Abstract
The quantitative analysis of polyunsaturated fatty acyl (PUFA) chain oxidation products in tissue samples by mass spectrometry is hindered by the lack of durable internal standards for the large number of possible products. To address this problem in a study of oxidative PUFA degradation in Alzheimer’s disease (AD) brain, uniformly 13C-labeled arachidonic acid (ARA) was produced biosynthetically, and allowed to oxidize under controlled conditions into a mixture of U-13C-labeled ARA oxidation products. The components of this mixture were characterized with respect to their partitioning behavior during lipid extraction, their durability during saponification, trends in mouse brain tissue concentrations during post mortem intervals, and their overall suitability as internal standards for multiple-reaction monitoring tandem mass spectrometry. This mixture has now been used as a set of internal standards to determine the relative abundance of ARA and 54 non-stereospecific oxidation products in milligram samples of brain tissue. Many of these oxidation products were recovered from both healthy mouse and healthy human brain, although some of them were unique to each source, and some have not heretofore been described. The list of oxidation products detected in AD brain tissue was the same as in healthy human brain, although simple hydroxy-eicosanoids were significantly increased in AD brain. while more complex oxidation products were not. These results are consistent with an increased level of chemically-mediated oxidative ARA degradation in Alzheimer’s disease. However, they also point to the existence of processes that selectively produce or eliminate specific oxidation products, and those processes may account for some of the inconsistencies in previously reported results.
Keywords: Alzheimer’s disease, Brain, Eicosanoids, Lipid Peroxidation, Mass Spectrometry, Oxidized Lipids
Graphical abstract
Introduction
Oxidative stress is caused by a dense, complex, and heterogeneous network of oxidizing reactions running counter to the reducing conditions that otherwise prevail in cells and tissues. It has been implicated in the pathogenesis of many human diseases including cancer, atherosclerosis, diabetes, and it is a particularly prominent biochemical feature of brain tissue in Alzheimer’s disease (AD) [1]. Lipids containing polyunsaturated fatty acyl (PUFA) chains are abundant in the brain, and among the most vulnerable compounds to oxidative stress [2]. There are well-known mechanisms of oxidative PUFA degradation that are mediated by hydroxyl radicals (•OH) and lead to both amyloid fibril formation [3, 4], and neurotoxic PUFA degradation products [5, 6]. Moreover, the amyloid plaques of AD are active centers of oxidative PUFA damage, and the site of protein modification by reactive PUFA oxidation products [7].
Heterogeneity among its chemical mechanisms renders oxidative stress challenging to characterize and quantify. It is common to assay one compound or class of compounds as representative of overall oxidative stress. For example, numerous studies of F2 isoprostanes (iPs) have been performed on material from people with AD and pre-AD syndromes [8–19]. There is some consensus that F2-iP concentrations are increased in the CSF of persons with AD, however, the results from brain tissue, plasma, and urine are less clear. Superimposed on any changes in the level of chemical oxidative stress in AD are changes in enzymatic activity [20], and lipid substrate concentrations [21–24].
Regardless of the clinical sample being assayed, it is clear that existing assays of PUFA oxidation products sample only a narrow subset of possible PUFA oxidation products, leaving many pathways of oxidative stress unexamined. The chief reason for this limitation has been that mass spectrometric methods rely on isotope-labeled internal standards. Relatively few such standards are commercially available, they are expensive, they are most reliable only when used to quantify the corresponding unlabeled compound, and the isotope labels are invariably deuterium that may be lost through hydrogen exchange during isolation procedures such as saponification.
To overcome these problems, a larger and more robust set of internal standards was created by cultivating an ARA-producing oceanic yeast in a medium containing U-13C-glucose as a sole carbon source [25]. Uniformly 13C-labeled ARA (U-13C-ARA) was extracted and purified from the cultures, which was then subjected to a controlled amount of air oxidation. The resulting mixture contained several dozen U-13C-labeled ARA oxidation products, which were characterized by retention time, partitioning behavior during lipid extraction procedure, stability during saponification, and tissue concentration changes during post mortem intervals.
This report describes the use of this mixture as a set of internal standards for the analysis of ARA and its oxidation products by multiple reaction monitoring liquid-chromatography tandem mass spectrometry (MRM-LC/MS/MS). Results show that many of the same ARA oxidation products are present in both mouse and human brain, but that there are some differences between mouse and human, as well as oxidation products that have not previously been described. Results also show that relatively simple ARA oxidation products are consistently increased in AD brain, but that more complex oxidation products are either not produced or preferentially cleared from brain tissue.
Materials and Methods
Reagents
ARA was purchased from Nu-Check Prep Inc. (Elysian, Mn). d8-ARA was purchased from Cayman Chemicals (Ann Arbor, Michigan). U-13C-ARA was produced biosynthetically as described previously [25] and stored at −80°C. Its isotopic purity was verified to be 95% by mass spectrometry.
HPLC and Mass spectrometry
20 μl samples were injected onto a 1 × 250 mm Zorbax 300SB 5 μm C18 column (Agilent, Santa Clara, CA). Solvent A was 8% acetonitrile, 92% water, and 0.1% formic acid. Solvent B was 100% acetonitrile and 0.1% formic acid. The mobile phase was pumped at 0.1 ml/min as the composition was changed linearly from 8% to 80% solvent B over 25 min, and held at 80% B for 9.5 min. The eluent was alkalinized post-column with 0.15 M ammonium hydroxide in methanol flowing at 50 μl/min before being introduced via electrospray ionization into an ABI 4000 mass spectrometer (Sciex, Toronto, Canada) operating in negative ion mode. The declustering potential was −70V, the ionization energy was −4500V, and the drying gas temperature was 200°C. The collision gas was nitrogen at 7 psi for multiple reaction monitoring (MRM) or product ion scanning mode, with the transitions and collision energy values listed in table 1. The collection time for each transition in MRM-LC/MSMS methods was 100 msec.
Table 1.
Molecular species referenced in this work, abbreviations, key characteristics, and reference locations.
| Appears in Figure5 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Abbreviation (example species)1 | 12C Transit ion | [M-H]− Ion2 | 13C Transit ion | Time3 | CE4 | Reference | 2 | 3 | 4 | 5 | 6 | S2 | S3 |
| ARA | 303.2/259.2 | C20H31O2 | 323.2/278.2 | 27 | 20 | This work | X | D | ACDE | AB | AB | X | A |
| 8-deoxy J2 ip | 315.2/95.2 | C20H27O3 | 335.2/101.2 | 14–18 | 20 | [29] | X | D | A | A | |||
| 12-deoxy J2 ip | 315.2/175.2 | C20H27O3 | 335.2/188.2 | 14–20 | 20 | [29] | X | A | A | ||||
| 15-deoxy J2 ip | 315.2/203.2 | C20H27O3 | 335.2/215.2 | 14–20 | 20 | [29] | X | D | A | X | A | ||
| 5-deoxy J2 ip | 315.2/217.2 | C20H27O3 | 335.2/230.2 | 14–20 | 20 | [29] | X | A | A | ||||
| Deoxy J2 ip | 315.2/271.2 | C20H27O3 | 335.2/290.2 | 14–20 | 20 | [29] | X | C | A | A | |||
| LA4 (LTA4) | 317.2/163.2 | C20H29O3 | 337.2/174.2 | 22.8 | 20 | [26] | X | D | A | ||||
| 5 HETE | 319.2/115.2 | C20H31O3 | 339.2/120.2 | 22.2 | 20 | [26] | X | B | A | A | A | ||
| 9 HETE | 319.2/151.2 | C20H31O3 | 339.2/160.2 | 22 | 20 | [26] | X | A | |||||
| 8 HETE | 319.2/155.2 | C20H31O3 | 339.2/163.2 | 21.8 | 20 | [26] | X | A | A | ||||
