Abstract
Background
We developed an HIV testing dashboard to complement the HIV care continuum in selected high-risk populations. Using NHBS data, we examined trends in HIV testing and care for men who have sex with men (MSM), persons who inject drugs (PWID) and heterosexuals at elevated risk (HET).
Methods
Between 2007–2015, 4,792 participants ≥18 years old completed a behavioral survey and were offered HIV testing. For the testing dashboard, proportions ever tested, tested in the past year, testing HIV-positive, and newly testing positive were calculated. An abbreviated care continuum for self-reported positives (SRP) included ever engagement in care, past year care, and current ARV use. The testing dashboard and care continuum were calculated separately for each population. Chi-square test for trend was used to assess significant trends over time.
Results
Among MSM, lifetime HIV testing and prevalence significantly increased from 96% to 98% (p=0.01) and 14% to 20% (p=0.02) over time; prevalence was highest among Black MSM at all time points. HIV prevalence among female PWID was significantly higher in 2015 vs. 2009 (27% and 13%; p<0.01). Among HET from 2010 to 2013, annual testing increased significantly (45% to 73%; p<0.001) and the proportion newly diagnosed decreased significantly (p<0.01). SRP MSM had high levels of care engagement and ARV use; among SRP PWID and HET, past year care engagement and ARV use increased over time.
Conclusions
The HIV testing dashboard can be used to complement the HIV care continuum to display improvements and disparities in HIV testing and care over time.
Keywords: HIV testing dashboard, HIV care continuum, National HIV Behavioral Surveillance, Washington, D.C
Introduction
Initiated in 2003, the Center for Disease and Prevention’s (CDC) National HIV Behavioral Surveillance (NHBS) system monitors HIV risk behaviors, testing and prevention service use among populations most at risk of HIV infection: men who have sex with men (MSM), persons who inject drugs (PWID), and heterosexuals at elevated risk (HET).1 Washington, DC began contributing data to NHBS in 2006 and has completed three rounds of data collection for each population. As the only continuous, routine HIV surveillance system in the U.S. that includes HIV negative persons at risk, newly diagnosed HIV-positive, and self-reported positive (SRP) persons, NHBS provides a unique opportunity to examine patterns of HIV testing and HIV care among populations at risk for HIV in Washington, DC over time.
HIV testing is the gateway to the HIV prevention continuum including important interventions such as pre-exposure prophylaxis (PrEP) and combination prevention.2 It also acts as the entry point to linkage to HIV care. Yet due to the nature of HIV core surveillance, it is difficult to know who is being tested for HIV, how many people are testing, and how frequently they are being tested. Nationally, among populations most at risk for HIV, high proportions report having ever been tested for HIV, but significant proportions are still not testing annually.3–5 In Washington, DC, a city with high HIV prevalence, the DC Department of Health has increased access to HIV testing over the last 10 years, conducting 163,000 publicly-funded HIV tests in 2014, a 4.3 fold increase since 2006.6–9 NHBS assesses lifetime and recent HIV testing in key populations, but these estimates have not been comprehensively examined locally to identify gaps in testing coverage and trends in testing over time.
In the realm of HIV care, the care continuum displays proportions of HIV-infected persons diagnosed, linked to and retained in care, and virally suppressed, and is helpful in identifying gaps in care coverage among persons living with HIV.6,10 In 2014, the CDC estimated 40% of those diagnosed were engaged in HIV care, 37% were prescribed antiretroviral (ARV) treatment and only 30% had achieved viral suppression.11 Since persons who are aware of their HIV infection and virally suppressed are less likely to transmit HIV, high levels of coverage at each step are integral to HIV prevention and reduction of HIV-related morbidity and mortality.12,13
Both HIV testing and care are now seen as necessary components of HIV prevention,2 yet few studies have examined these two side by side to present a wider view in a jurisdiction. We propose examining HIV testing and HIV prevalence as a testing “dashboard” in order to concisely display HIV testing behaviors and HIV prevalence among populations at high-risk for HIV over time. Using NHBS data, we aimed to identify trends, gaps, and needs regarding both HIV testing and HIV care in community-based populations between 2007 and 2015 for MSM, PWID and heterosexuals.
Methods
Sampling and recruitment
NHBS data were used from three data collection cycles each for MSM (MSM cycle 2, or MSM2: 2008; MSM3: 2011; MSM4: 2014), PWID (IDU2: 2009; IDU3: 2012; IDU4: 2015), and HET (HET1: 2007; HET2: 2010; HET3: 2013). NHBS methods have been described elsewhere.1 Briefly, cross-sectional behavioral and HIV testing data are collected in repeated, serial cross-sectional, community-based surveys among all three populations. Eligibility requirements included being ≥18 years old, a Washington, DC area resident, and able to complete the survey in English or Spanish. Conducting interviews in three populations also necessitated cycle-specific eligibility criteria. MSM had to be born and identify as male and report oral or anal sex with a male partner in the last year. PWID must have reported injection drug use in the last year, based on verification of visible injection site marks or the ability to describe injection practices. Heterosexuals at elevated risk for HIV in HET1 identified as male or female, reported vaginal or anal sex with an opposite sex partner in the last year, were 18–50 years old, and lived in high-risk areas, determined based on a combination of poverty and AIDS case rates. In the HET2 and HET3 cycles, participants must have identified as male or female, had an annual household income below federal poverty guidelines or a high school education or less, were 18–60 years old, and reported vaginal or anal sex with a person of the opposite-sex in the past year.
