Infections caused by carbapenemase-producing bacteria have experienced unprecedented intercontinental spread and proliferation and continue to be a therapeutic challenge. The genetic features that facilitate widespread dissemination are becoming increasingly understood. Control requires efficient laboratory detection and treatment and a coordinated international response.
Keywords: carbapenemase, carbapenemase detection tests, carbapenem-producing organisms, carbapenem-resistant Enterobacteriaceae, metallo-β-lactamase
Abstract
The dramatic increase in the prevalence and clinical impact of infections caused by bacteria producing carbapenemases is a global health concern. Carbapenemase production is especially problematic when encountered in members of the family Enterobacteriaceae. Due to their ability to readily spread and colonize patients in healthcare environments, preventing the transmission of these organisms is a major public health initiative and coordinated international effort are needed. Central to the treatment and control of carbapenemase-producing organisms (CPOs) are phenotypic (growth-/biochemical-dependent) and nucleic acid–based carbapenemase detection tests that identify carbapenemase activity directly or their associated molecular determinants. Importantly, bacterial isolates harboring carbapenemases are often resistant to multiple antibiotic classes, resulting in limited therapy options. Emerging agents, novel antibiotic combinations and treatment regimens offer promise for management of these infections. This review highlights our current understanding of CPOs with emphasis on their epidemiology, detection, treatment, and control.
One of the most concerning forms of antimicrobial resistance (AMR) is resistance to the carbapenems, especially when observed in members of the family Enterobacteriaceae. A primary mechanism of carbapenem resistance in gram-negative bacteria is acquired carbapenemases, enzymes that hydrolyze these antibiotics. In this review, the epidemiology, laboratory detection, approaches to combat widespread dissemination, and treatment strategies for carbapenemase-producing organisms (CPOs), especially carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE), will be discussed.
THE BIOLOGY AND EPIDEMIOLOGY OF CPOs
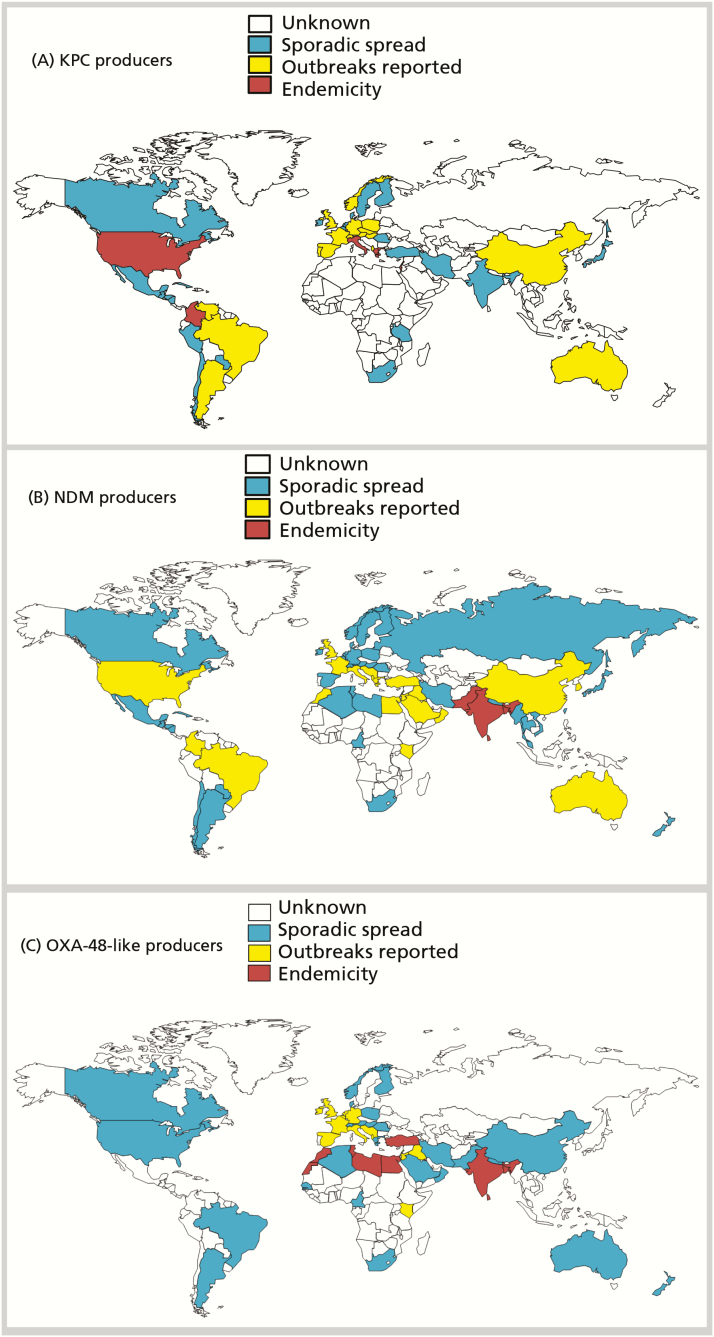
Phenotypic resistance to carbapenems in gram-negative bacteria commonly results from acquisition of carbapenemases, or production of cephalosporinases combined with mutations that decrease permeability of the bacterial cell wall to entry of carbapenems [1]. CPOs may exhibit significant variation in carbapenem minimum inhibitory concentration (MIC) values depending on their permeability status, the rate of carbapenem hydrolysis by the associated enzyme, and the level of gene expression [1]. Carbapenemases belong to Ambler classes A, B, or D, with class A and D enzymes possessing a serine-based hydrolytic mechanism, and class B enzymes requiring 1 or 2 zinc ions for their catalytic activity [1]. There is a rare instance of class C β-lactamase that is reported to hydrolyze imipenem (CMY-10) [2]. Globally distributed in many genera of bacteria, certain carbapenemases are typically associated with specific regions or countries (Figure 1). However, in an era of widespread international travel and exposure to medical care, the association between a specific resistance mechanism and a given region or country may change, creating an urgent need for routine local and national surveillance.
