Abstract
Inhibitors for polyamine oxidizing enzymes, spermine oxidase (SMOX) and N1-acetylpolyamine oxidase (PAOX), were designed and evaluated for their effectiveness in a photochemically induced thrombosis (PIT) mouse model. N1-Nonyl-1,4-diaminobutane (C9-4) and N1-tridecyl-1,4-diaminobutane (C13-4) competitively inhibited the activity of PAOX and SMOX in a manner comparable to N1,N4-bis(2,3-butadienyl)-1,4-butanediamine (MDL72527), an irreversible inhibitor of both enzymes. The two compounds were then tested for their effects in the PIT model. Both intraperitoneal (i.p.) and intracerebroventricular (i.c.v.) administration of C9-4 decreased infarct volumes significantly. By contrast, C13-4 reduced the volume of brain infarction by i.c.v. administration, but no reduction was observed after i.p. administration. C9-4 administered by i.p. injection reduced the volume of brain infarction significantly at doses of more than 3 mg/kg, and the dosage of 5 mg/kg or 10 mg/kg demonstrated the most potent effect and were more effective than equivalent doses of the other inhibitors such as MDL72527 and N-benzylhydroxylamine. I.P. injection of 5mg/kg of C9-4 provided a therapeutic time window of longer than 12 h. This report demonstrates that C9-4 is a potent inhibitor of the polyamine oxidizing enzymes and is useful lead compound for candidate drugs with a long therapeutic time window, to be used in the treatment of ischemic stroke.
Keywords: Spermine oxidase, N1-Acetylolyamine oxidase, Polyamine, Inhibitor, Stroke, Ischemia-reperfusion
Graphical abstract
Introduction
Ischemic stroke constitutes one of the major causes of morbidity and mortality in Japan and worldwide, which is usually caused by an embolic or thrombotic occlusion of a cerebral artery [1,2]. The pathophysiology of ischemic damage is complex and multifactorial. Early revascularization is a critical process rescuing salvageable tissues and causes better outcome and reduced mortality. One of the acute treatments available today, thrombolysis by recombinant tissue plasminogen activator, aims to restore blood flow to the ischemic area. Thrombolysis has a relatively short therapeutic time window (up to 4.5 h) and carries risk of severe adverse effects due to haemorrhage [3]. The other treatment is thrombectomy, which is the mechanical removal of the occluding blood clot, but the post-stroke recanalization of the vessel may be hampered by the occurrence of microvascular reperfusion failure [4]. Thus, new treatments with different mechanisms of action and wider therapeutic time window are required.
The polyamines spermine, spermidine, and their precursor putrescine (Put) are important in cell proliferation, differentiation, and survival [5]. Recently, there has been increasing interest in polyamine catabolism. Polyamine catabolism is mediated by three enzymes. Spermidine/spermine N1-acetyltransferase acetylates spermine and spermidine to produce N1-acetylated compounds, which are exported from cells or oxidized by the peroxisomal enzyme N1-acetylpolyamine oxidase (PAOX) to yield spermidine or putrescine, respectively, with H2O2 and 3-acetamidopropanal. The cytosolic and nuclear enzyme spermine oxidase (SMOX) can catalyze the oxidation of spermine directly to spermidine with H2O2 and 3-aminopropanal bypassing the necessity for acetylation. 3-Aminopropanal spontaneously eliminates acrolein. The acrolein mainly generated from spermine is strongly cytotoxic [6]. Saiki et al. reported that the increase of acrolein and polyamine oxidizing enzymes were observed in neuronal injury associated with neuropathological syndromes, including brain ischemia [7]. In that report, the polyamine oxidizing enzymes inhibitor, N1,N4-bis(2,3-butadienyl)-1,4-butanediamine (MDL72527), reduced the brain infarction volume in a mouse model of thrombosis when it was administered intraperitoneally at the onset of thrombosis, and interestingly, the scavenger of acrolein, N-benzylhydroxylamine reduced the brain infarction volume when it was administered intraperitoneally either at the onset of thrombosis or 6 h later. These data suggested that inhibitors of polyamine oxidizing enzymes are useful for the treatment of ischemic stroke with a wide therapeutic time window.
