Abstract
Purpose
Development of extra-nodal extension (ENE) has been associated with poor survival in patients with oral cavity squamous cell carcinoma (OSCC). Here we sought to confirm the role of ENE as a poor prognostic factor, and identify genomic and epigenetic markers of ENE in order to develop a predictive model and improve treatment selection.
Experimental design
An institutional cohort (University of Texas MD Anderson Cancer Center) was utilized to confirm the impact of ENE on clinical outcomes and evaluate the genomic signature of primary and ENE containing tissue. OSCC data from The Cancer Genome Atlas (TCGA) were analyzed for the presence of molecular events associated with nodal and ENE status.
Results
ENE was associated with decreased overall and disease free survival. Mutation of the TP53 gene was the most common event in ENE+ OSCC. The frequency of TP53 mutation in ENE+ tumors was higher compared to ENE- tumors and wild-type (wt) TP53 was highly-represented in pN0 tumors. pN+ENE+ patients had the highest proportion of high-risk TP53 mutations. Both primary tumors (PT) and lymph nodes with ENE (LN) exhibited a high rate of TP53 mutations (58.8%, 58.8% respectively) with no significant change in allele frequency between the two tissue sites.
Conclusions
ENE is one of the most significant markers of OSCC OS and DFS. There is a shift toward a more aggressive biological phenotype associated with high-risk mutations of the TP53 gene. Prospective clinical trials are required to determine whether TP53 mutational status can be used for personalized treatment decisions.
Keywords: extracapsular spread, extra nodal extension, head and neck cancer, tongue, p53
Introduction
Oral squamous cell carcinoma (OSCC) can be a devastating disease that affects nearly 30,000 patients each year in the US alone.(1) OSCC demonstrates a high propensity to metastasize to regional nodal basins which generally requires treatment escalation to include multi-modality treatment strategies.(2–4) Once OSCC has metastasized to lymph nodes patient survival is decreased by nearly 50%, and development of extra-nodal extension (ENE) is further associated with a negative impact on disease control and patient survival.(2,3,5) Fifteen years ago, we described the clinical impact of ENE on survival in patients with OSCC and identified it as a prognostic marker for regional recurrence and distant metastasis.(2) Since that time, we have witnessed significant improvements in the sensitivity and specificity of anatomic (computed tomography, CT; magnetic resonance imaging, MRI) and metabolic imaging (positron emission tomography, PET), in the delivery of radiotherapy, and an increased availability of conventional and targeted chemotherapeutic agents. In the current article, we update our previous analysis for ENE impact on survival, utilizing a larger patient cohort.
Recent advances in next generation sequencing (NGS) and multi-platform genomic and epigenetic characterization of solid tumors including OSCC have allowed us to better understand the molecular changes underlying OSCC development and progression.(6,7) In addition, this genomic information from OSCCs has led to the development of predictive biomarkers of the efficacy of radiation and/or chemotherapy in the adjuvant (post-surgical) setting.(8,9) Data from our group and others have confirmed the clear association of TP53 mutations with the development and progression of oral squamous cancer as well as SCC from other head and neck sites (HNSCC).(6,8–11) Moreover, we have demonstrated, in both patient cohorts and pre-clinical models of HNSCC, that the specific types of TP53 mutations can have a profound impact on tumor development, metastasis and response to treatment.(8,9,12–15) These novel discoveries are critical to continued refinement in the selection of more effective treatment strategies. To this end, we have developed an evolutionarily-based TP53 mutation grading system (named EAp53), which classifies TP53 mutations into low-risk missense, high-risk missense, and “other” (nonsense, splice site, frameshift, and insertion-deletion) mutations, and have demonstrated that HNSCC patients with low-risk TP53 mutations are associated with better treatment outcomes similar to those with wild type TP53 when compared to those with high-risk or “other” mutations.(8,9) While pathologic nodal status and the presence of ENE are our most reliable prognostic biomarkers for patients with OSCC, these can only be determined in the post-operative setting from histopathologic analysis of the neck dissection specimen. Since an increasing number of patients with HNSCC are treated non-surgically, prognostic biomarkers, which correlate with nodal status and presence of ENE, have become critically important. Therefore, in this study, we sought to identify a biological signature associated with ENE development in OSCC based on analysis of the TP53 mutational status in a biopsy of the primary tumor specimen.
In the current study, we aimed to address two translational questions. First, we sought to determine whether ENE is a poor prognostic indicator in the modern era of OSCC treatment. Second, we sought to identify a molecular signature associated with ENE development and aggressive clinical behavior in an effort to potentially triage patients more appropriately for multi-modality therapy in the absence of information about ENE that is available only after surgical removal of regional lymph nodes. To answer these questions, we evaluated data from a large institutional OSCC patient cohort as well as OSCC data from The Cancer Genome Atlas (TCGA).