NHBS uses two different recruitment methodologies, which have been described previously.1,14,15 Venue-based sampling (VBS) is used for the MSM cycle and involves random time–space sampling.14 Respondent-driven sampling (RDS) is used for the PWID and HET cycles. RDS is a chain-referral method which accesses hard to reach populations and provides estimates generalizable to the population of networks from which they are drawn.15
Data collection and HIV testing
Eligible and consenting participants completed an interviewer-administered survey and were offered a rapid HIV test. HIV testing was voluntary and was not required for study participation. All preliminary HIV reactive results and those self-reporting HIV-positive status were confirmed via Western Blot, except for the HET1 data collection cycle in which the preliminary rapid result was used.16 The NHBS survey assessed demographic characteristics, sexual and drug use risk behaviors, HIV testing history, and for SRP participants, engagement in care and ARV use. All activities were reviewed and approved by the George Washington University and DC Department of Health Institutional Review Boards.
Measures
Demographic and behavioral characteristics were assessed for each cycle including: age, gender, race, education, income, homelessness in the last year, injection drug use ever and in the last year, and non-injection drug use in the last year.
The HIV testing dashboard presents proportions of NHBS participants ever tested for HIV (lifetime testing), tested for HIV in the last 12 months, tested HIV-positive, and newly positive. Being ever tested for HIV was assessed in the NHBS core questionnaire among all participants. HIV testing in the last year was calculated for those who self-reported HIV-negative or unknown status and was based on month and year of participant’s last HIV test relative to the date of interview. HIV-positive status (i.e., HIV prevalence) was calculated as the number of persons with a confirmed NHBS HIV-positive test result (with the exception of the HET1 cycle as previously noted—see Magnus et al.)16 divided by the number of persons who completed HIV testing for NHBS. Newly-identified HIV-positive status was defined as being self-reported HIV-negative or of unknown HIV status in the core questionnaire and having a positive NHBS HIV test result.
Persons who self-reported HIV-positive status during the survey were asked questions about HIV care engagement. Based on available data, we generated an abbreviated HIV care continuum using self-reported data which included the proportion of SRP participants who were engaged in care (both ever and in the last 12 months), and who were currently on ARVs. Self-reported viral load was not asked until 2015, so viral suppression was excluded from this analysis. Engagement in care in the last 12 months was calculated based on the self-reported month and year of participant’s last appointment with an HIV provider relative to the interview date. Current ARV use was defined as having reported taking ARVs for HIV care at the time of interview. Not all SRP persons agreed to HIV testing or were confirmed HIV-positive by Western Blot (e.g., indeterminate result), so we assessed differences in the HIV continuum for SRP persons with and without serological confirmation. Although the number of unconfirmed SRP persons varied by cycle and year, HIV care continuum outcomes were not significantly different between those serologically confirmed and unconfirmed for all of the populations (data not shown). Therefore, we presented HIV care data for all SRP participants. The use of self-reported HIV care outcomes from NHBS is consistent with previous national publications.17
Data Analysis
Demographic characteristics were examined for each cycle. Descriptive statistics are presented for categorical variables, and medians and interquartile ranges are presented for continuous variables. To assess demographic differences across data collection years, chi-square tests were conducted for categorical variables and Wilcoxon rank sum tests for continuous variables. For RDS cycles, the RDS Analysis Tool (RDSAT) version 8.1 was used to create sample weights to adjust for referral patterns, network size and relationship to the recruiter in order to obtain population-based estimates which are unbiased to the underlying network.18,19 Data for the MSM cycles are unweighted due to lack of data on frequency of venue attendance across cycles.20–22 Unweighted analysis of MSM cycle data is consistent with previous national and local publications. 23–26
For the HIV testing dashboard, ever tested for HIV and HIV tested in the last 12 months were presented as proportions of the total cycle sample, while HIV-positive and newly positive are presented as proportions of those who completed NHBS HIV testing. Each variable in the abbreviated HIV care continuum for SRP participants was presented as the proportion of all SRP participants for that cycle. The HIV testing dashboard and care continuum were calculated separately for each population. Trends over time were assessed using the chi-square test for trend. To examine differences in HIV testing, data were analyzed by race (Black/White/Other) for MSM and gender (male/female) for PWID and HET. HIV care continuum data were analyzed by population. We did not assess between-population differences in order to focus on trends within each population over time. All analyses were completed using SAS version 9.3 (Cary, NC).
Results
Over 10 years, a total of 4,792 eligible participants completed a behavioral survey and were offered HIV testing. Table 1 presents demographic characteristics of each cycle completed between 2007 and 2015, by cycle and year. Among MSM in Washington, DC, median age was significantly higher at 32 years in 2011 compared to 30 in both 2008 and 2014 (p=0.0097). The proportions of White MSM recruited decreased significantly over time, from 48.2% in 2008 to 37.5% in 2014 (p=0.0003). The proportions of MSM reporting at least a college degree (56.0% in 2008 vs. 65.6% in 2011 and 64.5% in 2014; p=0.0003) and annual income above $50,000 (54.8% in 2008 vs. 60.2% in 2011 and 64.4% in 2014; p=0.01) were significantly higher in 2011 and 2014 compared to 2008.
Table 1.
