Abstract
Perinatal asphyxia results from the action of different risk factors like complications during pregnancy, preterm delivery, or long and difficult labor. Nowadays, it is still the leading cause of neonatal brain injury known as hypoxic-ischemic encephalopathy (HIE) and resulting neurological disorders. A temporal limitation of oxygen, glucose, and trophic factors supply results in alteration of neural cell differentiation and functioning and/or leads to their death. Among the affected cells are oligodendrocytes, responsible for myelinating the central nervous system (CNS) and formation of white matter. Therefore, one of the major consequences of the experienced HIE is leukodystrophic diseases resulting from oligodendrocyte deficiency or malfunctioning. The therapeutic strategies applied after perinatal asphyxia are aimed at reducing brain damage and promoting the endogenous neuroreparative mechanisms. In this review, we focus on the biology of oligodendrocytes and discuss present clinical treatments in the context of their efficiency in preserving white matter structure and preventing cognitive and behavioral deficits after perinatal asphyxia.
Keywords: Perinatal asphyxia, Neonatal hypoxia-ischemia, Oligodendrocyte progenitors, Myelinogenesis, Myelin structure, Electron microscopy, Neuroprotection, Cell-based therapies
Introduction
Perinatal asphyxia is the leading cause of neonatal brain injury known as hypoxic-ischemic encephalopathy (HIE). Accordingly, it is evoked by a temporarily limited (sometimes over a considerably prolonged period of time) supply of oxygen, which in turn leads to hypoxia or even anoxia in severe cases. The transiently reduced cerebral blood flow (ischemia) also results in the shortage of trophic support, especially the distribution of glucose. Perinatal asphyxia concerns about 4–6 of every 1000 full-term births and it is even more frequent in the case of children born prematurely [1]. Preterm delivery, which accounts for as much as 10% of newborns, as well as complications during labor are the major causes of birth asphyxia and make it to be the one of the leading causes of under-five child deaths [2–4]. Thanks to a constant progress in neonatal care programs, the mortality rate among newborn children has a tendency to decrease [5–7]. Nonetheless, the experienced deficiencies of oxygen and trophic support very often affect various body organs, including brain and trigger long-time consequences influencing the quality of life. Those include neurodevelopmental (neuromotor disorders, seizures, limb paresis) as well as cognitive and behavioral impairments [8–11].
Unfortunately, the cells constituting the neonatal nervous tissue are extremely sensitive to alterations in local homeostasis. In the perinatal period, a huge amount of neural progenitors arises in the effect of intense processes of neurogenesis and gliogenesis, contributing to the development of the central nervous system (CNS). The newly born neuroblasts give rise to specialized neurons like motoneurons, sensory neurons, or interneurons, which are responsible for behavioral and cognitive functions, as well as the interaction between those cells, respectively [12–14]. The physiological functioning of neurons is based on fast and efficient processing and transmitting signals within nervous system; therefore, any alterations usually lead to neurological disorders, which are pronounced to different extend. The process of signal transduction is highly energy-consuming and therefore neurons are supported by glial cells, which are present in at least an equal proportion to neurons, depending on a given brain region [15–17]. To enable salutatory conduction which is an efficient way of speeding-up propagation of impulses, axons are wrapped with myelin, elaborated by oligodendrocytes, the specialized glial cells.