Methods
Clinical data collection and analysis
Three patient cohorts were utilized for the data analysis; all studies were approved by the Institutional Review Board and all studies were conducted in accordance with recognized ethical guidelines (Declaration of Helsinki, CIOMS, Belmont Report and U.S. Common Rule). Waiver of written informed consent was provided as part of the approval process by the Institutional Review Board as is common for retrospectively conducted analyses. A cohort of 238 patients from The University of Texas MD Anderson Cancer Center (UTMDACC) was utilized to confirm the impact of ENE on clinical outcomes. Inclusion criteria included: OSCC diagnosis, primary surgical treatment (with or without adjuvant treatment) between July 2009 and December 2015. All patients underwent surgery including resection of primary tumor and simultaneous neck dissection. Exclusion criteria included: incomplete treatment, treatment for recurrent disease, and preoperative treatment with systemic therapy. Clinical-pathological information; demographics, tumor characteristics, status of lymph nodes, treatment characteristics, disease recurrence and survival were collected for all patients. Pathological data for the status of lymph nodes included the presence or absence of ENE. ENE was defined as consisting of tumor extension outside the nodal capsule and into the surrounding soft tissues. The presence of tumor cells in the capsule of the node was not considered ENE. HPV status was not known for the UTMDACC cohort due to the lack of routine testing for HPV in oral cavity patients and the low incidence of HPV positive OSCC in our patient population (6%).(16)
We extracted clinical and molecular data from The Cancer Genome Atlas (TCGA) for 230 HPV negative OSCC patients in order to confirm the impact of ENE on clinical outcomes. In addition, we mined genomic information of TCGA to discover the molecular markers relevant to ENE. These are obtained from FireBrowse.org website or TCGA.(17) Inclusion criteria included: OSCC diagnosis and primary surgical treatment (with or without adjuvant treatment). Exclusion criteria included the unavailability of either pathological or genomic information and presence of HPV expression by RNAseq.(17)
Finally, using the UTMDACC cohort, 17 OSCC patient specimens were used to examine differences in mutation status between primary tumors and lymph nodes with ENE. Inclusion criteria included: OSCC diagnosis, primary surgical treatment (with or without adjuvant treatment) with simultaneous neck dissection, demonstrated at least one pathologically metastatic node with ENE, and for which both primary tumor and paired ENE positive lymph node tissue were available.
Statistical analysis for clinical parameters
Statistical analysis for the impact of ENE on disease free survival (DFS) and overall survival (OS) was performed using Kaplan-Meier analysis (log-rank analysis for statistical significance). DFS time was defined as the interval between the date of the end of primary treatment and the date of the development of local, regional recurrence and distant metastasis after surgery. OS time was calculated from the date of initial examination to the date of death, to the date of last contact, or to the date of the 5-year follow up. The median follow up period was 28.4 months (range 1.6–60 months). Moreover, statistical significance of individual clinical parameters was confirmed using multi-variate analysis (version 22.0, SPSS, Inc., Chicago, IL).
Analysis of TCGA genomic data
Two hundred thirty patients were divided into three groups: pathologically negative nodes (pN0), pathologically positive node without ENE (pN+/ENE-), and pathologically positive node with ENE (pN+/ENE+). Fisher’s exact test was conducted for 5949 genes to compare mutation frequencies across these groups. The TP53 gene was also analyzed individually and mutations were characterized as “high-risk”, “low-risk” or “other” based on the previously described, validated Evolutionary Action scoring system (EAp53).(8,9) Missense mutations were scored using the EAp53 from 0 to 100 with higher scores representing alterations calculated to be more deleterious to protein function. Wild-type TP53 sequences were scored as 0 due to assumed normal function. The threshold used to define mutations as “high-risk” was 75.
Targeted sequencing of patient tumors
Seventeen OSCCs, identified from the UTMDACC tumor bank based on the above mentioned criteria, were examined by targeted next generation sequencing (NGS). Primary tumors and paired metastatic lymph nodes with ENE were re-evaluated by a trained head and neck pathologist (Diana Bell M.D.) to confirm the histologic diagnosis and the presence of ENE. Genomic DNA was isolated from frozen primary tumors and formalin-fixed paraffin-embedded (FFPE) tissues of entire lymph node metastasis including the site of ENE. As a control (to remove germline mutations), we used genomic DNA from frozen blood samples. Ten nanograms of genomic DNA were used as input for target DNA library preparation using the Ion AmpliSeq Library Kit, and sequenced in the Ion PGM Sequencer platform. Mutations were called with a custom pipeline in the Ion Reporter software.
