Abstract
Objective
To evaluate the magnetic susceptibility, Δχv as a surrogate marker of venous blood oxygen saturation, SvO2, in second- and third-trimester normal human fetuses.
Methods
Thirty-six pregnant women, having a mean gestational age (GA) of 31 2/7 weeks, underwent magnetic resonance imaging (MRI). Susceptibility weighted imaging (SWI) data from the fetal brain was acquired. Δχv, of the superior sagittal sinus (SSS) was quantified using MR susceptometry from the intra-vascular phase measurements. Assuming the magnetic property of fetal blood, Δχdo, is the same as that of adult blood, SvO2 was derived from the measured Δχv. The variation of Δχv and SvO2, as a function of GA, was statistically evaluated.
Results
The mean Δχv in the SSS in the second-trimester (n=8) and third-trimester fetuses (n=28) was found to be 0.34±0.06ppm and 0.49±0.05ppm, respectively. Correspondingly, the derived SvO2 values were 69.4%±3.27% and 62.6%±3.25%. Although not statistically significant, an increasing trend (p=0.08) in Δχv and a decreasing trend (p=0.22) in SvO2, with respect to advancing gestation was observed.
Conclusion
We report cerebral venous blood magnetic susceptibility and putative oxygen saturation in healthy human fetuses. Cerebral oxygen saturation in healthy human fetuses, despite a slight decreasing trend, does not change significantly with advancing gestation.
Keywords: susceptibility weighted imaging (SWI), fetal, brain, oxygen saturation, susceptometry
1. Introduction
Utilization of oxygen for aerobic metabolism is an integral part of the feto-placental growth process from the second trimester onward in human pregnancy [1]. Various conditions/risk factors during pregnancy can result in a reduction of the available oxygen that can lead to metabolic reprogramming of the feto-placental unit [2]. One of the aspects of this reprogramming involves hemodynamic adaptation in which preferential blood flow redistribution to principal organs such as the brain, heart, and spleen occurs to maintain oxygen and nutrient supply [3]. This redistribution happens at the expense of decreased blood supply to peripheral organs. The increased blood flow to the brain (referred to as the ‘brain sparing’ effect) is understood to be mediated by vasodilation in the cerebral circulation [4–9]. While Doppler ultrasound (US) can be used to measure changes in fetal cerebral blood flow [10], there are no clinical methods to evaluate cerebral fetal blood oxygenation, non-invasively, that are applicable during the second and third trimesters. Consequently, normal fetal cerebral blood oxygenation level during these gestations is not known. Fetal heart rate (FHR) has previously been used as an indirect measure for fetal oxygenation status [11–14]. However, due to the lack of sensitivity and the wide scatter in FHR values as a function of blood gases, it is only useful in identifying severe hypoxemic conditions [15]. Near infrared spectroscopy (NIRS) has been shown to be useful in the evaluation of fetal oxygenation status intrapartum [16]. Trans-abdominal NIRS was developed and applied in the recent past to evaluate fetal cerebral blood oxygenation in near-term fetuses [17]. However, due to issues related to penetration depth of the infrared light, NIRS cannot be used for evaluation of fetuses younger than 36 weeks.
Magnetic resonance imaging (MRI), on the other hand, does not suffer with such limitations and, therefore, is widely used to diagnose fetal conditions related to the brain and central nervous system [18–20]. MR spectroscopy has been used to identify the extent of anaerobic respiration in the growing fetus by measuring the lactate concentration in the brain [21, 22]. However, this is a downstream effect secondary to reduced oxygen supply. A more direct measure would be to study the blood oxygenation. Recently, MRI-based T2, transverse relaxation property, has been used to measure the peripheral blood oxygen saturation in late gestations [23]. While this quantitative technique is quite powerful, it can be affected by B1 inhomogeneities that are greater at 3.0 Tesla (T) field strength; it is also associated with a high specific absorption rate (SAR) [24, 25]. Deoxy-hemoglobin is paramagnetic in nature relative to surrounding tissue. This leads to a distinct intra-vascular phase change that has previously been used to non-invasively measure in-vivo blood oxygen saturation in adults [26, 27]. In comparison to the T2-based oximetry method, the phase change is insensitive to B1 inhomogeneities [28]. The intra-vascular phase-based method, dubbed as MR susceptometry, has recently been used in evaluating venous blood oxygenation (SvO2) in the brain of five healthy third-trimester fetuses [29]. The present work uses this MR susceptometry method to evaluate venous blood magnetic susceptibility (Δχv), and, consequently, the putative oxygen saturation, in normal fetuses in the second and third trimesters. Specifically, measurements are carried out in the superior sagittal sinus (SSS) that drains most of the superficial cerebral veins [30].
