Abstract
Aging is associated with progressive oxidation of the extracellular environment. The redox state of human plasma, defined by the concentrations of cysteine (Cys) and cystine (CySS), becomes more oxidized as we age. Recently, we showed that fibroblasts isolated from the lungs of young and old mice retain this differential phenotype; old cells produce and maintain a more oxidizing extracellular redox potential (Eh(Cys/CySS)) than young cells. Microarray analysis identified down-regulation of Slc7a11, the light subunit of the CySS/glutamate transporter, as a potential mediator of age-related oxidation in these cells. The purpose of the present study was to investigate the mechanistic link between Slc7a11 expression and extracellular Eh(Cys/CySS). Sulforaphane treatment or overexpression of Slc7a11 was used to increase Slc7a11 in lung fibroblasts from old mice, and sulfasalazine treatment or siRNA-mediated knock down was used to decrease Slc7a11 in young fibroblasts. Slc7a11 mRNA levels were measured by real-time PCR, Slc7a11 activity was determined by measuring the rate of glutamate release, Cys, CySS, glutathione (GSH) and its disulfide (GSSG) were measured by HPLC, and Eh(Cys/CySS) was calculated from the Nernst equation. The results showed that both Eh(Cys/CySS) and Eh(GSH/GSSG) were more oxidized in the conditioned media of old cells than in young cells. Up-regulation of Slc7a11 via overexpression or sulforaphane treatment restored extracellular Eh(Cys/CySS) in cultures of old cells, whereas down-regulation reproduced the oxidizing Eh(Cys/CySS) in young cells. Only sulforaphane treatment was able to increase total GSH and restore Eh(GSH/GSSG), whereas overexpression, knock down and sulfasalazine had no effect on these parameters. In addition, inhibition of GSH synthesis with buthionine sulfoximine had no effect on the ability of cells to restore their extracellular redox potential in response to an oxidative challenge. In conclusion, our study reveals Slc7a11 is the key regulator of age-dependent changes in extracellular Eh(Cys/CySS) in primary mouse lung fibroblasts, and its effects are not dependent on GSH synthesis.
Keywords: Slc7a11, aging, redox state, Cys/CySS, fibroblasts
Graphical abstract
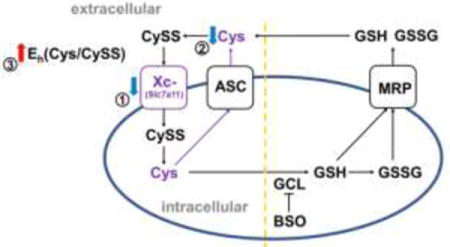
INTRODUCTION
Reversible reduction and oxidation (redox) of the sulfur-containing amino acid cysteine (Cys) is exploited for a large number of biological processes [1]. Redox reactive Cys can be found as the free amino acid, as part of the thiol antioxidant glutathione (γ-glutamylcysteinylglycine; GSH), or as functional/regulatory sites within proteins [2]. Cys and its oxidized form, cystine (CySS), constitute a redox couple that can be expressed in terms of its redox potential, or Eh value. Likewise, GSH and its disulfide form, abbreviated GSSG, comprise another redox couple. These 2 couples are functionally connected but differentially regulated [3]. Cys and CySS are present in greater concentrations than GSH and GSSG outside of cells, whereas GSH and GSSG predominate within the intracellular compartment [4, 5]. In addition, each couple and each compartment are maintained at different redox potentials [6]. Therefore, it is important to specify which compartment is being considered when reporting redox potentials. Both intracellularly and extracellularly Cys/CySS and GSH/GSSG function as redox buffers to maintain redox homeostasis [2] and resist or facilitate oxidation of protein thiols to change protein functions and transduce signals [7, 8]. Thus, changes in redox potential can have a dramatic effect on cellular function. For example, oxidation of extracellular Eh(Cys/CySS) suppressed proliferation and inhibited signal transduction in Caco2 cells [9, 10], increased pro-inflammatory IL-1β in human monocytic U937 cells [11], and stimulated proliferation and pro-fibrotic gene expression in mouse lung fibroblasts [12].
Oxidation of the extracellular space is reflected in changes in plasma redox potentials. In vivo studies have shown that plasma Eh(Cys/CySS) was oxidized in mice with bleomycin-induced lung injury [13], and in rats with kainic acid and pilocarpine-induced epilepsy [14]. In humans, plasma Eh(Cys/CySS) was found to be more oxidized in adults chronically exposed to arsenic [15], adults acutely exposed to acetaminophen [16], and in children with autism [17]. Thus, oxidation of the extracellular environment, or redox stress, is associated with disease processes and environmental or pharmacological exposures.
Aging is a risk factor for development of a number of chronic diseases. One way in which aging may promote disease development or progression is by changing the set-point of the redox buffering systems. Aging is associated with a steady oxidation of plasma Eh(Cys/CySS) [18], but the mechanisms responsible are unclear. Cells in culture maintain an Eh(Cys/CySS) remarkably close to the redox potential of plasma [10, 19, 20], suggesting that cells are actively involved in controlling their immediate extracellular redox environment. Recently, we found that lung fibroblasts from old mice (24 months old) produced an extracellular Eh(Cys/CySS) that was more oxidized than that produce by their young counterparts (2 months old) [21].
Differential gene expression analysis revealed that Slc7a11 was down-regulated in old mouse lung fibroblasts [21]. Slc7a11 (also called xCT) is the light chain of system Xc- which transports CySS into cells and exports glutamate with 1:1 as the exchange ratio [22]. Previous studies have suggested that Slc7a11 expression is linked to control of the extracellular Cys/CySS redox state. Mice lacking Slc7a11 have a more-oxidizing extracellular Eh(Cys/CySS), as evidenced by an increase in their plasma CySS concentrations that is not balanced by a corresponding increase in plasma Cys [23]. Conversely, stimulation of B cell differentiation is accompanied by an upregulation of Slc7a11 and an increase in extracellular Cys concentration [24]. In the latter study there was also an increase in intracellular GSH, consistent with other studies showing that Slc7a11 activity supports intracellular GSH levels by supplying Cys, which is the rate-limiting amino acid for its synthesis [25]. The purpose of the present study was to determine whether down-regulation of Slc7a11 in fibroblasts from old mice was sufficient to explain the oxidation of the extracellular redox environment associated with aging, and to determine whether synthesis of intracellular GSH was a pre-requisite for this effect.
MATERIALS AND METHODS
2.1. Reagents
Reagents were purchased from Sigma-Aldrich (St. Louis, MO) or Corning (Manassas, VA) unless otherwise specified.
2.2. Primary lung fibroblasts culture
Lung fibroblasts were isolated from young (3 months) or old (24 months) female C57BL/6 mice as described previously [21]. Animal use was approved by the Institutional Animal Care and Use Committee of the University of Louisville. DMEM with 10% FBS and 1% antibiotic-antimycotic solution were used for regular cell culture [26]. Fibroblasts between passage numbers 8 and 15 were used in the experiments.