Figure 1.
Worldwide distribution of carbapenemases. A, Klebsiella pneumoniae carbapenemase producers in Enterobacteriaceae and Pseudomonas aeruginosa. B, New Delhi metallo-β-lactamase producers in Enterobacteriaceae and P. aeruginosa. C, OXA-48–like producers in Enterobacteriaceae. Abbreviations: KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; OXA-48, oxacillinase-48.
The class A Klebsiella pneumoniae carbapenemase (KPC) has been extensively reported in K. pneumoniae and other Enterobacteriaceae, but has also been identified in other gram-negative pathogens including Pseudomonas aeruginosa [3]. KPC-producing K. pneumoniae is widespread in the United States, but is also endemic in some European countries such as Greece and Italy (Figure 1A) [4].
Class B β-lactamases, or metallo-β-lactamases (MBLs), are commonly identified in Enterobacteriaceae and Pseudomonas aeruginosa [5]. Among the MBLs, New Delhi metallo-β-lactamase (NDM) (Figure 1B), Verona integron-encoded metallo-β-lactamase (VIM), and imipenemase metallo-β-lactamase (IMP) enzymes are the most frequently identified worldwide [5]. IMP producing gram-negative bacteria are mainly detected in China, Japan, and Australia, mostly in Acinetobacter baumannii. VIM producers are most often found in Italy and Greece (Enterobacteriaceae) and in Russia (P. aeruginosa) [6, 7].
Acquired class D carbapenem-hydrolyzing β-lactamases are commonly reported in A. baumannii (mainly OXA [oxacillinase]-23, OXA-24/40-, and OXA-58–like enzymes), but not in P. aeruginosa. OXA-48 and derivatives (eg, OXA-181 and OXA-232) have been detected in Enterobacteriaceae, hydrolyze narrow-spectrum β-lactams and weakly hydrolyze carbapenems, but spare broad-spectrum cephalosporins (ceftazidime, cefepime) [8]. OXA-48–producing Enterobacteriaceae are endemic in Turkey (since 2004) and are frequently encountered in several European countries (eg, France and Belgium), and across North Africa (Figure 1C) [9]. Ten variants of OXA-48 β-lactamases are acknowledged and are increasingly reported worldwide [9], notably among nosocomial K. pneumoniae and community Escherichia coli isolates [10].
Carbapenemase genes are often located on mobile genetic elements, further enhancing their spread. For example, the widespread dissemination of the blaOXA-48 gene was shown to be related to a successful and epidemic plasmid that conjugates at high rates within Enterobacteriaceae [11].
Other less common carbapenemases belonging to a variety of molecular classes (eg, class A FRI-1 and IMI-like β-lactamases, class B SPM-1 and GIM-1, and class D OXA-198) are reported sporadically and are found in specific species, likely because the corresponding genes are located on narrow-host-range plasmids or chromosomes, which makes wide diffusion unlikely [10, 12].
LABORATORY DETECTION OF CPOs
Detection of carbapenemase-mediated carbapenem resistance is essential for patient management, infection control, and public health efforts. The diversity of these enzymes and the range of associated susceptibility phenotypes make detection challenging. Selection of a carbapenemase detection test (CDT) is contingent on several factors: epidemiology, diagnostic performance, labor intensity, complexity, and cost. The importance of turnaround time depends on whether the assay will be employed for therapeutic decision making and/or infection control or surveillance studies.