The active site information of the polyamine oxidizing enzymes, PAOX and SMOX, were reported by the research for their substrate activities of polyamine analogues [8–10]. These data suggested that both enzymes having substrate recognition site with two anionic centers with relatively large hydrophobic regions. Thus, the inhibitors were designed as N-alkyldiamines. The diamine was chosen 1,4-butanediamine over 1,3-diaminopropane, because a terminal 1,3-diaminopropane structure might produce acrolein through an amine oxidase reaction.
The aim of this study was the synthesis of inhibitors for the polyamine oxidizing enzymes and evaluation of their effect in the photochemically induced thrombosis (PIT) model mice.
Materials and methods
Chemistry
All reagents and solvents were purchased from commercial sources. Analytical TLC was performed on silica coated plates (silica gel 60 F-254, Merck) and compounds were visualized under UV light. Column chromatography was carried out using silica gel (Wakogel C-200, Wako). All melting points were determined using a Yanagimoto micro-hot stage and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded on a Varian 400-MR 400 MHz spectrometer using tetramethylsilane as the internal standard. MS spectra were measured using a JEOL JMS-700 spectrometer. Elemental analyses were carried out on a Yanaco CHN MT-6 elemental analyzer. MDL72527 was synthesized according to the previously reported method [11]. The inhibitors, N1-hexyl-1,4-diaminobutane (C6-4), N1-nonyl-1,4-diaminobutane (C9-4), N1-tridecyl-1,4-diaminobutane (C13-4) were synthesized from 1,4-diaminobutane and aldehyde (hexanal, nonanal, tridecanal) according to the reported method by in situ reduction of the intermediate Schiff base of primary amine group and aldehyde with NaBH4 [12].
N1-Hexyl-1,4-butanediamine (C6-4)
Yield 52.1%. White scaly crystal. mp 265°C < (dec.). 1H-NMR (D2O, 400 MHz) δ: 2.95-2.85 (6H, m, NCH2), 1.67-1.57 (4H, m, CH2), 1.57-1.48 (2H, m, CH2), 1.27-1.10 (6H, m, CH2), 0.72 (3H, t, J = 7.1 Hz. CH3). 13C-NMR (D2O, 100 MHz) δ: 47.6, 46.6, 38.7, 30.3, 25.3, 25.2, 23.9, 22.6, 21.6, 13.1. MS (Fast atom bombardment (FAB)) m/z 173 [M+H]+. Anal. Calcd for C10H26N2Cl2: C, 48.98; H, 10.69; N, 11.42. Found: C, 48.74; H, 10.60; N, 11.38.
N1-Nonyl-1,4-butanediamine (C9-4)
Yield 77.2%. White scaly crystal. mp 265°C < (dec.). 1H-NMR (D2O, 400 MHz) δ: 2.95-2.85 (6H, m, NCH2), 1.68-1.57 (4H, m, CH2), 1.57-1.48 (2H, m, CH2), 1.27-1.08 (12H, m, CH2), 0.71 (3H, t, J = 7.0 Hz. CH3). 13C-NMR (D2O, 100 MHz) δ: 47.6, 46.6, 38.7, 31.0, 28.3, 28.2, 28.1, 25.6, 25.4, 23.9, 22.6, 21.9, 13.3. MS (FAB) m/z 215 [M+H]+. Anal. Calcd for C13H32N2Cl2: C, 54.34; H, 11.23; N, 9.75. Found: C, 54.16; H, 11.16; N, 9.68.
N1-Tridecyl-1,4-butanediamine (C13-4)
Yield 86.4%. White scaly crystal. mp 265°C < (dec.). 1H-NMR (D2O, 400 MHz) δ: 2.98-2.84 (6H, m, NCH2), 1.72-1.50 (6H, m, CH2), 1.27-1.08 (20H, m, CH2), 0.72 (3H, t, J = 7.0 Hz. CH3). 13C-NMR (D2O, 100 MHz) δ: 47.7, 46.7, 38.7, 31.2, 28.84, 28.83, 28.8, 28.7, 28.6, 28.5, 28.2, 25.7, 25.5, 23.9, 22.7, 21.1, 13.4. MS (FAB) m/z 271 [M+H]+. Anal. Calcd for C17H40N2Cl2: C, 59.46; H, 11.74; N, 8.16. Found: C, 59.41; H, 11.73; N, 8.14.