2.3 Preparation of redox media
Redox media refers to media with specific Cys and CySS concentrations to achieve a specific redox state. DMEM without Met or Cys was used to make redox media. For 0 mV (oxidizing) media, 99.75 μM CySS and 0.5 μM Cys were added. All redox media were freshly prepared immediately before use.
2.4 Pharmacological treatment of fibroblasts
For sulforaphane experiments, primary lung fibroblasts from old mice were seeded in 6-well plates at the density of 1,000,000 cells/well in DMEM containing 10% FBS and 1% antibiotic-antimycotic solution. After 24 hours, the media was changed in the induction group to fresh DMEM with 5 μM sulforaphane. In the controls, media was changed to fresh DMEM. After incubation for 4 hours, media was changed to 0 mV redox media and incubated for another 24 hours. Afterwards, media was collected for HPLC analysis and cells for qPCR analysis.
For sulfasalazine experiments, young fibroblasts were used. Twenty four hours after plating, media were changed to 0 mV redox media with or without 300 μM sulfasalazine. Four hours later, cells and media were collected.
For L-buthionine sulfoximine experiments, young fibroblasts were incubated with or without 20 μM L-buthionine sulfoximine for 24 hours followed by 4 hours 0 mV redox media incubation. Afterwards, cells and media were collected.
2.5 Genetic manipulation of Slc7a11 expression in lung fibroblasts
Plasmid transfection was used to over-express Slc7a11 in old fibroblasts, while siRNA was used to knock down Slc7a11 in young fibroblasts. Plasmid encoding mouse Slc7a11 was from Origene Technologies, Inc. (Rockville, MD), and siRNA was from Dharmacon (Lafayette, CO). Plasmid LacZ encoding for beta-D-galactosidase and non-targeted NT2 were used as plasmid and si-RNA control. Two μg of plasmid and 30 pmol of siRNA were used for electroporation. Electroporation was conducted using Mode U-023 in Nucleofector™ 2b Device (Lonza, Allendale, NJ) following the protocol in the Amaxa™ Basic Nucleofector™ Kit for Primary Mammalian Fibroblasts (Lonza, Allendale, NJ). One million fibroblasts were used for each electroporation and then plated in 6-well plates. Serum-free and antibiotic-free DMEM were used to incubate the transfected cells. After 24 hours recovery, DMEM were changed to 0 mV media for 4 hours incubation. Then, media and cells were collected.
2.6 Media derivatization and HPLC analysis
Collected media were centrifuged at 800g for 4 minutes to pellet the suspended fibroblasts. Five hundred μl cell-free media were combined with 500 μl ice-cold 10% (w/v) perchloric acid, 0.2 M boric acid and 20 μM γ-glutamyl glutamate [27]. These samples were derivatized by iodoacetic acid and dansyl chloride, and then analyzed by HPLC (Waters Corporation, Millford, MA) as previously described [28]. Concentrations of Cys, CySS, GSH, GSSG, CySSG were measured by integration relative to the internal standard γ-glutamyl glutamate. Total Cys concentration = [Cys]+2*[CySS]+[CySSG] and total GSH concentration = [GSH]+2*[GSSG]+[CySSG]. Eh of Cys/CySS and GSH/GSSG were calculated according to the Nernst equation for pH 7.4: Eh(Cys/CySS) = −250+30*log([CySS]/[Cys]2); Eh(GSH/GSSG) = −264+30*log([GSSG]/[GSH]2) [29].
2.7 Measurement of Slc7a11, Gclc and Nqo1 mRNA level
RNAqueous®-4PCR Kit (Thermo Fisher Scientific, Waltham, MA) was used for DNA-free RNA isolation from primary mouse lung fibroblasts. SuperScript™ VILO™ cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA) was used for cDNA synthesis. Real-time quantitative PCR (qPCR) was conducted to measure Slc7a11, Gclc, Nqo1 and Gapdh mRNA expression with TaqMan probes (TaqMan® Gene Expression Assay Mm00442530_m1, Mm00802655_m1, Mm01253561_m1, Mm99999915_g1; Applied Biosystems), according to the manufacturer’s protocol (TaqMan Universal Master Mix II; Applied Biosystems). Step One Plus Real Time PCR System (Applied Biosystems) was used for qPCR with the parameters: 50°C 2 minutes, 95°C 10 minutes, followed by 40 cycles of 95°C 15 seconds and 60°C 1 minute. Results were analyzed using Step One Software version 2.3 (Applied Biosystems). The amplification curves were analyzed by the mathematical equation of the second derivative, and the amounts of Slc7a11, Gclc, Nqo1 mRNA expression were normalized to the housekeeping gene Gapdh mRNA expression. The 2−ΔΔCT method was used to calculate relative quantification [30].
2.8 Measurement of Slc7a11 activity
Slc7a11 activity was measured as the rate of glutamate release. Fresh 0 mV media containing 100 μM CySS and 0 μM glutamate was added to cells, and aliquots of conditioned media were removed at 15 minute intervals. Glutamate concentrations in media were measured using the Glutamate/Glutamate Oxidase assay kit from Molecular Probes (Waltham, MA) according to manufacturer’s recommendations.
2.9 Statistical analysis
Data were presented as mean ± standard deviation. Significance was evaluated by one-way ANOVA and unpaired two-tailed t-test. Linear regression was used to assess differences between rates of glutamate release.
RESULTS
Manipulation of Slc7a11 by pharmacological agents
Consistent with our previous studies [21], primary lung fibroblasts from old mice had lower expression of Slc7a11 (Figure 1A) and more oxidized extracellular Eh(Cys/CySS) redox potential (Figure 1B) relative to fibroblasts from young mice. To begin to assess whether expression level of Slc7a11 was responsible for the observed differences in the extracellular redox states of young and old fibroblasts, we treated old fibroblasts with sulforaphane, an Nrf2 inducer known to increase expression of Slc7a11 [31]. Sulforaphane increased Slc7a11 expression in old cells to the level seen in young cells (Figure 1A). Sulforaphane also reduced extracellular Eh(Cys/CySS) to the value seen in young cells (Figure 1B). There was no significant difference in the concentration of CySS in the conditioned media from cultures of young and old fibroblasts (Figure 1C). Rather, a decrease in the amount of Cys (Figure 1D) that accumulated in the media was responsible for the 30 mV oxidation of old fibroblasts conditioned media.
Figure 1. Sulforaphane treatment of old fibroblasts restored Slc7a11 expression and extracellular Eh(Cys/CySS) to the levels seen in young fibroblasts.