2. Material and Methods
2.1 Patient Recruitment
Thirty-six pregnant women receiving routine obstetrical care at Hutzel Women’s Hospital, Detroit, Michigan, USA, were non-consecutively recruited in this study. Pregnant women (age = 24.7 ± 4.2 years), who were between 20 weeks and 40 weeks of gestation, were approached for recruitment. Singleton, uncomplicated pregnancies with normal ultrasound examinations and no reporting of contraindications for MRI were eligible to participate. Normal US examination implies estimated fetal weight to be within 10-90th centile for gestational age, umbilical artery pulsatility index < 95th centile and a normal anatomical evaluation. The fetal weight was estimated using the equation provided in Hadlock et. al., [31] which involved US based evaluation of 4 fetal biometric parameters: biparietal diameter, head circumference, abdominal circumference and femur length. The imaging study was approved by the Institutional Review Boards and was compliant with HIPAA regulations. Written informed consent was obtained from each participant before the research MRI scan. Relevant MR imaging data was collected as part of an ongoing study where the scanning duration was limited to 60 minutes. The median and inter-quartile range of the gestational age (GA) of the fetuses included in this study are 31 1/7 weeks and 8.8 weeks, respectively.
2.2 MR Imaging
Fetal MR imaging was carried out on a 3.0T Siemens Verio system (Erlangen, Germany) using a six-channel body flex array coil, along with a spine coil. The modified fully flow-compensated susceptibility weighted imaging (SWI) sequence was used in this study for both two-dimensional (2D) and/or three-dimensional (3D) data acquisition [29].
2D SWI
A single-echo 2D SWI sequence was used with the following MRI parameters: repetition time (TR) = 280 ms, echo time (TE) = 15-18.7 ms, bandwidth (BW) = 199 Hz/pixel, flip angle (FA) = 32°, matrix size = 448 × 168 – 448 × 175, in-plane resolution = 0.78 × 1.56 mm2 and 0.85 × 1.7 mm2, and slice thickness (ST) = 3.5 mm. Images were interpolated to a square pixel size in-plane to either 0.78 × 0.78 mm2 or 0.85 × 0.85 mm2. A total of 10-11 slices were collected within a total acquisition time of 22-24 seconds.
3D SWI
The following MRI parameters were used for a single echo 3D SWI sequence: TR = 23 ms, TE = 13.5 or 17.3 ms, FA = 10°, BW = 219 Hz/pixel, in-plane resolution = 0.78 × 1.56 mm2 with a ST of 3 mm or 3.5 mm, and acquisition matrix size = 448 × 175. Images were interpolated to a square pixel size in-plane to 0.78 × 0.78 mm2. A total of 16 slices were acquired. In addition to a parallel imaging factor of 2, partial-Fourier reconstruction in both phase and slice direction was used to reduce the total data acquisition time to 22-24 seconds.
2.3 Oxygen saturation quantification in SSS
Fetal-SWI image data were first reviewed for general quality, fetal motion, and proper delineation of SSS in both magnitude and phase images. Multiple series of 2D and/or 3D scans were acquired within the available scan time, particularly when fetal motion was encountered or if there was inadequate coverage of the fetal brain. On an average, a fetal SWI scan (2D or 3D) was applied 5 times in any given fetus. SWI acquisition volumes were placed such that the slice was perpendicular to the long axis of the vessel at the occipital aspect of the fetal brain [29].
Raw phase images from the fetal-SWI data were first filtered using a homodyne high pass filter [32] of size 48 × 48 to remove bulk background field inhomogeneities. In all the fetuses, two or three, with a minimum of two, consecutive slices were selected such that there was no displacement of the SSS vessel lumen between the slices. This was to ensure that measurement was carried out along the straight segment of the SSS. Phase images were zoomed twice using nearest neighborhood algorithm, and a region of interest (ROI) encompassing the vessel was manually drawn avoiding the boundary voxels to mitigate partial volume effects. Mean and standard deviation of the phase from the SSS and the immediate background parenchymal region were measured to obtain, Δφsss=φvessel − φbackground. The fetal head could be in any arbitrary orientation within the mother’s uterus. This makes the vessel angle, θ, between the long axis of the SSS with respect to the main field, Bo, arbitrary. However, with the vessel being perpendicular to the slice plane, the angle θ can be evaluated using the slice normal vector from the image DICOM header (Digital Imaging and Communications in Medicine DICOM standard). Vessel Δχv, was then obtained using the following equation [29]:
| (1) |
where TE is the echo time and γ – the gyromagnetic ratio.