Alterations in Oligodendrocyte Development
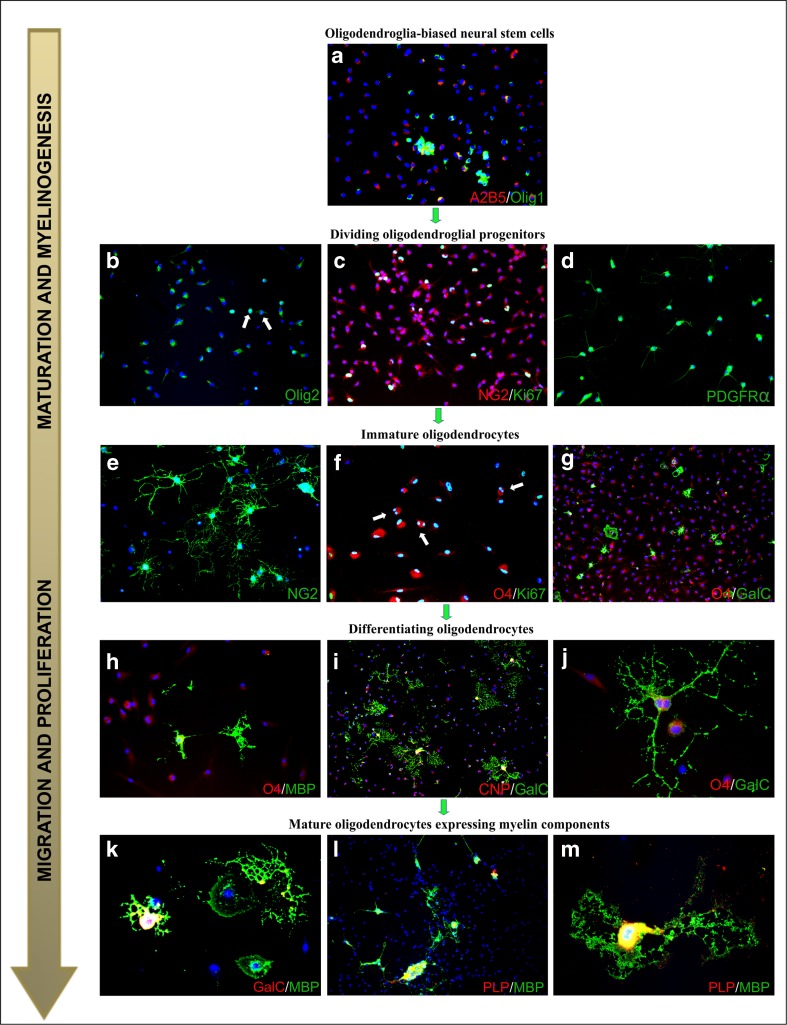
Myelin, which insulates axons and facilitates signal conduction, is essentially a compact multilamellar and highly organized structure [18, 19]. Generally, it is an extended and modified plasma membrane of oligodendrocytes, which are the cells responsible for myelinating CNS. Their precursors arise from neuroectoderma and populate the developing nervous system starting from approximately mid-gestation [20–22]. Gliogenesis is known to peak at the perinatal period and it proceeds intensely during the first postnatal months, in the course of constant neurogenesis. However, to gain capability for myelinogenesis, oligodendrocytes undergo a multistep process of maturation, which could be described by the expression of overlapping cell-specific markers (Fig. 1). Accordingly, the glial commitment of the neural stem cells is associated with the presence on their surface, the A2B5 marker (Fig. 1a), corresponding to ganglioside GT3 and its O-acetylated derivative epitope [23]. Oligodendrocyte progenitors cells (OPCs) (Fig. 1c–d) are commonly distinguished by the expression of transmembrane chondroitin sulfate proteoglycans (also known as NG2: neuron-glial antigen 2), which has been shown to be engaged in the cell migration and response to pathological signals [24–26]. The oligodendroglia-biased progenitors could be also distinguished by the expression of lineage-specific transcription factors Nkx2.2 and Olig2 [27–29]. Different localization of Olig1/Olig2 (either nuclear or cytosolic) is associated with the regulation of myelin genes and—after phosphorylation and acetylation—in process outgrowth [30, 31]. Interestingly, a significant number of NG2-positive cells, sometimes with already well-elaborated cell processes and therefore identified as polydendrocytes (Fig. 1e) [32, 33], remains in their undifferentiated state and is scattered in the brain parenchyma, both in the white and the gray matter [34]. They demonstrate a high proliferative potential and were shown to be the major population of cycling cells within the CNS [35–37].