CDTs are broadly differentiated into 2 groups: phenotypic (growth-/biochemical-dependent) and nucleic acid-based. Phenotypic assays monitor carbapenemase activity through a variety of methods: growth of a susceptible reporter strain following drug inactivation by a carbapenemase-producing test strain, observation of a pH change after β-lactam ring hydrolysis, detection of carbapenem hydrolysis products, or via inhibition with small molecules. In contrast, nucleic acid assays detect genetic determinants associated with carbapenemases.
The modified Hodge test (MHT) is probably the most extensively described CDT used in Enterobacteriaceae. This assay demonstrates acceptable sensitivity for most carbapenemases, especially KPC enzymes, but low sensitivity for NDM-producing strains [13, 14]. Additionally, it has poor specificity as isolates producing cephalosporinases in conjunction with porin mutations are often false-positive [13, 15]. Although the MHT is inexpensive and uncomplicated to perform, it is often difficult to interpret and requires an additional 24-hour growth step after antimicrobial susceptibility test (AST) results are obtained.
Conceptually akin to the MHT, the carbapenem inactivation method (CIM) assesses growth of a susceptible reporter strain around a carbapenem disk previously incubated with a suspension of a suspected carbapenemase-producing test strain [16]. If the test strain produces a carbapenemase, drug in the disk will be inactivated, thus allowing growth of the reporter strain up to the edge of the disk. In contrast, a zone of growth inhibition indicates the antibiotic in the disk remains active and the test strain lacks carbapenemase activity. CIM sensitivity is reported to be between 98% and 100% [16, 17], but again this technique typically requires an overnight culture step. A modified version of the CIM (mCIM) was evaluated in a multicenter study, demonstrating 97% sensitivity and 99% specificity for detection of carbapenemase production in Enterobacteriaceae [18]. Based on those data, the mCIM was added to the Clinical and Laboratory Standards Institute M100 document as a reliable method for detection of carbapenemase production in Enterobacteriaceae [19].
The Carba NP test (RAPIDEC CARBA NP, bioMérieux, Durham, North Carolina), its derivatives, and matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) monitor the hydrolysis of carbapenems using bacterial extracts and produce same-day results [20, 21]. In the Carba NP test, carbapenemase-dependent hydrolysis of imipenem causes a decrease in pH, registered by a pH indicator as a color change. The test exhibits excellent sensitivity [20], although the recognition of OXA-48–producing isolates may be challenging [17, 22]. To aid in early identification, the Carba NP test has been successfully extended to detect the presence of CP-CRE in positive blood cultures even before isolation of organism on solid media [23].
MALDI-TOF MS can identify carbapenem degradation products following incubation of a bacterial protein extract with a carbapenem substrate. Overall, the sensitivity of MALDI-TOF MS for this purpose is high, and sensitivity for OXA-48–producing isolates is enhanced by inclusion of bicarbonate in the reaction buffer [22]. Despite the potential of mass spectrometry–based assays, because they are complex to perform and interpret, widespread implementation in clinical microbiology laboratories may be unfeasible.
Conventional AST methods such as broth microdilution, disk diffusion, and gradient diffusion can be modified to detect different classes of carbapenemases by performing them in the absence and presence of small molecule inhibitors, including phenylboronic acid, which inhibits serine active site enzymes, and ethylenediaminetetraacetic acid, an inhibitor of MBL activity. These assays have reportedly high sensitivities and specificities [24–28] and are inexpensive and generally easy to implement and interpret, but require overnight incubation.
Nucleic acid–based CDTs include commercially available and laboratory-developed polymerase chain reaction (PCR) and microarray platforms to detect carbapenemase genes in bacterial isolates or directly from clinical specimens. They exhibit clinically relevant sensitivities and specificities and have same-day turnaround times [29–33] but are typically associated with high costs. PCR- and microarray-based platforms only detect certain carbapenemase genes and thus would not detect the emergence of new or previously uncommon carbapenemases.
Whole-genome sequencing (WGS) platforms potentially represent the ultimate molecular CDT by interrogating the entire genomic content, chromosomal and extrachromosomal, of a bacterium to identify carbapenem resistance determinants [34–36]. Furthermore, WGS data provide an opportunity to query for extra information, including strain relatedness, plasmid types encoding the carbapenemase, other factors influencing carbapenem resistance (eg, porin mutations), and presence of additional resistance factors, and data can be analyzed in near real-time or archived for future inquiry. Notwithstanding the power and promise of WGS, these assays are still the purview of advanced clinical microbiology and public health laboratories, and require considerable expertise to perform and interpret. As algorithms improve, costs decrease, and commercialized options are brought to market, the clinical workforce is likely to become increasingly proficient at performing and interpreting these data, allowing WGS to gain wider acceptance.