| 8,9 EET | 319.2/163.2 | C20H31O3 | 339.2/173.2 | 23.8 | 20 | [26] | X | A | X | ||||
| 11,12 EET | 319.2/167.2 | C20H31O3 | 339.2/177.2 | 23.8 | 20 | [26] | X | A | A | X | |||
| 11 HETE | 319.2/167.2 | C20H31O3 | 339.2/177.2 | 21.7 | 20 | [26] | X | B | A | AB | A | A | |
| 12 HETE | 319.2/179.2 | C20H31O3 | 339.2/190.2 | 21.9 | 20 | [26] | X | A | A | A | X | ||
| 5,6 EET | 319.2/191.2 | C20H31O3 | 339.2/205.2 | 24 | 20 | [26] | X | X | |||||
| 15 HETE | 319.2/219.2 | C20H31O3 | 339.2/233.2 | 21.2 | 20 | [26] | X | B | B | AB | A | ||
| 14,15 EET | 319.2/219.2 | C20H31O3 | 339.2/233.2 | 23.1 | 20 | [26, 30] | X | ||||||
| B2 ip (PGB2) | 333.2/175.2 | C20H29O4 | 353.2/184.2 | 18–20 | 30 | [26] | X | BCDE | X | A | |||
| J2 ip (PGJ2) | 333.2/189.2 | C20H29O4 | 353.2/202.2 | 18.2 | 20 | [26, 27] | X | D | A | A | A | ||
| A2 ip (PGA2) | 333.2/271.2 | C20H29O4 | 353.2/290.2 | 14–20 | 20 | [26, 27] | X | C | ACDE | A | X | A | |
| LB4 (LTB4) | 335.2/195.2 | C20H31O4 | 355.2/209.2 | 18.5 | 20 | [26] | X | A | A | ||||
| 15 k E2 ip (15 keto PGE2) | 349.2/113.2 | C20H30O5 | 369.2/120.2 | 14.5 | 40 | [27] | X | C | A | A | A | X | A |
| U17 | 349.2/129.2 | C20H30O5 | 369.5/135.2 | 15.6 | 20 | This work | X | C | ACDE | A | X | A | |
| K2 ip (PGK2) | 349.2/205.2 | C20H30O5 | 369.2/219.2 | 15.2 | 30 | [30] | X | C | A | X | A | ||
| D2/E2/H2 ip (PGD2/PGE2/PGH2) | 351.2/189.2 | C20H31O5 | 371.2/202.2 | 13.7 | 20 | [26, 31] | X | C | A | A | X | A | |
| I2 ip (PGI2) | 351.2/215.2 | C20H31O5 | 371.2/230.2 | 15.1 | 30 | [26] | X | A | A | ||||
| 15 k F2 ip (15 keto PGF2α) | 351.2/219.2 | C20H31O5 | 371.2/234.2 | 14–18 | 20 | [30] | X | C | A | ||||
| D2 ip (PGD2) | 351.2/233.2 | C20H31O5 | 371.2/247.2 | 13.8 | 20 | [26] | X | C | A | A | |||
| D2/E2 ip (PGD2/PGE2) | 351.2/271.2 | C20H31O5 | 371.2/290.2 | 13.7 | 20 | [26, 27, 30] | X | C | A | A | X | ||
| E2/15 k F2 ip (15 keto PGF2α) | 351.2/315.2 | C20H31O5 | 371.2/335.2 | 13.7–16 | 20 | [27] | X | C | A | AB | A | X | A |
| diHk E2 ip (13,14-dihydro, 15-keto PGE2) | 351.2/333.2 | C20H31O5 | 371.2/353.2 | 13.7 | 20 | [27] | X | D | A | X | A | ||
| 5 F2 ip | 353.2/115.2 | C20H33O5 | 373.2/120.2 | 16 | 30 | [32] | X | C | A | AB | AB | A | |
| 8 F2 ip | 353.2/127.2 | C20H33O5 | 373.2/134.2 | 15.1 | 30 | [32] | X | D | A | AB | AB | A | |
| U11 | 353.2/144.2 | C20H33O5 | 373.2/151.8 | 17.2 | 20 | This work | X | A | A | A | |||
| 12 F2 ip | 353.2/151.2 | C20H33O5 | 373.2/162.2 | 14.8 | 30 | [32] | X | A | A | A | |||
| U1 | 353.2/167.2 | C20H33O5 | 373.2/177.2 | 14.7 | 30 | This work | X | C | A | AB | AB | ||
| 15 F2 ip (PGF2α) | 353.2/193.2 | C20H33O5 | 373.2/205.2 | 15.4 | 20 | [26, 27, 30] | X | D | A | B | A | X | A |
| G2 ip (PGG2) | 367.2/173.2 | C20H31O6 | 387.2/185.2 | 14.8 | 20 | This work | X | C | A | A | |||
| U16 | 367.2/245.2 | C20H31O6 | 387.2/262.2 | 16.1 | 30 | This work | X | C | A | AB | B | X | A |
| TB2 (TXB2) | 369.2/169.2 | C20H33O6 | 387.2/178.2 | 12–16 | 20 | [30] | X | C | ACDE | X | A | ||
| Isofurans | 369.2/351.2 | C20H33O6 | 389.2/371.2 | 11–16 | 20 | [28] | X | C | ACDE | X | A | ||
| U2 | 385.2/149.2 | C20H33O7 | 405.2/159.2 | 13.9 | 20 | This work | X | C | A | AC | A | A | |
| U3 | 385.2/245.2 | C20H33O7 | 405.2/262.2 | 15.4 | 30 | This work | X | C | A | A | A | ||
| U15 | 385.2/331.2 | C20H33O7 | 405.2/351.2 | 16.5 | 20 | This work | X | D | A | AB | X | A | |
| U5 | 395.2/115.2 | C20H27O8 | 415.2/120.2 | 15.3 | 20 | This work | X | C | A | AC | A | ||
| U4a | 395.2/269.2 | C20H27O8 | 415.2/288.2 | 15.8 | 20 | This work | X | C | A | A | A | ||
| U4b | 395.2/269.2 | C20H27O8 | 415.2/288.2 | 18 | 20 | This work | X | A | |||||
| U14 | 399.2/145.2 | C20H31O8 | 419.2/151.2 | 8–14 | 20 | This work | X | C | A | X | A | ||
| U13 | 399.2/191.2 | C20H31O8 | 419.2/203.2 | 10.5 | 20 | This work | X | C | ACDE | AB | X | A | |
| U6 | 399.2/245.2 | C20H31O8 | 419.2/262.2 | 16.2 | 40 | This work | X | C | B | AB | AB | A | |
| U7 | 403.2/289.2 | C20H35O8 | 423.2/307.2 | 16 | 30 | This work | X | A | A | A | |||
| U9 | 413.2/129.2 | C20H29O9 | 433.2/135.2 | 15.6 | 20 | This work | X | C | A | A | A | ||
| U8 | 413.2/149.2 | C20H29O9 | 433.2/159.2 | 11.8 | 40 | This work | X | C | A | C | B | A | |
| U12 | 419.2/237.2 | C20H35O9 | 439.2/253.2 | 2.2 | 20 | This work | X | A | A | A | |||
| U10 | 431.2/109.2 | C20H31O10 | 451.2/116.2 | 14.8 | 40 | This work | X | C | A | C | A | ||
Notes:
The abbreviations used for each molecular species throughout this article are listed, and all should be interpreted as non-stereospecific. For transitions that correspond to known biologically active stereospecific compound, the common abbreviation for that species is given in parentheses.
Parent ion chemical formula.
Elution time (min).
Collision energy (volts).
Cross-reference to locate which species appear in various figures; an X is entered for single-panel figures, whereas A–E designate individual panels within a figure.
Generation of transition sets for MRM methods and internal standards
An initial MRM-LC/MSMS method was created by accumulating a list of known transitions from the literature [26–33]. If the name assigned to a transition was inclusive of all possible stereoisomers, it was listed as such in Table 1. If the literature name corresponded to a specific stereoisomer (e.g. it was enzymatically-derived, or it had biological activity), then a stereo-inclusive abbreviation was assigned, and the name of the stereoisomer is listed in parentheses as an “example species”. To this list, transitions were added when oxidation products were identified in 2 mM solutions of ARA in 10 mM HEPES pH 7.4 that had been incubated at room temperature for 1 to 8 days. The incubations were performed in vials containing 100 μl volumes in 350 μl inserts made from reduced surface activity glass (MicroSolv, Eatontown, NJ). They were tightly capped, but contained 250 μl of air above the solution. This incubation is referenced below as “air oxidation”. Each mixture was separated by HPLC and Q1 scans from 300 to 450 were recorded every 150 milliseconds. Each parent ion observed by Q1 scan was subsequently analyzed by product ion scanning to identify product ions, determine optimal collision energies, and verify elution times. Species that have no corresponding transition in the literature are designated “U” species in Table 1, and total ioncurrent chromatograms (TICs) for these species are provided as supporting information (figure S1). Several hydroxy-eicosanoid (HETE) and epoxy-eicosanoid (EET) species have identical transitions but different elution times; the earlier-eluting species was designated the HETE [34, 35].
U-13C-ARA was incubated in the same way as ordinary ARA to create a corresponding array of U-13C-labeled oxidation products, and the elution profile of this material was shown to be identical to that of the 12C products. A 600 μl batch of “internal standard mixture” (ISM) was prepared by mixing 200 μl of ARA subjected to air oxidation for 2 days, 300 μl subjected for 4 days, and 100 μl subjected for 8 days. Mixing batches oxidized for different intervals in this way provided for a mixture that contained molecular species that appeared early and then degraded into other species at longer times, as well as more complex species that only appeared with longer oxidation times. The corresponding set of transitions for MRM-LC/MSMS was created by adding 20 m/z to the parent ion m/z and an appropriate value to the fragment ion m/z (table 1). Thus, Table 1 constitutes a list of abbreviations for the molecular species examined in this work, which are defined by (a) parent ion m/z ratio, (b) fragment ion m/z ratio, (c) chemical formula, (d) retention time, and (e) co-eluting parent/fragment ions in the ISM bearing the expected extra mass of U-13C-labels. Common abbreviations for the stereospecific form of a molecular species are listed in parentheses after the abbreviation, and further information about such forms may be obtained from the listed reference.