Fig. 1.
Differentiation of rat oligodendrocytes in a primary culture. Cell nuclei are stained with Hoechst 33258 (blue). a Neural stem cells, clearly discernible due to the expression of A2B5 marker (red), which are already oligodendroglia-biased (Olig-1 marker, green). b Oligodendroglial progenitors characterized by either nuclear (arrows) or cytosolic presence of transcription factor Olig-2 (green) after 24 h of in vitro culture. c Dividing OPCs expressing NG2 (red) and Ki67 (green) markers, indicating proliferating cells. d Visualization of PDGF-AA receptor (PDGFαR, green) characteristic for OPCs. e Immature NG2-positive cells (green), which are characterized by branched cell processes (polydendrocytes). f Immature oligodendrocytes recognized by their typical marker O4 (red), which are still able to divide, as indicated by Ki67 staining (green). g After 48 h of in vitro culturing, differentiating O4+ (red) oligodendrocytes express the GalC antigen (green). h The next step of oligodendrocyte (GalC+, red) differentiation associated with the expression of myelin components (MBP+, green). i Maturating cells recognized by their two most characteristic markers: CNP (red) and GalC (green). j Vanishing O4 presence (red) is replaced by GalC (green) expression in multibranched cells with long cellular extensions. k Cells with complex morphology, characterized by the presence of GalC (red) and MBP (green). l Mature oligodendroroglia expressing major myelin proteins: PLP (red) and MBP (green). m Magnification of double-labeled differentiated (PLP-red, MBP-green) myelinating oligodendrocyte on day 5 of in vitro culture
OPCs however are the ultimate precursors of myelinating cells. The progress is their differentiation is associated with the appearance on the cell surface, the O4 and O1 markers (Fig. 1f–h), which are sulfatides attributed to immature cells, often termed pre-oligodendrocytes [38–41]. More advanced stages of oligodendrocyte maturation could also be identified by the intracellular presence of 2′,3′-cyclic nucleotide-3′-phosphodiesterase of CNPase (Fig. 1i), an enzyme engaged in myelin synthesis and maintenance [42, 43]. Differentiated cells are stained with a common marker against galactosylceramidase (GalC) (Fig. 1i–k), an enzyme hydrolyzing certain galactolipids, which are integrative myelin molecules [44–46]. Initiating myelin component synthesis opens up a new opportunity for immunostaining the mature oligodendrocytes. The antibodies against myelin basic protein (MBP), proteolipid protein (PLP) (Fig. 1k–m), myelin-associated glycoprotein (MAG), and myelin oligodendrocyte glycoprotein (MOG) are commonly used to visualize both the cells and the formed myelin sheaths [47–52]. Gained ability for myelinogenesis is the endpoint of oligodendrocyte maturation.