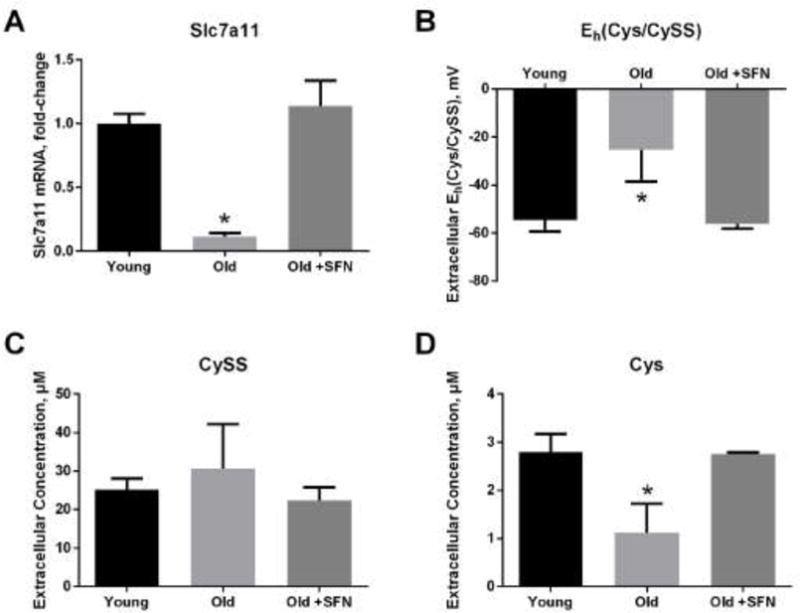
Primary lung fibroblasts from young and old mice were incubated in DMEM with or without 5 μM sulforaphane for 4 hours followed by 24 hours incubation in 0 mV redox media. (A) Slc7a11 mRNA expression, (B) extracellular Eh(Cys/CySS), (C) extracellular Cys concentration, and (D) extracellular CySS concentration, were measured as described in Materials and Methods. Data are expressed as mean ± standard deviation of 3 independent replicates. * - Indicates p<0.05 compared to untreated young fibroblasts.
Extracellular glutathione concentrations were also different between cultures of young and old fibroblasts. GSH, GSSG and the mixed disulfide between CySS and GSH (abbreviated CySSG) were all lower in the conditioned media from old cells compared to young cells (Figure 2A). There was a larger decrease in GSH than in GSSG; as a result, the redox potential of this couple (Eh(GSH/GSSG)) was 30 mV more oxidized in the old cultures than in the young (Figure 2B). GSH was not present in the 0 mV redox media initially. Therefore, the appearance of GSH indicated that it was released from the cells. To facilitate analysis of GSH export, the total GSH pool size was calculated by combining GSH contributed by all 3 forms: GSH, GSSG and CySSG. As shown in Figure 2C, total GSH was 4-fold lower in old fibroblasts than in young fibroblasts. Sulforaphane treatment restored extracellular GSH, GSSG, CySSG, total GSH and Eh(GSH/GSSG) in old cultures to the levels seen in cultures of young fibroblasts (Figure 2A–C).
Figure 2. Glutathione was less abundant and more oxidized in the conditioned media of old fibroblasts, and sulforaphane corrected these deficiencies.
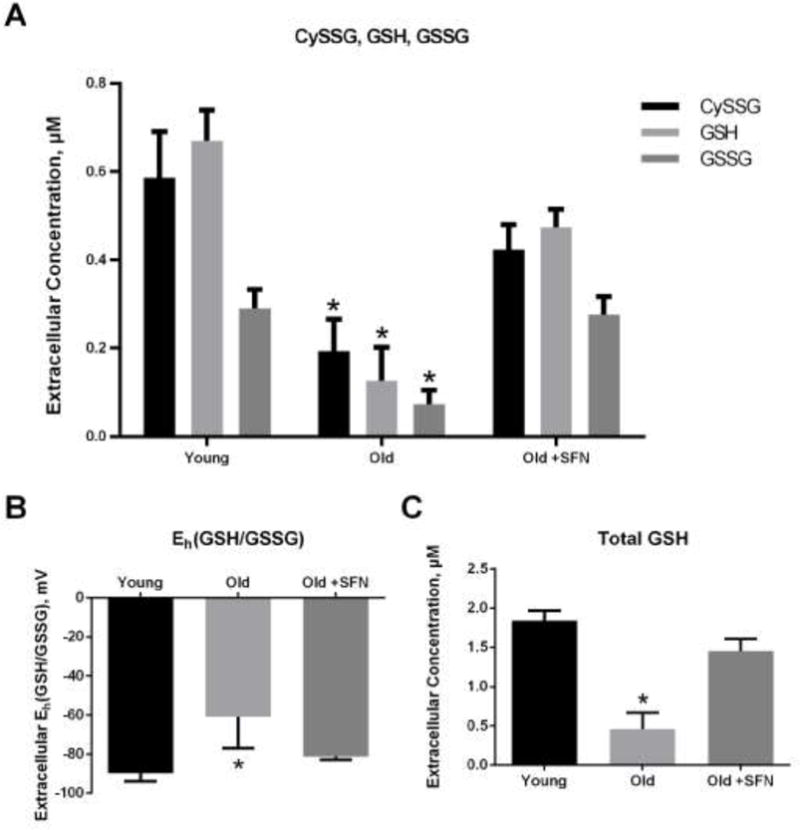
Primary lung fibroblasts from young and old mice were incubated in DMEM with or without 5 μM sulforaphane for 4 hours followed by 24 hours incubation in 0 mV redox media. (A) CySSG, GSH and GSSG, were measured in the conditioned medium by HPLC. (B) Extracellular Eh(GSH/GSSG) was calculated from the Nernst equation. (C) Total GSH concentration was calculated according to the formula: Total Cys = CySSG + GSH + 2*GSSG. Data are expressed as mean ± standard deviation of 3 independent replicates. * - Indicates p<0.05 compared to untreated young fibroblasts.
Sulforaphane is known to induce the expression of a number of antioxidant genes via its activation of the transcription factor Nrf2 [32]. To determine the extent to which the effects of sulforaphane on extracellular Eh(Cys/CySS) are mediated by Slc7a11 as opposed to its other transcriptional targets, cells were first transfected with siRNA to knock down Slc7a11 and then treated with sulforaphane. The results in Figure 3 showed that extracellular Eh(Cys/CySS) no longer became more reduced in response to sulforaphane in cells depleted of Slc7a11 (Figure 3A). Expression of Slc7a11 was still induced by sulforaphane in the knock down cells, but not to the level seen in control cells (Figure 3B). In contrast, other Nrf2 target genes (Gclc and Nqo1) were induced equally well in both control and knock down cells (Figures 3C and 3D).
Figure 3. Reduction of extracellular Eh(Cys/CySS) in response to sulforaphane is mediated by Slc7a11.