Losses during saponification
ARA (10 mm) was subjected to 7 days of air oxidation and a 10 μl aliquot of this solution and a calibrated aliquot of d8-ARA was added to 90 μl 1:1 methanol:water and analyzed by MRM-LC/MSMS with the U-12C transition set. A second 10 μl aliquot of this solution was saponified in 85% methanol and 0.5 M NaOH at 37°C for 40 minutes. The sample was cooled to room temperature, acidified using HCl, dried under argon, and reconstituted in 100 μl 1:1 methanol:water with a calibrated aliquot of d8-ARA. Analysis was performed by MRM-LC/MSMS with the U-12C transition set and the fold change of each oxidation product between saponified and unsaponified sample was determined.
Mouse brain tissue processing
B6SJLF1/J mice were obtained from the Jackson Laboratory (Bar Harbor, ME), aged for 16–18 months, and sacrificed by CO2 asphyxiation. All experimental procedures and animal care were in compliance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals. The brain tissue was harvested, immediately frozen on dry ice, and subjected to modified Bligh-Dyer extraction (BDx) as described previously [7]. Tissue samples (~20 mg) were homogenized with a tip sonicator (F60 Sonic Dismembrator, Fisher Scientific, Pittsburgh, PA) for 60 sec in an autosampler vial containing 760 μl BDx monophase (400 μl methanol, 200 μl dichloromethane, and 160 μl of 5 mM ammonium chloride, pH 5.6), 10 μl of 100 μM BHT, and a calibrated aliquot of the ISM. A single batch of ISM was used for all of the results reported herein. Following sonication, 200 μl dichloromethane and 160 μl water were added to break the monophase, the vial was briefly vortexed, and the resulting phases were clarified by 2 minutes of low speed centrifugation. The lower (mostly dichloromethane) and upper (methanol and water) phases were separated and evaporated under nitrogen. Residue from upper phase (BDx-U) was redissolved in 100 μl of 1:1 methanol:water. Residue from the lower phase (BDx-L) was redissolved in 750 μl MeOH and 250 μl of 2M NaOH. A second calibrated aliquot of the ISM was added to the BDx-L since nearly all components of the original ISM partitioned into the BDx-U and the sample was saponified at 37°C for 40 minutes. Following saponification the sample was acidified with 150 μl of 0.5M HCl, evaporated under nitrogen, and redissolved in 100 μl 1:1 methanol:water.
Human AD and control brain tissues
Frontal cortex from 6 human patients with documented severe AD histopathology, and 3 normal human brains with the characteristics listed in table 2, were obtained from the Center for Neurodegenerative Disease Research at the University of Pennsylvania. Tissue processing was performed as described for mouse brain tissues.
Table 2.
Human Brain Samples
| Sex | Age | Pathology | PMI (hours) | ApoE isoform |
|---|---|---|---|---|
| M | 67 | AD | 10 | E4/E4 |
| M | 74 | AD | 18 | E4/E4 |
| M | 86 | AD | 17 | E3/E3 |
| F | 80 | AD | 6 | E3/E4 |
| F | 80 | AD | 4.5 | E4/E4 |
| F | 81 | AD | 4 | E4/E4 |
| M | 83 | Healthy | 6 | E3/E3 |
| M | 72 | Healthy | 13.5 | E3/E4 |
| M | 68 | Healthy | 14 | NA |
Statistical analysis
Statistical significance was determined using Student’s two-tailed t-test with unequal variances. The Benjamini-Hochberg correction was applied to the data with a false discovery rate of 0.2 when indicated. All error bars represent standard errors of the mean.
Results
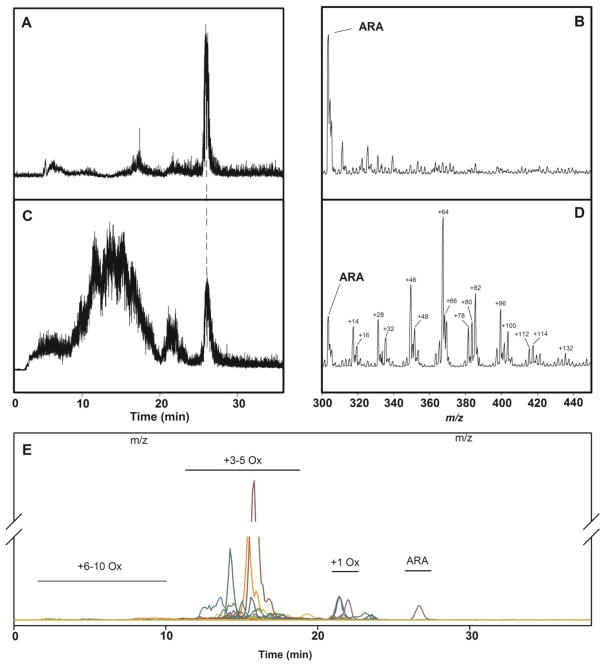
Neat ARA eluted in a sharp peak at 23 min (figure 1A) with an m/z of 303.2 in a negative mode Q1 scan (figure 1B). The same material after air oxidation for 7 days yielded several broad peaks eluting between 2 and 28 min (figure 1C). ARA with an m/z = 303.2 still eluted at 23 min, but its signal was relatively small. Larger peaks 48 and 64 m/z higher than 303.2 suggest that 3 or 4 oxygen atoms had been added to ARA (figure 1D), while other peaks suggest that in addition to oxygen additions, rings have formed (−2) and/or waters have been lost (−18).
Figure 1.
Total ion current chromatograms (TIC) and negative-mode Q1 mass spectra from m/z 300–450 and 0–30 min of ARA before and after air oxidation. A. TIC of ARA before air oxidation. B. Q1 mass spectrum of ARA before air oxidation. C. TIC of ARA after 6 days of air oxidation. D. Q1 mass spectrum of ARA after 6 days of air oxidation. E. Extracted ion chromatograms of the 12C MRM transition set.
A search of the literature for MRM transitions assigned to known ARA oxidation products, and product ion scans of all molecular species observed in the Q1 scan, yielded a set of 55 transitions for ARA and related monoisotopic (i.e. U-12C) species (table 1). Within this U-12C transition set, 18 did not correspond to any known molecular species and were designated unidentified or “U” species (table 1). Given that the starting material was neat ARA, and there were no reactants containing nitrogen, sulfur, halogens, or light-metal cations present during oxidation, a chemical formula could be assigned unambiguously to each parent/product ion pair, including “U” species. However, each molecular species may have multiple stereoisomers, and these are not resolved by the methods employed herein. Therefore, each of the molecular species in table 1 has been assigned a non-stereospecific designation. When the MRM transition corresponds to a biologically active stereoisomer, its designation is included in parentheses.
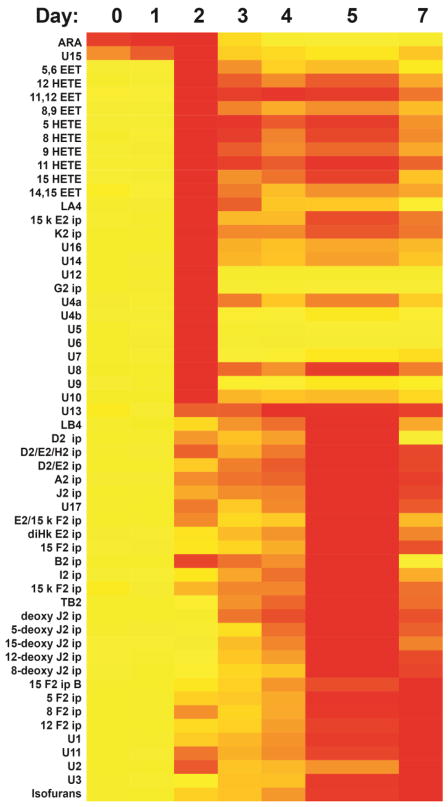
When a pure sample of ARA was examined with an MRM-LC/MSMS method based on this U-12C transition set, there were negligible signals from molecular species other than ARA, and narrow peaks for each transition after 7 days of air oxidation (figure 1E). Samples examined after 1, 3, 5, and 7 days of air oxidation revealed the expected evolution of oxidation products, with signals from molecular species arising from single oxygen additions appearing early and then fading, while those arising from multiple oxygen additions appearing later and increasing (figure 2 and table S1). The evolution of signals from these oxidation products was effectively halted by the addition of BHT (final concentration 10 μM) or by freezing at −80°C (figures S2 and table S2).
Figure 2.
Heat map of AA and its oxidation products over 7 days of air oxidation. Red blocks indicate the day on which the maximum MRM signal was recorded, with a gradient to yellow representing no signal. An aliquot of d8-ARA was added prior to each analysis and used to normalize signals from the various MRM transitions. Numerical results and statistical significance estimates are provided as supplementary information.