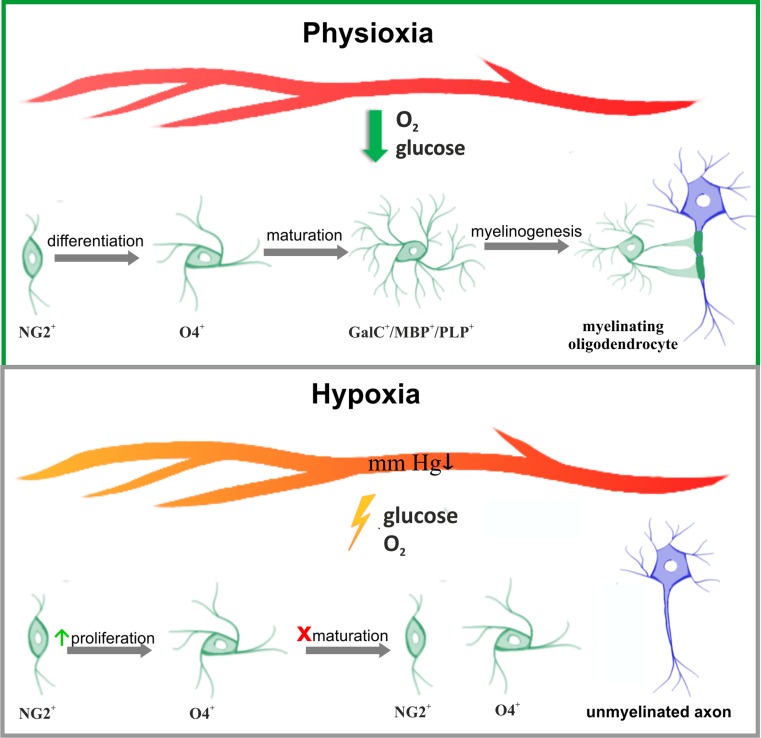
However, differentiation of progenitors into oligodendrocytes with extended and branched cell processes is an energy-consuming process. It requires constant trophic support to sustain the maturation process; otherwise, it could be inefficient or even arrested. As mentioned above, capability for myelinogenesis is associated with the activation of the set of genes coding for specific protein and lipid components and elaboration of several layers of lipid-rich layers of tightly compacted membrane. Poor vasculature or temporal limitation of oxygen and metabolic substrates due to reduced blood pressure might result in transient hypoxia, subsequently leading to activating the Wnt signaling pathway by upregulating the expression of hypoxia-inducible factors (HIFs) in oligodendrocytes [53]. Accordingly, in physiological oxygen level (also termed as “physioxia” and corresponding to 2–5% in nervous tissue), perinatal OPC-expressed HIF arrests cell differentiation yet promoting angiogenesis through Wnt7 signaling pathway in a paracrine manner. Once the microvasculature is established, HIF1α and HIF2α are deactivated by specific oxygen-dependent enzymes (asparaginyl and prolyl hydroxylases) and oligodendrocyte differentiation proceeds.
There is a growing list of evidence that oligodendrocytes are extremely sensitive to the alteration in local homoestasis and they respond to various kinds of pathological signals by either limited survival and arrested maturation process [54–57] or by increasing their proliferation rate and migrating towards the site of injury [37, 58, 59].
Myelination of CNS by Mature Oligodendrocytes
Competed oligodendrocyte maturation means acquiring an ability to express myelin components, their intracellular transport, and incorporation into forming myelin sheaths. Although myelin is an extension of oligodendroglial cell membrane, its composition is highly modified in context of protein to lipid proportion. Whereas in cell membrane, this ratio is approximately 1:1, in myelin lipids constitute up to 70–85% lipids of dry mass, comprising predominantly cholesterol, galactosylceramide, and ethanolamine plasmalogen, which enable the close packing and tight organization of molecules within the membrane [60, 61]. Thus, numerous genes have to be activated (some of them are regulated by HIFs and therefore depend on the local level of oxygen) to express specific myelin components. The processes of membrane modification and generation have to be orchestrated and very efficient since large myelin quantities are elaborated by a given oligodendrocyte. In the CNS, almost all axons with diameters greater than 0.2 μm are myelinated and a large myelinated axon may have up to 250 to 300 turns of myelin wrapping around it [62]. Accordingly, the ratio between axon diameter and that of the total nerve fiber (axon and myelin) has been established to be about 0.6–0.7. Moreover, one oligodendroglial cell is responsible for myelinating several axons (making even 40 myelin segments) and is able to produce as much as 5–50 × 103 μm2 of membrane a day [63]. Once established, myelin sheath is maintained by oligodendrocytes throughout adulthood, contributing to accelerating signal transduction even about 50–100-fold (up to 70–120 m/s for axons with diameter about 2–20 μm) [62].