Gene expression by RNA sequencing
We generated a box plot to compare the overall gene expression among samples and the 3 groups listed above from the RNAseq data available within the Head and Neck TCGA cohort. Hierarchical clustering as well as principle component analysis (PCA) were used to evaluate sample quality and differences between samples. Genes with the expression value of less than 5 (corresponding to 31 reads) in all samples were filtered out. One-way ANOVA was applied on a gene-by-gene basis to test for difference among the 3 ENE groups. The Benjamini-Hochberg method was applied to the resulting p-values, computed from test statistics, to adjust for multiple testing. Pair-wise comparisons between different groups were done using Tukey’s Honest Significance (HSD) test with 95% family-wise confidence level.
Copy number
Copy number alterations were evaluated for each gene. Fisher’s exact test was conducted for each gene to compare copy number across the groups to test for association between copy number and the ENE groups. We generated three groups for each gene: deletion, normal, and amplification. Fisher’s exact test was then conducted for this version of copy number values. Benjamini-Hochberg correction was applied to adjust for multiple testing. With the amplification peaks output by GISTIC (Genomic Identification of Significant Targets in Cancer; www.broadinstitute.org/cancer/cga/gistic), we obtained genes from each peak and retrieved their copy number values for each patient sample. Then, we considered the genes from the same peak as a group and assigned a copy number value to each patient. Samples were separated into two groups, a group without gains (−2, −1, 0) and a group with gains (1, 2), and compared the difference across the groups. Deletions were evaluated in a similar manner.
Results
Patient characteristics
A total of 238 patients with OSCC were analyzed as part of the UTMDACC cohort. One hundred twenty eight of the 238 patients (53.8%) had negative nodes (pN0), 40 (16.8%) had a pathologically positive node without ENE (pN+/ENE-), and 70 (29.4%) had a pathologically positive node with ENE (pN+/ENE+). The UTMDACC patient clinical-pathological information are summarized in Supplementary Table 1. A total of 230 OSCC patients’ data were analyzed as part of the TCGA cohort. One hundred thirteen of the 230 patients (49.1%) were pN0, 62 (27.0%) were pN+/ENE-, and 55 (23.9%) were pN+/ENE+. TCGA patient clinical-pathological information are summarized in Supplementary Table 2.
Impact of ENE on survival (UTMDACC cohort)
To assess the impact of ENE on survival, the survival data of the patients were compared between the three groups, pN0, pN+/ENE-, and pN+/ENE+. Kaplan-Meier survival curves of DFS and OS for the UTMDACC cohort are presented in Figure 1A and 1B, respectively. The 3-year and 5-year DFS rates are 76.6% and 71.9% for pN0, 70.2% and 62.4% for pN+/ENE-, 49.7% and 45.9% for pN+/ENE+ patients. The 3-year and 5-year OS rates are 83.0% and 75.2% for pN0, 73.0% and 59.0% for pN+/ENE-, 47.8% and 45.0% for pN+/ENE+ patients. These results demonstrate the adverse impact of pN+/ENE+ compared to pN0 and pN+/ENE- on DFS (log rank test, p<0.0001 and p=0.036 respectively) and OS rates (log rank test, p<0.0001 and p= 0.043 respectively). Multivariate Cox proportional hazards analysis, including clinical-pathological factors revealed that smoking and ENE were independent predictors of DFS (HR= 0.598 and 2.629, 95% confidence interval= 0.375–0.954 and 1.650–4.189, p= 0.031 and p<0.0001 respectively; Supplementary Table 3). With regard to OS, pathological T stage and presence of ENE showed a statistically significant prognostic value (HR= 1.981 and 2.652, 95% confidence interval= 1.201–3.270 and 1.625–4.327, p= 0.007 and p<0.0001 respectively; Supplementary Table 3). Therefore, the presence of ENE is the only significant independent predictor of both DFS and OS.
Figure 1.
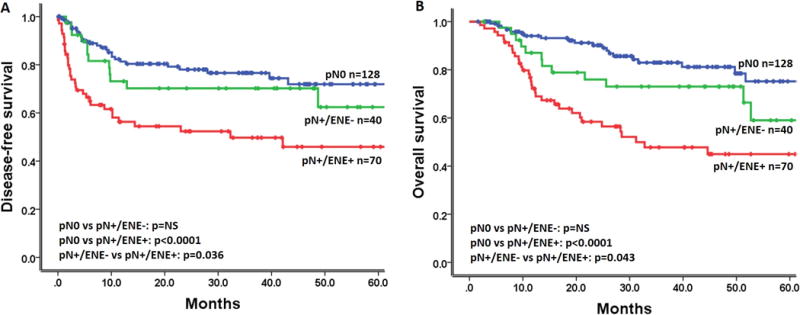
Impact of ENE on survival (UTMDACC cohort). Kaplan-Meier plots for A) disease-free and B) overall survival according to lymph node status. P-values were calculated by using the log-rank test.