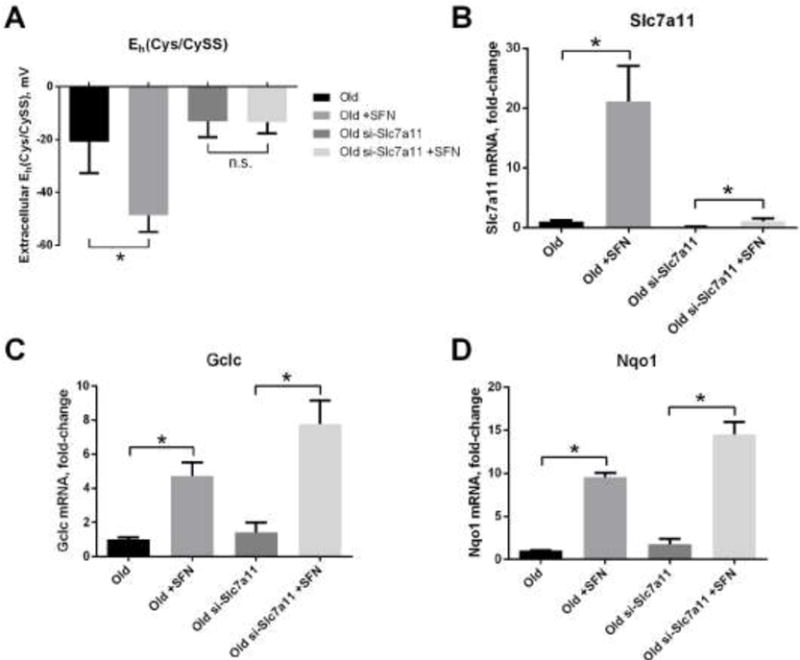
Primary lung fibroblasts from old mice were transfected with siRNA targeting Slc7a11 (si-Slc7a11) via electroporation. Non-targeting siRNA was electroporated as control. Fibroblasts were plated, and 24 hours later the media were changed to fresh DMEM with or without 5 μM sulforaphane for 4 hours. After that, media were changed to 0 mV redox media for 4 hours. (A) Extracellular Eh(Cys/CySS), (B) Slc7a11 mRNA expression, (C) Gclc mRNA expression, and (D) Nqo1 mRNA expression, were measured as described in the legends to Figures 1 and 2. Data are expressed as mean ± standard deviation of 3 independent replicates. * - Indicates p<0.05 between sulforaphane-treated and untreated fibroblasts.
Having shown that a drug that increased Slc7a11 expression also reduced extracellular Eh(Cys/CySS), we sought to demonstrate that the inverse was also true: that inhibition of system Slc7a11 activity would oxidize extracellular Eh(Cys/CySS). For this purpose, we used sulfasalazine, an inhibitor of CySS transport that acts on the Slc7a11 subunit of system Xc- [33]. As predicted, sulfasalazine treatment of young fibroblasts oxidized extracellular Eh(Cys/CySS) by 30 mV (Figure 4A). This oxidation was due to a significant decrease in the concentration of extracellular Cys, while extracellular CySS concentration was unaffected by sulfasalazine (Figure 4B and 4C). Inhibition of Slc7a11 activity had no effect on accumulation of total GSH in the conditioned media (Figure 4D).
Figure 4. Slc7a11 inhibition by sulfasalazine in young mice lung fibroblasts resulted in oxidation of extracellular Eh(Cys/CySS).
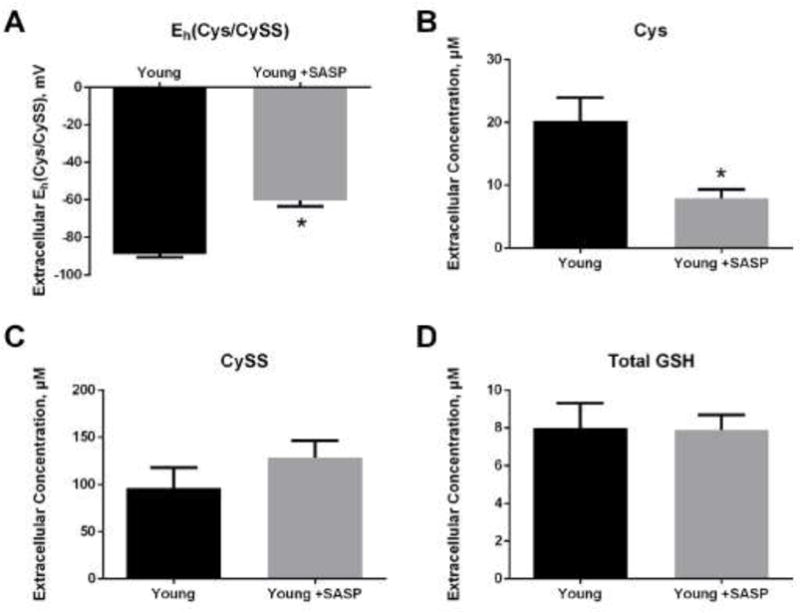
Primary lung fibroblasts from young mice were incubated in 0 mV redox media with or without 300 μM sulfasalazine (SASP) for 4 hours. Conditioned media were collected for analysis by HPLC. (A) Eh(Cys/CySS), (B) Cys concentration, (C) CySS concentration and (D) total GSH concentration were determined as described in the legends to Figures 1 and 2. Data are expressed as mean ± standard deviation of 3 independent replicates. * - Indicates p<0.05 compared to untreated young fibroblasts.
Not only Slc7a11 mRNA, but also the activity of Slc7a11-mediated transport was lower in fibroblasts from old mice (Figure 5). Because Slc7a11 exports a glutamate for every CySS it imports, we measured accumulation of glutamate in the media as a function of time to assess transport activity. This approach avoided having to account for the multiple fates of CySS once imported into the cell. Using this approach, we could also demonstrate that our pharmacological manipulations translated to changes in activity. Sulforaphane increased Slc7a11 transporter activity (Figure 5A), whereas sulfasalazine inhibited activity (Figure 5B).
Figure 5. Slc7a11 transport activity was increased by sulforaphane and inhibited by sulfasalazine.
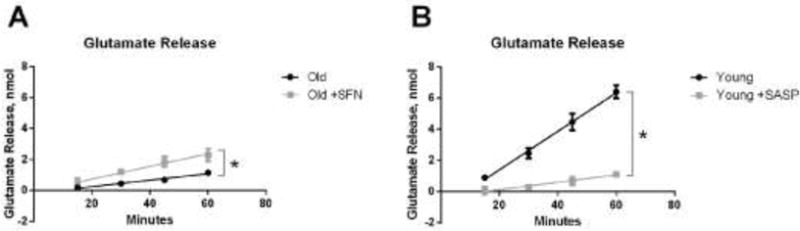
(A) Glutamate release by old fibroblasts with or without sulforaphane treatment. Primary lung fibroblasts from old mice were incubated in DMEM with or without 5 μM sulforaphane for 4 hours followed by 20 hours incubation in 0 mV redox media. After that, media were changed to fresh 0 mV media, and media was collected at 15 min, 30 min, 45 min and 60 min for measuring extracellular glutamate as described in Materials and Methods. (B) Glutamate release by young fibroblasts with or without sulfasalazine treatment. Primary lung fibroblasts from young mice were incubated in 0 mV media with or without 300 μM sulfasalazine. Media were collected at 15 min, 30 min, 45 min and 60 min for measuring extracellular glutamate. Data are expressed as mean ± standard deviation of 3 independent replicates. * - Indicates p<0.05 between treated and untreated fibroblasts.