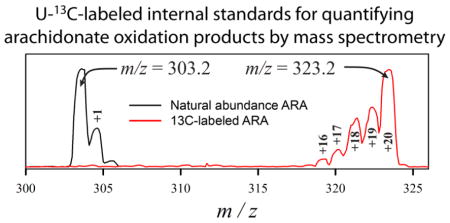
A U-13C transition set, analogous to the U-12C set, was created by adding 20 m/z to the parent ion m/z of each transition in the U-12C set, and a number corresponding to the expected number of carbon atoms to each product ion m/z. When air-oxidized U-13C-ARA was analyzed with this U-13C transition set, it yielded a TIC essentially identical to the TIC obtained when air-oxidized ARA was analyzed with the U-12C transition set. Most importantly, air-oxidized U-13C-ARA yielded no measurable signals when analyzed with the U-12C transition set, indicating that none of the U-13C-labeled molecular species contributed to U-12C oxidation product signals being measured. These results indicate that the 54 oxidation products derived from air oxidation of U-13C-ARA could be identified by their characteristic retention time and MRM transitions, and that they might be useful as an internal standard mixture (ISM) for the quantitation of oxidation products by mass spectrometry in brain tissue (where ARA is concentrated). To examine their possible utility as internal standards, several aspects of oxidation product analysis, extraction, and isolation were examined.
First, the ISM was examined by MRM-LC/MSMS with the U-12C transition set. There were small but measureable signals for many of the transitions, indicating that adding the ISM to a sample would add small amounts of U-12C-ARA oxidation products to the background of any quantitative analysis. Moreover, the ISM was a mixture of products from 3 different oxidation times, so that different correction factors would be required for each molecular species. Hence, the ratio of signals from an U-12C molecular species to that of the corresponding U-13C molecular species, rx,s, was calculated according to
| (1) |
where Ix is the area of the MRM peak for chemical species x in the ISM, Tx,s is the area of the MRM peak for chemical species x in tissue sample s, and Ms is the mass of tissue sample s. In each case, the superscript preceding the symbol indicates whether the measurement was made with the 12C or the 13C transition set. The ratios were normalized by tissue sample mass because the amount of ISM added to each sample was constant in the studies described below.
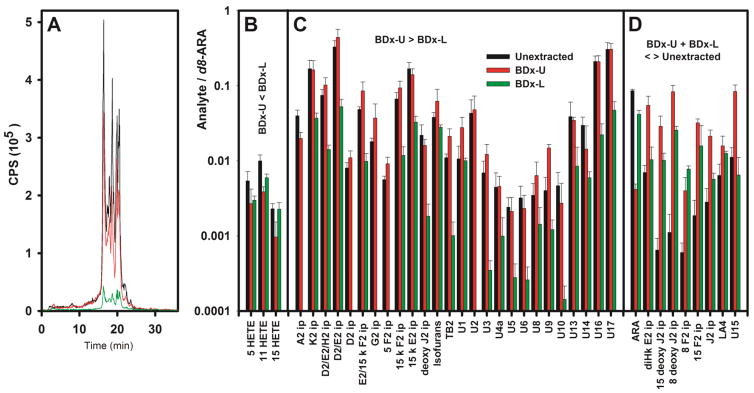
Second, the partitioning of U-13C-ARA oxidation products in a Bligh-Dyer extract (BDx) was examined. 100 μl aliquots of 2 mM U-13C-ARA solutions in HEPES buffer were subjected to air oxidation for 7 days, followed by the addition of a calibrated aliquot of d8-ARA. A 10 μl aliquot of this solution was analyzed by MRM-LC/MSMS with the U-13C transition set without further processing (the “unextracted” sample). A second 10 μl aliquot of this solution was added to a ~20 mg of mouse cortical brain tissue. The tissue was then homogenized in the presence of BHT and subjected to Bligh-Dyer extraction. The monophase was broken into upper (BDx-U) and lower (BDx-L) phases as described above, and separated. Both phases were then analyzed by MRM-LC/MSMS with the U-13C transition set. The unextracted homogenate of mouse brain tissue did not contain a detectable amount of any U-13C-ARA oxidation products (data not shown), so the use of brain tissue in this experiment was solely for the purpose of assessing the recovery U-13C-ARA oxidation products from this type of sample (i.e. U-12C oxidation products in the homogenate were not being quantified in this experiment).
Among the 37 U-13C-ARA oxidation products recovered from the BDx, 3 partitioned primarily into the BDx-L including 3 HETEs (figure 3B and table S3). Most of the other oxidation products (26 of 37) partitioned into the BDx-U, and the amount of each oxidation product recovered from the BDx-U and BDx-L combined closely matched the amount recovered from the unextracted sample (figure 3C).
Figure 3.
Recovery of U-13C-ARA oxidation products added to a brain tissue homogenate. An aliquot of the U-13C-ARA after a 7 day air oxidation was analyzed immediately (the “unextracted” sample). A second aliquot was added to a homogenized 20 mg sample of mouse brain that was subjected to BDx followed by separation of the BDx-U and BDx-L, drying under N2, and dissolving in 1:1 methanol:water. Neither the BDx-U nor the BDx-L were saponified, and signals from brain that did not have U-13C-ARA oxidation products did not yield measurable signals. Therefore, these analyses reveal the extent to which ARA and its oxidation products that are known to be present in brain tissue can be recovered after BDx. A. TIC for the U-13C-labeled ARA oxidation products recorded from the unextracted tissue homogenate (black), the BDx-U (red) of the homogenate, and the BDx-L (green) of the homogenate. For panels B–D, a uniform aliquot of d8-ARA was added prior to each analysis as an internal standard, and the peak area for each species was divided by the d8-ARA peak area to obtain the relative concentrations of that species in the unextracted, BDx-U, and BDx-L. Results from the BDx-U and BDx-L were added together, and compared to the corresponding result from the unextracted sample. Statistical significance was evaluated using the Benjamini-Hochberg procedure with a false discovery rate of 20%. B. Species for which BDx-U + BDx-L ≈ unextracted, but BDx-U ≥ BDx-L. C. Species for which BDx-U + BDx-L ≈ unextracted, but BDx-U < BDx-L. D. Species for which the sum of the results from BDx-U and BDx-L does not equal the results from unextracted. Note the use of a vertical log scale so that widely disparate results may be represented on the same graph. Numerical and statistical results are provided as supplementary information.
In 9 cases (ARA and 8 oxidation products), however, there was a significant discrepancy between the total amount of a molecular species recovered from the BDx-U and BDx-L, and the amount present in the original unextracted solution (figure 3D). In the case of ARA, 64% of material in the unextracted sample was recovered in the BDx-L, and 11% was recovered from the BDx-U, suggesting a loss of 25%. In 8 cases, the oxidation product partitioned primarily into the BDx-U, and the amount recovered from the BDx-U and BDx-L exceeded the amount in the unextracted sample. Altogether, the loss of ARA and the increase in some oxidation products suggest that some oxidative degradation still occurs during BD extraction despite the presence of BHT throughout.
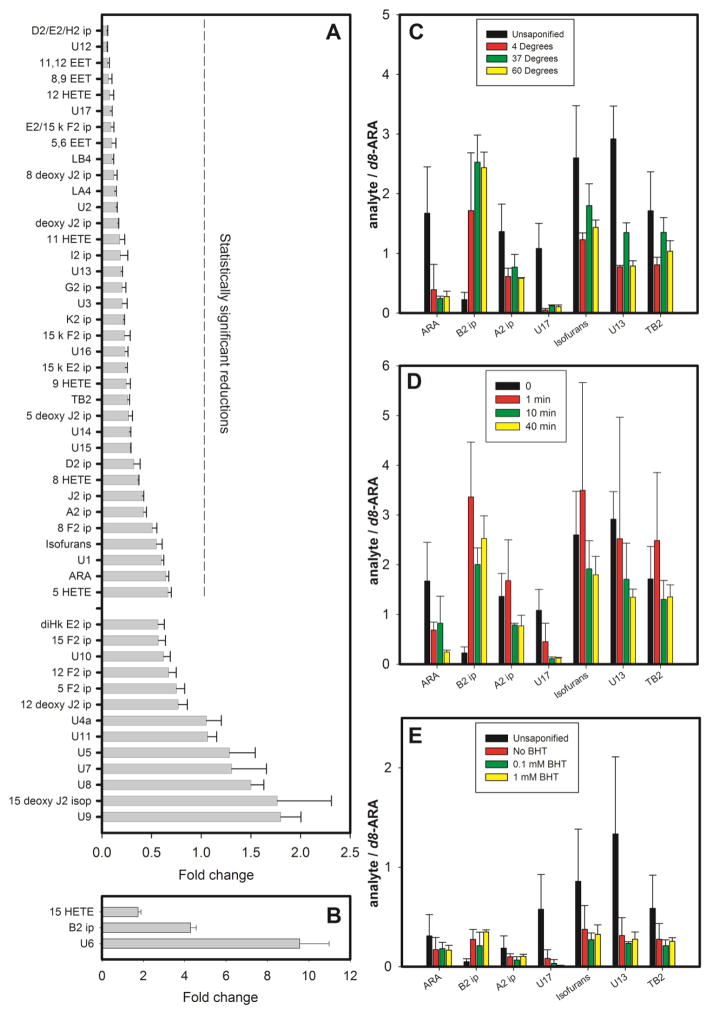
Third, the stability of ARA oxidation products during mild alkaline saponification was examined. To enhance the detection of oxidation products in these experiments, the concentration of ARA subjected to 7 days air oxidation was increased from 2 mM to 10 mM, which increased the number of molecular species detected to 51. Analysis was performed by MRM-LC/MSMS with the U-12C transition set and the fold change of each oxidation product between saponified and unsaponified sample was determined. Significant losses were observed for ARA and 35 of its oxidation products, while 13 products exhibited statistically insignificant or minimal losses (figure 4A and table S4). Selected oxidation products were examined under alkaline conditions, in the presence of BHT, and at various temperatures, but losses were still observed (figure 4D,E). When they were subjected to 37°C for 40 min in non-alkaline conditions, however, the losses were negligible (figure 4C). Therefore, the losses during saponification were due primarily to an exposure to alkaline pH. Acid hydrolysis yielded much poorer recoveries overall (data not shown). The concentration of 3 oxidation products (15-HETE, B2 ip, U6) increased during saponification (figure 4B).