Myelinated axons, besides being able to efficiently and rapidly propagate signals, are also protected by myelin from exogenous noxious stimuli. Neurodegenerative disorders developing in a consequence of CNS hypo/demyelination are actually based on unmyelinated axon dystrophy and their malfunctioning. A lesson learned from animal models point to the wide spectrum of neurological symptoms resulting from insufficient CNS myelination. They are pronounced to different extend, strongly depending on the severity of CNS dys/demyelination and include among others body tremor, focal sensory loss, limb paresis, and ataxia. Taking into consideration that during evolution, the amount of white matter tremendously increased achieving in primates and humans about 60% of brain volume (versus about 10% in rodents) [15], precise and efficient myelination seems to be crucial for correct CNS functioning.
Leukodystrophic Disorders Resulting from Perinatal Asphyxia
Delayed and/or disturbed maturation of oligodendrocytes, triggered by temporal limitation of oxygen and trophic support due to perinatal hypoxic-ischemic event, results in CNS hypomyelination and contributes to development of leukodystrophic diseases (Fig. 2). Since pathological insults affect various brain regions to a different extent, also the functions of oligodendrocytes would be either retained or altered and white matter lesions are diffused. The local intensity of inflammatory processes and the density of an extant vasculature have a significant impact on oligodendrocyte maturation and their efficiency in properly assembling myelin layers. It has been reported that endothelial cells associated with vessels play an important role in promoting the proliferation and survival of oligodendrocytes by secreting trophic stimuli to local microenvironment, defined as “oligovascular niche” [64–66]. Moreover, the enhanced vascularization process observed after stroke promotes oligodendrocyte survival and maturation—areas of the highest vessel density were also characterized by greater number of differentiated oligodendrocytes—suggesting a crucial role of constant trophic supply in physiological maturation of oligodendrocytes [67, 68]. Trophic coupling between endothelium and oligodendrocytes had been shown to contribute to maintaining the brain-blood barrier integrity [69]. Altogether, angiogenesis supporting cell survival, proliferation, and paracrine activity seems to be part of a strategic response to pathological clues leading to white matter injury.
Fig. 2.
Impact of temporal hypoxia on the biology of oligodendrocytes. The processes of oligodendroglial differentiation, maturation and the capability for myelinogenesis are highly energy-consuming and are supported by metabolites provided by circulating, oxygenated blood (upper panel). Perinatal asphyxia leads to a decrease in blood pressure and a temporal limitation in oxygen and glucose supply (lower panel). Maturation of oligodendrocytes is arrested and myelinogenesis is altered/delayed
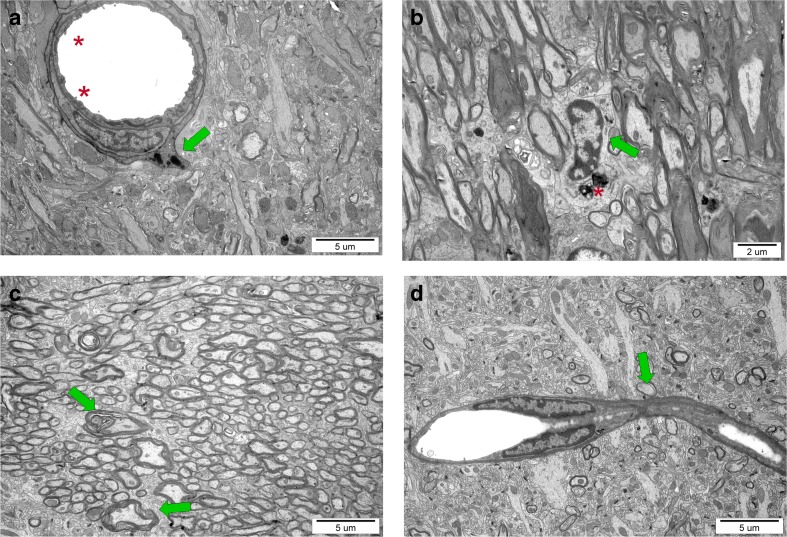
In our in vivo studies on impact of perinatal asphyxia on oligodendrocyte survival and maturation in rat model, pathological changes in nervous tissue of various brain regions (hippocampus, striatum, corpus callosum, cerebral cortex) were observed even several weeks post insult. Accordingly, the ultrastructural examination by means of electron microscopy revealed many symptoms of locally ongoing inflammatory process like neuropil edema, collapsed small blood vessels, and macrophage infiltration (Fig. 3). However, the angiogenesis in the traumatized tissue was also detected, usually as the bridging vessels. The areas significantly depleted from mature oligodendrocytes were notified as well, which might correspond to the development of the diffuse white matter injury (DWMI)—one of the most characteristic outcomes of the HI episode [57, 70, 71].