Purification of the recombinant enzymes
The BL21 (DE3) strain of Escherichia coli containing the pET15b/PAOh1/SMO plasmid [13] or pET15b/hPAO1 plasmid [14] were cultured. Following isopropyl-β-D-1-thiogalactopyranoside (IPTG) induction of the protein expression, the cells were collected and the enzyme proteins were purified by His-tag affinity column (TARON) according to manufacturer’s protocol (Takara Bio.). Eluted imidazole containing fractions were de-salted by PD-10 column (Bio-Rad), and aliquots were stored at −80°C and used as the enzyme source.
Inhibition of the polyamine oxidizing enzyme activity
PAOX and SMOX activities were assayed by measuring the amount of H2O2 generated by the enzyme reaction [15]. The standard incubation mixture (final volume, 100 μL) contained the enzyme solution, 0.2 mM N1-acetylspermine for PAOX or 0.4 mM spermine for SMOX, 0.56 mM aminoguanidine, 0.036 mM pargyline, 1 mM EDTA, 0.04 μg horseradish peroxidase, 0.1 mg homovanillic acid in 0.1 M Tris–HCl buffer (pH 8.0 for PAOX or pH 7.2 for SMOX). Before the addition of homovanillic acid and N1-acetylspermine/spermine, the mixtures were preincubated for 5 min at 37°C. After preincubation, homovanillic acid and N1-acetylspermine or spermine were added, the mixtures were incubated 10 min at 37°C and the reaction was stopped by the addition of 100 μL of 1 M NaOH solution. The inhibitors were added with the substrates. The resulting fluorescence of homovanillic acid dimer was measured at Ex 315 nm/Em 425 nm using a microplate reader (Molecular Devices SPECTRA MAX M2).
Animal experiment (PIT mouse model)
All experimental procedures using animals in this study were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee (College of Pharmacy, Nihon University, Japan). The male ddY mice (Sankyo Labo Service Co Inc. Japan) weighing 30 to 35 g were used for all experiments. Mice were housed singly in a calm, identical place with food and water ad libitum in polycarbonate cages at a room temperature of 24 ± 1 °C with a relative humidity of 55 ± 5% maintained under a 12 h light–dark cycle. Mice were anesthetized with 2% isoflurane, the thrombotic occlusion of the middle cerebral artery was induced by the photochemical reaction: an incision was made between the left orbit and the external auditory canal, and the temporalis muscle was detached from dura mater to expose the proximal section of the middle cerebral artery. Immediately after intravenous injection of photosensitizer, Rose Bengal (20 mg/kg), through a vena caudalis, green light (wavelength: 540 nm) emitted from a xenon lamp illuminated the middle cerebral artery for 10 minutes. At 24 hours after the induction of PIT stroke, the brain was removed and sectioned into 2-mm thick coronal slices. Each slice was incubated with 2 % triphenyltetrazolium chloride (TTC) solution at 37°C for 15 minutes. Volume of infarction was analyzed using ImageJ.
Polyamine oxidizing enzymes inhibitor or saline as vehicle were administered by interperitoneal (5 mg/kg, 100 μL/animal) or intracerebroventricular (2 mg/kg, 3 μL/animal) injections under anesthesia with 2% isoflurane. All experiments using the PIT model used each 5 – 16 mice.
Statistics
Data were analyzed by ANOVA followed by the Bonferoni multiple comparison test and significant difference from control value at *P < 0.05.
Results
Newly synthesized compounds as inhibitors of polyamine oxidizing enzymes
Compounds designed and prepared based on the information of substrate recognition site of the oxidizing enzymes, C6-4, C9-4 and C13-4, including putrescine and MDL72527 as reference compounds, were evaluated for their inhibitory activity against PAOX and SMOX. Their structures and IC50 values are summarized in Table 1. Elongation of the alkyl chain was effective in enhancing the inhibitory activity against PAOX or SMOX. C9-4 and C13-4 were found to inhibit the enzymes at a comparable potency to MDL72527.
Table 1.