Demographic characteristics by National HIV Behavioral Surveillance data collection cycle (MSM, PWID, HET) and year, Washington, DC, 2007–2015
| MSM-2 (2008) (n=500) |
MSM-3 (2011) (n=503) |
MSM-4 (2014) (n=510) |
p-value | IDU-2 (2009) N=553 |
IDU-3 (2012) N=518 |
IDU-4 (2015) N=517 |
p-value | HET-1 (2007) N=750 |
HET-2 (2010) N=482 |
HET-3 (2013) N=459 |
p-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%)* | n | (%)* | n | (%)* | n | (%)* | n | (%)* | n | (%)* | ||||
| Median Age (IQR)** | 30 (24–39) | 32 (25–41) | 30 (26–36) | 0.0097 | 52 (48–56) | 54 (49–58) | 55 (50–59) | <0.0001 | 36 (24–43) | 38.5 (24–48) | 35 (24–47) | <0.0001 | |||||||||
| Gender | - | 0.486 | 0.066 | ||||||||||||||||||
| Male | 500 | (100.0) | 503 | (100.0) | 510 | (100.0) | 342 | (62.7) | 319 | (61.4) | 324 | (61.2) | 310 | (39.6) | 225 | (46.0) | 215 | (40.7) | |||
| Female | - | - | - | - | - | - | 206 | (36.4) | 195 | (38.2) | 191 | (38.6) | 440 | (60.4) | 257 | (54.0) | 244 | (59.3) | |||
| Transgender | - | - | - | - | - | - | 5 | (1.0) | 4 | (0.4) | 2 | (0.2) | - | - | - | - | - | - | |||
| Race | 0.0003 | <0.0001 | 0.002 | ||||||||||||||||||
| Black | 158 | (31.6) | 150 | (29.8) | 213 | (41.8) | 532 | (96.6) | 504 | (97.4) | 494 | (92.3) | 700 | (94.7) | 431 | (89.7) | 426 | (92.6) | |||
| White | 241 | (48.2) | 237 | (47.1) | 191 | (37.5) | 5 | (0.7) | 2 | (0.4) | 8 | (5.1) | 2 | (1.0) | 6 | (1.6) | 0 | (0.0) | |||
| Other | 101 | (20.2) | 116 | (23.1) | 106 | (20.8) | 16 | (2.9) | 12 | (2.2) | 15 | (2.6) | 48 | (4.3) | 45 | (8.6) | 33 | (7.4) | |||
| Education | <0.0001 | n=517 | <0.0001 | 0.003 | |||||||||||||||||
| HS/GED or less | 93 | (18.6) | 85 | (16.9) | 54 | (10.6) | 413 | (67.3) | 418 | (80.9) | 407 | (65.6) | 629 | (86.9) | 442 | (88.8) | 409 | (87.2) | |||
| Some college | 127 | (25.4) | 88 | (17.5) | 127 | (24.9) | 126 | (23.5) | 89 | (16.0) | 104 | (27.0) | 112 | (12.4) | 37 | (8.0) | 47 | (11.4) | |||
| Bachelor’s degree or greater | 280 | (56.0) | 330 | (65.6) | 329 | (64.5) | 14 | (9.1) | 10 | (3.1) | 6 | (7.3) | 9 | (0.7) | 3 | (3.1) | 3 | (1.4) | |||
| Income | (n=491) | (n=500) | (n=508) | 0.014 | (n=549) | (n=503) | (n=512) | <0.0001 | (n=716) | (n=470) | (n=453) | 0.059 | |||||||||
| <$20,000 | 74 | (15.1) | 82 | (16.4) | 67 | (13.2) | 461 | (74.9) | 434 | (90.2) | 442 | (91.5) | 549 | (81.1) | 394 | (85.8) | 378 | (87.2) | |||
| $20–49,999 | 148 | (30.1) | 117 | (23.4) | 114 | (22.4) | 68 | (22.9) | 56 | (7.0) | 59 | (6.9) | 145 | (16.4) | 64 | (12.2) | 61 | (10.8) | |||
| $50,000+ | 269 | (54.8) | 301 | (60.2) | 327 | (64.4) | 20 | (2.2) | 13 | (2.8) | 11 | (1.6) | 22 | (2.5) | 12 | (2.0) | 14 | (2.1) | |||
| Been homeless in the past 12 months | 24 | (4.8) | 23 | (4.6) | 18 | (3.5) | 0.568 | 313 | (47.8) | 212 | (41.8) | 177 | (31.7) | <0.0001 | 151 | (22.8) | 183 | (41.9) | 92 | (22.5) | <0.0001 |
| Injection drug use, ever | 20 | (4.0) | 22 | (4.4) | 21 | (4.1) | 0.955 | 553 | (100.0) | 518 | (100.0) | 517 | (100.0) | - | 92 | (13.6) | 56 | (13.1) | 26 | (10.9) | 0.386 |
| Injection drug use in the past 12 months | 5 | (1.0) | 8 | (1.6) | 12 | (2.4) | 0.239 | 553 | (100.0) | 518 | (100.0) | 517 | (100.0) | - | - | - | - | - | - | - | - |
| Non-injection drug use in the past 12 months | 259 | (51.8) | 234 | (46.5) | 276 | (54.1) | 0.047 | 369 | (67.6) | 273 | (45.5) | 348 | (57.2) | <0.0001 | 473 | (59.1) | 305 | (59.3) | 298 | (61.3) | 0.744 |
| Completed NHBS HIV testing | 484 | (96.8) | 490 | (97.4) | 455 | (89.2) | - | 549 | (99.3) | 508 | (98.1) | 513 | (99.2) | - | 719 | (95.9) | 480 | (99.6) | 456 | (99.3) | - |
Weighted %
Age restricted to ≤50 years for HET1 and ≤60 years for HET2/HET3
MSM = men who have sex with men data collection cycle
PWID = people who inject drugs
IDU = injection drug user data collection cycle
HET = heterosexuals at elevated risk for HIV data collection cycle
Among PWID between 2009 and 2015, there were several significant differences. Median age was higher over time, at 52 years in 2009 to 54 and 55 years in 2012 and 2015, respectively (p<0.0001). Across the years, most PWID self-identified as being Black; however, the proportion of white PWID sampled increased from 0.7% in 2009 to 5.1% in 2015 (p<0.0001). The proportion of PWID reporting a high school education or less was highest in 2012 (80.9%) compared to 2009 and 2015 (67.3% and 65.6%, respectively, p<0.0001). Additionally, in 2009 67.6% of PWID also reported using non-injection drugs in the past year, compared to 45.5% in 2012 and 57.2% in 2015 (p<0.0001).
