteria-Infected Mice or Cultured Macrophages Can Recruit and Activate Immune Cells In Vitro and In Vivo. J. Immunol. 2012;189:777–785. doi: 10.4049/jimmunol.1103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walters SB, Kieckbusch J, Nagalingam G, Swain A, Latham SL, Grau GER, Britton WJ, Combes V, Saunders BM. Microparticles from Mycobacteria-Infected Macrophages Promote Inflammation and Cellular Migration. J. Immunol. 2012;190:669–677. doi: 10.4049/jimmunol.1201856. [DOI] [PubMed] [Google Scholar]
- 52.Nathan C, Ding A. Nonresolving Inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 53.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, Patton JG, Weaver AM. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016;15:978–987. doi: 10.1016/j.celrep.2016.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shurtleff M, Karfilis KV, Temoche-Diaz M, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lougheed M, Lum CM, Ling W, Suzuki H, Kodama T, Steinbrecher U. High affinity saturable uptake of oxidized low density lipoprotein by macrophages from mice lacking the scavenger receptor class A type I/II. J. Biol. Chem. 1997;16:12938–12944. doi: 10.1074/jbc.272.20.12938. [DOI] [PubMed] [Google Scholar]
- 59.Wagschal A, Najafi-Shoushtari SH, Wang L, et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat. Med. 2015;21:1290–1297. doi: 10.1038/nm.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taganov KD, Boldin MP, Chang K, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saba R, Sorensen DL, Booth SA. MicroRNA-146a: A dominant, negative regulator of the innate immune response. Front. Immunol. 2014;5:1–11. doi: 10.3389/fimmu.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li K, Ching D, Luk FS, Raffai RL. Apolipoprotein e Enhances MicroRNA-146a in Monocytes and Macrophages to Suppress Nuclear Factor-κB-Driven Inflammation and Atherosclerosis. Circ. Res. 2015;117:e1–e11. doi: 10.1161/CIRCRESAHA.117.305844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang K, He YS, Wang XQ, Lu L, Chen QJ, Liu J, Sun Z, Shen WF. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011;585:854–860. doi: 10.1016/j.febslet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Cheng HS, Besla R, Li A, et al. Paradoxical suppression of atherosclerosis in the absence of microRNA-146a. Circ. Res. 2017 doi: 10.1161/CIRCRESAHA.116.310529. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raitoharju E, Lyytikäinen LP, Levula M, Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kähönen M, Karhunen PJ, Laaksonen R, Lehtimaki T. MiR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 66.Xiong XD, Cho M, Cai XP, Cheng J, Jing X, Cen JM, Liu X, Yang XL, Suh Y. A common variant in pre-miR-146 is associated with coronary artery disease risk and its mature miRNA expression. Mutat. Res. 2014;761:15–20. doi: 10.1016/j.mrfmmm.2014.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.