The IC50 values indices for PAOX and SMOX of the polyamine oxidases inhibitors.
| Compound | Structure | PAOX | SMOX |
|---|---|---|---|
| PUT |
|
>10000 μM | >10000 μM |
| C6-4 |
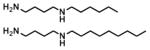
|
480 ± 25 μM | 1733 ± 252 μM |
| C9-4 | 2.6 ± 0.2 μM | 88 ± 9.0 μM | |
| C13-4 |
|
1.1 ± 0.3 μM | 5.5 ± 0.3 μM |
| MDL72527 | 9.8 ± 0.2 μM | 53 ± 1.0 μM |
Effects of synthesized compounds on the volume of brain infarction in PIT model mice
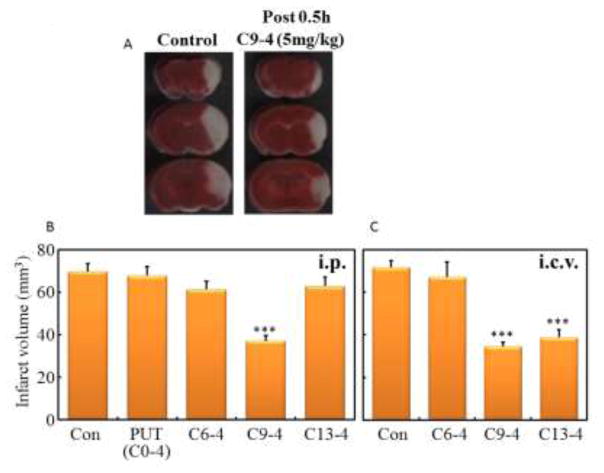
Hydrochloride salts of C6-4, C9-4, C13-4 or Put were administered by intraperitoneal (i.p.) or intracerebroventricular (i.c.v.) injection at 5 mg/kg (i.p) or 2 mg/kg (i.c.v.) dose 0.5 h after induction of ischemia in the PIT mice. The brain infarct volume at 24 h after the induction of infarction in each mouse was determined based on the sum of infarct area in all brain slices. Their infarct volumes were shown in Fig. 1. Both i.p. and i.c.v. administration of C9-4 reduced the infarct volume significantly. C13-4 similarly reduced the volume of i.c.v. administration.
Fig. 1.
Effect of the polyamine oxidases inhibitors on the volume of brain infarction in the PIT mouse model.
Inhibitors were administered by interperitoneal (i.p., 5 mg/kg) or intracerebroventricular (i.c.v., 2 mg/kg) injections 0.5 h after the ischemia. (A) Representative TTC-stained coronal sections at −2 mm, 0 mm and +2 mm from the bregma of the control and C9-4 treated mouse. (B) The infarct volume at 24 hours after the ischemia. The infarct volume in each mouse was determined from the sum (N = 5 ~ 16) of infarct area in all brain slices. Significant difference from control: ***P<0.001
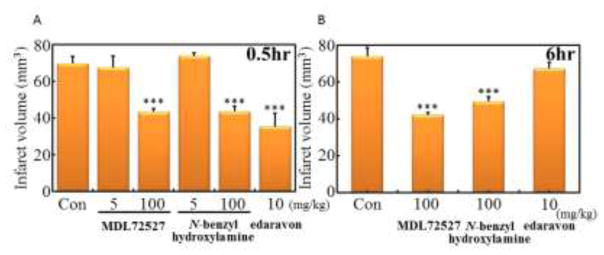
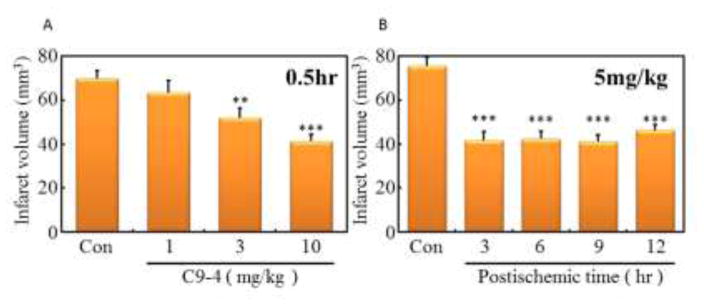
For the confirmation of the PIT model, MDL72527, N-benzylhydroxylamine and edaravon, a scavenger of ROS, were administered by i.p. injection 0.5 h or 6 h after the ischemia at indicated dosage (Fig. 2). MDL72527 and N-benzylhydroxylamine reduced infarction volume at 100 mg/kg dosage, but 5 mg/kg dosage had no effect. In addition, the administration of MDL72527 or N-benzylhydroxylamine at 100 mg/kg reduced infarction volume even 6 h after the onset of thrombosis. Edaravon could not reduce the infarction volume at 10 mg/kg administration 6 h after the ischemia, the dose which reduced the volume 0.5 h after the ischemia. These results suggested that C9-4 was the most promising compound of those tested. Dose-dependency of C9-4 on the reduction of infarct volume was then examined by i.p. injection 0.5 h after ischemia (Fig. 3A). C9-4 significantly reduced the volume of brain infarction at doses of more than 3 mg/kg, and the doses of 5 mg/kg and 10 mg/kg demonstrated similar effects. Next, time-dependency of the administration of C9-4 after the ischemia on the reduction of infarct volume was examined at the constant dose of 5 mg/kg by i.p. injection (Fig. 3B). A significant reduction of the infarct volume was observed until 12 h of post-ischemic time. These results suggested the utility of C9-4 as a candidate drug having a therapeutic time window at least for 12 h.