Impact of ENE on survival (TCGA cohort)
To validate the impact of ENE on survival, we analyzed data from the TCGA patient cohort. The 3-year and 5-year OS rates were 64.0%, 56.4% for pN0, 66.8%, 61.7% for pN+/ENE-, and both 13.9% for pN+/ENE+ patients respectively (Figure 2). We confirmed the adverse impact on OS of pN+/ENE+ compared with pN0 and pN+/ENE- (log rank test, p<0.0001). Multivariate Cox proportional hazards analysis, including clinical-pathological factors and TP53 mutation status (wild-type/”low-risk” versus “high-risk”/”other”), revealed that pathological T-stage, and ENE were independently correlated with OS (HR= 2.607 and 3.090, 95% confidence interval= 1.558–4.362 and 1.982–4.819, p<0.0001 and p<0.0001 respectively; Supplementary Table 4). DFS rates were not analyzed for this cohort due to the limited recurrence details collected by TCGA.
Figure 2.
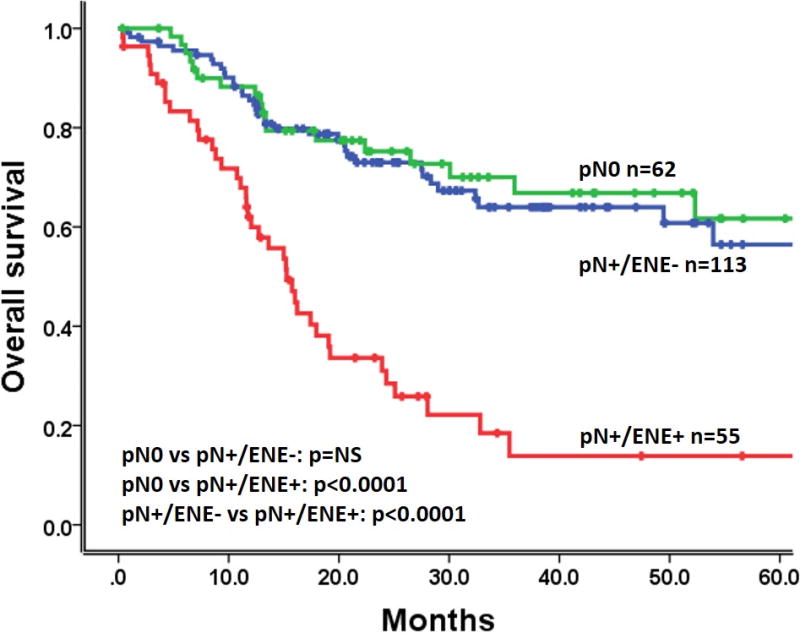
Impact of ENE on survival (TCGA cohort). Kaplan-Meier plot for overall survival according to lymph node status. P-values were calculated by using the log-rank test.
TP53 mutational analysis (TCGA cohort)
Sequencing information for the TCGA OSCC tumors is summarized in Supplementary Tables 5 and 6. A total of 181 (78.7%) patients exhibited any mutations in TP53 gene and 113 (49.1%) patients had missense mutations. Forty patients had more than one mutation in TP53 and 21 patients had the combination of missense mutations and other mutations. In these cases, the representative type of mutation for the each patient was defined using the missense mutation. We classified missense mutations into two groups: “low-risk” mutations and “high-risk” mutations with the previously described and validated Evolutionary Action scoring system.(8,9) Consequently, a total of 45 patients had “low-risk” mutations, 68 patients had “high-risk” mutations, and 68 patients had “other” mutations. Eight patients whose tumors had both “low-risk” mutations and “high-risk” mutations were classified as having “high-risk” mutations.
Correlation between TP53 mutational status and ENE (TCGA cohort)
We divided patients into 3 groups: pN0, pN+/ENE-, and pN+/ENE+; 49 out of 55 (89.1%) patients with ENE exhibited mutations in TP53. The frequency of TP53 mutation in pN+/ENE+ (89.1%) was higher compared to both pN0 (72.4%) and pN+/ENE- (80.6%). Moreover, when we classified TP53 status into 4 groups (wild-type, “low-risk” mutations, “high-risk” mutations, and “other” mutations), “high-risk” and “other” mutations were over-represented in pN+/ENE+ tumors. Among pN+/ENE+ patients with mutations, 24 out of 49 (49.0%) had “high-risk” and 20 (40.8%) had “other” mutations (Supplementary Table 5, Figure 3). The EAp53 scoring system only calculates a score for missense mutations, therefore, non-missense mutations are classified as “other”.
Figure 3.