WGS FOR INVESTIGATION OF THE EPIDEMIOLOGY AND DIVERSITY OF CPOs
Recent studies indicate that WGS, combined with hospital epidemiology, may facilitate the tracking of transmissions within healthcare facilities with the level of precision necessary to guide the modification of infection control procedures and limit the spread of healthcare-associated infections [34–39]. One example is the National Institutes of Health Clinical Center outbreak in which a single patient colonized on admission with KPC-producing K. pneumoniae was eventually linked to CP-CRE colonization in 18 additional patients. The epidemiologic data could not discriminate between undetected transmission from the index patient or introduction of a second strain. The extensive genetic similarity among KPC-producing K. pneumoniae in the United States prevented a definitive match to the index patient using standard outbreak investigation tools such as pulsed-field gel electrophoresis or repetitive-element PCR. WGS revealed direct linkage of the index patient, with transmission originating from different anatomic sites [34], indicating silent colonization, even in immunocompromised patients. In another healthcare-related outbreak, WGS was instrumental in identifying limited healthcare-associated transmission of CP-CRE against a background of sporadic introduction of multiple other strains [36]. In other studies, WGS was key in determining the phylogeny of carbapenem-resistant Enterobacter species and how gene regulation by insertion sequence elements impacted carbapenem and multidrug resistance in A. baumannii [40, 41]. WGS has also been used to create a reference set capturing the diversity of plasmids and mobile elements that carry the KPC gene [36, 42].
NOVEL TREATMENT STRATEGIES FOR CPOs
Treatment of CPO, especially CP-CRE, remains difficult. Patients with CP-CRE infection suffer unacceptably high mortality, emphasizing the need for novel diagnostics and therapies. Studies performed to date demonstrate a bias to report trials of successful combination chemotherapy, informed largely by results from in vitro studies. In most trials targeting CP-CRE, combination therapies have included the use of (i) colistin (polymyxin E) and a carbapenem; (ii) colistin and tigecycline, or colistin and fosfomycin; or (iii) double carbapenem therapy. Interestingly, it was also shown in vitro that dual carbapenem combinations might work against carbapenemase-producing strains, with significant synergies observed when using imipenem and another carbapenem [43].
In an early study performed at a tertiary care center, Qureshi and colleagues reported that 28-day mortality was 13.3% in the combination therapy group (colistin and another agent) compared with 57.8% in the monotherapy group (P = .01) and that combination regimens were independently associated with better survival (P = .02) [44]. Additionally, a multicenter retrospective cohort study conducted in 3 large Italian teaching hospitals examined death within 30 days of the first positive blood culture among 125 patients with bloodstream infections caused by KPC-producing K. pneumoniae [45]. That investigation found 54.3% mortality in the monotherapy arm vs 34.1% mortality in the combination therapy group (P = .02); triple combination therapy (tigecycline, colistin, and meropenem) was associated with lowest mortality (P = .01). This study also revealed that patients infected by CP-CRE with imipenem MIC values of ≥4 µg/mL had worse outcomes than patients whose isolates had an MIC value of ≤2 µg/mL. The “dividing line” appears to be an MIC value between 2 and 4 µg/mL, and predicted differences in mortality were notable (16.1% vs 76.9%; P < .01); each imipenem MIC doubling dilution increased the probability of death 2-fold.
In a subsequent review of 20 clinical studies involving 414 patients, Tzouvelekis and colleagues reported that a single active agent resulted in mortality rates not significantly different from those observed in patients administered no active therapy [46]. Consistent with the notions reported above, combination therapy with 2 or more agents active in vitro was superior to monotherapy, providing a clear survival benefit (mortality rate, 27.4% vs 38.7%; P < .001). The lowest mortality rate (18.8%) was observed in patients treated with carbapenem-containing combinations.
In contrast, Falagas and partners in 2014 reported the largest meta-analysis performed to date [47], examining 20 studies involving 692 patients. Surprisingly, the authors reported 50% mortality in patients treated with tigecycline and gentamicin, 64% mortality for tigecycline and colistin, and 67% mortality for carbapenems and colistin. This comprehensive analysis called into question the conclusions drawn from the earlier retrospective, nonrandomized studies, and emphasized that unexplained molecular heterogeneity and nonuniform microbiology testing might be confounding results. These differences suggest that studies concluding the superiority of combination therapy over monotherapy may not be sufficiently rigorous for us to accept their conclusions.
