Polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes (POEMS) syndrome is a rare plasma cell dyscrasia characterized by high serum levels of vascular endothelial growth factor (VEGF)1,2. Bone marrow plasma cells are the likely source of this angiogenic cytokine, which is responsible for the characteristic symptoms of this disorder, including extra-vascular volume overload, hemagiomata and papilledema. A reduction of VEGF level after treatment usually correlates with symptomatic improvement3–6. The main treatment strategy is to target plasma cell clones, and monoclonal protein and VEGF levels are used to monitor disease activity.
Hematologic complete response (CRH) has proved to be a significant predictor of disease outcome. However, some patients without CRH still show reasonable survival rates, suggesting that this group is highly heterogenous and requires better prognostic indicators7. VEGF response after treatment showed good prognostic value in a retrospective study of 20 patients. Those achieving a normal serum VEGF value after treatment attained prolonged relapse-free survival8. However, previous studies were limited by patient number and follow-up time, and lacked OS outcome and comparison with hematologic response.
A total of 476 patients were newly-diagnosed POEMS syndrome and treated at our institute between January 2000 and October 2016. Of these, 190 patients (39.9%, and baseline clinical characteristics were not significantly different from all patients), who had both baseline and post-treatment serum M protein and VEGF data, and whose post-treatment serum samples were collected for half a year since the treatment began, were enrolled in the present study. All patients had been followed for at least 6 months and had elevated baseline serum VEGF levels ( > 600 pg/mL). All patients met the diagnostic criteria proposed by Dispenzieri2. Primary therapies included autologous stem cell transplantation (77 patients), melphalan-based therapy (21 patients) and novel agent-based (thalidomide, lenalidomide, bortezomib) therapy (92 patients). The median length of follow-up was 32 months (range, 6–179 months). Detailed clinical features and laboratory information were collected at the time of diagnosis, as described previously9. (Online Supplementary Table 1) Serum VEGF was measured with a human Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA)10. All patients provided informed consent, and the study was approved by the Institutional Review Board of Peking Union Medical College Hospital, in accordance with the Declaration of Helsinki.
The upper limit of the normal serum VEGF range was 600 pg/mL in our institute, as previously described10. The complete response of VEGF (CRV) was a normalization of serum VEGF levels. A VEGF partial response (PRV) was a reduction of > 50% (baseline must ≥ 1200 pg/mL, 3% patients were between 600 and 1200 pg/mL at baseline). Others were considered as VEGF non-response (NRV) patients. Hematologic response included CRH (a confirmed negative immunofixation electrophoresis (IFE) test and for patients with only light-chain secreting clone also undetectable light chain with serum and urine samples) and no CRH (did not meet the criteria for a complete response).
Analyses were performed with SPSS 23 (SPSS Inc., Chicago, IL, USA). The Pearson χ2 test or Fisher’s exact test were used to ascertain differences between categorical variables. Relationships between baseline factors and VEGF response was compared using a logistic multivariate regression model. Progression-free survival (PFS) and OS were calculated from the start of treatment. Progression was defined as the recurrence or deterioration of clinical symptoms. OS was defined as the time from transplantation to death from any cause. Survival curves were plotted with the Kaplan–Meier method and compared with a log-rank test. Risk factors were analyzed utilizing Cox multivariate models, and the threshold for statistical significance was set at p = 0.10. Variates which met criteria in univariate analysis, or factors reported prognostic previously were included in multivariate models. Data with p-values < 0.05 were considered statistically significant.
There were 17 deaths during follow-up, and other 18 patients had disease progressions. The 3-year PFS was 81.7% and the 3-year OS was 92.8%. Eighty patients (42.1%) achieved CRH, and the remaining patients (57.9%) had no CRH. The median time from diagnosis to complete hematologic response was 10 months (range, 1–179 months). If CRH was achieved, patients had superior progression-free (p = 0.016) and OS rates (p = 0.001) compared with patients with no CRH. (Online Supplementary Figure 1) In CRH and non-CRH patients, the estimated 3-year PFS was 90.1% and 74.9%, while the 3-year OS were 100.0% and 87.1%, respectively.