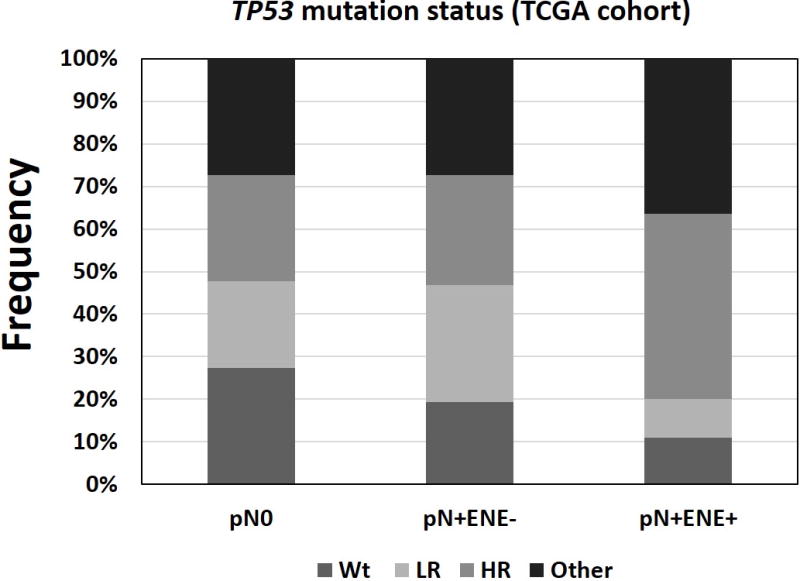
Distribution of TP53 mutation types (TCGA cohort). Mutations were classified according to the EAp53 scoring system and grouped by lymph node status.
Chi-square analysis across the 3 patient groups showed TP53 “high-risk” mutations were significantly correlated with pN+/ENE+ group (p = 0.014). When we classified TP53 status into 2 groups: wild-type/”low-risk” mutations and “high-risk”/”other” mutations, a further statistical association was seen between “high-risk”/”other” mutations and pN+/ENE+ group (Chi-square test: p = 0.001). Multivariate logistic regression analysis, including clinical-pathological factors, TP53 mutation status, showed that smoking habit, pathological T-stage, and TP53 mutation status (wild-type/”low-risk” versus “high-risk”/”other”) were independently significant factors of ENE (odds ratio = 2.757, 3.528, and 3.683, 95% confidence interval= 1.172–6.484, 1.655–7.519, and 1.725–7.863, p=0.020, 0.001, and 0.001, respectively, hit ratio = 75.9%; Table 1).
Table 1.
Multivariate logistic regression analysis for ENE (TCGA cohort).
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Age | — | — | NS |
| Sex | — | — | NS |
| Alcohol | — | — | NS |
| Smoking | 2.757 | 1.172―6.484 | 0.020 |
| Pathological T stage | 3.528 | 1.655―7.519 | 0.001 |
| TP53 mutation (EA score) | 3.683 | 1.725―7.863 | 0.001 |
OR= odds ratio; CI= confidence interval
Impact of TP53 mutations on the outcome of TCGA cohort
Kaplan-Meier survival curves for OS according to TP53 mutation type are presented in Supplementary Figure 1. The 3-year and 5-year OS rate were both 67.7% for patients with wild type, both 54.1% for those with “low-risk”, 52.8%, 46.2% for those with “high-risk”, and 45.4%, 36.1% for those with “other” mutations, respectively (log-rank test, wild type versus “other” mutations: p= 0.036). When we classified TP53 status into 2 groups: wild-type/”low-risk” mutations and “high-risk”/”other” mutations, the 3-year and 5-year OS rates were both 60.0% for patients with wild type/“low-risk” mutations, and 48.9% and 40.9% for those with “high-risk”/”other” mutations respectively (log-rank test. p= NS).
TP53 mutations in ENE
The sequencing results for OSCC primary tumors and paired lymph node metastasis of 17 UTMDACC patients with ENE are shown in Table 2 and Supplementary Table 7. We found that 12 (70.6%) out of 17 patients had a TP53 mutation in the primary tumor (PT) and/or lymph node metastasis with ENE (LN). Eight patients (66.7%, 8/12) had concordant TP53 mutations in both PT and paired LN. Another 4 patients (33.3%, 4/12) were shown to have discordant mutations between the PT and paired LN. TP53 mutation scores were equally distributed when comparing all PT and LN samples.
Table 2.