What about new drugs in development? Avibactam is a synthetic non-β-lactam, bicyclic diazabicyclooctane (DBO) β-lactamase inhibitor that inhibits the activities of Ambler class A and class C β-lactamases and some Ambler class D enzymes. Avibactam closely resembles portions of the cephem bicyclic ring system and has been shown to bond covalently to β-lactamases. Against carbapenemase-producing K. pneumoniae, the addition of avibactam significantly improves the activity of ceftazidime in vitro (~4-fold MIC reduction). In surveillance studies, the combination of ceftazidime with avibactam restores in vitro susceptibility against all extended-spectrum β-lactamases and most KPCs tested. Studies comparing outcomes of infections with KPC-producing gram-negative bacteria treated with ceftazidime-avibactam as monotherapy or in combination with colistin are ongoing. An important study comparing the outcomes of patients infected with CP-CRE treated with colistin vs ceftazidime-avibactam was recently performed [48]. Patients initially treated with either ceftazidime-avibactam or colistin for CP-CRE infections were selected from the Consortium on Resistance Against Carbapenems in Klebsiella and other Enterobacteriaceae (CRACKLE), a prospective, multicenter, observational study. Thirty-eight patients were treated first with ceftazidime-avibactam and 99 with colistin either as monotherapy or combination therapy. Patients treated with ceftazidime-avibactam vs colistin (monotherapy or combination) had a higher probability of a better outcome as compared to patients treated with colistin. This study strengthens the notion that treatment with a highly active agent as monotherapy in the appropriate clinical setting may be better than therapy with a less desirable agent singly or in combination.
Relebactam, also a DBO, combined with imipenem/cilistatin, will soon be evaluated in clinical studies [49]. In vitro studies indicate that imipenem/cilistatin-relebactam is comparable to ceftazidime-avibactam. The role of the combination of imipenem vs ceftazidime with different DBOs remains to be defined.
The US Food and Drug Administration (FDA) recently approved ceftazidime-avibactam based on data obtained in Phase 2/3 trials of complicated urinary tract infections and intra-abdominal infections (ceftazidime-avibactam combined with metronidazole). Despite encouraging results, the FDA cautioned that ceftazidime-avibactam should be reserved for situations when there are limited or no alternative drugs for treating an infection. The concern was that resistance to ceftazidime-avibactam would emerge in KPC-producing strains. Regrettably, resistance is already being reported due to mutations occurring in the KPC enzyme and porin changes [50, 51]
In summary, combination chemotherapies seem to be effective against KPC-producing bacteria (Table 1) [49], but we still need to design the right trial to answer the fundamental question as to why. We also need to carefully examine new drugs in the pipeline, and use clinical trials to define their best use. Other drugs in development are summarized in Table 2. The reader will note that there are some drugs specifically targeted for MBL producers (aztreonam-avibactam and cefidericol); these developments are awaited in earnest. Novel combinations (ceftazidime-avibactam paired with aztreonam) are also being explored [52]. In addition, the optimization of pharmacokinetic and pharmacodynamic parameters is essential for ensuring efficacy in difficult-to-treat infections. Activities such as testing in hollow fiber models, prolonged infusion, or continuous infusion are being aggressively evaluated to optimize drug dosing [53–55].
Table 1.
Clinical Regimens Used in Observational Studies for Treating Carbapenem-Resistant Klebsiella pneumoniae Where Carbapenemase Is Identified
| β-Lactamases Present | Regimen | Improved Survival vs Monotherapy |
|---|---|---|
| KPC- and MBL- producing Klebsiella pneumoniae | •Carbapenem and tigecycline, plus aminoglycoside or colistin •Carbapenem and tigecycline •Carbapenem and aminoglycoside •Carbapenem and colistin |
Yes |
| KPC-producing K. pneumoniae | •Colistin and aminoglycoside •Colistin and tigecycline •Colistin and quinolone •Colistin and carbapenem •Carbapenem and carbapenem |
Yes |
Abbreviations: KPC, Klebsiella pneumoniae carbapenemase; MBL, metallo-β-lactamase.
Table 2.
Novel Agents in Development for Treating Carbapenem-Resistant Organisms, Including Carbapenemase-Producing Organisms and Those Resistant to Carbapenems by Other Mechanisms
| Antibiotic | Drug Class | Intended Indication/Activity/Comments |
|---|---|---|
| Aztreonam-avibactam | Monocyclic-β-lactam and DBO BLI | Gram-negative bacteria expressing ESBLs, serine-based carbapenemases, and MBLs |
| Cefiderocol | Siderophore-β-lactam (cephalosporin) | •cUTI, carbapenem-resistant gram-negative bacterial infections •Active against MBL-producing strains |
| Ceftaroline fosamil-avibactam | Cephalosporin and DBO BLI | Currently undefined, likely CAP |
| Eravacycline | Tetracycline | •cIAI and cUTI •Multidrug-resistant gram-negative rods |
| Imipenem/cilistatin-relebactam | Carbapenem and DBO BLI | •cUTI •cIAI •HAP •Active against ESBLs and KPCs |
| LYS228 | Monobactam | MBL-producing Enterobacteriaceae including CRE |
| Meropenem-vaborbactam | Carbapenem and cyclic boronic acid BLI | •cUTI •CRBSI •HAP •VAP •cIAI due to CRE |
| Plazomycin | Aminoglycoside | •cUTI •CRBSI •HAP •VAP •cIAI due to CPOs and CRE |
Abbreviations: BLI, β-lactamase inhibitor; CAP, community acquired pneumonia; cIAI, complicated intra-abdominal infection; CPO, carbapenemase-producing organism; CRBSI, catheter-related bloodstream infection; CRE, carbapenem-resistant Enterobacteriaceae; cUTI, complicated urinary tract infection; DBO, diazabicyclooctane; ESBL, extended-spectrum β-lactamase; HAP, hospital-acquired pneumonia; KPC, Klebsiella pneumoniae carbapenemase; MBL, metallo-β-lactamase; VAP, ventilator-associated bacterial pneumonia.