From 2007 to 2013, heterosexuals at elevated risk for HIV were majority female and reported income below $20,000 annually. In 2013, the median age was significantly lower (35 years old) than in previous cycles (36 and 38.5 years old in 2007 and 2010, respectively, p<0.0001). Although most heterosexuals sampled identified as Black, the proportion identified as other race was significantly higher in 2010 (8.6%) compared to 4.3% and 7.4% in 2007 and 2013, respectively (p=0.002). In 2010, a higher proportion of heterosexuals had experienced homelessness in the last year compared to 22.8% and 22.5%, respectively, in 2007 and 2013 (p<0.0001).
HIV Testing Dashboard
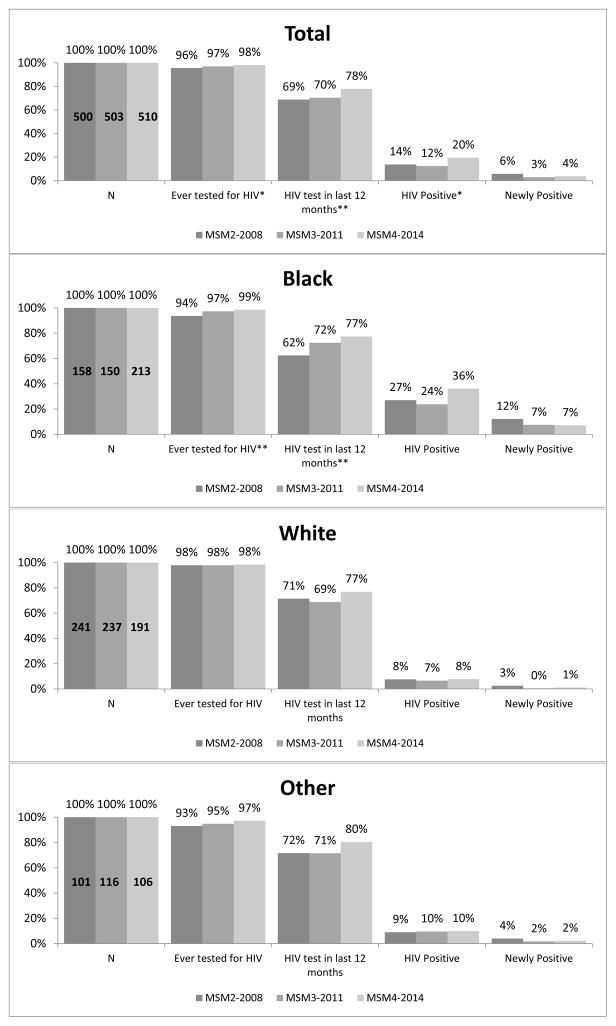
The HIV testing dashboard for MSM (Figure 1) revealed high proportions of lifetime HIV testing, with a small but significant increase between 2008 and 2014 (96% to 98%, p=0.01). Past year testing also was significantly higher comparing 2008 and 2014 (69% vs. 78%, respectively; p=0.003). Among MSM overall, HIV prevalence was highest in 2014 compared to 2008 and 2011 (20% vs. 14% and 12%, respectively; p=0.02), but the proportions of newly HIV-positive MSM over this period were not significantly different (6% in 2014 vs. 4% in 2008; p=0.11). Compared to MSM of other races, Black MSM reported the largest significant increases, from 2008 to 2014, in lifetime (94% to 99%; p=0.001) and past year HIV testing (62% to 77%; p=0.005). Although not statistically significant, among Black MSM, HIV prevalence was higher in 2014 compared to 2008 and 2011 (36% vs. 27% and 24%, respectively; p=0.06) and the proportion newly positive was lower in later years (7% and 7%, 2011 and 2014, respectively) compared to 2008 (12%; p=0.12).
Figure 1. HIV Testing Dashboard for men who have sex with men by NHBS cycle year, Washington, DC.
*p<0.05, **p<0.01
MSM = men who have sex with men data collection cycle
Other = Asian, American Indian/Alaska Native, Hispanic, Native Hawaiian, Multiracial
Note: HIV test in the last 12 months proportions are of those who self-reported negative/unknown HIV status (MSM2: n=448; MSM3: n=439; MSM4: n=434).
HIV positive and newly positive proportions are of those who completed NHBS testing
(MSM2: n=484; MSM3: n=490; MSM4: n=455).
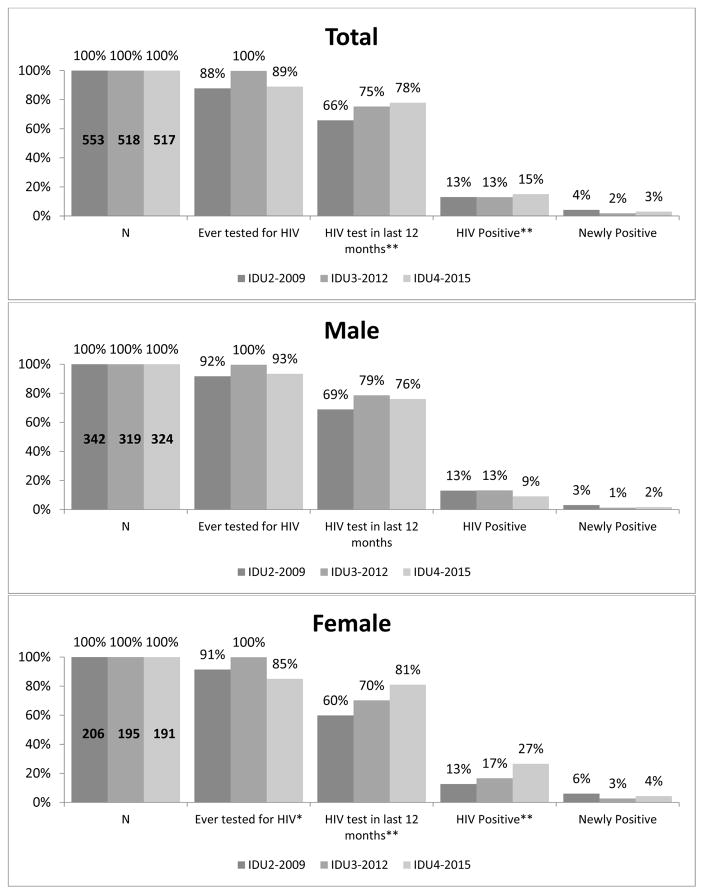
Among PWID sampled (Figure 2), being ever tested for HIV did not differ significantly over time, while HIV testing in the past year was significantly higher in 2015 compared to 2009 (78% vs. 66%, respectively p<0.0001). Estimated HIV prevalence was 13% in both 2009 and 2012, and was significantly different in 2015 at 15% (p<0.0001). Across most time points, a higher proportion of male PWID had ever been HIV tested than female PWID. Among female PWID, a significantly lower proportion reported ever testing for HIV in 2015 compared to 2009 and 2012 (85% vs. 91% and 100%, respectively, p=0.045), but past year HIV testing was significantly higher in 2015 vs 2009 (81% vs. 60%, respectively, p<0.001). Estimated HIV prevalence among female PWID was significantly higher in later years (13% in 2009 vs. 17% in 2012 and 27% in 2015; p=0.0005); the HIV prevalence for male PWID did not differ over time.