Figure 4.
Recovery of ARA and its oxidation products after alkaline saponification. A 10 mM solution of ARA was subjected to air oxidation for 7 days, and 51 oxidation products were detected. None of the 14 additional products detected in this more concentrated preparation were detected in tissue samples (where 37 products were identified), but the higher concentrations and larger number of products facilitated a broader characterization of the effects of saponification. The mixture of oxidation products was divided into two portions. One portion was subjected to saponification conditions (0.5M NaOH at 37°C for 40 min) and then neutralized with HCl, while the second was not. Both portions were dried under N2, and reconstituted in 1:1 methanol:water. Identical calibrated aliquots of d8-ARA were added to both portions as internal standards. For panels A and B, the peak area for each analyte in each portion was normalized by the peak area of its internal standard, and the ratio of normalized peak areas in the unsaponified and saponified portions was calculated to determine the “fold change” during saponification. Statistical analysis was done using the Benjamini-Hochberg procedure with a false discovery rate of 20%. A. The fold change for oxidation products that decreased (fold change < 1) or that did not significantly change during saponification. B. The fold change for 3 oxidation products that increased significantly during saponification (fold change > 1). For panels C–E, the vertical axis represents normalized peak areas, i.e. the peak area for each analyte divided by the peak area of the internal standard. C. Results for selected oxidation products before and after saponification at different temperatures. D. Results for selected oxidation products before and after saponification at 37 °C for different lengths of time. E. Results for selected oxidation products before and after saponification at 37 °C for 30 min in the presence of different BHT concentrations. Numerical results and are provided as supplementary information.
Fourth, the efficiency of extracting these compounds from 85% methanol in water with isooctane (isoO) was examined. Aliquots (100 μl) of 2 mM ARA in HEPES buffer were subjected to air oxidation for 7 days. The parent solution was acidified with HCl and extracted with 3 equal volumes of isoO. ARA and U9 partitioned almost entirely into the isoO phase, while U14 remained entirely in the methanol-water phase. 42 oxidation products partitioned to varying extents into both phases (figure S3A and table S5). The extent to which they partitioned into isoO roughly correlated with their calculated partition coefficients (figure S3B).
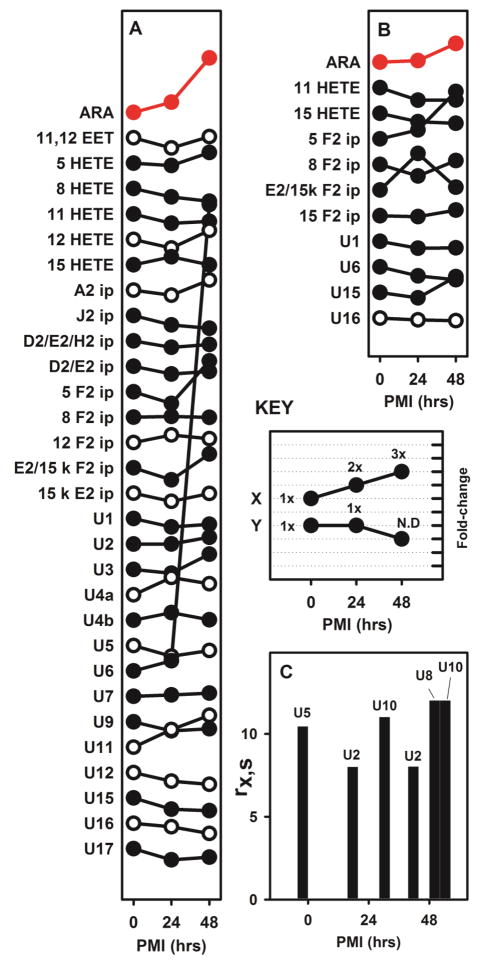
Finally, the effects of PMI on the recovery of various ARA oxidation products from brain tissue were examined by sacrificing wild-type mice and storing the intact carcasses at 4°C for 0, 24, and 48 hours. After those intervals, the brains were harvested, flash frozen, homogenized and subjected to BDx. The BDx-L from each brain was saponified and acidified, then both the BDx-U and the acidified BDx-L were dried and redissolved in 1:1 methanol:water. An aliquot of the ISM was added to the tissue prior to homogenization. Because most components of the ISM partitioned into the BDx-U phase, a second aliquot of the ISM was added to the BDx-L prior to saponification. A hybrid MRM-LC/MSMS method that included both the U-12C and U-13C transition sets was created to analyze these samples.
ARA and 29 of its oxidation products were detected in the unsaponified BDx-U (figure 5A and table S6). Among these, 4 decreased after PMIs of 24h and 48h (two D2/E2 ip, J2 ip and 8-HETE), 2 decreased at 24h but returned to their original relative concentration after a 48h PMI (U1 and U5), and 1 increased after 48h (U6). ARA increased after PMIs of 24h and 48h.
Figure 5.
The changes in concentration of ARA and its oxidation products after 24h and 48h PMIs. The results from cortex and cerebellum were not significantly different, and were therefore combined. The value of rx,s (equation 1) was determined for ARA and each of its oxidation products, at each PMI interval, and divided by the value of rx,s at PMI = 0. The “key” panel illustrates results for hypothetical species ‘X’ and ‘Y’. Both initial values at PMI = 0 indicate a 1x change, but they are displaced vertically by 2 units. For species ‘X’, the symbols at 24 and 48 hours represent 2x, and 3x the initial concentration. For species ‘Y’, the symbols at 24 and 48 hours represent a concentration that is unchanged at 24 hours (i.e. 1x) and undetectable at 48 h (i.e. ‘0x’). The data are presented in this manner to show that there are no consistent trends across the majority of species, or even among closely related species; some species increase with PMI and others decrease, while the large variance within this data obscures significant differences. Symbols in red (for ARA) indicate species for which values at 24 and 48 hours are statistically significant changes from PMI = 0 after Benjamini-Hochberg analysis. Open vs closed circles alternate along the vertical axis for clarity, and have no other significance.
A. ARA and its oxidation products in the unsaponified BDx-U. B. ARA and its oxidation products in the saponified BDx-L. C. ARA oxidation products in the BDx-L that were not recovered at all 3 PMIs. Results in panel C could not be divided by PMI = 0 and are therefore presented as values of rx,s. Numerical results and statistical analyses are provided as supplementary information.
ARA and 10 oxidation products were detected in the saponified BDx-L (figure 5B). ARA and U16 increased after a 48h PMI. There were 4 additional oxidation products detected inconsistently (figure 5C). U5 was only detected at day 0, U8 was only detected after 48H, while U2 and U10 were detected only after 24h and 48h PMI.
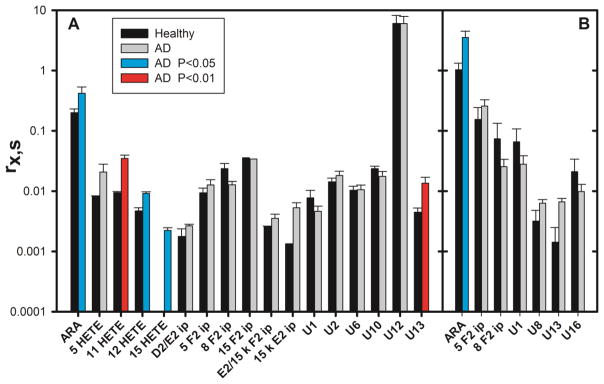
With the foregoing information about the recoverability of ARA oxidation products, brain tissue from the frontal cortex of 6 human patients with Alzheimer’s disease (AD) and 3 healthy controls was examined. The tissue was subjected to BDx, the phases were separated, a calibrated aliquot of the ISM was added to each phase, and the BDx-L was saponified. ARA and 16 oxidation products were detected in the BDx-U (figure 6A and table S7). Of the 16 oxidation products, 14 had been previously detected in mouse brain tissues; U10 and U13 were only detected in human samples. The amount of ARA recovered from the BDx-U was significantly greater in AD brain, as were the amounts of 11-HETE, 12-HETE, 15-HETE, and U13. None of these 4 oxidation products increased in vitro during BDx, and amounts of the 3 HETEs did not vary with PMI in mice. ARA and 6 oxidation products were also detected in the saponified BDx-L of human brain samples (figure 6B and table S7). Again, the amount of ARA recovered was significantly greater in AD brain, but there were no differences between healthy and AD brain among the 6 oxidation products.