MONITORING AND CONTROL OF CPOs
Approaches to addressing the rapid intercontinental spread of CPOs and other multidrug-resistant organisms include surveillance and judicious use of infection prevention and control (IPC) practices. There is evidence that IPC efforts at the local and country-wide levels are effective in reducing transmission of CPOs [56], and the role of IPC in the overall control of CPOs cannot be overemphasized. Regarding surveillance at a global level, the Global Antimicrobial Resistance Surveillance System (GLASS) program was launched in 2015 as part of the WHO Global Action Plan on AMR to support a standardized approach to collection, analysis, and sharing of AMR data to inform local and national decision making, and provide the evidence base for action and advocacy. Another approach that has been suggested is the application of the International Health Regulations (IHR), which represents a legal framework for international efforts to reduce the risk from public health threats that may spread between countries [57]. The IHR requires countries to report certain disease outbreaks, including smallpox, wild-type poliomyelitis, severe acute respiratory syndrome, new types of influenza, or any public health event of international concern (PHEIC), which may include “new or emerging antibiotic resistance” [57]. The rationale for declaring AMR, specifically CPOs, as a PHEIC has been reported previously [58] and includes multidrug resistance, propensity for rapid spread, absence of geographic/political boundaries, presence in E. coli (the most common cause of urinary tract infection globally), presence in microbes of high public health importance (namely Salmonella, Shigella, and Vibrio species), and carriage of resistance traits on very mobile broad-host-range plasmids [59]. The emergence of plasmid-mediated colistin resistance in CP-CRE has created a potential scenario of pan-resistant Enterobacteriaceae [60].
Although application of IHR to CPOs may have potential benefits including increased surveillance and response capacities to address the spread of AMR on a global basis [58], a counter-reaction argues that it is difficult to appreciate how the global spread of AMR constitutes an “extraordinary event” and that it is neither pragmatic nor within the framework of the IHR to consider it a PHEIC [61]. The only PHEICs declared to date include H1N1 2009 global influenza pandemic, Ebola virus disease in 2014, and the recent clusters of microcephaly and neurological abnormalities associated with Zika virus. In addition to global efforts under way, country-specific guidelines, including the “Combating Antibiotic-Resistant Bacteria” report and the President’s Council of Advisors on Science and Technology strategic plans, provide practical recommendations to the United States government to facilitate addressing the problem of AMR. Canada and the European Union have made similar commitments.
Notes
Acknowledgments. The authors thank Jean B. Patel, Office of Antimicrobial Resistance, US Centers for Disease Control and Prevention (CDC), for thoughtful review of the manuscript.
Disclaimer. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the CDC, the National Institutes of Health, or the Department of Health and Human Services.