Figure 2. HIV Testing Dashboard for persons who inject drugs (PWID) by NHBS cycle year, Washington, DC.
*p<0.05, **p<0.01
IDU = injection drug user (or PWID) data collection cycle
Note: HIV test in the last 12 months proportions are of those who self-reported negative/unknown HIV status
(IDU2: n=504; IDU3: n=484; IDU4: n=476).
HIV positive and newly positive proportions are of those who completed NHBS testing
(IDU2: n=549 ; IDU3: n=508; IDU4: n=513)
All proportions presented are weighted for respondent driven sampling.
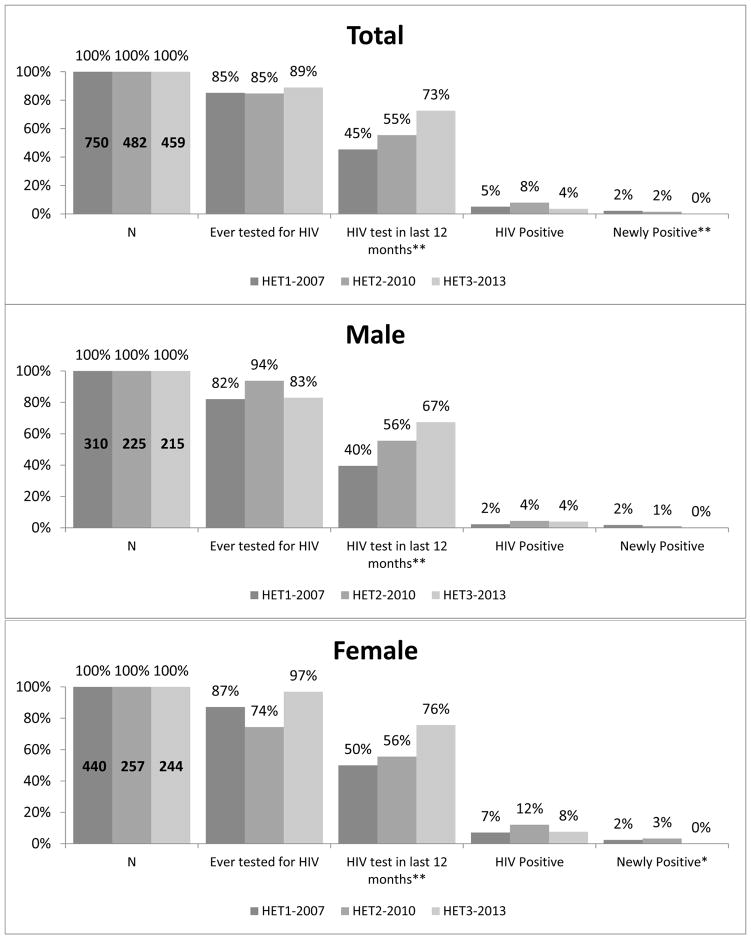
Among sampled heterosexuals at elevated risk for HIV, the proportion who had ever been HIV tested remained stable from 2007 to 2013 (Figure 3) (85% to 89%, p=0.12), while past year testing rose significantly (45% to 73%, p<0.001). HIV prevalence in HET overall ranged from 4% to 8% across years; the proportion newly diagnosed as HIV-positive decreased significantly between 2007 and 2013 (2% to 0%, respectively; p=0.003). Stratifying by gender, male and female HET participants both reported significant increases in past year testing between 2007 and 2013 (male: 40% to 67%, p<0.0001; female: 50% to 76%, p<0.0001). Estimated prevalence of newly identified HIV infections in both HET males and females decreased from 2% in 2007 to 0% in 2013, but this was only statistically significant among HET females (p=0.04).
Figure 3. HIV Testing Dashboard for heterosexuals at elevated risk for HIV by NHBS cycle year, Washington, DC.
*p<0.05, **p<0.01
HET = Heterosexual at elevated risk for HIV data collection cycle
Note: HIV test in the last 12 months proportions are of those who self-reported negative/unknown HIV status
(HET1: n=735; HET2: n=453; HET3: n=435).
HIV positive and newly positive proportions are of those who completed NHBS testing
(HET1: n=719; HET2: n=480; HET3: n= 456)
All proportions presented are weighted for respondent driven sampling.