Modulation of Slc7a11 expression via genetic methods
Because pharmacological agents can have off-target effects, we next attempted to verify the above results using transfection to overexpress and knock down Slc7a11 in old and young fibroblasts. Similar to the results with induction by sulforaphane, transfection of old fibroblasts with a Slc7a11-encoding plasmid increased Slc7a11 mRNA expression by three-fold (Figure 6A) and reduced the extracellular Eh(Cys/CySS) by 30 mV (Figure 6B). Conversely, siRNA-mediated knock down of Slc7a11 in young fibroblasts decreased Slc7a11 mRNA level by three-fold (Figure 6C). Correspondingly, extracellular Eh(Cys/CySS) became 45 mV more oxidized after Slc7a11 knock-down (Figure 6D). The changes in extracellular Eh(Cys/CySS) were largely driven by differences in Cys concentrations. Neither overexpression nor knock down had a significant effect on extracellular CySS concentrations (Figure 7A and 7B). However, Slc7a11 over-expression in old cells significantly increased extracellular Cys from 2 μM to 5 μM while Slc7a11 knock-down in young cells significantly decreased extracellular Cys from 6 μM to 1 μM (Figure 7C and 7D). Neither overexpression nor knock down of Slc7a11 had a significant effect on the accumulation of extracellular GSH (Figure 7E and 7F).
Figure 6. Effect of genetic manipulation of Slc7a11 on extracellular Eh(Cys/CySS).
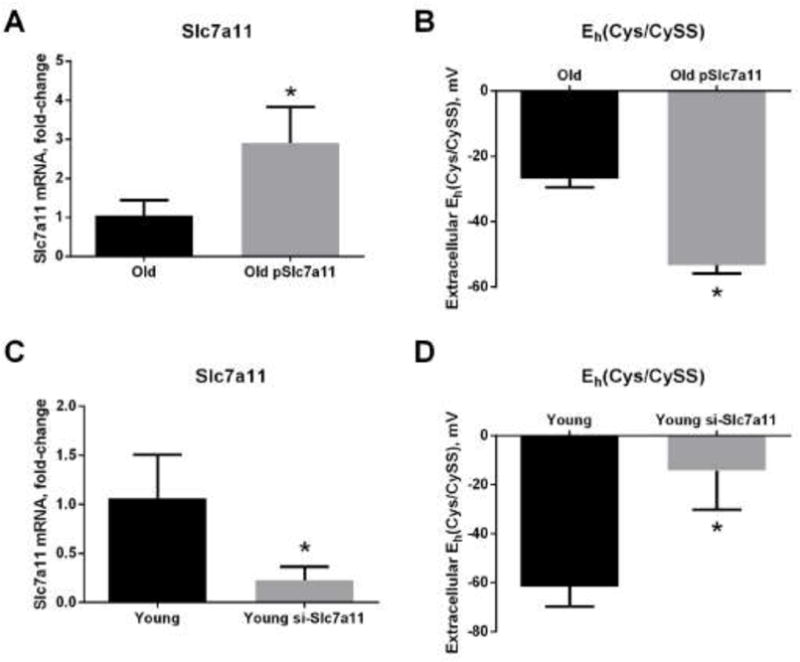
Primary lung fibroblasts from old and young mice were transfected with either an Slc7a11-encoding plasmid (pSlc7a11) or siRNA targeting Slc7a11 (si-Slc7a11) via electroporation. Controls were electroporated with pLacZ or non-targeting siRNA. Fibroblasts were plated, and 24 hours later the media were changed to 0mV redox media for 4 hours. (A) Slc7a11 mRNA expression and (B) extracellular Eh(Cys/CySS) in old fibroblasts with and without overexpression of Slc7a11. (C) Slc7a11 mRNA expression and (D) extracellular Eh(Cys/CySS) of young fibroblasts with and without knock down of Slc7a11. Data are expressed as mean ± standard deviation of 3 independent replicates. * - Indicates p<0.05 compared to controls.
Figure 7. Overexpression of Slc7a11 increased, and knock down of Slc7a11 decreased, extracellular Cys concentrations.
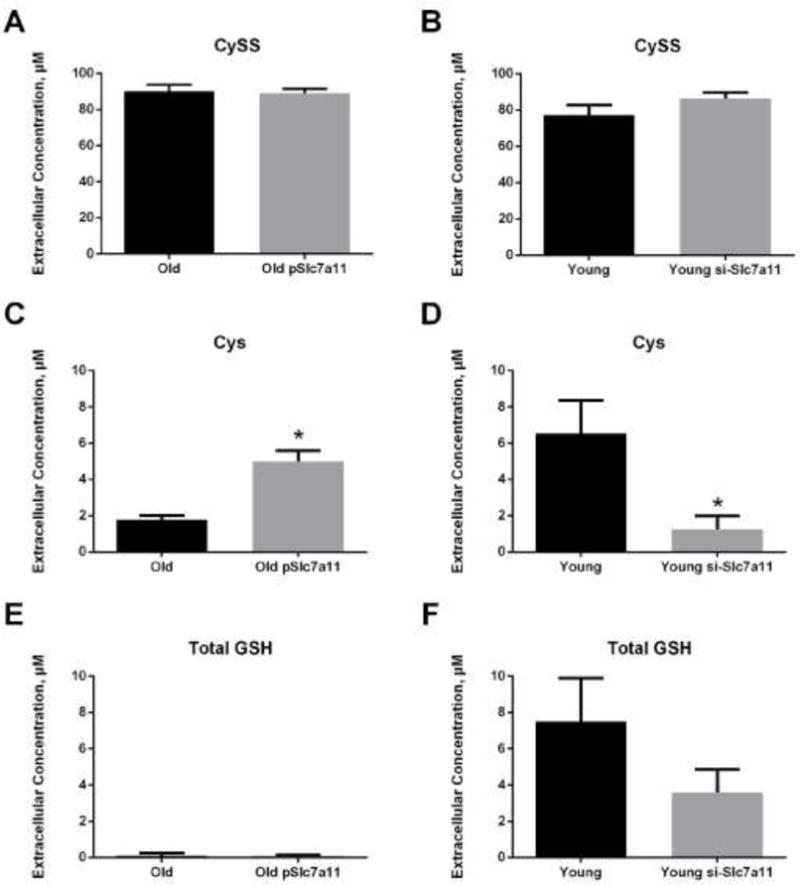
Primary lung fibroblasts from old and young mice were transfected with either pSlc7a11 (overexpression) or si-Slc7a11 (knock down), as described in the legend to Figure 4. (A and B) Extracellular CySS concentrations, (C and D) extracellular Cys concentrations, and (E and F) total extracellular GSH concentrations in old and young fibroblasts, respectively. Data are expressed as mean ± standard deviation of 3 independent replicates. * - Indicates p<0.05 compared to controls.