Putative SvO2, was subsequently calculated using the following relation from [27]:
| (2) |
where Δχdo- the magnetic susceptibility difference between fully oxygenated and deoxygenated fetal blood and Hct is the fetal blood hematocrit. Hct value corresponding to a given gestation is obtained from established nomograms [33]. Assuming that Δχdo of fetal blood is same as that of adult blood (Δχdo=0.27ppm, cgs units) [34, 35], equation (2) was used to calculate SvO2.
Vessel Δχv values as well as corresponding SvO2 values from all slices within a given volume and all volumes obtained within a given scan session were averaged to obtain the mean Δχv and SvO2 value for a given fetus. Corresponding standard error of the mean was also evaluated from the measurement error associated with each given slice [29].
2.4 Statistics
We used a generalized linear model to examine the association between Δχv and gestation and to determine the trend in SvO2 across GA. Based upon previous ex-vivo umbilical vein blood oximetry studies that observed a linear relationship between blood oxygen saturation and GA in human fetuses, a linear regression model was chosen [36]. We also evaluated the difference in Δχv and SvO2, between fetuses in their second vs. third trimester using two-tailed student’s t-test. Statistical significance was defined by p < 0.05. The R3.2.2 script was used to perform the analysis.
3. Results
SWI data from healthy fetuses (n = 36) was analyzed and the Δχv and corresponding SvO2 were obtained. Figure-1 shows the number of fetuses vs. gestational age distribution of the studied cohort. A representative set of fetal SWI magnitude and phase images are shown in Figure-2. The SSS vein can clearly be seen in the posterior aspect of the brain on both the magnitude and phase images. Figure-3 shows the distribution of Δχv of the venous blood in the SSS across gestation. The mean Δχv was 0.46±0.06 ppm in the entire fetal cohort studied, with the mean in second-trimester fetuses being 0.34±0.06 ppm (n = 8) and 0.49±0.05 ppm in third-trimester fetuses (n = 28). Thus, an increasing trend of Δχv vs. gestation was observed (Figure-3). However, this trend was not statistically significant (slope = 0.01±0.01, p=0.08). The corresponding mean SvO2 in the entire cohort studied was 64.1%±3.3%. A decreasing trend in the fetal SvO2 as a function of GA was observed (Figure-3) with the mean value of the second trimester being 69.4%±3.27% and of the third trimester 61.8±3.3%. However, this trend was also not statistically significant (slope = −0.51±0.41, p=0.22) (Figure-3). While we see a difference in the mean Δχv and SvO2 between second and third trimester fetuses, they were not statistically significant: p = 0.06 and p = 0.2, respectively.
Figure-1.
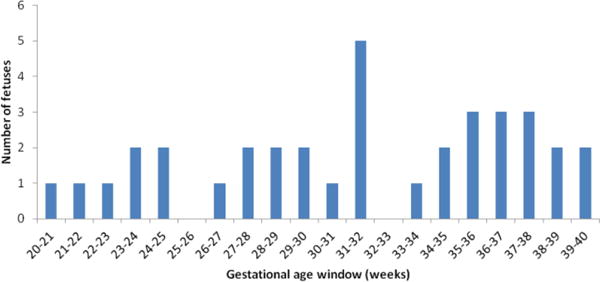
A plot showing the distribution of the number of fetuses vs. gestational age of the studied cohort.
Figure-2.