HIV Care Continuum
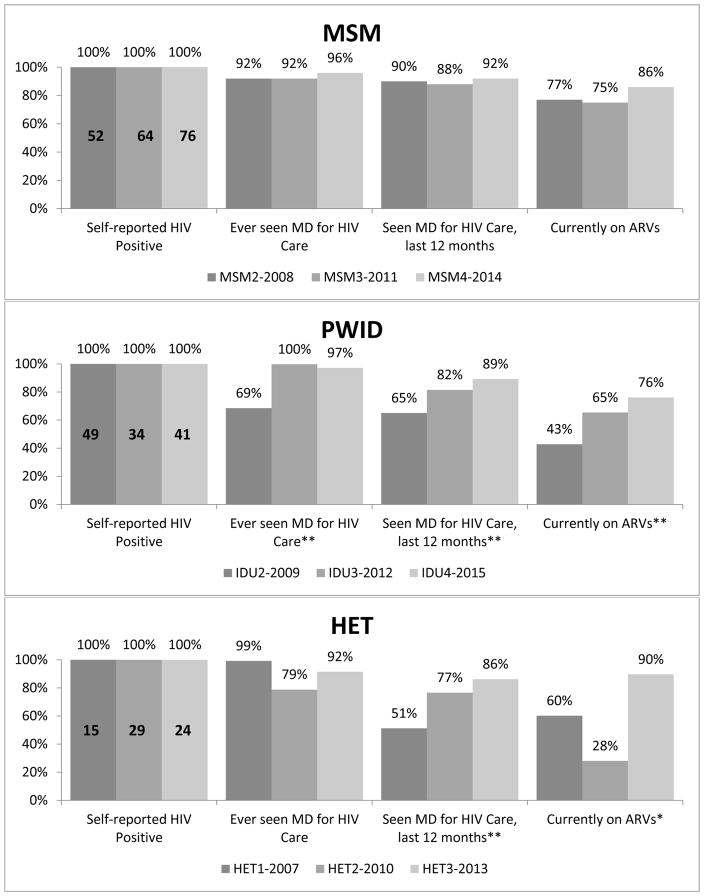
In Figure 4, among SRP MSM, the proportion who had ever seen an HIV care provider was high but did not differ significantly across time (92% to 96%, p=0.36), as was the proportion who had seen an HIV care provider in the past 12 months (90% to 92%, p=0.68). Among SRP PWID, significantly higher proportions had ever been in care in 2012 and 2015 compared to 2009 (100% and 97% vs. 69%, respectively, p<0.0001). Additionally, there were significant increases from 2009 to 2015 in the proportion who had seen a medical provider for HIV care in the past year (65% vs. 89%, p=0.003) and on ARV treatment at the time of interview (43% vs. 76%, p=0.0003). Among SRP HET participants, having seen a medical provider for HIV care in the past year increased from 51% in 2007 to 86% in 2013 (p=0.007). While currently being on ARV treatment was higher in 2007 compared to 2013 (60% vs. 90%, respectively; p=0.045), ARV use in 2010 was particularly low at 28%.
Figure 4. Abbreviated HIV Care Continuum by NHBS data collection cycle and year, Washington, DC.
*p<0.05, **p<0.01
MSM = men who have sex with men data collection cycle
PWID = people who inject drugs; IDU = injection drug user data collection cycle
HET = heterosexuals at elevated risk for HIV data collection cycle
Note: IDU and HET proportions presented are weighted for respondent driven sampling.
Discussion
We developed an HIV testing dashboard to provide key information on HIV testing coverage in high-risk populations to complement the HIV care continuum. The dashboard highlights gaps and trends in HIV testing and prevalence in Washington, DC from 2007 to 2015 and may also indicate the potential impact of expanded HIV testing programs. HIV testing, both lifetime and past year, was highest for all populations in the most recent cycles compared to the first cycle, suggesting important increases in HIV testing coverage over time. This reflects national trends, in which MSM reported the highest proportions of past year HIV testing and HIV prevalence, compared to PWID and HET.3,4,27 Higher HIV prevalence was observed among MSM and PWID in later data collection years, and observed HIV prevalence was lower in later years among HET. The abbreviated care continuum showed that MSM also had higher levels of engagement in care, both ever and in the past year, and ARV use, compared to PWID and heterosexuals.
Among MSM, PWID, and heterosexuals, >80% reported ever testing for HIV, and there were significant increases over time in the proportion tested for HIV in the past year. An analysis among cities funded through CDC’s Expanded HIV Testing Initiative found a similar increase in recent testing between 2008 and 2011 among MSM,28 and Washington, DC reports among the highest levels of past year testing in all populations.3,4,27 Over time we observed successively higher proportions of engagement in care and ARV use among heterosexuals and PWID. This may suggest the increased identification of HIV-positive individuals who are linked into care as a result of the expansion of HIV testing and linkage services in Washington, DC in the past decade.8,18 In addition, much research and programming to increase HIV care engagement has been conducted in Washington, DC, which may also have contributed to increased proportions of HIV care engagement observed across all populations over time.6,29–35
Despite these promising trends, gaps in annual HIV testing and disparities in HIV prevalence persist in each key population, particularly among Black MSM and women. While Washington, DC has been successful in increasing HIV testing overall in these populations,7 our findings among Black MSM highlight the continued racial disparity in HIV prevalence among MSM, seen both nationally and in Washington, DC.9,36 In addition, differences in HIV prevalence persisted across time among women compared to men in both the HET and PWID cycles in Washington, DC. Participants in the PWID and HET cycles predominantly identify as Black, which may partially explain these disparities, as Black women continue to be highly affected by HIV.37 Combined, these observed disparities signal the need for continued development and implementation of prevention interventions focusing on these highly impacted populations.