TP53 mutation status (UTMDACC ENE cohort).
| PTa | LNb | |||||
|---|---|---|---|---|---|---|
| Patient | Amino Acid | EA Score | Typec | Amino Acid | EA Score | Typec |
| 1 | R248Q | 78.95 | HR | 0 | Wt | |
| 2 | R342* | Other | R342* | Other | ||
| 3 | E286A | 87.02 | HR | E286A | 87.02 | HR |
| 4 | 0 | Wt | 0 | Wt | ||
| 5 | 0 | Wt | L111fs, Y205C | 77.88 | HR | |
| 6 | G245S | 86.45 | HR | G245S | 86.45 | HR |
| 7 | 0 | Wt | 0 | Wt | ||
| 8 | L137fs | Other | L137fs | Other | ||
| 9 | 0 | Wt | 0 | Wt | ||
| 10 | Y107* | Other | Y107* | Other | ||
| 11 | 0 | Wt | 0 | Wt | ||
| 12 | R248Q | 78.95 | HR | R248Q | 78.95 | HR |
| 13 | Y220C | 72.52 | LR | 0 | Wt | |
| 14 | 0 | Wt | 0 | Wt | ||
| 15 | 0 | Wt | E285K | 69.87 | LR | |
| 16 | D207fs | Other | D207fs | Other | ||
| 17 | H179R | 81.91 | HR | H179R | 81.91 | HR |
PT: Primary Tumor
LN: Lymph Node Metastasis
TP53 mutations divided into 4 groups based on EA system: Wt (Wild Type), LR (Low-risk missense mutation), HR (High-risk missense mutation), Other (any type of mutations except missense mutations)
Non-TP53 mutations in the TCGA cohort
The TCGA data was mined for mutation frequency across all OSCC specimens which met inclusion criteria. The most commonly mutated genes were FAT1 (28%), CDKN2A (27%), NOTCH1 (21%), CASP8 (18%) and PIK3CA (16%). An unbiased analysis of mutational frequency and ENE status did not identify any mutated genes other than TP53 significantly associated with ENE (no gene with FDR of <0.05).
Other genomic alterations
Copy number analysis was performed to determine if any copy number alterations were associated with ENE. In order to improve the statistical power only significant GISTIC regions from the full HNSC TCGA analysis were analyzed (Firebrowse.org). We found that the regions of 11q23.1 and 19p13.3 demonstrated an association between ENE and copy number loss (adjusted p=0.0246 and p=0.02509, respectively) (Supplementary Table 8). 11q23.1 contains the gene SDHD and is lost in 55% of ENE+ patients compared to 24% of pN0 and 42% of pN+/ENE-. 19p13.3 contains LKB1/STK11 and is lost in 55% of ENE+ cases compared to 26% of pN0 and 35% of pN+/ENE-. Global gene expression analysis identified 9 genes differentially expressed between the ENE groups (Supplementary Table 9). Hierarchical clustering of the samples based on the expression of these genes identified a cluster enriched for LN metastasis (94% vs 44%, 29/31 vs 88/199), but not ENE specifically (Supplementary Figure 2). No pathways were enriched among these genes.
Discussion
Despite significant technological and scientific advances over the last 3 decades, survival for patients with advanced OSCC remains poor (Figures 1, 2). The data summarized here, confirms that ENE is an important biomarker of OSCC prognosis. Given that these data match quite precisely findings from our previous institutional cohort despite lack of any patient overlap, we consider ENE to be one of the most reliable prognostic markers of OSCC clinical outcomes.(2) Our institutional findings are further strengthened by confirmation of ENE importance in the TCGA patient cohort. In fact, clinical outcomes for pN+ENE+ OSCC patients in the TCGA cohort are even worse, with 2-year survival at approximately 25%. Whether this represents simply a statistical anomaly, selection bias in patients in whom samples were collected for the TCGA, or variation of clinical treatments for advanced OSCC at the many centers that provided specimens is unclear and will need to be further investigated. Inclusion of HPV positive patients in the TCGA cohort did not alter the overall results and/or conclusions.
Since OSCC is primarily treated as a surgical disease, ascertaining pN+ and ENE status will continue to represent an important component of standard of care post-surgical adjuvant treatment paradigms. In light of the recent publication by D’Cruz et al. on the diagnostic and clinical impact of elective neck dissection for OSCC tumors compared to therapeutic node dissection, it is likely that the majority of patients with OSCC will continue to undergo surgical management which can reliably generate pN+ and ENE status.(18) In contrast to OSCC, other subsites (i.e. oropharynx, larynx) within the head and neck are increasingly treated using non-surgical treatment algorithms.(19–23) As a result, pN+ and ENE status and their impact on clinical prognosis cannot be reliably and consistently ascertained. Therefore, there is a critical need to define the biological signature associated with pN+ and ENE in order to incorporate this prognostic information into non-surgical treatment algorithms
The impact of TP53 mutation on HNSCC biology and response to treatment has been thoroughly documented by our group and others using both pre-clinical disease models and retrospective patient cohorts.(8,9,11,13,15,24–26) TP53 mutations can behave in a heterogeneous manner with response to both tumorigenesis and treatment response.(8,9,14) Most recently, we developed an evolutionarily-based scoring system to classify TP53 mutations as: 1) “low-risk” missense mutations, 2) “high-risk” missense mutations, and 3) “other” TP53 mutations (e.g. nonsense, splice site, insertion-deletion). Although this classification overlaps some of the older systems (i.e. disruptive vs non-disruptive), it has been shown to correlate with both prognosis and treatment response in both pre-clinical models and patient cohorts.(8,9,14) Here, we demonstrate that “high-risk” TP53 mutations are also associated with pN+ENE+ status in 2 distinct patient cohorts. Whether “high-risk” TP53 mutations actually drive pN+ENE+ development cannot be addressed via retrospective analysis and will require additional investigation using existing pre-clinical disease models. However, if both ENE development and treatment resistance are in fact partially driven by “high-risk” TP53 mutations it is possible that “high-risk” TP53 mutations may represent a robust biomarker of ENE development and adverse clinical outcomes.