Both Cys and GSH can be exported by cells and used to reduce an oxidized extracellular Eh(Cys/CySS). To determine whether metabolism of Cys to GSH is necessary for cells to reduce an oxidizing extracellular Eh(Cys/CySS), buthionine sulfoximine (BSO) was used to inhibit GSH synthesis by γ-glutamylcysteinyl ligase. As shown in Figure 8, 24 hours pre-treatment with BSO dramatically lowered intracellular GSH (Figure 8A). The inhibition of GSH synthesis by BSO also led to a dramatic decrease in extracellular GSH concentrations (Figure 8B). BSO treatment had no effect on extracellular Cys concentration (Figure 8C) or the ability of cells to restore extracellular Eh(Cys/CySS) within 4 hours of challenge with 0 mV media (Figure 8D), suggesting that GSH synthesis was not required for the normalization of an oxidizing extracellular Eh(Cys/CySS).
Figure 8. Glutathione depletion with BSO did not affect extracellular Cys concentration or Eh(Cys/CySS).
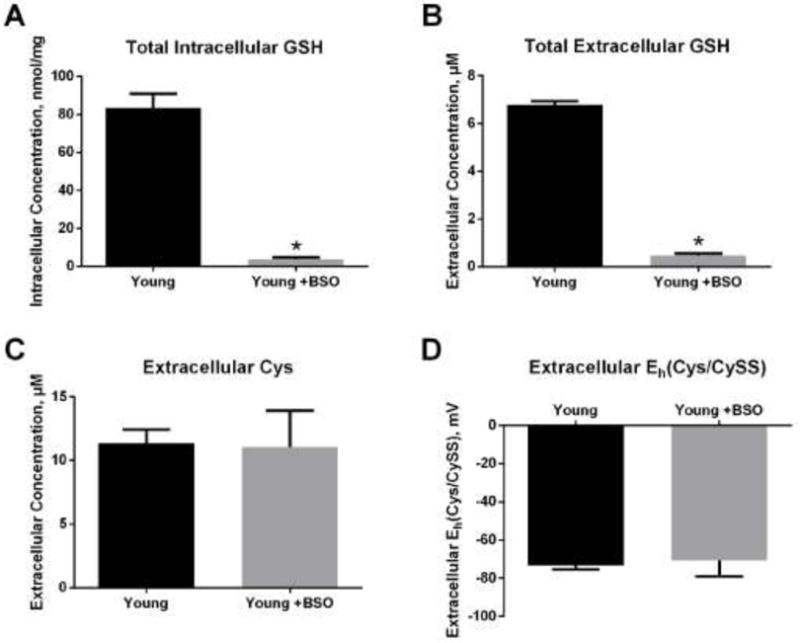
Primary lung fibroblasts from young mice were incubated in 0 mV redox media with or without 20 μM L-buthionine sulfoximine (BSO) for 24 hours. (A) Total intracellular GSH. (B) Total extracellular GSH. (C) Extracellular Cys concentration. (D) Extracellular Eh(Cys/CySS). Data are expressed as mean ± standard deviation of 3 independent replicates. * - Indicates p<0.05 compared to controls.
DISCUSSION
The current studies confirm the finding that Slc7a11 expression was lower in primary lung fibroblasts from old mice than in those from young mice, and that this was associated with increased oxidation of extracellular Eh(Cys/CySS) redox potential [21]. We have now extended those findings by investigating the mechanistic link between these two observations. We found that up-regulation of Slc7a11 expression by either sulforaphane treatment or transient transfection was sufficient to restore the ability of lung fibroblasts from old mice to reduce their extracellular Eh(Cys/CySS) to the level achieved by those from young mice. Conversely, inhibition of Slc7a11 activity by either sulfasalazine treatment or siRNA-mediated knock down produced young fibroblasts that resembled old fibroblasts in terms of their extracellular redox potential. Taken together, these findings show that Slc7a11 activity is the major determinant of the extracellular redox environment produced and maintained by primary lung fibroblasts.
In each of the studies presented here, Slc7a11 was positively correlated with extracellular Cys concentrations. This suggests that intracellular reduction of CySS to Cys, and the subsequent export of Cys, is limited by the rate of delivery of CySS to cells. Differences in Slc7a11 activity had no effect on extracellular CySS concentrations under the culture conditions used in this study. This is most likely a reflection of the process by which cells regulate their extracellular redox environment. CySS cannot be reduced to Cys in the extracellular space. Therefore, cells adjust the relative proportions of extracellular CySS and Cys by importing CySS, reducing it to Cys, and then exporting Cys via system ASC [34-37]. To achieve physiological redox potential of about −80 mV [18], only a fraction of the media CySS needed to be reduced to Cys. Because each molecule of CySS yields 2 molecules of Cys, very little CySS is consumed in the process of normalizing the extracellular redox potential. Thus, CySS import via Slc7a11 appears to be the rate-limiting factor for intracellular Cys formation and export, just as it is rate-limiting for GSH synthesis in some cell types [38, 39].
An increase in extracellular Cys, but not CySS, in response to increased Slc7a11 activity has been observed previously. Overexpression of Slc7a11 in Burkitt’s Lymphoma cells was associated with increased extracellular Cys concentrations [40], similar to our results. In contrast, knock out mice had elevated plasma CySS, but no change in plasma Cys [23]. The discrepancy between these findings and our results with sulfasalazine or siRNA-mediated knock down of Slc7a11 may have been due to the fact that the fibroblasts used in the current studies do not completely lack Slc7a11, or it could be the presence of other cell types in the mice that may remove Cys from the plasma [41].
Of the 3 component amino acids of GSH, Cys is usually present at the lowest concentrations within cells and limits the rate at which GSH can by synthesized [25]. Therefore, changes in Slc7a11 activity can affect intracellular GSH production. In cancer cells, elevated Slc7a11 expression is associated with increased resistance to chemotherapy drugs [42]. Conversely, inhibition of CySS transport can sensitize cancer cells to radiation therapy [43]. The age-related decrease in Slc7a11 expression observed in the current study may limit GSH synthesis and contribute to lower extracellular concentrations of GSH.
