Abstract
Survivors of acute respiratory distress syndrome (ARDS) experience severe muscle wasting. Upper arm anthropometrics can provide a quick, non-invasive estimate of muscle status, but its accuracy is unknown. This study examines the accuracy of upper arm percent muscle area (UAMA) with reference measures of lean mass from dual energy X-ray absorptiometry (DXA). Data are from 120 ARDS survivors participating in a multi-center national study. Receiver Operating Characteristic (ROC) Curves, by patient sex, demonstrated that UAMA did no better than chance in discriminating low appendicular skeletal muscle mass identified using DXA findings (c-statistics, 6m: 0.50-0.59, 12m: 0.54-0.57). Modest correlations of UAMA with DXA measures (whole-body: r=0.46-0.49, arm-specific: r=0.50-0.51, p<0.001) and Bland-Altman plots indicate poor precision. UAMA is not an appropriate screening measure for estimating muscle mass when compared to a DXA reference standard. Alternate screening measures should be evaluated in ARDS survivors.
Keywords: lean mass, anthropometrics, dual energy X-ray absorptiometry (DXA), critical illness survivors, acute respiratory distress syndrome (ARDS)
Introduction
Patients with acute respiratory distress syndrome (ARDS) experience substantial muscle wasting within the first week of admission to the intensive care unit(1). By hospital discharge, ARDS survivors had an 18% mean loss in body weight(2). Monitoring changes in body composition post-discharge is critical given the muscle loss and the associated functional impairments that are commonly observed in this population(3,4). Furthermore, evaluations of nutrition and exercise interventions aimed at improving muscle mass, strength, and physical functioning in these patients, require accurate measures of muscle mass.
Upper arm anthropometrics can provide quick, non-invasive screening measures of muscle area(5). However, it is unclear how well upper arm percent muscle area (UAMA) performs relative to measures of lean mass from reference standards, such as dual energy X-ray absorptiometry (DXA). If UAMA accurately replicates DXA measures, it can be a practical and valid option for monitoring muscle mass in ARDS survivors. Using data from a national, multicenter prospective cohort of ARDS survivors, this study examines the accuracy of UAMA relative to DXA-based estimates of lean mass.
Methods
Study Population
Data were collected as part of the ARDS Network (ARDSNet) Long-Term Outcome Study (ALTOS), a national multicenter prospective study that longitudinally evaluated survivors 6 and 12 months after ARDS. Supplemental funding (R01HL091760-02S1) provided for DXA scans at 5 ALTOS study centers Consent for DXA assessments were provided as part of written informed consent. Institutional review boards at Johns Hopkins University and participating study centers approved the study. The online methods supplement provides details on study population and DXA assessments.
Measurements
Dual energy X-ray absorptiometry
DXA-based percent lean mass for the whole body and right arm was evaluated at 6m and 12m. Of 120 ARDS survivors with ≥1 DXA scan, 97 had a scan at 6m and 91 at 12m. Appendicular skeletal muscle mass (ASMM), a DXA-based measure, was calculated as the sum of lean mass in the arms and legs (kg) divided by height squared (m2). Low ASMM (<7.26 kg/m2 in men and <5.45 kg/m2 in women) has been used to indicate sarcopenia(6).
Percent Muscle Area1
UAMA(7) was calculated as the mean of three triceps skinfold measurements and three mid-arm circumference measurements, all made on the right arm. Research personnel underwent rigorous in-person training and ongoing quality assurance assessments for completing anthropometric assessment.
Statistical Analysis
We examined the cross-sectional correlation of DXA whole body and right arm percent lean mass with UAMA. Additionally, Bland-Altman plots, comparing UAMA with corresponding DXA right arm percent lean mass, was created to assess measurement bias and accuracy. We used Receiver Operating Characteristic (ROC) Curves to evaluate, by patient sex, the ability of UAMA to discriminate between clinically different groups: survivors with vs. without low ASMM, which represents one aspect of sarcopenia. C-statistic of 1.0 indicates perfect discrimination; 0.5 reflects poor discrimination. Analyses were repeated for each follow-up and by baseline obesity status (obese = BMI ≥30). SAS® 9.4 was used for all analyses.
Results
Survivors with low ASMM were older, primarily white, had lower BMI, and longer hospital length of stay (Table 1). Correlation was modest between both UAMA and DXA percent lean mass of whole body (6m: r=0.46, 95%CI: 0.28, 0.61; 12m: r=0.49, 95%CI: 0.31, 0.64) and of the right arm (6m: r=0.50, 95%CI: 0.32, 0.65; 12m: r=0.51, 95%CI: 0.32, 0.66). Bland-Altman plots confirm the low accuracy of UAMA for replicating DXA right arm percent lean mass, with wide 95% limits of agreement for the mean difference between these methods. DXA estimates were higher than UAMA estimates (6m: 7.7, 95%CI: -12.0, 27.5; 12m: 6.8, 95%CI: -14.1, 27.7), although this bias was not significant at either follow-up. Bland-Altman plots and scatterplots are provided in the online data supplement (Appendix Figure A1-2).