The National HIV/AIDS Strategy (NHAS) aims to increase retention in care and viral suppression among persons living with HIV.38 Our findings suggest that Washington, DC has succeeded in increasing engagement in care among HIV-positive MSM, PWID, and heterosexuals in the past decade. Specifically, the NHAS aims to increase retention in HIV care to 90% for persons living with HIV by 2020. Among MSM surveyed in 2014 for NHBS, Washington, DC successfully achieved this goal, and was close to 90% among heterosexuals and PWID, based on data from 2013 and 2015, respectively. In the most recent data collection cycles, SRP MSM, PWID, and heterosexuals reported higher proportions of engagement in care and ARV use compared to national estimates, but it should be noted that there are methodological differences between the data used from NHBS and those from the National HIV Surveillance System and Medical Monitoring Project.11
While 10 years of behavioral surveillance data for three high-risk populations provide numerous insights into the spectrum of HIV testing and care, these findings should be considered in light of their limitations. First, although data for PWID and HET were RDS-weighted, data from the MSM cycles were unweighted due to lack of information on venue attendance; therefore, these data may not be representative of venue-attending MSM or MSM who do not attend venues due to the nature of the sampling scheme. While HIV status is confirmed by serologic testing, HIV testing and care data are based on self-report, and therefore they may be subject to recall and social desirability bias. Although interviewers were highly trained and routine data quality checks were conducted, there was the possibility of recording errors and intra/inter-interviewer differences in questionnaire administration While eligibility and recruitment criteria were consistent across PWID and MSM cycles, the eligibility and recruitment criteria differed slightly between HET1 and HET2 as noted previously. Lastly, the abbreviated HIV care continuum was based on a small sample size and does not include a measure of viral suppression.
Conclusion
The HIV testing dashboard provides a concise view of key HIV testing indicators in high-risk populations within a jurisdiction that are unique to NHBS data. Together with the abbreviated HIV care continuum, the dashboard provides a wider lens through which a more complete picture of HIV prevention and treatment activities can be viewed.
Acknowledgments
Source of Funding: This study was funded through a cooperative agreement between the Centers for Disease Control and Prevention and the District of Columbia Department of Health and through a contract from the District of Columbia Department of Health (contract number: DCPO2011-C-0073 and CDC Grant 5U1BPS003261). This publication resulted (in part) from support from the District of Columbia Center for AIDS Research, an NIH funded program (AI117970), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, NIGMS, NIDDK, and OAR.
We would like to thank our partners at the George Washington University and DC Department of Health, as well as community members, and participants who have made National HIV Behavioral Surveillance in Washington, DC possible over the last decade.
National HIV Behavioral Surveillance in DC is funded through a public health/academic partnership between the District of Columbia Department of Health and The George Washington University Milken Institute School of Public Health (Contract number: DCPO2011-C-0073 and CDC Grant 5U1BPS003261).
Footnotes
Conflicts of Interest: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Findings from this study were presented at the HIV Research for Prevention Conference in Chicago, IL on October 19, 2016.
References
- 1.Lansky A, Abdul-Quader AS, Cribbin M, et al. Developing an HIV behavioral surveillance system for injecting drug users: the National HIV Behavioral Surveillance System. Public health reports (Washington, DC : 1974) 2007;122(Suppl 1):48–55. doi: 10.1177/00333549071220S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNairy ML, El-Sadr WM. A paradigm shift: focus on the HIV prevention continuum. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(Suppl 1):S12–15. doi: 10.1093/cid/ciu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV Infection, Risk, Prevention, and Testing Behaviors among Persons Who Inject Drugs: National HIV Behavioral Surveillance Injection Drug Use 20 U.S. Cities. Atlanta, GA: Department of Health and Human Services; 2015. [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV Infection Risk, Prevention, and Testing Behaviors among Men Who Have Sex with Men: National HIV Behavioral Surveillance, 20 U.S. Cities. Atlanta, GA: Department of Health and Human Services; 2016. [Google Scholar]
- 5.Centers for Disease Control and Prevention. HIV Testing Trends in the United States, 2000–2011. Atlanta, GA: Department of Health and Human Services; 2013. [Google Scholar]
- 6.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health Aff (Millwood) 2009;28(6):1677–1687. doi: 10.1377/hlthaff.28.6.1677. [DOI] [PubMed] [Google Scholar]
- 7.Castel AD, Greenberg AE, Befus M, et al. Temporal association between expanded HIV testing and improvements in population-based HIV/AIDS clinical outcomes, District of Columbia. AIDS Care. 2014;26(6):785–789. doi: 10.1080/09540121.2013.855296. [DOI] [PubMed] [Google Scholar]
- 8.Castel AD, Magnus M, Peterson J, et al. Implementing a novel citywide rapid HIV testing campaign in Washington, D.C.: findings and lessons learned. Public health reports (Washington, DC : 1974) 2012;127(4):422–431. doi: 10.1177/003335491212700410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.District of Columbia Department of Health HIV/AIDS Hepatitis STDs and Tuberculosis Administration. District of Columbia Department of Health HIV/AIDS, Hepatitis, STD and TB Epidemiology Annual Report. Washington, DC: District of Columbia Department of Health; 2015. [Google Scholar]
- 10.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley H, Hall HI, Wolitski RJ, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morbidity and mortality weekly report. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–181. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. HIV Infection, Risk, Prevention, and Testing Behaviors: National HIV Behavioral Surveillance Men Who Have Sex with Men 20 U.S. Cities, 2011. Atlanta, GA: Department of Health and Human Services; 2014. [Google Scholar]