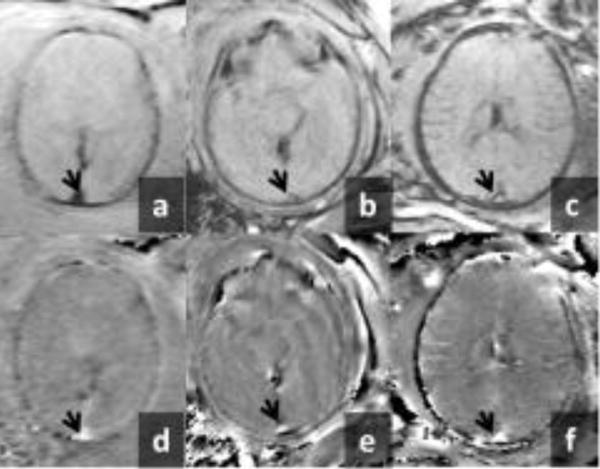
Representative examples of magnitude and phase images from fetal SWI data sets. Top row shows the magnitude image of the fetal brain from a fetus at a: 22 2/7 weeks, b: 31 2/7 weeks and c: 37 3/7 weeks of gestation. Bottom row shows the corresponding phase images in d, e and f, respectively. The arrow head points to the superior-sagittal-sinus. The intra-vascular phase associated with the magnetic susceptibility of the superior-sagittal-sinus vein (arrow) is evident in all the phase images.
Figure-3.
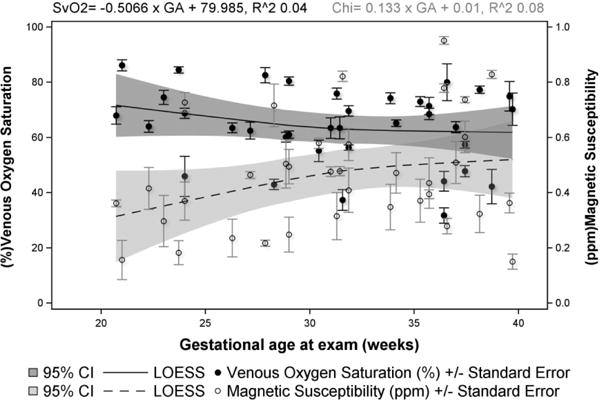
Magnetic susceptibility, Δχv (ppm) of the superior-sagittal-sinus (SSS) vein and the putative blood oxygen saturation, SvO2 (%) in normal human fetuses are plotted across their corresponding gestational age (weeks). A linearly increasing trend is observed in Δχv but was not found to be significant (p = 0.08). The SvO2 in the SSS is obtained using a Δχdo = 0.27ppm. Average SvO2 across the entire cohort was found to be 64.1%±3.3%. Although not statistically significant (p = 0.22), a decreasing trend in SvO2 with advancing gestation is observed.
4. Discussion
In this study, we quantified the SvO2 from Δχv in the SSS vein of the fetal brain in a cross-sectional cohort of second- and third-trimester normal fetuses. The MR susceptometry method was used for this quantification, which has been validated and used in adults [26, 27] and neonates previously [37] and recently applied in the human fetus as well [29]. Previous ex-vivo studies have shown that umbilical venous blood oxygenation decreases marginally from 24 weeks to 37 weeks [36]. Concurrently, the metabolic demand of the fetus increases from the second trimester to the third trimester [38]. Taken together, it is reasonable to expect an increased oxygen extraction fraction (OEF) (i.e., decreasing oxygen saturation – SvO2) with advancing gestation, provided fetal cerebral blood flow conditions do not change. Indeed, our results do suggest a decreasing trend in cerebral venous blood SvO2 across gestation (SvO2 = − 0.56*GA + 80), although it is not statistically significant (p = 0.22). The lack of statistical significance could be a consequence of a) the large variability seen in the measured SvO2 even within a given gestational week and b) the relatively small sample size studied. The large variability observed in estimated SvO2 (or the Δχv) could indeed be physiologically driven as cerebral blood flow rates within the fetal brain are known to be variable even within a given gestational age [39].
A recent work by Zhu et al., reported cerebral OEF by measuring the difference in arterial vs. venous blood T2 (transverse relaxation) values at the major thoracic vessels feeding and draining the cranium in third-trimester fetuses [23]. T2 measurements were carried out from the aorta for the arterial and superior vena-cava (SVC) for venous oxygenation. Converting the T2 values presented to oxygen saturation, using the cited calibration curve [40], the SvO2 value of the SVC turns out to be 49.4%. This value is considerably different than the putative SvO2 from SSS measured in our study. On the other hand, in a recent report [41], cerebral venous blood SvO2 in term-born neonates, measured in the SSS, was found to be 63.9%±8.2%, closely comparable to the values from third-trimester fetuses in our study. The mean SvO2 from third trimester fetuses in our study is also in good agreement with the 66% ± 9.6% value recently reported using MR susceptometry [29], albeit in a small cohort of 5 fetuses. Similarly, the mean oxygen saturation of 57.74% ± 4.2% from 8 fetuses between 36 and 40 weeks gestation in our cohort, also compares well with the 61% ± 14% obtained from 5 fetuses in similar gestational window using trans-abdominal NIRS [17]. Furthermore, fetal cerebral oxygenation measured intra-partum in late-gestation fetuses using NIRS varied between 50%-70% [42–44]. Our SvO2 values from the SSS are within this range, in concordance with these previous works.