In addition, “other” mutations are likely to be functionally inactivating and could be given a score of 100. In support of this, they performed similarly to the “high-risk” mutations in the data presented here. However, “high-risk” mutations may not be strictly loss-of-function, rather, many of them have been shown to demonstrate gain-of-function properties, and there can be differences between the “high-risk” and “other” groups depending on the patient cohort or in vitro phenotype being studied.(8,9,11,13–15) For this reason the “other” mutations should be considered a separate group and not automatically combined with the “high-risk” group. Additionally, since “other” mutations are loss-of-function they are considered recessive to “low-risk” or “high-risk” mutations in patients with 2 mutations in different groups.
Although Wang et al. reported on a gene signature associated with ENE development in OSCC, our analysis did not independently identify a gene expression profile that consistently associates with ENE in the TCGA cohort.(27) The institutional patient cohort analysis was primarily focused on TP53 mutations and therefore did not provide gene expression information. The lack of complete concordance between TP53 mutational status of the tumor and corresponding metastatic lymph nodes is likely related to tumor evolution and heterogeneity. It has been shown that different regions of primary tumors can have diverse mutational profiles, likely due to divergent evolution from a single cell into multiple clonal populations in the primary tumor.(28) Sampling of the primary tumor away from a clone represented in the metastatic population would account for a discordance as would clonal expansion after metastasis of a clone carrying the mutation found in the lymph node not being found in the primary tumor. A more thorough study, focusing on just this endpoint, should be performed with additional sampling from the primary and nodal regions in order to get a better understanding for the heterogeneity present and whether this will impact the sensitivity of genomic biomarker testing.
Identification of copy number changes in LKB1 and SDHD generates intriguing hypotheses regarding a role for metabolism in ENE development, which will require dedicated investigation in future studies. We have previously shown that TP53 mutations generate a profound effect on HNSCC tumor metabolism; the presence of high-risk TP53 mutations can potentially link ENE with alterations in the tumor metabolic profile. This gives rise to a metabolic phenotype which supports a more aggressive biological phenotype, but also uncovers potential targeting strategies based on lack of metabolic flexibility. Given that our group has previously shown that TP53 mutant tumor cells exhibit decreased metabolic flexibility and enhanced susceptibility to metabolic inhibition, this may represent a viable therapeutic strategy.(12,14,29,30)
Supplementary Material
Statement of translational relevance.
Oral squamous cell carcinoma (OSCC) has a high mortality rate and cervical lymph node metastasis and development of extra nodal extension (ENE) are clearly associated with the development of regional relapse, distant metastases, and death from disease. The factors which contribute to ENE development remain poorly understood. Therefore, we evaluated the genomic profile of OSCC clinical samples to determine whether ENE development is associated with a unique, identifiable biomarker signature. Our data demonstrate a robust association between ENE development and the presence of high-risk TP53 mutations previously shown to be associated with treatment resistance in both pre-clinical models and OSCC patients. Our findings suggest an urgent clinical need to understand the mechanisms by which high-risk TP53 mutations drive disease progression and treatment response in order to improve clinical outcomes for OSCC patients.
Acknowledgments
This work was supported by National Institute of Dental and Craniofacial Research 5 R01 DE014613 12 (J. Myers).