Table 1. Participant Characteristics.
| Variables | Total N=120 | Low ASMM† N=30 | Normal ASMM N=50 | p-value |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
|
| ||||
| Age, mean (sd) | 50 (15) | 57 (14) | 46 (14) | <.001 |
|
| ||||
| Male, n (%) | 57 (48) | 15 (50) | 20 (40) | 0.383 |
|
| ||||
| White Race, n (%) | 103 (86) | 29 (97) | 38 (76) | 0.015 |
|
| ||||
| Comorbidities and Baseline Status | ||||
|
| ||||
| Charlson Comorbidity Index, mean (sd) | 1.2 (1.7) | 1.2 (1.3) | 1.1 (1.7) | 0.769 |
|
| ||||
| Functional Comorbidity Index, mean (sd) | 1.9 (1.5) | 1.6 (1.3) | 1.9 (1.4) | 0.294 |
|
| ||||
| COPD, n (%) | 6 (5) | 4 (13) | 0 (0) | 0.008 |
|
| ||||
| Rheumatologic comorbidity*, n (%) | 5 (7) | 2 (11) | 1 (3) | 0.322 |
|
| ||||
| Neurologic comorbidity, n (%) | 9 (8) | 3 (10) | 6 (12) | 0.784 |
|
| ||||
| BMI in hospital WHO category, n (%) | <.001 | |||
| < 18.5 | 1 (<1) | 1 (3) | 0 (0) | |
| 18.5 - < 25 | 20 (17) | 9 (30) | 5 (10) | |
| 25 - <30 | 37 (31) | 16 (53) | 12 (24) | |
| ≥30 | 62 (52) | 4 (13) | 33 (66) | |
|
| ||||
| Normalized SF-36 PF, mean (sd) | 44 (13) | 42 (13) | 45 (13) | 0.229 |
|
| ||||
| ICU and Hospital Exposures | ||||
|
| ||||
| APACHE III severity of illness score, mean (sd) | 86 (28) | 90 (30) | 81 (26) | 0.206 |
|
| ||||
| Days with organ failure, mean (sd) | 0.7 (0.1) | 0.7 (0.2) | 0.7 (0.1) | 0.508 |
|
| ||||
| Ever used steroids, n (%) | 53 (44) | 15 (50) | 22 (44) | 0.602 |
|
| ||||
| Ever used neuromuscular blockade, n (%) | 30 (25) | 8 (27) | 12 (24) | 0.790 |
|
| ||||
| ICU length of stay, mean (sd) | 15 (11) | 17 (11) | 14 (10) | 0.175 |
|
| ||||
| Hospital length of stay, mean (sd) | 22 (15) | 27 (16) | 19 (11) | 0.020 |
COPD= Chronic Obstructive Pulmonary Disease, BMI= body-mass index, ICU=Intensive Care Unit, WHO=World Health Organization, SF-36 PF= Short Form-36 physical function, APACHE= Acute Physiologic Assessment and Chronic Health Evaluation;
Low appendicular skeletal muscle mass (ASMM) was used to reflect low muscle mass, which is one component of sarcopenia.30 patients had low ASMM at either 6m or 12m follow-up and 50 patients had normal ASMM at either follow-up.
Rheumatologic comorbidity data not collected in the SAIL study, percentage based on N=74.
ROC analysis indicated that UAMA performed no better than chance (c-statistics: 6m=0.50-0.59; 12m=0.54-0.57) in identifying survivors with low ASMM for either males or females (Figure).
Figure.
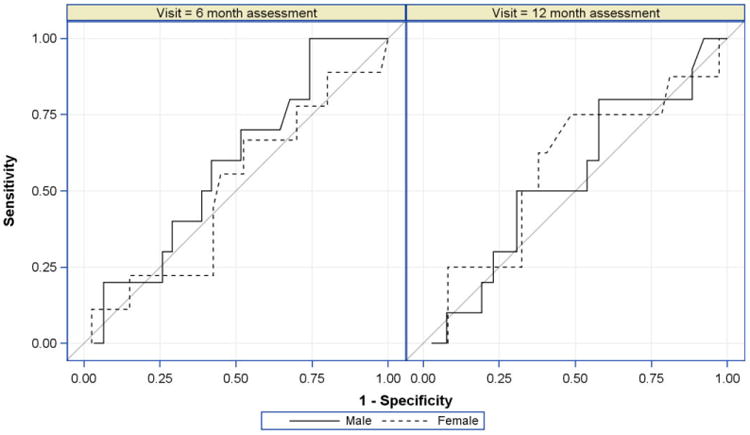
Ability of Percent Upper Arm Muscle Area (UAMA) to Discriminate Low ASMM, Receiver Operating Characteristic Curves by Patient Sex at 6m and 12m Follow-up. Male (6m: N=41, 12m: N=36), Female (6m: N=49, 12m: N=45).