Fetal hemoglobin (HbF) has different oxygen affinity compared to adult hemoglobin (HbA), owing to the difference in its molecular structure [45]. Therefore, it may be that the magnetic susceptibility of HbF is slightly different from that of HbA. The relative fractions of HbA and HbF within fetal whole blood also change with GA [46]. This, in turn, may lead to fetal Δχdo, becoming a function of the GA. However, as there is no information about this potential variation, we have used the values for adult blood. Two different values have been quoted in the literature for Δχdo of adult blood – 0.18ppm [47] or 0.27ppm [35] cgs units. In this work, we assumed that Δχdo of fetal blood is the same as that of adult blood and is equal to 0.27 ppm. If the value of 0.18ppm were to be used, the presented SvO2 values would correspondingly shift to lower values and the mean SvO2 of the cohort comes out to be 48.9% ± 5.2%. Nevertheless, Δχdo value only influences the absolute value of SvO2 and not its relative change from one gestation to another. Thus, the conclusions of this study are not influenced by the Δχdo value.
In principle, there is little to no difference in the quantitative phase values between 2D SWI with zero slice-gap and 3D SWI for similar voxel sizes and echo times [48]. However, for a fixed imaging time, 3D offers slightly better SNR per unit time compared to 2D multi-slice imaging [49]. Conversely, 3D acquisition is more susceptible to motion artifacts compared to 2D multi-slice acquisition due to partition encoding along the slice direction. In view of these factors, we used both 2D and 3D modes of acquisition for fetal SWI. Fetal motion and maternal breathing continue to remain a challenge in robust and consistent data acquisition. We used the modified version of SWI with optimized parameters as suggested in Neelavalli et al [29] to keep the scan time short, in the order of 30 seconds. Nevertheless, in this study too, the success rate of obtaining measurable fetal SWI data has remained similar to the previously reported value of 26% [29]. Hence the data presented here comprises a subset of the fetuses within a larger cohort, in whom venous phase measurements could be performed successfully. The choice of a longer TR in the SWI sequences was deliberate, with the intention of reducing the SAR. The SARs in both the 2D and 3D scans were maintained below 0.6 W/Kg [50]. The choice of the longer TE was to improve the phase signal-to-noise, but it also increases the susceptibility artifacts from the maternal abdominal tissue. One approach to overcome the need for a longer TE would be the use of the quantitative susceptibility mapping (QSM) approach, which takes into account both the phase inside and outside a vessel, thereby improving the estimations. An added advantage of using QSM is that this method is independent of the model and orientation.
5. Conclusion
In conclusion, we have quantified the magnetic susceptibility and the putative cerebral oxygen saturation values in normal second- and third-trimester human fetuses using MR susceptometry. This is the first study of fetal cerebral SvO2 from both second- and third-trimester fetuses. Although not statistically significant, our results suggest a decreasing trend in the fetal cerebral SvO2 with advancing gestation. This work opens up the potential for using cerebral SvO2 to non-invasively assess fetal hypoxia.
Key points.
Cerebral venous magnetic susceptibility and oxygenation in human fetuses can be quantified.
Cerebral venous oxygenation was not different between second- and third-trimester fetuses.
Fetal cerebral venous oxygenation does not change significantly with advancing gestation.
Acknowledgments
The authors would like to thank Maria Cabrera and the research staff at the PRB for their help in volunteer recruitment.
Funding:
This research was supported, in part, by the Perinatology Research Branch (PRB), Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C; and an STTR grant from the NHLBI number 1R42HL112580- 01A1.