Fig. 2.
Effects of MDL72527, N-benzylhydroxylamine and edaravon on the volume of brain infarction in the PIT mouse model.
MDL72527, N-benzylhydroxylamine and edaravon were administered by interperitoneal (i.p.) injections 0.5 h (A) or 6 h (B) after the ischemia. The infarct volume in each mouse was determined from the sum (N = 6 ~ 16) of infarct area in all brain slices. Significant difference from control: ***P<0.001
Fig. 3.
Effects of C9-4 with different doses or various times on the volume of brain infarction in PIT model mice.
C9-4 (1, 3 or 10 mg/kg) were administered by interperitoneal (i.p.) injections 0.5 h after ischemia (A) or C9-4 (5mg/kg) were administered 3, 6, 9 or 12 h after the ischemia (B). The infarct volume in each mouse was determined from the sum (N = 5 ~ 10) of infarct area in all brain slices. Significant difference from control: ***P<0.001, **P<0.05
Discussion
One of the major goals of stroke research has been to develop neuroprotectants that could reduce ischemic damage without the use of the recombinant tissue plasminogen activator, since little preclinical research using it has translated into effective stroke therapies. One of the reasons for the lack of treatment success is the complexity of the mechanisms involved in ischemic neuronal death and the fact that current available neuroprotective agents (e.g. NMDA receptor antagonists) have a short time window of effectiveness [16]. Therefore, new treatments with different mechanisms of action has been focused on downstream signaling pathways that may provide both improved specificity and wider therapeutic window of opportunity [17].
In this report, we chose the polyamine oxidizing enzymes, SMOX and PAOX, as targets because Saiki et al. reported MDL72527 reduced the brain infarction volume in thrombosis model mice when it was administered intraperitoneally at 6 h later of thrombosis. Recently, Uemura et al. reported that the activities of the polyamine back conversion enzymes, SMOX, PAOX, SSAT, were induced in brain infarctions [18]. This also suggested that the polyamine back conversion pathway is an important drug target for stroke therapy. Recently, Persichini’s groups reported that HIV-tat induced neurotoxicity was mediated by NMDA receptor-elicited SMOX activation in SH-SY5Y cells [19, 20]. In that reports, chlorhexidine was used as polyamine oxidizing enzyme inhibitor and prevented the neuronal cell death [21]. These data suggested that SMOX was downstream of NMDA signaling pathway.
Further, the central administration of the polyamine back conversion enzyme inhibitor, berenil (diminazene aceturate) [22], was reported to exert a reduction in cerebral infarct size and the mechanism involved ACE2 activation [23]. This effect might be caused by polyamine oxidizing enzymes inhibition. Other polyamine related compounds, such as N1-(quinolin-2-ylmethyl)butane-1,4-diamine [24], 2(E)-N-[3-({4-[(3-aminopropyl)amino]-cyclohexyl}amino)propyl]-3-(4-hydroxyphenyl) prop-2-enamide [25], were evaluated and reported their effects on the ischemic model, however, their administrations were before the ischemia.