Findings were similar by obesity status, with modest correlations (obese: r=0.46-0.55; non-obese: r=0.48-0.60) and low accuracy for UAMA based on Bland-Altman plots. We did not conduct ROC analysis by subgroup as there were too few obese patients with low ASMM. See online data supplement Appendix B for full results.
Discussion
In our multi-center national study of 120 ARDS survivors, UAMA did not accurately replicate whole-body or arm-specific estimates from DXA, a reference method for assessing lean mass. These findings indicate that UAMA, despite its practicality, does not provide an adequate estimate of muscle mass in ARDS survivors.
Concerns regarding the accuracy and reproducibility of muscle area anthropometric measures have been raised in studies of elderly patients(8). Our findings suggest that alternative measures of muscle mass are also needed for ARDS survivors. Our multi-center sample, while typical of ARDS survivors, was generally younger with a higher BMI than samples included in the prior review(8). Although we found similar correlations between UAMA and DXA measures in both obesity groups, there were too few obese survivors with low ASMM for valid ROC analysis. Future studies should investigate whether similar findings would be observed in other populations of critical illness survivors, including obese patients. Finally, all body composition assessment methods, including DXA, have limitations when used during non-steady state conditions, which could not be confirmed in our study.
Our findings have practical implications. BMI and UAMA based on anthropometric measurements are commonly used in dietetic practice to assess malnutrition risk and changes in body composition. While practical tools are necessary for dietetic assessments, our findings suggest caution is needed in interpreting UAMA as indicators of ARDS survivors' muscle status.
Accurate estimates of muscle mass, along with muscle function (e.g, strength), is important for determining functional sarcopenia(9). More reliable and practical tools are needed to help identify survivors who may benefit from nutrition and rehabilitation interventions and improve outcomes of ARDS survivors.
Supplementary Material
Acknowledgments
This research was supported by the NHLBI (R24 HL111895, R01HL091760, R01HL091760-02S1, R01HL096504, R01HL88045 and P050HL73994), the Johns Hopkins Institute for Clinical and Translational Research (ICTR) (UL1 TR 000424-06), and the ALTA, EDEN, OMEGA and SAILS trials (contracts for sites participating in this study: HSN268200536170C, HHSN268200536171C, HHSN268200536173C, HHSN268200536174C, HSN268200536175C, and HHSN268200536179C).
Footnotes
Percent muscle area is calculated as UAMA/TUA where Upper Arm Muscle Area (UAMA) = [C − (Ts × π)]2 / (4 × π) and Total upper arm area (TUA) = C2/ (4 × π); C = mid-arm circumference, Ts = triceps skinfold.
KSC, MM and DMN conceived and designed the study. All authors contributed to the analysis plan. MM, CLH, EWE, PEM, ROH, DMN, and VDD acquired the data and LAF performed all analyses. All authors were involved in the interpretation of study results. KSC and MM drafted the manuscript and all authors critically revised it for important intellectual content and approved the final version to be submitted.
Financial disclosure(s): None declared.
Conflicts of interest: None declared.