Footnotes
Conflict of interest: the authors have no conflict of interests to disclose
References
- 1.Society AC; Society AC. Cancer Facts & Figures 2016. 2016. [Google Scholar]
- 2.Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92(12):3030–6. doi: 10.1002/1097-0142(20011215)92:12<3030::aid-cncr10148>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Chinn SB, Myers JN. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J Clin Oncol. 2015;33(29):3269–76. doi: 10.1200/JCO.2015.61.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michikawa C, Uzawa N, Kayamori K, Sonoda I, Ohyama Y, Okada N, et al. Clinical significance of lymphatic and blood vessel invasion in oral tongue squamous cell carcinomas. Oral Oncol. 2012;48(4):320–4. doi: 10.1016/j.oraloncology.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26(3–4):645–62. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakata Y, Uzawa N, Takahashi K, Sumino J, Michikawa C, Sato H, et al. EGFR gene copy number alteration is a better prognostic indicator than protein overexpression in oral tongue squamous cell carcinomas. Eur J Cancer. 2011;47(15):2364–72. doi: 10.1016/j.ejca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Neskey DM, Osman AA, Ow TJ, Katsonis P, McDonald T, Hicks SC, et al. Evolutionary Action Score of TP53 Identifies High-Risk Mutations Associated with Decreased Survival and Increased Distant Metastases in Head and Neck Cancer. Cancer Res. 2015;75(7):1527–36. doi: 10.1158/0008-5472.CAN-14-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osman AA, Neskey DM, Katsonis P, Patel AA, Ward AM, Hsu TK, et al. Evolutionary Action Score of TP53 Coding Variants Is Predictive of Platinum Response in Head and Neck Cancer Patients. Cancer Res. 2015;75(7):1205–15. doi: 10.1158/0008-5472.CAN-14-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3(7):770–81. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552–61. doi: 10.1056/NEJMoa073770. doi 357/25/2552 [pii] 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandulache VC, Skinner HD, Ow TJ, Zhang A, Xia X, Luchak JM, et al. Individualizing antimetabolic treatment strategies for head and neck squamous cell carcinoma based on TP53 mutational status. Cancer. 2012;118(3):711–21. doi: 10.1002/cncr.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sano D, Xie TX, Ow TJ, Zhao M, Pickering CR, Zhou G, et al. Disruptive TP53 mutation is associated with aggressive disease characteristics in an orthotopic murine model of oral tongue cancer. Clin Cancer Res. 2011;17(21):6658–70. doi: 10.1158/1078-0432.CCR-11-0046. doi 1078-0432.CCR-11-0046 [pii] 10.1158/1078-0432.CCR-11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skinner HD, Sandulache VC, Ow TJ, Meyn RE, Yordy JS, Beadle BM, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res. 2012;18(1):290–300. doi: 10.1158/1078-0432.CCR-11-2260. doi 1078-0432.CCR-11-2260 [pii] 10.1158/1078-0432.CCR-11-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou G, Wang J, Zhao M, Xie TX, Tanaka N, Sano D, et al. Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol Cell. 2014;54(6):960–74. doi: 10.1016/j.molcel.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zafereo ME, Xu L, Dahlstrom KR, Viamonte CA, El-Naggar AK, Wei Q, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol. 2016;56:47–53. doi: 10.1016/j.oraloncology.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center BITGDA; Harvard BIoMa. Analysis Overview for Head and Neck Squamous Cell Carcinoma (Primary solid tumor cohort) 2016 [Google Scholar]
- 18.D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N Engl J Med. 2015;373(6):521–9. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 19.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlstrom KR, Calzada G, Hanby JD, Garden AS, Glisson BS, Li G, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119(1):81–9. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garden AS, Kies MS, Morrison WH, Weber RS, Frank SJ, Glisson BS, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol. 2013;8:21. doi: 10.1186/1748-717X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandulache VC, Hamblin J, Lai S, Pezzi T, Skinner HD, Khan NA, et al. Oropharyngeal squamous cell carcinoma in the veteran population: Association with traditional carcinogen exposure and poor clinical outcomes. Head Neck. 2015;37(9):1246–53. doi: 10.1002/hed.23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grover S, Swisher-McClure S, Mitra N, Li J, Cohen RB, Ahn PH, et al. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. Int J Radiat Oncol Biol Phys. 2015;92(3):594–601. doi: 10.1016/j.ijrobp.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, et al. Assembly And Initial Characterization Of A Panel Of 85 Genomically Validated Cell Lines From Diverse Head And Neck Tumor Sites. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandulache VC, Chen Y, Skinner HD, Lu T, Feng L, Court LE, et al. Acute Tumor Lactate Perturbations as a Biomarker of Genotoxic Stress: Development of a Biochemical Model. Mol Cancer Ther. 2015;14(12):2901–8. doi: 10.1158/1535-7163.MCT-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandulache VC, Chen Y, Feng L, William WN, Skinner HD, Myers JN, et al. Metabolic interrogation as a tool to optimize chemotherapeutic regimens. Oncotarget. 2017;8(11):18154–65. doi: 10.18632/oncotarget.15186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Lim WK, Leong HS, Chong FT, Lim TK, Tan DS, et al. An eleven gene molecular signature for extra-capsular spread in oral squamous cell carcinoma serves as a prognosticator of outcome in patients without nodal metastases. Oral Oncol. 2015;51(4):355–62. doi: 10.1016/j.oraloncology.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandulache VC, Ow TJ, Pickering CR, Frederick MJ, Zhou G, Fokt I, et al. Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer. 2011;117(13):2926–38. doi: 10.1002/cncr.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41–5. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