CySS and GSH have many fates both extracellularly and intracellularly, complicating efforts to account for contributions of metabolism and transport to changes in concentrations in any given location. Extracellular GSH can be used to increase extracellular Cys concentrations by two mechanisms: it can undergo thiol-disulfide exchange with extracellular CySS to yield Cys and the mixed disulfide CySSG, or it can be catabolized enzymatically by gamma-glutamyltransferase and dipeptidase to yield Cys and the other two component amino acids of GSH, glutamate and glycine [25]. GSH can also be oxidized to GSSG and used to glutathionylate extracellular proteins. Similarly, CySS can cysteinylate proteins. Extracellular Cys itself can be taken up by some cell types through other amino acid transporters such as systems ASC, EAAT and LAT2 [44, 45]. Once CySS is imported, it can be reduced to Cys either non-enzymatically through thiol-disulfide exchange with GSH or enzymatically by Txnrd1 or Txndc17 [36]. Intracellular Cys can be used to synthesize proteins or GSH, which can then by oxidized, glutathionylate proteins, conjugate to electrophilic metabolites, or be exported. Despite the complexity of these interconnected pathways, our data point to a rather straightforward relationship between Slc7a11 activity and extracellular Eh(Cys/CySS). Importantly, we found that GSH was not involved in regulation of extracellular Eh(Cys/CySS), and that accumulation of Cys in the extracellular space was directly related to the level of Slc7a11 activity.
Extracellular GSH was less abundant and more oxidized in cultures of lung fibroblasts from old mice. Whereas sulforaphane treatment corrected these defects, Slc7a11 overexpression did not. A likely explanation for this discrepancy is that sulforaphane activates a much broader antioxidant response than does Slc7a11 overexpression alone. For example, our study confirmed that, in addition to Slc7a11 induction, sulforaphane upregulated the expression of Gclc, the catalytic subunit of the rate-limiting enzyme in GSH synthesis. Both Slc7a11 and Gclc are transcriptionally regulated by the transcription factor Nrf2. Sulforaphane is an electrophile that interacts directly with nucleophilic residues on Keap1, thereby activating Nrf2 [46]. In cancer cells, sulforaphane can induce anticancer responses driven in part through production of reactive oxygen species via interactions with mitochondrial respiratory complex I [47]. Oxidative stress produced in this way can promote the formation of 4-hydroxynonenal, an endogenous electrophilic activator of Nrf2 [48]. However, untransformed cells, such as the primary fibroblasts used in the present study, are typically protected from the anticancer effects of sulforaphane [49].
In conclusion, oxidative stress has been well recognized in aging. While oxidative stress can be measured in many ways, our studies focused on oxidation of the extracellular Eh(Cys/CySS) redox potential. Oxidation of extracellular Eh(Cys/CySS) has been linked to age-dependent lung matrix remodeling and changes in the phenotype of lung fibroblasts [12, 21], as well as phenotypic changes in other cell types [50-52]. Identification of Slc7a11 as a critical factor in the regulation of the extracellular redox environment will undoubtedly lead to novel approaches to understanding the effects of aging in health and disease.
Supplementary Material
Supplementary Figure S1. SASP, BSO, SFN treatments do not affect fibroblast viability. Primary lung fibroblasts from young and old mice were plated in a 96-well plate (20×103 cells/well). After recovery overnight, fibroblasts were treated with fresh DMEM with or without 300 mM sulfasalazine (SASP), 20 μM L-buthionine sulfoximine (BSO) or 5 μM sulforaphane (SFN) for 24 hours. MTT solution was then added to make the final concentration 0.2 mg/ml. After 30 min incubation, DMSO was added and absorbance was measured at 540 nm. Data are expressed as mean ± standard deviation of 3 independent replicates. There is no significant difference between groups using one-way ANOVA.
Highlights.
Aging is associated with decreased expression and activity of Slc7a11.
Decreased Slc7a11 is responsible for oxidation of extracellular Eh(Cys/CySS).
Slc7a11 restoration in old cells is sufficient to reduce extracellular Eh(Cys/CySS).
Regulation of extracellular Eh(Cys/CySS) by Slc7a11 is not dependent on glutathione.
Acknowledgments
Research reported in this publication was supported by Veterans Affairs Grant 5I01 BX000216-02 (Roman) and by R01 AA019953 (Roman), U01 HL121807 (Roman), the National Institute on Alcohol Abuse and Alcoholism under Award no. P50AA024337-8305 (Roman), and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant no. P20GM113226-6176 (Watson).
ABBREVIATIONS
- Cys
cysteine
- CySS
cystine
- GSH
glutathione
- GSSG
glutathione disulfide
- CySSG
cysteine-glutathione mixed disulfide
- HPLC
high performance liquid chromatography
- Eh
redox potential
- IL-1β
interleukin-1β
- qPCR
real-time quantitative polymerase chain reaction
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Slc7a11
solute carrier family 7 (cationic amino acid transporter, y+ system), member 11
- Gclc
glutamate-cysteine ligase, catalytic subunit
- Nqo1
NAD(P)H dehydrogenase, quinone 1
- Nrf2
nuclear factor-erythroid 2
- SFN
sulforaphane
- SASP
sulfasalazine
- BSO
L-buthionine sulfoximine
- Txnrd1
thioredoxin reductase 1
- Txndc17
thioredoxin domain containing 17
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP. Thioredoxin and its role in toxicology. Toxicol Sci. 2004;78(1):3–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- 2.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 3.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9–10):1865–79. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 4.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Jr, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18(11):1246–8. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 5.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28(4):625–35. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 6.Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50(4):495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go YM, Jones DP. The redox proteome. J Biol Chem. 2013;288(37):26512–20. doi: 10.1074/jbc.R113.464131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson WH, Chen Y, Jones DP. Redox state of glutathione and thioredoxin in differentiation and apoptosis. BioFactors. 2003;17(1–4):307–14. doi: 10.1002/biof.5520170130. [DOI] [PubMed] [Google Scholar]
- 9.Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33(11):1499–506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 10.Nkabyo YS, Go YM, Ziegler TR, Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G70–8. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- 11.Iyer SS, Accardi CJ, Ziegler TR, Blanco RA, Ritzenthaler JD, Rojas M, Roman J, Jones DP. Cysteine redox potential determines pro-inflammatory IL-1beta levels. PLoS One. 2009;4(3):e5017. doi: 10.1371/journal.pone.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez A, Ramadan B, Ritzenthaler JD, Rivera HN, Jones DP, Roman J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293(4):L972–81. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