Figure 6.
ARA and its oxidation products in normal and AD human brain. Tissues from the frontal cortex of 6 AD patients and 3 control healthy brains were homogenized in BDx monophase, 10 μl of 100 μM BHT and 10 μl of the ISM were added to the homogenate, the monophase was broken, the BDx-U and BDx-L were separated, and an additional 10 μl of the ISM was added to the BDx-L prior to saponification. A. Results from the BDx-U. The absence of a black bar for 15 HETE in healthy controls indicates none detected. B. Results from the saponified BDx-L. Note the use of a vertical log scale. Results from AD brain that are significantly different from normal brain are indicated by cyan bars (P > 0.05) or a red bar (P > 0.01). Statistical analysis was done using the Benjamini-Hochberg procedure with a false discovery rate of 20%. Numerical results and are provided as supplementary information.
Discussion
The key result reported above is that the concentrations of several oxidative ARA degradation products are higher in AD brain compared to healthy brain, but the products with elevated concentrations were chiefly HETEs, not the more complex oxidation products more typically measured when characterizing oxidative stress. HETEs are relatively simple oxidation products because they are created through a single oxidation event. In contrast, and with only one exception (U13), the concentrations of more complex oxidation products (e.g. isoprostanes) were similar in AD and healthy brain.
Tissue concentrations are determined by the rates at which compounds are produced, less the rates at which they are cleared or converted into something else. In the case of ARA oxidation products, the same chemical process that produces a HETE is capable of converting a HETE into more complex oxidation products. It follows that an increase in the rate of that process will result in a much greater increase in the steady-state concentrations of a few compounds formed via single oxidation events (e.g. HETEs), rather than any one of a larger number of compounds formed via multiple oxidation events (e.g. isoprostanes). Thus, the finding that only HETE concentrations are elevated in AD is consistent with increased production of ARA oxidation products by non-enzymatic chemical reactions. There may indeed be increases in more complex products, but demonstrating those increases may require enantiomer-specific methods.
When many comparisons are made between two large datasets in the manner reported above, some of the apparent differences are likely to be falsely identified at a rate that is related to the stringency of the statistical tests that have been applied to determine significance. For that reason, various statistical procedures (e.g. Benjamini-Hochberg) and corrections (e.g. Bonferroni) are applied to gauge the false discovery rate. However, these approaches generally assume that the underlying data is uncorrelated. There are clear chemical relationships among the oxidation products described above, suggesting that correlations should be expected. Finding that all 4 of the HETEs assayed in this work exhibited higher concentrations in AD brain is an example of the kind of correlation expected because all 4 HETEs can be produced by the same chemical mechanism: 11-HETE was elevated with P < 0.01, 12-HETE and 15-HETE were elevated with P < 0.05, and 5-HETE was elevated but with P > 0.05. On the other hand, the elevated concentration of compound U13 in AD brain (figure 6) is suspect because it is the only complex oxidative degradation product among many that is elevated in AD brain. As applied in this work, the Benjamini-Hochberg procedure is consistent with this appraisal, suggesting that one of the “significant” increases has been falsely “discovered”.
Stereospecific forms of 5-, 11-, 12-, and 15-HETE can be produced enzymatically through the activity of their corresponding lipoxygenases [36]. At least some of these enzymes have increased activity in TG mice, and they may be involved in mediating tau pathology, synaptic integrity, β secretase expression, and mitochondrial damage [37–40]. Stereospecific HETEs are known to accumulate in the CSF of subjects with mild cognitive impairment and AD [41], as well as in subarachnoid hemorrhage, multiple sclerosis and traumatic brain injuries [42, 43]. The foregoing analyses do not distinguish between enzymatic and chemical products, so it is possible that increased HETE concentrations are due to an increase in the enzymatic oxidation of ARA during AD as previously reported [44]. However, HETEs tend to be minor products of these enzymatic processes, and there were no increases observed in their major products. Therefore, it seems most likely that the differences in HETE concentrations between healthy and AD brain are due to an increase in the kind of chemical oxidation that can produce all 4 HETEs.
It follows that elevated HETE concentrations in AD brain may be due to higher concentrations of their chemical precursor, namely ARA. Alternatively, elevated ARA concentrations in AD brain may be a metabolic response to increased oxidative consumption of ARA, or to the presence of oxidation products. The concentration of ARA was higher in human AD brain tissue, compared to healthy controls, in contrast to earlier reports where either a decrease or no differences were found [52–54]. The discrepancy may be due to differences in PMI, since some earlier reports in which no difference was found included brain tissue with PMIs greater than 100 hours [53]. ARA concentrations did increase with PMI in mouse brain (figure 5), consistent with findings by others in rats [55]. However, the PMIs for the human brain samples were relatively short and did not differ significantly between AD and healthy brain (table 2). The finding that ARA concentrations were increased in AD brain is consistent with results from a PET scan study of living human patients in which the incorporation of 1-11C-ARA was greater in AD brain tissue compared to controls [56]. In addition, higher concentrations of ARA have been reported in a mouse model of AD, where PMI is essentially zero [57], and in the erythrocytes of persons with preclinical AD [58]. Aside from ARA at 24 hours post mortem, and some trends that did not reach statistical significance, the concentrations of ARA and its oxidation products in mouse and human brain tissues did not vary with PMI.
The BD and Folch procedures are common lipid extraction techniques [45], but little attention has been given to the partitioning behavior of PUFA oxidation products during these procedures. Their relatively high polarity causes many PUFA oxidation products to partition into the polar, upper phase of the BDx (figure 3C). Thus, efforts to measure their concentration in tissues must consider all phases of an extract. Saponification of the BDx-L, where esterified PUFA chains tend to partition, is complicated by losses due to chemical alteration of the oxidation products. The results above show that the losses are due primarily to the alkaline conditions (figure 4). Shorter times, lower temperatures, and the addition of BHT did not meaningfully alleviate these losses, and acid saponification was inefficient. Some have used phospholipase-A2 to avoid chemical damage while releasing esterified PUFA chains [29, 46], but that approach introduces its own large array of uncertainties about enzyme activity and substrate access, and it would not liberate PUFA chains from triglycerides, cardiolipins, cholesterol esters, or the sn1 position of PE, PA, and PS phospholipids [47].
The mass spectrometric methods employed in this work could detect ARA and 54 of its oxidation products in an internal standard mixture, but only 18 of those products were detected in human brain tissue (figure 6). Some of the missing oxidation products may have been destroyed during saponification (figure 4), but losses during saponification do not explain an inability to recover compounds that resist damage during saponification (e.g. iP B2, U5, U7, and 15-deoxy-iP J2). Moreover, some compounds were readily detected in mouse brain but not in human brain, and vice versa. These results suggest that pathways for their production and/or clearance may exhibit specificity for some oxidative ARA degradation products. This possibility is consistent with data showing that AD brain tissue has higher concentrations of some, but not all iPs [48].
The detection of a molecular species in these investigations does not necessarily imply its presence in brain tissue. For example, simple acid- or base-catalyzed dehydrations in aqueous media may convert PGD2 into products like PGJ2 [49], and PGE2 into products like PGA2 and PGB2 [50]. The possibility that these conversions have occurred ex vivo must be kept in mind when considering the significance of detecting A2 iPs in the BDx-U of mouse brain (figure 5A), but not the BDx-L (figure 5B), as well as the post mortem losses of D2- and E2-series iPs (figure 5A). Another possible confounding reaction is that J2/A2 iPs are Michael acceptors, rendering them vulnerable to spontaneous adduct formation with thiols and other nucleophiles [29].
Likewise, the absence of a molecular species in these investigations does not imply its absence in brain, because these analyses only included eicosanoid species for which a U-13C form was produced by air oxidation in vitro; the list of molecular species that appears in Table 1 is not exhaustive. Although this approach characterized 18 previously unknown products of air-oxidized ARA by retention time and fragmentation pattern, and 8 of these molecular species were identified in human brain tissue, there are undoubtedly ARA oxidation products (e.g. oxo-EET’s and HpETE’s) that were not produced by air oxidation in quantities sufficient for detection.
It has been consistently reported that F2-iP concentrations are higher in the cerebrospinal fluid of AD brain [8, 11, 15, 17], but mixed results have been reported from brain tissue [48, 51]. The analytical approach described herein was able to detect 5-, 8-, and 15-F2 iPs, and reproducibly determine differences in their concentrations, but no differences in concentration were found between healthy and AD brain. 12-F2-iP was readily measured in normal mouse brain tissue, but not detected in healthy or AD human brain tissue. This finding underscores the suggestion, mentioned above, that pathways for the production and/or clearance of oxidative ARA degradation products appear to exhibit some specificity, and it highlights one of several differences observed between ARA oxidation products in mouse and human brain tissues.