The median value of baseline VEGF was 4 764 pg/mL (range, 660–14 328 pg/mL). The mean number of serum VEGF tests carried on any individual patient was 4, and the median interval between each measurement was 7 months. The median time from diagnosis to best VEGF response was 6 months (range, 1–125 months). A total of 112 (58.9%) patients achieved CRV after treatment. Fifty-three patients (27.9%) attained PRV and the remaining 25 patients (13.2%) had NRV. According to logistic multivariate analysis, patients with lymphoadenopathy (odds ratio [OR], 0.41; 95% confidence intervals [CI], 0.20–0.84, p = 0.014) and IgA type monoclonal protein (OR, 0.45; 95% CI, 0.22–0.93; p = 0.032) were less likely to achieve CRV. These clinical variables, may not be specific but are known to reflect disease burden, and imply that patients with heavy disease are still somehow refractory to modern treatment.
Patients with CRV showed better PFS compared with PRV patients (p = 0.030). However, PRV patients had no significant difference compared with NRV patients in terms of PFS (p = 0.054). NRV group included patients with VEGF 600-1200 pg/mL at baseline. The estimated 3-year PFS rates were 87.7%, 79.9%, and 54.8% in CRV, PRV and NRV patients, respectively. Patients with CRV also had superior OS compared to PRV patients (p = 0.004), as did PRV patients compared with NRV patients (p = 0.035). The estimated 3-year OS in CRV, PRV and NRV patients were 97.4%, 95.1% and 62.8%, respectively. (Online supplementary Figure 2) According to Cox multivariate analysis, the group with CRV had superior PFS (HR, 0.38; 95% CI, 0.19–0.77; p = 0.008) and OS (HR, 0.12; 95% CI, 0.03–0.44; p = 0.001), independent of baseline factors reported previously. (Online supplementary Table 2 & 3)
Next, we compared hematologic and VEGF response by showing the hematologic response distribution in each VEGF response group and vice versa. The proportion of hematologic complete response was similar to VEGF response depth, with 62 (55.3%), 17 (32.1%) and 1 (4.0%) patients attaining CRH in CRV, PRV and NRV groups, respectively. Almost all patients but one case with CRH attained CRV/PRV. Nearly half of the patients (45.5%) with no CRH had CRV. (Fig. 1) These results suggest that monoclonal plasma cells are still the base of VEGF production but may not be the direct origin. In accordance with this clinical finding, Wang et al. suggested that bone marrow monoclonal plasma cells secret interleukin-6 to promote the proliferation of polyclonal plasma cells and consequent VEGF production in a paracrine circuit11. Therefore, targeting plasma cells is the first step in the inhibition of VEGF secretion, however, whether the treatment depth of VEGF inhibition is sufficient for long-term benefit remains unknown.
Fig. 1.
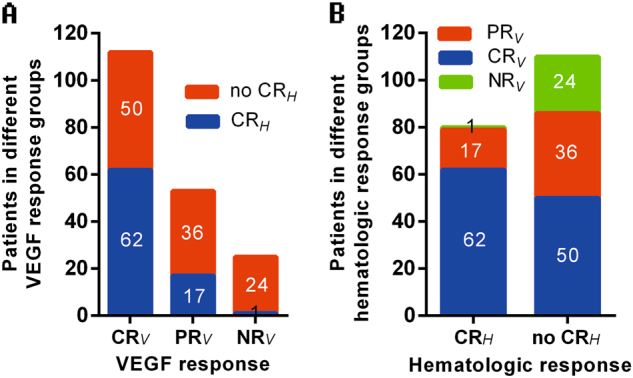
Composition of hematologic and VEGF response groups: (a) Hematologic response composition in different VEGF response groups, (b) VEGF response composition in different hematologic response groups
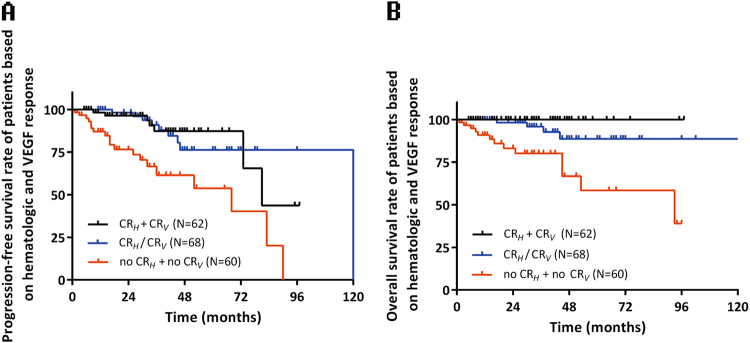
We combined PRV and NRV into a non-CRV group to dichotomize VEGF response and combine it with CRH to produce a more well-rounded model for prognostic prediction. Patients attained either CRH or CRV were combined into one group in the Kaplan–Meier survival curve. This group of patients had similar PFS (p = 0.932) and OS (p = 0.064) compared to patients achieving both CRH and CRV, and showed clearly better progression-free (p < 0.001) and OS (p = 0.002) than patients with neither CRH nor CRV. (Fig. 2) As mortality is usually caused by organ dysfunction, the normalization of VEGF is supposed to prevent organ damage arising from increased vascular permeability and angiogenesis, which will translate into a long-term benefit. It may be considered that either the complete eradication of plasma cells or the inhibition of VEGF production can derive clinical benefits.