- 13.Iyer SS, Ramirez AM, Ritzenthaler JD, Torres-Gonzalez E, Roser-Page S, Mora AL, Brigham KL, Jones DP, Roman J, Rojas M. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296(1):L37–45. doi: 10.1152/ajplung.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang LP, Patel M. Plasma cysteine/cystine redox couple disruption in animal models of temporal lobe epilepsy. Redox Biol. 2016;9:45–49. doi: 10.1016/j.redox.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, Slavkovich V, Ilievski V, Levy D, Siddique AB, Parvez F, Mey JL, van Geen A, Graziano J, Gamble MV. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in bangladeshi adults. Environ Health Perspect. 2013;121(9):1068–74. doi: 10.1289/ehp.1205727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannery YO, Ziegler TR, Park Y, Jones DP. Oxidation of plasma cysteine/cystine and GSH/GSSG redox potentials by acetaminophen and sulfur amino acid insufficiency in humans. J Pharmacol Exp Ther. 2010;333(3):939–47. doi: 10.1124/jpet.110.166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose S, Melnyk S, Trusty TA, Pavliv O, Seidel L, Li J, Nick T, James SJ. Intracellular and extracellular redox status and free radical generation in primary immune cells from children with autism. Autism Res Treat. 2012;2012:986519. doi: 10.1155/2012/986519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33(9):1290–300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 19.Jonas CR, Gu LH, Nkabyo YS, Mannery YO, Avissar NE, Sax HC, Jones DP, Ziegler TR. Glutamine and KGF each regulate extracellular thiol/disulfide redox and enhance proliferation in Caco-2 cells. Am J Physiol Regul Integr Comp Physiol. 2003;285(6):R1421–9. doi: 10.1152/ajpregu.00702.2002. [DOI] [PubMed] [Google Scholar]
- 20.Miller LT, Watson WH, Kirlin WG, Ziegler TR, Jones DP. Oxidation of the glutathione/glutathione disulfide redox state is induced by cysteine deficiency in human colon carcinoma HT29 cells. J Nutr. 2002;132(8):2303–6. doi: 10.1093/jn/132.8.2303. [DOI] [PubMed] [Google Scholar]
- 21.Watson WH, Burke TJ, Zelko IN, Torres-Gonzalez E, Ritzenthaler JD, Roman J. Differential Regulation of the Extracellular Cysteine/Cystine Redox State (EhCySS) by Lung Fibroblasts from Young and Old Mice. Oxid Med Cell Longev. 2016;2016:1561305. doi: 10.1155/2016/1561305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. The Journal of biological chemistry. 1999;274(17):11455–8. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 23.Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T, Takahashi S, Bannai S. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280(45):37423–9. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 24.Vene R, Delfino L, Castellani P, Balza E, Bertolotti M, Sitia R, Rubartelli A. Redox remodeling allows and controls B-cell activation and differentiation. Antioxid Redox Signal. 2010;13(8):1145–55. doi: 10.1089/ars.2009.3078. [DOI] [PubMed] [Google Scholar]
- 25.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–53. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roman J, Ritzenthaler JD, Gil-Acosta A, Rivera HN, Roser-Page S. Nicotine and fibronectin expression in lung fibroblasts: implications for tobacco-related lung tissue remodeling. FASEB J. 2004;18(12):1436–8. doi: 10.1096/fj.03-0826fje. [DOI] [PubMed] [Google Scholar]
- 27.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 28.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275(2):175–84. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 29.Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283(6):G1352–9. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Mann GE. Nrf2-mediated redox signalling in vascular health and disease. Free Radic Biol Med. 2014;75(Suppl 1):S1. doi: 10.1016/j.freeradbiomed.2014.10.595. [DOI] [PubMed] [Google Scholar]
- 32.Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci Technol. 2017;69(Pt B):257–269. doi: 10.1016/j.tifs.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bridges RJ, Natale NR, Patel SA. System xc(-)cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165(1):20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bannai S, Ishii T. Formation of sulfhydryl groups in the culture medium by human diploid fibroblasts. J Cell Physiol. 1980;104(2):215–23. doi: 10.1002/jcp.1041040211. [DOI] [PubMed] [Google Scholar]
- 35.Bannai S, Ishii T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J Cell Physiol. 1982;112(2):265–72. doi: 10.1002/jcp.1041120216. [DOI] [PubMed] [Google Scholar]
- 36.Pader I, Sengupta R, Cebula M, Xu J, Lundberg JO, Holmgren A, Johansson K, Arner ES. Thioredoxin-related protein of 14 kDa is an efficient L-cystine reductase and S-denitrosylase. Proc Natl Acad Sci U S A. 2014;111(19):6964–9. doi: 10.1073/pnas.1317320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson CL, Iyer SS, Ziegler TR, Jones DP. Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1069–75. doi: 10.1152/ajpregu.00195.2007. [DOI] [PubMed] [Google Scholar]
- 38.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdo J, Dargusch R, Schubert D. Distribution of the cystine/glutamate antiporter system xc- in the brain, kidney, and duodenum. J Histochem Cytochem. 2006;54(5):549–57. doi: 10.1369/jhc.5A6840.2006. [DOI] [PubMed] [Google Scholar]
- 40.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kolle P, Tschoep K, Issels RD, Daniel PT, Conrad M, Bornkamm GW. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27(11):1618–28. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 41.Ishii T, Mann GE. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014;2:786–94. doi: 10.1016/j.redox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuno S, Sato H, Kuriyama-Matsumura K, Tamba M, Wang H, Sohda S, Hamada H, Yoshikawa H, Kondo T, Bannai S. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br J Cancer. 2003;88(6):951–6. doi: 10.1038/sj.bjc.6600786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sleire L, Skeie BS, Netland IA, Forde HE, Dodoo E, Selheim F, Leiss L, Heggdal JI, Pedersen PH, Wang J, Enger PO. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc-, leading to glutathione depletion. Oncogene. 2015;34(49):5951–9. doi: 10.1038/onc.2015.60. [DOI] [PubMed] [Google Scholar]
- 44.Balthasar C, Stangl H, Widhalm R, Granitzer S, Hengstschlager M, Gundacker C. Methylmercury Uptake into BeWo Cells Depends on LAT2-4F2hc, a System L Amino Acid Transporter. Int J Mol Sci. 2017;18(8) doi: 10.3390/ijms18081730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P. The cystine/glutamate antiporter system x(c) (−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18(5):522–55. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244(1):66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sestili P, Fimognari C. Cytotoxic and Antitumor Activity of Sulforaphane: The Role of Reactive Oxygen Species. Biomed Res Int. 2015;2015:402386. doi: 10.1155/2015/402386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leone A, Diorio G, Sexton W, Schell M, Alexandrow M, Fahey JW, Kumar NB. Sulforaphane for the chemoprevention of bladder cancer: molecular mechanism targeted approach. Oncotarget. 2017;8(21):35412–35424. doi: 10.18632/oncotarget.16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veeranki OL, Bhattacharya A, Tang L, Marshall JR, Zhang Y. Cruciferous vegetables, isothiocyanates, and prevention of bladder cancer. Curr Pharmacol Rep. 2015;1(4):272–282. doi: 10.1007/s40495-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111(22):2973–80. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 51.Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424(3):491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- 52.Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99(3):1491–6. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. SASP, BSO, SFN treatments do not affect fibroblast viability. Primary lung fibroblasts from young and old mice were plated in a 96-well plate (20×103 cells/well). After recovery overnight, fibroblasts were treated with fresh DMEM with or without 300 mM sulfasalazine (SASP), 20 μM L-buthionine sulfoximine (BSO) or 5 μM sulforaphane (SFN) for 24 hours. MTT solution was then added to make the final concentration 0.2 mg/ml. After 30 min incubation, DMSO was added and absorbance was measured at 540 nm. Data are expressed as mean ± standard deviation of 3 independent replicates. There is no significant difference between groups using one-way ANOVA.