Each of the 18 previously unknown ARA oxidation products contains five or more oxygens, and they elute relatively early on a reversed-phase HPLC column. Some appear early and transiently in the course of air oxidation, while others appear relatively late (figure 2). One subset of these 18 compounds is relatively resistant to saponification, and another subset is detected in human brain, but there is little overlap between these two subsets. One of these molecular species (U13) had significantly increased concentrations in human AD brain, despite ~75% losses during saponification (figure 4), and undetectable concentrations in mouse brain (figure 5). The U-12C form of this molecular species yielded a fragment that was isobaric with a U-12C fragment of 5,6-EET, but the corresponding U-13C form of U13 did not yield a fragment corresponding to U-13C-5,6-EET. As mentioned above, despite a high level of statistical significance for the concentration difference of U13 in healthy and AD brain, its true significance is suspect because it is the only complex oxidation product to exhibit such a difference.
The U-13C-ARA-derived ISM used in this work affords many advantages for quantitative mass spec analysis. Chief among these advantages is chemical stability of the isotopic label; there are significant losses of ARA and most of its oxidation products in mild saponification conditions, and these conditions are also likely to cause the exchange of deuterium for hydrogen, which would cause an overestimation of analyte concentrations if deuterium-labeled internal standards were used. This problem is eliminated with U-13C-ARA and its oxidation products. Another advantage is that a large number of internal standards could be created, facilitating the parallel determinations of over 50 oxidation products. Valid determinations of relative quantities require the same ISM to be used for each group to be compared, but once determined, relative quantities are independent of batch-to-batch differences in ISM composition because signals from the ISM cancel when the ratio of results from different groups are calculated. Similar procedures to that used for preparing U-13C-ARA and its oxidation products have been used to make U-13C-DHA (unpublished data). Thus, the results reported herein for eicosanoid oxidation products are a foundation for an analogous future study of docosanoid oxidation products.
As applied in this work, the ISM only yielded relative quantities. However, the ISM also facilitate the determination of absolute quantities when needed because it allows any compound to be used as a reference standard to calibrate the ISM; they do not have to be isotope-labeled because the ISM is isotope-labeled. When the ISM is calibrated with the reference standards of interest, absolute quantities for multiple species may be obtained from a single run. Absolute quantities of specific enantiomers may be obtained by adding reference standards to fractions of the effluent and subjecting them to a second chromatographic separation. U-13C-ARA-derived internal standards control for losses during saponification, but not for saponification efficiency, which is of particular concern when mild saponification conditions are used (as in this investigation) to avoid chemical losses. Esterified U-13C compounds would be needed to gauge saponification efficiency.
In summary, AD brain contained elevated concentrations of several HETE species compared to healthy human brain, but the concentrations of more complex oxidation products were unchanged. These findings are consistent with the oxidative degradation of ARA by random chemical processes that not only created simple oxidation products, but also converted them into more complex products. However, the oxidative degradation products recovered from tissues suggest that their production and/or clearance is subject to some specificity, and this specificity appears to differ between mouse and human brain. This analysis illustrates the utility of U-13C-ARA-derived internal standards, which provide a chemically robust means for the parallel quantitative analysis of over 50 oxidative ARA degradation products.
Supplementary Material
Highlights.
U-13C-labeled compounds are valuable as internal standards for mass spectrometry
Over 50 U-13C-labeled arachidonate oxidation products were prepared
The ability to recover them from brain tissue is highly variable
Mouse and human brain differ in kinds of oxidation products that can be recovered
Arachidonate and some simple oxidation products are increased in Alzheimer’s disease
Acknowledgments
This work was supported by grants from the NIH (NS74178 and GM76201), the Alzheimer’s Association, and the American Health Assistance Foundation (to P.H.A.). The authors would also like to acknowledge helpful discussions with Robert C. Murphy and Ian A. Blair.
Abbreviations
- ARA
arachidonic acid
- ISM
internal standard mixture
- BHT
butylated hydroxyl-toluene
- BDx
Bligh-Dyer extract
- BDx-U
Upper aqueous phase of a Bligh-Dyer extract
- BDx-L
lower organic phase of a Bligh-Dyer extract
- HETE
hydroxyeicosanoid
- EET
epoxyeicosanoid
- iP
isoprostanes
- PMI
post mortem interval
- MRM
multiple reaction monitoring
- LC/MS/MS
liquid chromatography tandem mass spectrometry
- TIC
total ioncurrent chromatogram
- XIC
extracted ion chromatogram
- TBARS
thiobarbituric acid reactive substances
- CSF
cerebrospinal fluid
Footnotes
Conflict of Interest
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Axelsen PH, Komatsu H, Murray IVJ. Oxidative Stress and Cell Membranes in the Pathogenesis of Alzheimer’s Disease. Physiol. 2011;26:54–69. doi: 10.1152/physiol.00024.2010. [DOI] [PubMed] [Google Scholar]
- 2.Buettner GR. The Pecking Order of Free-Radicals and Antioxidants - Lipid-Peroxidation, Alpha-Tocopherol, and Ascorbate. Arch Biochem Biophys. 1993;300:535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 3.Koppaka V, Axelsen PH. Accelerated accumulation of amyloid beta proteins on oxidatively damaged lipid membranes. Biochem. 2000;39:10011–6. doi: 10.1021/bi000619d. [DOI] [PubMed] [Google Scholar]
- 4.Koppaka V, Paul C, Murray IVJ, Axelsen PH. Early synergy between Aβ42 and oxidatively damaged membranes in promoting amyloid fibril formation by Aβ40. J Biol Chem. 2003;278:36277–84. doi: 10.1074/jbc.M301334200. [DOI] [PubMed] [Google Scholar]
- 5.Murray IVJ, Sindoni ME, Axelsen PH. Promotion of oxidative lipid membrane damage by amyloid beta proteins. Biochem. 2005;44:12606–13. doi: 10.1021/bi050926p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray IVJ, Liu L, Komatsu H, Uryu K, Xiao G, Lawson JA, et al. Membrane mediated amyloidogenesis and the promotion of oxidative lipid damage by amyloid beta proteins. J Biol Chem. 2007;282:9335–45. doi: 10.1074/jbc.M608589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furman R, Murray IVJ, Schall HE, Liu Q, Ghiwot Y, Axelsen PH. Amyloid Plaque-Associated Oxidative Degradation of Uniformly Radiolabeled Arachidonic Acid. ACS Chem Neurosci. 2016;7:367–77. doi: 10.1021/acschemneuro.5b00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ. Cerebrospinal fluid F-2-isoprostane levels are increased in Alzheimer’s disease. Ann Neurol. 1998;44:410–3. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- 9.Pratico D, Uryu K, Leight S, Trojanowswki JQ, Lee VMY. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–7. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuppo EE, Forman LJ, Spur BW, Chan-Ting RE, Chopra A, Cavalieri TA. Sign of lipid peroxidation as measured in the urine of patients with probable Alzheimer’s disease. Brain Res Bull. 2001;54:565–8. doi: 10.1016/s0361-9230(01)00450-6. [DOI] [PubMed] [Google Scholar]
- 11.Pratico D, Clark CM, Liun F, Lee VMY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment - A possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–6. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 12.Montine TJ, Quinn JF, Milatovic D, Silbert LC, Dang T, Sanchez S, et al. Peripheral F-2-isoprostanes and F-4-neuroprostanes are not increased in Alzheimer’s disease. Ann Neurol. 2002;52:175–9. doi: 10.1002/ana.10272. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Zhukareva V, Sung S, Clark CM, Rokach J, Lee VMY, et al. Enhanced brain levels of 8,12-iso-iPF(2 alpha)-VI differentiate AD from frontotemporal dementia. Neurol. 2003;61:475–8. doi: 10.1212/01.wnl.0000070185.02546.5d. [DOI] [PubMed] [Google Scholar]
- 14.Quinn JF, Montine KS, Moore M, Morrow JD, Kaye JA, Montine TJ. Suppression of longitudinal increase in CSFF2-isoprostanes in Alzheimer’s disease. J Alz Dis. 2004;6:93–7. doi: 10.3233/jad-2004-6110. [DOI] [PubMed] [Google Scholar]
- 15.Montine TJ, Montine KS, McMahan W, Markesbery WR, Quinn JF, Morrow JD. F-2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid Redox Signal. 2005;7:269–75. doi: 10.1089/ars.2005.7.269. [DOI] [PubMed] [Google Scholar]
- 16.Irizarrya MC, Yao Y, Hyman BT, Growdon JH, Pratico D. Plasma F2A lsoprostane levels in Alzheimer’s and Parkinson’s disease. Neurodeg Dis. 2007;4:403–5. doi: 10.1159/000107699. [DOI] [PubMed] [Google Scholar]
- 17.Montine TJ, Quinn J, Kaye J, Morrow JD. F-2-isoprostanes as biomarkers of late-onset Alzheimer’s disease. J Mol Neurosci. 2007;33:114–9. doi: 10.1007/s12031-007-0044-1. [DOI] [PubMed] [Google Scholar]