The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network: University of Washington, Harborview (*L. Hudson, S. Gundel, C. Hough, M. Neff, K. Sims, A. Ungar, T. Watkins); Baystate Medical Center (*J. Steingrub, M. Tidswell, E. Braden, L. DeSouza, C. Kardos, L. Kozikowski, S. Ouellette); Baylor College of Medicine (K. Guntupalli, V. Bandi, C. Pope, C. Ross); Johns Hopkins University (*R. Brower, H. Fessler, D. Hager, P. Mendez-Tellez, D. Needham, K. Oakjones); Johns Hopkins Bayview Medical Center (J. Sevransky, A. Workneh); University of Maryland (C. Shanholtz, D. Herr, H. Howes, G. Netzer, P. Rock, A. Sampaio, J. Titus); Union Memorial Hospital (P. Sloane, T. Beck, D. Highfield, S. King); Washington Hospital Center (B. Lee, N. Bolouri); Cleveland Clinic Foundation (*H.P. Wiedemann, R.W. Ashton, D.A. Culver, T. Frederick, J.A. Guzman, J.J. Komara Jr, A.J. Reddy); University Hospitals of Cleveland (R. Hejal, M. Andrews, D. Haney); MetroHealth Medical Center (A.F. Connors, S. Lasalvia, J.D. Thornton, E.L. Warren); University of Colorado Hospital, Aurora (*M. Moss, E.L. Burnham, L. Gray, J. Maloney, M. Mealer); Denver Health Medical Center (I. Douglas, K. Overdier, K. Thompson, R. Wolken); Rose Medical Center (S. Frankel, J. McKeehan); Swedish Medical Center (M.L. Warner); Saint Anthony's Hospital (T. Bost, C. Higgins, K. Hodgin); Duke University (*N. MacIntyre, L. Brown, C. Cox, M. Gentile, J. Govert, N. Knudsen); University of North Carolina (S. Carson, L. Chang, S. Choudhury, W. Hall, J. Lanier); Vanderbilt University (*A.P. Wheeler, G.R. Bernard, M. Hays, S. Mogan, T.W. Rice); Wake Forest University (*R.D. Hite, A. Harvey, P.E. Morris, Mary Ragusky); Moses Cone Memorial Hospital (P. Wright, S. Groce, J. McLean, A. Overton); University of Virginia (J. Truwit, K. Enfield, M. Marshall); Intermountain Medical Center (*A. Morris, *C. Grissom, A. Austin, S. Barney, S. Brown, J. Ferguson, H. Gallo, T. Graydon, E. Hirshberg, A. Jephson, N. Kumar, M. Lanspa, R. Miller, D. Murphy, J. Orme, A. Stowe, L. Struck, F. Thomas, D. Ward,); LDS Hospital (P. Bailey, W. Beninati, L. Bezdjian, T. Clemmer, S. Rimkus, R. Tanaka, L. Weaver); McKay Dee Hospital (C. Lawton, D. Hanselman); Utah Valley Regional Medical Center (K. Sundar, W. Alward, C. Bishop, D. Eckley, D. Harris, T. Hill, B. Jensen, K. Ludwig, D. Nielsen, M. Pearce); University of California, San Francisco (*M.A. Matthay, C. Calfee, B. Daniel, M. Eisner, O. Garcia, K. Kordesch, K. Liu, N. Shum, H. Zhou); University of California, San Francisco, Fresno (M.W. Peterson, J. Blaauw, K. Van Gundy); San Francisco General Hospital (R. Kallet and E. Johnson); University of California, Davis (T. Albertson, B. Morrissey, E. Vlastelin); Louisiana State University Health Sciences Center-New Orleans (*B. deBoisblanc, A. Antoine, D. Charbonnet, J. Hunt, P. Lauto, A. Marr, G. Meyaski, C. Romaine,); Earl K. Long Medical Center (S. Brierre, J. Byrne, T. Jagneaux, C. LeBlanc, K. Moreau, C. Thomas); Ochsner Clinic Foundation (S. Jain, D. Taylor, L. Seoane); Our Lady of the Lake Medical Center (C. Hebert, J. Thompson); Tulane Medical Center (F. Simeone, J. Fearon). Clinical Coordinating Center: Massachusetts General Hospital and Harvard Medical School (*D. Schoenfeld, N. Dong, M. Guha, E. Hammond, P. Lazar, R. Morse, C. Oldmixon, N. Ringwood, E. Smoot, B.T. Thompson, R. Wilson). National Heart, Lung and Blood Institute: A. Harabin, S. Bredow, M. Waclawiw, G. Weinmann. Data and Safety Monitoring Board: R. G. Spragg (chair), A. Slutsky, M. Levy, B. Markovitz, E. Petkova, C. Weijer. Protocol Review Committee: J. Sznajder (chair), M. Begg, L. Gilbert-McClain E. Israel, J. Lewis, S. McClave, P. Parsons.
*Principal investigator.
References
- 1.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute Skeletal Muscle Wasting in Critical Illness. J Am Med Assoc. 2013:1–10. doi: 10.1001/jama.2013.278481. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24108501. [DOI] [PubMed]
- 2.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22492988%5Cnhttp://www.nejm.org/doi/full/10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 4.Pfoh ER, Wozniak AW, Colantuoni E, Dinglas VD, Tellez PAM, Shanholtz C, et al. Intensive Care Med. 10. Vol. 42. Springer; Berlin Heidelberg: 2016. Physical declines occurring after hospital discharge in ARDS survivors: a 5 - year longitudinal study; pp. 1557–66. [DOI] [PubMed] [Google Scholar]
- 5.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez Pa, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014 Apr;42(4):849–59. doi: 10.1097/CCM.0000000000000040. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24247473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9554417. [DOI] [PubMed] [Google Scholar]
- 7.Frisancho AR. Anthropometric Standards for the Assessment of Growth and Nutritional Status. Ann Arbor, MI: University of Michigan Press; 1990. [Google Scholar]
- 8.Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. Aging Clin Exp Res. 6. Vol. 28. Springer International Publishing; 2016. The role of DXA in sarcopenia; pp. 1047–60. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(April):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