Fig. 2.
Kaplan–Meier survival curves according to hematologic and VEGF response: (a) Progression-free survival curve according to hematologic and VEGF response, (b) Overall survival curve according to hematologic and VEGF response
In summary, we demonstrated the prognostic value of VEGF response alone, as well as combined with hematologic response, in POEMS patients, not only re-affirming the importance of regularly monitoring VEGF in routine practice, but also supporting its use as a surrogate endpoint in clinical trials.
Electronic supplementary material
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Hao Zhao and Hao Cai.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41408-018-0073-8).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li J, Zhou Db. New advances in the diagnosis and treatment of POEMS syndrome. Br. J. Haematol. 2013;161:303–315. doi: 10.1111/bjh.12236. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A. POEMS syndrome: 2017 Update on diagnosis, risk stratification, and management. Am. J. Hematol. 2017;92:814–829. doi: 10.1002/ajh.24802. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza A, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood. 2011;118:4663–4665. doi: 10.1182/blood-2011-06-362392. [DOI] [PubMed] [Google Scholar]
- 4.Soubrier M, et al. Growth factors in POEMS syndrome: Evidence for a marked increase in circulating vascular endothelial growth factor. Arthritis Rheum. 1997;40:786–787. doi: 10.1002/art.1780400430. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe O, et al. Overproduction of vascular endothelial growth factor - Vascular permeability factor is causative in Crow-Fukase (POEMS) syndrome. Muscle & Nerve. 1998;21:1390–1397. doi: 10.1002/(SICI)1097-4598(199811)21:11<1390::AID-MUS5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Dyck PJ, Engelstad J, Dispenzieri A. Vascular endothelial growth factor and POEMS. Neurology. 2006;66:10–12. doi: 10.1212/01.wnl.0000194614.56025.ec. [DOI] [PubMed] [Google Scholar]
- 7.Kourelis TV, et al. Long-term outcome of patients with POEMS syndrome: An update of the Mayo Clinic experience. Am. J. Hematol. 2016;91:585–589. doi: 10.1002/ajh.24356. [DOI] [PubMed] [Google Scholar]
- 8.Misawa S, et al. Vascular endothelial growth factor as a predictive marker for POEMS syndrome treatment response: retrospective cohort study. BMJ Open. 2015;5:e009157. doi: 10.1136/bmjopen-2015-009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, et al. Combination of melphalan and dexamethasone for patients with newly diagnosed POEMS syndrome. Blood. 2011;117:6445–6449. doi: 10.1182/blood-2010-12-328112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, et al. Markedly elevated serum total N-terminal propeptide of type I collagen is a novel marker for the diagnosis and follow up of patients with POEMS syndrome. Haematologica. 2014;99:e78–e80. doi: 10.3324/haematol.2013.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, et al. Remarkable expression of vascular endothelial growth factor in bone marrow plasma cells of patients with POEMS syndrome. Leuk. Res. 2016;50:78–84. doi: 10.1016/j.leukres.2016.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.