- 15.Heckathorn DD. Respondent-driven sampling II: Deriving valid population estimates from chain-referral samples of hidden populations. Social Problems. 2002;49(1):11–34. [Google Scholar]
- 16.Magnus M, Kuo I, Shelley K, et al. Risk factors driving the emergence of a generalized heterosexual HIV epidemic in Washington, District of Columbia networks at risk. AIDS. 2009;23(10):1277–1284. doi: 10.1097/QAD.0b013e32832b51da. [DOI] [PubMed] [Google Scholar]
- 17.Hoots BE, Finlayson TJ, Wejnert C, Paz-Bailey G, Group NS. Early Linkage to HIV Care and Antiretroviral Treatment among Men Who Have Sex with Men--20 Cities, United States, 2008 and 2011. PLoS One. 2015;10(7):e0132962. doi: 10.1371/journal.pone.0132962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo I, Magnus M, Phillips G, 2nd, et al. HIV testing among heterosexuals at elevated risk for HIV in the District of Columbia: has anything changed over time? AIDS Behav. 2014;18(Suppl 3):333–339. doi: 10.1007/s10461-013-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnus M, Kuo I, Phillips G, 2nd, et al. Differing HIV risks and prevention needs among men and women injection drug users (IDU) in the District of Columbia. J Urban Health. 2013;90(1):157–166. doi: 10.1007/s11524-012-9687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafson P, Gilbert M, Xia M, et al. Impact of Statistical Adjustment for Frequency of Venue Attendance in a Venue-based Survey of Men Who Have Sex With Men. American Journal of Epidemiology. 2013;177(10):1157–1164. doi: 10.1093/aje/kws358. [DOI] [PubMed] [Google Scholar]
- 21.MacKellar DA, Gallagher KM, Finlayson T, Sanchez T, Lansky A, Sullivan PS. Surveillance of HIV risk and prevention behaviors of men who have sex with men--a national application of venue-based, time-space sampling. Public health reports (Washington, DC : 1974) 2007;122(Suppl 1):39–47. doi: 10.1177/00333549071220S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKellar D, Valleroy L, Karon J, Lemp G, Janssen R. The Young Men’s Survey: methods for estimating HIV seroprevalence and risk factors among young men who have sex with men. Public health reports (Washington, DC : 1974) 1996;111(Suppl 1):138–144. [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips G, 2nd, Magnus M, Kuo I, et al. Correlates of group sex among a community-based sample of men who have sex with men (MSM) in Washington, DC. AIDS Behav. 2014;18(8):1413–1419. doi: 10.1007/s10461-013-0527-8. [DOI] [PubMed] [Google Scholar]
- 24.Magnus M, Kuo I, Phillips G, 2nd, et al. Elevated HIV prevalence despite lower rates of sexual risk behaviors among black men in the District of Columbia who have sex with men. AIDS Patient Care STDS. 2010;24(10):615–622. doi: 10.1089/apc.2010.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balaji AB, Bowles KE, Le BC, Paz-Bailey G, Oster AM, Group NS. High HIV incidence and prevalence and associated factors among young MSM, 2008. AIDS. 2013;27(2):269–278. doi: 10.1097/QAD.0b013e32835ad489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.German D, Sifakis F, Maulsby C, et al. Persistently high prevalence and unrecognized HIV infection among men who have sex with men in Baltimore: the BESURE study. Journal of acquired immune deficiency syndromes (1999) 2011;57(1):77–87. doi: 10.1097/QAI.0b013e318211b41e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. HIV Infection, Risk, Prevention, and Testing Behaviors Among Heterosexuals at Increased Risk of HIV Infection National HIV Behavioral Surveillance 20 U.S. Cities. Atlanta, GA: Department of Health and Human Services; 2015. [Google Scholar]
- 28.Cooley LA, Wejnert C, Rose CE, Paz-Bailey G National HIVBSSG. Increases in recent HIV testing among men who have sex with men coincide with the Centers for Disease Control and Prevention’s expanded testing initiative. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(3):483–485. doi: 10.1093/cid/ciu851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DC Appleseed Center. HIV/AIDS in the Nation’s Capital Report Card No. 9. Washington, DC: DC Appelseed Center; 2014. [Google Scholar]
- 30.District of Columbia Department of Health HIV/AIDS Hepatitis STDs and Tuberculosis Administration. 90/90/90/50 Plan: Ending the HIV Epidemic in the District of Columbia by 2020. Washington, DC: District of Columbia Department of Health; 2015. [Google Scholar]
- 31.Octane Public Relations. DC Takes on HIV: Public Awareness, Resident Engagement, and a Call to Action. Washington, DC: District of Columbia Department of Health; 2014. [Google Scholar]
- 32.Willis S, Castel AD, Ahmed T, Olejemeh C, Frison L, Kharfen M. Linkage, engagement, and viral suppression rates among HIV-infected persons receiving care at medical case management programs in Washington, DC. Journal of acquired immune deficiency syndromes. 1999;64(Suppl 1(0 1)):S33–41. doi: 10.1097/QAI.0b013e3182a99b67. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchacz K, Farrior J, Beauchamp G, et al. Changing Clinician Practices and Attitudes Regarding the Use of Antiretroviral Therapy for HIV Treatment and Prevention. Journal of the International Association of Providers of AIDS Care. 2017;16(1):81–90. doi: 10.1177/2325957416671410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurth A, Kuo I, Peterson J, et al. Information and Communication Technology to Link Criminal Justice Reentrants to HIV Care in the Community. AIDS Res Treat. 2013;2013:547381. doi: 10.1155/2013/547381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurth AE, Mayer K, Beauchamp G, et al. Clinician practices and attitudes regarding early antiretroviral therapy in the United States. Journal of acquired immune deficiency syndromes (1999) 2012;61(5):e65–69. doi: 10.1097/QAI.0b013e31826a184c.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. HIV Surveillance Report, 2014. 2015. [Google Scholar]
- 37.Centers for Disease Control and Prevention. [Accessed October 5, 2016];HIV among Women. http://www.cdc.gov/hiv/group/gender/women/index.html.
- 38.White House. [Accessed May 01, 2016];National HIV/AIDS Strategy for the United States: Updated to 2020. 2015 https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update/index.html.