- 18.Mufson EJ, Leurgans S. Inability of Plasma and Urine F2A-Isoprostane Levels to Differentiate Mild Cognitive Impairment from Alzheimer’s Disease. Neurodeg Dis. 2010;7:139–42. doi: 10.1159/000289224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montine TJ, Peskind ER, Quinn JF, Wilson AM, Montine KS, Galasko D. Increased Cerebrospinal Fluid F-2-Isoprostanes are Associated with Aging and Latent Alzheimer’s Disease as Identified by Biomarkers. Neuromol Med. 2011;13:37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farooqui AA, Rapoport SI, Horrocks LA. Membrane phospholipid alterations in Alzheimer’s disease: Deficiency of ethanolamine plasmalogens. Neurochem Res. 1997;22:523–7. doi: 10.1023/a:1027380331807. [DOI] [PubMed] [Google Scholar]
- 21.Han XL, Holtzman DM, McKeel DW. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77:1168–80. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 22.Han XL, Holtzman DM, McKeel DW, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–18. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 23.Han X. Lipid Alterations in the Earliest Clinically Recognizable Stage of Alzheimer’s Disease: Implication of the Role of Lipids in the Pathogenesis of Alzheimer’s Disease. Curr Alzheimer Res. 2005;2:65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- 24.Han XL. Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stage of Alzheimer’s disease: a tale of shotgun lipidomics. J Neurochem. 2007;103:171–9. doi: 10.1111/j.1471-4159.2007.04708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JV, Furman R, Axelsen PH. Biosynthesis of uniformly labeled C-13- and C-14-arachidonic acid in Mortierella alpina. Biores Technol. 2017;227:142–6. doi: 10.1016/j.biortech.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy RC, Barkley RM, Berry KZ, Hankin J, Harrison K, Johnson C, et al. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346:1–42. doi: 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 27.Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rap Comm Mass Spec. 2008;22:75–83. doi: 10.1002/rcm.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song WL, Lawson JA, Reilly D, Rokach J, Chang CT, Giasson BI, et al. Neurofurans, novel indices of oxidant stress derived from docosahexaenoic acid. J Biol Chem. 2008;283:6–16. doi: 10.1074/jbc.M706124200. [DOI] [PubMed] [Google Scholar]
- 29.Hardy KD, Cox BE, Milne GL, Yin HY, Roberts LJ. Nonenzymatic free radical-catalyzed generation of 15-deoxy-Delta(12,14)-prostaglandin J(2)-like compounds (deoxy-J(2)-isoprostanes) in vivo. J Lipid Res. 2011;52:113–24. doi: 10.1194/jlr.M010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strassburg K, Huijbrechts AML, Kortekaas KA, Lindeman JH, Pedersen TL, Dane A, et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal Bioanal Chem. 2012;404:1413–26. doi: 10.1007/s00216-012-6226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoang HD, Prasain JK, Dorand D, Miller MA. A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Caenorhabditis elegans Reproductive Tract. Plos Genetics. 2013;9 doi: 10.1371/journal.pgen.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigor C, Bertrand-Michel J, Pinot E, Oger C, Vercauteren J, Le Faouder P, et al. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J Chrom B. 2014;964:65–78. doi: 10.1016/j.jchromb.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Grozer Z, Toth A, Toth R, Kecskemeti A, Vagvolgyi C, Nosanchuk JD, et al. Candida parapsilosis produces prostaglandins from exogenous arachidonic acid and OLE2 is not required for their synthesis. Virulence. 2015;6:85–92. doi: 10.4161/21505594.2014.988097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller TM, Donnelly MK, Crago EA, Roman DM, Sherwood PR, Horowitz MB, et al. Rapid, simultaneous quantitation of mono and dioxygenated metabolites of arachidonic acid in human CSF and rat brain. J Chrom B. 2009;877:3991–4000. doi: 10.1016/j.jchromb.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yue HF, Jansen SA, Strauss KI, Borenstein MR, Barbe MF, Rossi LJ, et al. A liquid chromatography/mass spectrometric method for simultaneous analysis of arachidonic acid and its endogenous eicosanoid metabolites prostaglandins, dihydroxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and epoxyeicosatrienoic acids in rat brain tissue. J Pharm Biomed Anal. 2007;43:1122–34. doi: 10.1016/j.jpba.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hecker M, Ullrich V, Fischer C, Meese CO. Identification of Novel Arachidonic-Acid Metabolites Formed by Prostaglandin-H Synthase. Eur J Biochem. 1987;169:113–23. doi: 10.1111/j.1432-1033.1987.tb13587.x. [DOI] [PubMed] [Google Scholar]
- 37.Giannopoulos PF, Joshi YB, Chu J, Pratico D. The 12-15-lipoxygenase is a modulator of Alzheimer’s-related tau pathology in vivo. Aging Cell. 2013;12:1082–90. doi: 10.1111/acel.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tajima Y, Ishikawa M, Maekawa K, Murayama M, Senoo Y, Nishimaki-Mogami T, et al. Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer’s disease. Lipids Health Dis. 2013;12 doi: 10.1186/1476-511X-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu J, Zhuo JM, Pratico D. Transcriptional regulation of beta secretase-1 by 12/15-lipoxygenase results in enhanced amyloidogenesis and cognitive impairments. Ann Neurol. 2012;71:57–67. doi: 10.1002/ana.22625. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Pallast S, Arai K, Wang XY, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem. 2009;111:882–9. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao YM, Clark CM, Trojanowski JQ, Lee VMY, Pratico D. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann Neurol. 2005;58:623–6. doi: 10.1002/ana.20558. [DOI] [PubMed] [Google Scholar]
- 42.Mattsson N, Yaong M, Rosengren L, Blennow K, Mansson JE, Andersen O, et al. Elevated cerebrospinal fluid levels of prostaglandin E2 and 15-(S)-hydroxyeicosatetraenoic acid in multiple sclerosis. J Int Med. 2009;265:459–64. doi: 10.1111/j.1365-2796.2008.02035.x. [DOI] [PubMed] [Google Scholar]
- 43.Poloyac SM, Reynolds RB, Yonas H, Kerr ME. Identification and quantification of the hydroxyeicosatetraenoic acids, 20-HETE and 12-HETE, in the cerebrospinal fluid after subarachnoid hemorrhage. J Neurosci Meth. 2005;144:257–63. doi: 10.1016/j.jneumeth.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou YU, Halabisky B, et al. Phospholipase A(2) reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11:1311–8. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puppolo M, Varma D, Jansen SA. A review of analytical methods for eicosanoids in brain tissue. J Chrom B. 2014;964:50–64. doi: 10.1016/j.jchromb.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Spaargaren M, Wissink S, Defize LHK, Delaat SW, Boonstra J. Characterization and Identification of An Epidermal-Growth-Factor-Activated Phospholipase-A2. Biochem J. 1992;287:37–43. doi: 10.1042/bj2870037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Axelsen PH, Murphy RC. Quantitative Analysis of Phospholipids Containing Arachidonate and Docosahexaenoate Chains in Microdissected Regions of Mouse Brain. J Lipid Res. 2010;51:660–71. doi: 10.1194/jlr.D001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pratico D, Lee VMY, Trojanowski JQ, Rokach J, FitzGerald GA. Increased F-2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–83. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 49.Fitzpatrick FA, Wynalda MA. Albumin-Catalyzed Metabolism of Prostaglandin-D2 - Identification of Products Formed Invitro. J Biol Chem. 1983;258:11713–8. [PubMed] [Google Scholar]
- 50.Jones RL. 15-Hydroxy-9-Oxoprosta-11,13-Dienoic Acid As Product of A Prostaglandin Isomerase. J Lipid Res. 1972;13:511. [PubMed] [Google Scholar]
- 51.Reich EE, Markesbery WR, Roberts LJ, Swift LL, Morrow JD, Montine TJ. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Am J Path. 2001;158:293–7. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snowden SG, Ebshiana AA, Hye A, An Y, Pletnikova O, O’Brien R, et al. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology., and cognitive performance: A nontargeted metabolomic study. Plos Medicine. 2017;14 doi: 10.1371/journal.pmed.1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraser T, Tayler H, Love S. Fatty Acid Composition of Frontal, Temporal and Parietal Neocortex in the Normal Human Brain and in Alzheimer’s Disease. Neurochem Res. 2010;35:503–13. doi: 10.1007/s11064-009-0087-5. [DOI] [PubMed] [Google Scholar]
- 54.Nourooz-Zadeh J, Liu EHC, Yhlen B, Anggard EE, Halliwell B. F-4-isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer’s disease. J Neurochem. 1999;72:734–40. doi: 10.1046/j.1471-4159.1999.0720734.x. [DOI] [PubMed] [Google Scholar]
- 55.Marion J, Wolfe LS. Origin of the Arachidonic-Acid Released Postmortem in Rat Forebrain. Biochim Biophys Acta. 1979;574:25–32. doi: 10.1016/0005-2760(79)90080-8. [DOI] [PubMed] [Google Scholar]
- 56.Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, et al. Imaging neuroinflammation in Alzheimer’s disease with radiolabeled arachidonic acid and PET. J Nucl Med. 2008;49:1414–21. doi: 10.2967/jnumed.107.049619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou YU, Halabisky B, et al. Phospholipase A(2) reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11:1311–8. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goozee K, Chatterjee P, James I, Shen K, Sohrabi HR, Asih PR, et al. Alterations in erythrocyte fatty acid composition in preclinical Alzheimer’s disease. Sci Rep. 2017;7 doi: 10.1038/s41598-017-00751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.