Abbreviations
- SWI
Susceptibility weighted imaging
- MRI
Magnetic resonance imaging
- SSS
Superior sagittal sinus
- SvO2
Venous oxygen saturation
- Δχv
Magnetic susceptibility
- Δχdo
Magnetic susceptibility difference between fully oxygenated and deoxygenated fetal blood
- Hct
Hematocrit
- HbF
Fetal hemoglobin
- HbA
Adult hemoglobin
- FHR
Fetal heart rate
- GA
gestational age
Footnotes
Compliance with ethical standards:
Guarantor:
The scientific guarantor of this publication is Dr. Jaladhar Neelavalli, Ph.D.
Conflict of interest:
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry:
One of the authors has significant statistical expertise.
Informed consent:
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval:
Institutional Review Board approval was obtained.
- prospective
- experimental
- performed at one institution
References
- 1.Schneider H. Oxygenation of the placental–fetal unit in humans. Respiratory physiology & neurobiology. 2011;178(1):51–58. doi: 10.1016/j.resp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Baschat AA. Pathophysiology of fetal growth restriction: implications for diagnosis and surveillance. Obstetrical & gynecological survey. 2004;59(8):617–627. doi: 10.1097/01.ogx.0000133943.54530.76. [DOI] [PubMed] [Google Scholar]
- 3.Baschat DAA. Fetal responses to placental insufficiency: an update. BJOG: An International Journal of Obstetrics & Gynaecology. 2004;111(10):1031–1041. doi: 10.1111/j.1471-0528.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 4.Valcamonico A, et al. Absent end-diastolic velocity in umbilical artery: risk of neonatal morbidity and brain damage. American journal of obstetrics and gynecology. 1994;170(3):796–801. doi: 10.1016/s0002-9378(94)70285-3. [DOI] [PubMed] [Google Scholar]
- 5.Gramellini D, et al. Cerebral-umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstetrics & Gynecology. 1992;79(3):416–420. doi: 10.1097/00006250-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Hecher K, et al. Potential for diagnosing imminent risk to appropriate- and small- for- gestational- age fetuses by Doppler sonographic examination of umbilical and cerebral arterial blood flow. Ultrasound in Obstetrics & Gynecology. 1992;2(4):266–271. doi: 10.1046/j.1469-0705.1992.02040266.x. [DOI] [PubMed] [Google Scholar]
- 7.AL- GHAZALI W, et al. Evidence of redistribution of cardiac output in asymmetrical growth retardation. BJOG: An International Journal of Obstetrics & Gynaecology. 1989;96(6):697–704. doi: 10.1111/j.1471-0528.1989.tb03285.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferrazzi E, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth- restricted fetus. Ultrasound in Obstetrics and Gynecology. 2002;19(2):140–146. doi: 10.1046/j.0960-7692.2002.00627.x. [DOI] [PubMed] [Google Scholar]
- 9.Dubiel M, Gunnarsson G, Gudmundsson S. Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound in Obstetrics and Gynecology. 2002;20(2):117–121. doi: 10.1046/j.1469-0705.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- 10.Wladimiroff J, Tonge H, Stewart P. Doppler ultrasound assessment of cerebral blood flow in the human fetus. BJOG: An International Journal of Obstetrics & Gynaecology. 1986;93(5):471–475. [PubMed] [Google Scholar]
- 11.Rosen K, Kjellmer I. Changes in the fetal heart rate and ECG during hypoxia. Acta Physiologica. 1975;93(1):59–66. doi: 10.1111/j.1748-1716.1975.tb05790.x. [DOI] [PubMed] [Google Scholar]
- 12.Parer J, et al. Increased fetal heart rate variability with acute hypoxia in chronically instrumented sheep. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1980;10(6):393–399. doi: 10.1016/0028-2243(80)90025-8. [DOI] [PubMed] [Google Scholar]
- 13.Smith J, et al. Antenatal fetal heart rate variation in relation to the respiratory and metabolic status of the compromised human fetus. BJOG: An International Journal of Obstetrics & Gynaecology. 1988;95(10):980–989. doi: 10.1111/j.1471-0528.1988.tb06501.x. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon R, Johnston L, Murotsuki J. Fetal placental embolization in the late-gestation ovine fetus: alterations in umbilical blood flow and fetal heart rate patterns. American journal of obstetrics and gynecology. 1996;175(1):63–72. doi: 10.1016/s0002-9378(96)70252-1. [DOI] [PubMed] [Google Scholar]