Potential conflicts of interest. L. P. is co-inventor of the Carba NP, for which an international patent has been filled on behalf of INSERM (Paris, France). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 2007; 20:440–58, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim JY, Jung HI, An YJ et al. Structural basis for the extended substrate spectrum of CMY-10, a plasmid-encoded class C beta-lactamase. Mol Microbiol 2006; 60:907–16. [DOI] [PubMed] [Google Scholar]
- 3. Naas T, Bonnin RA, Cuzon G, Villegas MV, Nordmann P. Complete sequence of two KPC-harbouring plasmids from Pseudomonas aeruginosa. J Antimicrob Chemother 2013; 68:1757–62. [DOI] [PubMed] [Google Scholar]
- 4. Munoz-Price LS, Poirel L, Bonomo RA et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palzkill T. Metallo-β-lactamase structure and function. Ann N Y Acad Sci 2013; 1277:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: the quiet before the storm?Clin Microbiol Rev 2005; 18:306–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edelstein MV, Skleenova EN, Shevchenko OV et al. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect Dis 2013; 13:867–76. [DOI] [PubMed] [Google Scholar]
- 8. Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 2012; 67:1597–606. [DOI] [PubMed] [Google Scholar]
- 9. Potron A, Poirel L, Dortet L, Nordmann P. Characterisation of OXA-244, a chromosomally-encoded OXA-48-like β-lactamase from Escherichia coli. Int J Antimicrob Agents 2016; 47:102–3. [DOI] [PubMed] [Google Scholar]
- 10. Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect 2014; 20:821–30. [DOI] [PubMed] [Google Scholar]
- 11. Potron A, Poirel L, Nordmann P. Derepressed transfer properties leading to the efficient spread of the plasmid encoding carbapenemase OXA-48. Antimicrob Agents Chemother 2014; 58:467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 2015; 45:568–85. [DOI] [PubMed] [Google Scholar]
- 13. Girlich D, Poirel L, Nordmann P. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol 2012; 50:477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vasoo S, Cunningham SA, Kohner PC et al. Comparison of a novel, rapid chromogenic biochemical assay, the Carba NP test, with the modified Hodge test for detection of carbapenemase-producing gram-negative bacilli. J Clin Microbiol 2013; 51:3097–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carvalhaes CG, Picão RC, Nicoletti AG, Xavier DE, Gales AC. Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: be aware of false positive results. J Antimicrob Chemother 2010; 65:249–51. [DOI] [PubMed] [Google Scholar]
- 16. van der Zwaluw K, de Haan A, Pluister GN, Bootsma HJ, de Neeling AJ, Schouls LM. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One 2015; 10:e0123690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tijet N, Patel SN, Melano RG. Detection of carbapenemase activity in Enterobacteriaceae: comparison of the carbapenem inactivation method versus the Carba NP test. J Antimicrob Chemother 2016; 71:274–6. [DOI] [PubMed] [Google Scholar]
- 18. Pierce VM, Simner PJ, Lonsway DR et al. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol 2017; 55:2321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 27th ed Supplement M100. Wayne, PA: CLSI, 2017. [Google Scholar]
- 20. Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2012; 18:1503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hrabák J, Studentová V, Walková R et al. Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2012; 50:2441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papagiannitsis CC, Študentová V, Izdebski R et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry meropenem hydrolysis assay with NH4HCO3, a reliable tool for direct detection of carbapenemase activity. J Clin Microbiol 2015; 53:1731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dortet L, Bréchard L, Poirel L, Nordmann P. Rapid detection of carbapenemase-producing Enterobacteriaceae from blood cultures. Clin Microbiol Infect 2014; 20:340–4. [DOI] [PubMed] [Google Scholar]
- 24. Migliavacca R, Docquier JD, Mugnaioli C et al. Simple microdilution test for detection of metallo-beta-lactamase production in Pseudomonas aeruginosa. J Clin Microbiol 2002; 40:4388–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsakris A, Poulou A, Pournaras S et al. A simple phenotypic method for the differentiation of metallo-beta-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J Antimicrob Chemother 2010; 65:1664–71. [DOI] [PubMed] [Google Scholar]
- 26. Miriagou V, Tzelepi E, Kotsakis SD, Daikos GL, Bou Casals J, Tzouvelekis LS. Combined disc methods for the detection of KPC- and/or VIM-positive Klebsiella pneumoniae: improving reliability for the double carbapenemase producers. Clin Microbiol Infect 2013; 19:E412–5. [DOI] [PubMed] [Google Scholar]
- 27. Girlich D, Halimi D, Zambardi G, Nordmann P. Evaluation of Etest strips for detection of KPC and metallo-carbapenemases in Enterobacteriaceae. Diagn Microbiol Infect Dis 2013; 77:200–1. [DOI] [PubMed] [Google Scholar]
- 28. van Dijk K, Voets GM, Scharringa J et al. A disc diffusion assay for detection of class A, B and OXA-48 carbapenemases in Enterobacteriaceae using phenyl boronic acid, dipicolinic acid and temocillin. Clin Microbiol Infect 2014; 20:345–9. [DOI] [PubMed] [Google Scholar]
- 29. Cuzon G, Naas T, Bogaerts P, Glupczynski Y, Nordmann P. Evaluation of a DNA microarray for the rapid detection of extended-spectrum β-lactamases (TEM, SHV and CTX-M), plasmid-mediated cephalosporinases (CMY-2-like, DHA, FOX, ACC-1, ACT/MIR and CMY-1-like/MOX) and carbapenemases (KPC, OXA-48, VIM, IMP and NDM). J Antimicrob Chemother 2012; 67:1865–9. [DOI] [PubMed] [Google Scholar]