In this report, we found C9-4 had the most potent effect on the amelioration of brain infarction size and a long therapeutic time window of at least 12 h. In vitro experiments, C13-4 inhibited PAOX and SMOX more potently than C9-4, but in PIT model experiments C13-4 showed a weaker effect than C9-4. The difference may be due to the difference in blood-brain barrier penetration, suggesting that permeability of C13-4 is lower than that of C9-4. Pajouhesh and Lenz [26] reported the attributes of a successful central nervous system drug properties, one of them was Clog P value < 5. ClogP value for C13-4 was more than 5 (5.53 by calculation using ChemBio 3D Ultra) and ClogP value of C9-4 was 3.41. This might support those differences of the effects.
In summary, the data presented above indicate that C9-4 is a potent inhibitor of both PAOX and SMOX. Since polyamine catabolism has been linked the pathologies of ischemic brain injury, this compound represents an exciting lead compound for the treatment of ischemic stroke. Importantly, the data also indicate that this compound has a long therapeutic time window, thus improving the potential of successfully treating strokes in a clinical setting.
Highlights.
Inhibitors for polyamine oxidizing enzymes, spermine oxidase (SMOX) and N1-acetylpolyamine oxidase (PAOX), were synthesized.
N1-Nonyl-1,4-diaminobutane (C9-4) and N1-tridecyl-1,4-diaminobutane (C13-4) were identified as potent inhibitor of PAOX and SMOX.
Intraperitoneal and intracerebroventricular (i.c.v.) injection of C9-4 and the i.c.v. injection of C13-4 at 0.5 or 6 h after the ischemia decreased an infarct volume significantly in the PIT model mice.
C9-4 is a useful candidate drug for the ischemic stroke with a long therapeutic time window.
Acknowledgments
This work was partially supported by NIH Grant NCI CA204345.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke, Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Takashima N, Arima H, Kita Y, Fujii T, Miyamatsu N, Komori M, Sugimoto Y, Nagata S, Miura K, Nozaki K. Incidence, Management and Short-Term Outcome of Stroke in a General Population of 1.4 Million Japanese- Shiga Stroke Registry. Circ J. 2017 doi: 10.1253/circj.CJ-17-0177. in press. [DOI] [PubMed] [Google Scholar]
- 3.Minematsu K, Toyoda K, Hirano T, Kimura K, Kondo R, Mori E, Nakagawara J, Sakai N, Shiokawa Y, Tanahashi N, Yasaka M, Katayama Y, Miyamoto S, Ogawa A, Sasaki M, Suga S, Yamaguchi T. Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis. 2013;22:571–600. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 4.El Amki M, Wegener S. Improving cerebral blood flow after arterial recanalization: A novel therapeutic strategy in stroke. Int J Mol Sci. 2017;18:E2669. doi: 10.3390/ijms18122669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–94. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pegg AE. Toxicity of polyamines and their metabolic products. Chem Res Toxicol. 2013;26:1782–800. doi: 10.1021/tx400316s. [DOI] [PubMed] [Google Scholar]
- 7.Saiki R, Park H, Ishii I, Yoshida M, Nishimura K, Toida T, Tatsukawa H, Kojima S, Ikeguchi Y, Pegg AE, Kashiwagi K, Igarashi K. Brain infarction correlates more closely with acrolein than with reactive oxygen species. Biochem Biophys Res Commun. 2011;404:1044–1049. doi: 10.1016/j.bbrc.2010.12.107. [DOI] [PubMed] [Google Scholar]
- 8.Moriya S, Miura T, Takao K, Sugita Y, Samejima K, Hiramatsu K, Kawakita M. Development of irreversible inactivators of spermine oxidase and N1-acetylpolyamine oxidase. Biol Pharm Bull. 2014;37:475–480. doi: 10.1248/bpb.b13-00913. [DOI] [PubMed] [Google Scholar]
- 9.Takao K, Shirahata A, Samejima K, Casero RA, Jr, Igarashi K, Sugita Y. Pentamines as substrate for human spermine oxidase. Biol Pharm Bull. 2013;36:407–411. doi: 10.1248/bpb.b12-00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takao K, Shibata S, Ozawa T, Wada M, Sugitia Y, Samejima K, Shirahata A. A conceptual model of the polyamine binding site of N1-acetylpolyamine oxidase developed from a study of polyamine derivatives. Amino Acids. 2009;37:401–405. doi: 10.1007/s00726-008-0168-9. [DOI] [PubMed] [Google Scholar]