- 15.RIBBERT LS, et al. Relation of fetal blood gases and data from computer- assisted analysis of fetal heart rate patterns in small for gestation fetuses. BJOG: An International Journal of Obstetrics & Gynaecology. 1991;98(8):820–823. doi: 10.1111/j.1471-0528.1991.tb13489.x. [DOI] [PubMed] [Google Scholar]
- 16.Chipchase J, et al. Cerebral hemoglobin concentration and oxygen saturation measured by intensity modulated optical spectroscopy in the human fetus during labor. Journal of perinatal medicine. 2002;30(6):502–509. doi: 10.1515/JPM.2002.078. [DOI] [PubMed] [Google Scholar]
- 17.Vintzileos AM, et al. Transabdominal fetal pulse oximetry with near-infrared spectroscopy. American journal of obstetrics and gynecology. 2005;192(1):129–133. doi: 10.1016/j.ajog.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Rutherford M, et al. MR imaging methods for assessing fetal brain development. Developmental neurobiology. 2008;68(6):700–711. doi: 10.1002/dneu.20614. [DOI] [PubMed] [Google Scholar]
- 19.Levine D, et al. Fast MR imaging of fetal central nervous system abnormalities 1. Radiology. 2003;229(1):51–61. doi: 10.1148/radiol.2291020770. [DOI] [PubMed] [Google Scholar]
- 20.Whitby E, et al. Comparison of ultrasound and magnetic resonance imaging in 100 singleton pregnancies with suspected brain abnormalities. BJOG: An International Journal of Obstetrics & Gynaecology. 2004;111(8):784–792. doi: 10.1111/j.1471-0528.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 21.Wolfberg AJ, et al. Identification of fetal cerebral lactate using magnetic resonance spectroscopy. American journal of obstetrics and gynecology. 2007;196(1):e9–e11. doi: 10.1016/j.ajog.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Cetin I, et al. Lactate detection in the brain of growth-restricted fetuses with magnetic resonance spectroscopy. American journal of obstetrics and gynecology. 2011;205(4):350. e1–350. e7. doi: 10.1016/j.ajog.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Zhu MY, et al. The hemodynamics of late-onset intrauterine growth restriction by MRI. American journal of obstetrics and gynecology. 2016;214(3):367. e1–367. e17. doi: 10.1016/j.ajog.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Stuber M, et al. Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magnetic resonance in medicine. 2002;48(3):425–429. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 25.Frayne R, et al. Magnetic resonance imaging at 3.0 Tesla: challenges and advantages in clinical neurological imaging. Investigative radiology. 2003;38(7):385–402. doi: 10.1097/01.rli.0000073442.88269.c9. [DOI] [PubMed] [Google Scholar]
- 26.Haacke EM, et al. In vivo measurement of blood oxygen saturation using magnetic resonance imaging: A direct validation of the blood oxygen level- dependent concept in functional brain imaging. Human brain mapping. 1997;5(5):341–346. doi: 10.1002/(SICI)1097-0193(1997)5:5<341::AID-HBM2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Fernández- Seara MA, et al. MR susceptometry for measuring global brain oxygen extraction. Magnetic resonance in medicine. 2006;55(5):967–973. doi: 10.1002/mrm.20892. [DOI] [PubMed] [Google Scholar]
- 28.Vegh V, et al. Selective channel combination of MRI signal phase. Magnetic resonance in medicine. 2015 doi: 10.1002/mrm.26057. [DOI] [PubMed] [Google Scholar]
- 29.Neelavalli J, et al. Measuring venous blood oxygenation in fetal brain using susceptibility- weighted imaging. Journal of Magnetic Resonance Imaging. 2014;39(4):998–1006. doi: 10.1002/jmri.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheema R, et al. Fetal cerebral venous Doppler velocimetry in normal and high- risk pregnancy. Ultrasound in obstetrics & gynecology. 2004;24(2):147–153. doi: 10.1002/uog.1117. [DOI] [PubMed] [Google Scholar]
- 31.Hadlock FP, et al. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. American journal of obstetrics and gynecology. 1985;151(3):333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Artery and vein separation using susceptibility-dependent phase in contrast-enhanced MRA. J Magn Reson Imaging. 2000;12(5):661–70. doi: 10.1002/1522-2586(200011)12:5<661::aid-jmri2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Boulot P, et al. Hematologic values of fetal blood obtained by means of cordocentesis. Fetal diagnosis and therapy. 1993;8(5):309–316. doi: 10.1159/000263845. [DOI] [PubMed] [Google Scholar]