- 30. Kaase M, Szabados F, Wassill L, Gatermann SG. Detection of carbapenemases in Enterobacteriaceae by a commercial multiplex PCR. J Clin Microbiol 2012; 50:3115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dodémont M, De Mendonça R, Nonhoff C, Roisin S, Denis O. Performance of the Verigene gram-negative blood culture assay for rapid detection of bacteria and resistance determinants. J Clin Microbiol 2014; 52:3085–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salimnia H, Fairfax MR, Lephart PR et al. Evaluation of the FilmArray blood culture identification panel: results of a multicenter controlled trial. J Clin Microbiol 2016; 54:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tato M, Ruiz-Garbajosa P, Traczewski M et al. Multisite evaluation of Cepheid Xpert Carba-R assay for detection of carbapenemase-producing organisms in rectal swabs. J Clin Microbiol 2016; 54:1814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Snitkin ES, Zelazny AM, Thomas PJ et al. NISC Comparative Sequencing Program Group Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 2012; 4:148ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mathers AJ, Stoesser N, Sheppard AE et al. Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae at a single institution: insights into endemicity from whole-genome sequencing. Antimicrob Agents Chemother 2015; 59:1656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pecora ND, Li N, Allard M et al. Genomically informed surveillance for carbapenem-resistant Enterobacteriaceae in a health care system. MBio 2015; 6:e01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Köser CU, Holden MT, Ellington MJ et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med 2012; 366:2267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harris SR, Cartwright EJ, Török ME et al. Whole-genome sequencing for analysis of an outbreak of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect Dis 2013; 13:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Köser CU, Ellington MJ, Cartwright EJ et al. Routine use of microbial whole genome sequencing in diagnostic and public health microbiology. PLoS Pathog 2012; 8:e1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wright MS, Jacobs MR, Bonomo RA, Adams MD. Transcriptome remodeling of Acinetobacter baumannii during infection and treatment. MBio 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wright MS, Iovleva A, Jacobs MR, Bonomo RA, Adams MD. Genome dynamics of multidrug-resistant Acinetobacter baumannii during infection and treatment. Genome Med 2016; 8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conlan S, Thomas PJ, Deming C et al. NISC Comparative Sequencing Program Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci Transl Med 2014; 6:254ra126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poirel L, Kieffer N, Nordmann P. In vitro evaluation of dual carbapenem combinations against carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 2016; 71:156–61. [DOI] [PubMed] [Google Scholar]
- 44. Qureshi ZA, Paterson DL, Potoski BA et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tumbarello M, Viale P, Viscoli C et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–50. [DOI] [PubMed] [Google Scholar]
- 46. Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2014; 20:862–72. [DOI] [PubMed] [Google Scholar]
- 47. Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 2014; 58:654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Duin D, Lok J, Earley M et al. Colistin vs. ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae [manuscript published online ahead of print 4 September 2017]. Clin Infect Dis 2017. doi:10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy?Expert Opin Pharmacother 2016; 17:761–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shields RK, Potoski BA, Haidar G et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spellberg B, Bonomo RA. Editorial commentary: ceftazidime-avibactam and carbapenem-resistant Enterobacteriaceae: “We’re gonna need a bigger boat.” Clin Infect Dis 2016; 63:1619–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Papp-Wallace KM, Bonomo RA. New β-lactamase inhibitors in the clinic. Infect Dis Clin North Am 2016; 30:441–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Drusano GL. From lead optimization to NDA approval for a new antimicrobial: use of pre-clinical effect models and pharmacokinetic/pharmacodynamic mathematical modeling. Bioorg Med Chem 2016; 24:6401–8. [DOI] [PubMed] [Google Scholar]
- 54. Carpentier A, Metellus P, Ursu R et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol 2010; 12:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mattoes HM, Kuti JL, Drusano GL, Nicolau DP. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin Ther 2004; 26:1187–98. [DOI] [PubMed] [Google Scholar]
- 56. Schwaber MJ, Lev B, Israeli A et al. Israel Carbapenem-Resistant Enterobacteriaceae Working Group Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally-implemented intervention. Clin Infect Dis 2011; 52:1–8. [DOI] [PubMed] [Google Scholar]
- 57. World Health Organization. International health regulations (2005). Geneva, Switzerland: WHO, 2008. Available at: http://www.who.int/ihr/9789241596664/en/. Accessed 15 December 2016. [Google Scholar]
- 58. Wernli D, Haustein T, Conly J, Carmeli Y, Kickbusch I, Harbarth S. A call for action: the application of the International Health Regulations to the global threat of antimicrobial resistance. PLoS Med 2011; 8:e1001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 2011; 11:355–62. [DOI] [PubMed] [Google Scholar]
- 60. Liu YY, Wang Y, Walsh TR et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2016; 16:161–8. [DOI] [PubMed] [Google Scholar]
- 61. Kamradt-Scott A. A public health emergency of international concern? Response to a proposal to apply the International Health regulations to antimicrobial resistance. PLoS Med 2011; 8:e1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]