- 11.Bey P, Bolkenius FN, Seiler N, Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985;28:1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- 12.Niitsu M, Samejima K. Syntheses of a series of linear pentaamines with three and four methylene chain intervals. Chem Pham Bull. 1986;34:1032–1038. [Google Scholar]
- 13.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA., Jr Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304:605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Hacker A, Murray-Stewart T, Frydman B, Valasinas A, Fraser AV, Woster PM, Casero RA., Jr Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): potential role in determining drug sensitivity. Cancer Chemother Pharmacol. 2005;56:83–90. doi: 10.1007/s00280-004-0936-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA., Jr Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–5373. [PubMed] [Google Scholar]
- 16.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu T, Dietz RM, Cruz-Torres I, Strnad F, Garske AK, Moreno M, Venna VR, Quillinan N, Herson PS. Extended therapeutic window of a novel peptide inhibitor of TRPM2 channels following focal cerebral ischemia. Exp Neurol. 2016;283:151–156. doi: 10.1016/j.expneurol.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uemura T, Watanabe K, Ishibashi M, Saiki R, Kuni K, Nishimura K, Toida T, Kashiwagi K, Igarashi K. Aggravation of brain infarction through an increase in acrolein production and a decrease in glutathione with aging. Biochem Biophys Res Commun. 2016;473:630–635. doi: 10.1016/j.bbrc.2016.03.137. [DOI] [PubMed] [Google Scholar]
- 19.Capone C, Cervelli M, Angelucci E, Colasanti M, Macone A, Mariottini P, Persichini T. A role for spermine oxidase as a mediator of reactive oxygen species production in HIV-Tat-induced neuronal toxicity. Free Radic Biol Med. 2013;63:99–107. doi: 10.1016/j.freeradbiomed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Mastrantonio R, Cervelli M, Pietropaoli S, Mariottini P, Colasanti M, Persichini T. HIV-Tat Induces the Nrf2/ARE Pathway through NMDA Receptor-Elicited Spermine Oxidase Activation in Human Neuroblastoma Cells. PLoS One. 2016;11:e0149802. doi: 10.1371/journal.pone.0149802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervelli M, Polticelli F, Fiorucci L, Angelucci E, Federico R, Mariottini P. Inhibition of acetylpolyamine and spermine oxidases by the polyamine analogue chlorhexidine. J Enzyme Inhib Med Chem. 2013;28:463–467. doi: 10.3109/14756366.2011.650691. [DOI] [PubMed] [Google Scholar]
- 22.Libby PR, Porter CW. Inhibition of enzymes of polyamine back-conversion by pentamidine and berenil. Biochem Pharmacol. 1992;44:830–832. doi: 10.1016/0006-2952(92)90424-h. [DOI] [PubMed] [Google Scholar]
- 23.Mecca AP, Regenhardt RW, O’Connor TE, Joseph JP, Raizada MK, Katovich MJ, Sumners C. Cerebroprotection by angiotensin-(1–7) in endothelin-1-induced ischaemic stroke. Exp Physiol. 2011;96:1084–1096. doi: 10.1113/expphysiol.2011.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cen J, Liu L, He L, Liu M, Wang CJ, Ji BS. N1-(quinolin-2-ylmethyl)butane-1,4-diamine, a polyamine analogue, attenuated injury in in vitro and in vivo models of cerebral ischemia. Int J Dev Neurosci. 2012;30:584–595. doi: 10.1016/j.ijdevneu.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Henman MC, Atkinson J, Fixon-Owoo S, Tatlisumak T, Shaw GG, Doyle KM. The pre-ischaemic neuroprotective effects of the polyamine analogues BU43b and BU36b in permanent and transient focal cerebral ischaemia models in mice. Brain Res. 2006;1076:209–215. doi: 10.1016/j.brainres.2005.12.097. [DOI] [PubMed] [Google Scholar]
- 26.Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2:541–553. doi: 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]