- 34.Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab. 2010;30(9):1598–607. doi: 10.1038/jcbfm.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spees WM, et al. Water proton MR properties of human blood at 1.5 Tesla: Magnetic susceptibility, T1, T2, T* 2, and non- Lorentzian signal behavior. Magnetic resonance in medicine. 2001;45(4):533–542. doi: 10.1002/mrm.1072. [DOI] [PubMed] [Google Scholar]
- 36.Schroter B, et al. Normal value curves for intrauterine fetal blood gas and acid-base parameters in the 2nd and 3rd trimester. Gynakol Geburtshilfliche Rundsch. 1997;37(3):130–5. doi: 10.1159/000272842. Article in German. [DOI] [PubMed] [Google Scholar]
- 37.Jain V, et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. Journal of Cerebral Blood Flow & Metabolism. 2014;34(3):380–388. doi: 10.1038/jcbfm.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton G, Jaunaiux E. Maternal vascularisation of the human placenta: does the embryo develop in a hypoxic environment? Gynécologie obstétrique & fertilité. 2001;29(7):503–508. doi: 10.1016/s1297-9589(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 39.Veille JC, Hanson R, Tatum K. Longitudinal quantitation of middle cerebral artery blood flow in normal human fetuses. American journal of obstetrics and gynecology. 1993;169(6):1393–1398. doi: 10.1016/0002-9378(93)90406-9. [DOI] [PubMed] [Google Scholar]
- 40.Nield LE, et al. MRI-based blood oxygen saturation measurements in infants and children with congenital heart disease. Pediatric radiology. 2002;32(7):518–522. doi: 10.1007/s00247-001-0652-9. [DOI] [PubMed] [Google Scholar]
- 41.Liu P, et al. Quantitative assessment of global cerebral metabolic rate of oxygen (CMRO2) in neonates using MRI. NMR in Biomedicine. 2014;27(3):332–340. doi: 10.1002/nbm.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chua S, et al. Fetal oxygen saturation during labour. BJOG: An International Journal of Obstetrics & Gynaecology. 1997;104(9):1080–1083. doi: 10.1111/j.1471-0528.1997.tb12071.x. [DOI] [PubMed] [Google Scholar]
- 43.Dildy GA, Loucks CA, Clark SL. Intrapartum fetal pulse oximetry in the presence of fetal cardiac arrhythmia. American journal of obstetrics and gynecology. 1993;169(6):1609–1611. doi: 10.1016/0002-9378(93)90446-p. [DOI] [PubMed] [Google Scholar]
- 44.Dildy GA, et al. The relationship between oxygen saturation and pH in umbilical blood: implications for intrapartum fetal oxygen saturation monitoring. American Journal of obstetrics and gynecology. 1996;175(3 Pt 1):682–687. doi: 10.1053/ob.1996.v175.a74922. [DOI] [PubMed] [Google Scholar]
- 45.Avni R, et al. MR Imaging–derived Oxygen-Hemoglobin Dissociation Curves and Fetal-Placental Oxygen-Hemoglobin Affinities. Radiology. 2016;280(1):68–77. doi: 10.1148/radiol.2015150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bard H, et al. The relative rates of synthesis of hemoglobins A and F in immature red cells of newborn infants. Pediatrics. 1970;45(5):766–772. [PubMed] [Google Scholar]
- 47.Weisskoff RM, Kiihne S. MRI susceptometry: Image- based measurement of absolute susceptibility of MR contrast agents and human blood. Magnetic Resonance in Medicine. 1992;24(2):375–383. doi: 10.1002/mrm.1910240219. [DOI] [PubMed] [Google Scholar]
- 48.Liu C, et al. Susceptibility- weighted imaging and quantitative susceptibility mapping in the brain. Journal of magnetic resonance imaging. 2015;42(1):23–41. doi: 10.1002/jmri.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson G, Wadghiri YZ, Turnbull DH. 2 D multislice and 3 D MRI sequences are often equally sensitive. Magnetic resonance in medicine. 1999;41(4):824–828. doi: 10.1002/(sici)1522-2594(199904)41:4<824::aid-mrm23>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 50.Krishnamurthy U, et al. MR imaging of the fetal brain at 1.5 T and 3.0 T field strengths: comparing specific absorption rate (SAR) and image quality. Journal of perinatal medicine. 2015;43(2):209–220. doi: 10.1515/jpm-2014-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]