Fig. 3.
Ultrastructure of nervous tissue obtained from control and experimental rat brains 7 weeks after perinatal asphyxia performed in 7-day-old rat. a Corpus callosum of H-I rat: characteristic microvilli on the endothelium surface (red asterisk) and a macrophage cell residing in the blood vessel wall (green arrow) suggesting temporal interruption of blood-brain barrier. b Activated microglial cells (green arrow) with numerous lysosomes (asterisk), filled with hydrolytic enzymes, in the hippocampus of H-I rats. c Malformed myelin sheaths with splitting lamellae in striatum of injured rats. d The bridging vessel (green arrow) in H-I rats indicating the ongoing angiogenesis, conducive to processes of neurorestoration
Apart from limitation of trophic support and development of inflammatory process, the oxidative stress is thought to play a pivotal role in oligodendrocyte survival and differentiation after the hypoxic-ischemic insult. Increase in the level of free radicals (molecules containing an unpaired electron in an atomic orbital), especially the reactive oxygen species (ROS) and the reactive nitrogen species (RNS), strongly affects cell functioning and leads to the imbalance in local homeostasis. While at the physiological levels, ROS and RNS, generated during adenosine triphosphate (ATP) production by mitochondria, contribute to regulation of the cell survival and proliferation; their excess exerts a negative impact, especially by directly impairing mitochondrial function and often leading to an apoptotic cell death [72, 73].
In this way, oxidative stress is particularly harmful to oligodendrocytes due to their high metabolic demands and mitochondrial activity associated with generation and maintenance of myelin membranes. Since the process is energy dependent, significant amounts of ATP and oxygen are utilized, contributing to the increase in the ROS and RNS levels due to intense metabolism. Additionally, the high iron content, characteristic for oligodendrocytes and necessary for myelinogenesis, might contribute to generation of free radicals and subsequent lipid peroxidation. Free radical formation is also enhanced by cytokines associated with inflammatory process, which in reciprocal manner might enhance the ongoing inflammation, since hydrogen peroxide and ROS are used by the immune system as cytotoxic mediators [74]. It has been shown that pro-inflammatory cytokine-mediated downregulation of myelin genes is redox sensitive [75]. Free radicals might also participate in disrupting oligodendrocyte maturation by upregulating the expression of differentiation-inhibiting genes (like ID2, ID4) and downregulating the expression of genes promoting oligodendrocyte maturation (Sox10, Olig1, Olig2), by epigenetic mechanisms involving histone acetylation (repression of gene coding histone deacetylase 3, HDAC3) [76].
Physiologically, the detrimental effects of free radicals are prevented by several enzymes of antioxidant defense comprising superoxide dismutase-1 and dismutase-2 (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione-S-transferase (GST). Oligodendrocytes, especially in the developing brain, are however characterized by the low concentrations of glutathione and SODs which would prevent either oxidative or nitrosative injury [77, 78]. Thus, supplementation with antioxidants which act as free radical scavengers seems to be indispensable for sustaining oligodendrogial functions after perinatal asphyxia.
Development of oxidative stress as a consequence of the experienced incident of perinatal asphyxia and its potential severity might be evaluated by determination of different biochemical markers, which usually are the products of reactions triggered by the excess of free radicals. While some of them are useful as indicators of lipid peroxidation (which also indirectly might provide information about myelin damage, like the presence of F2-dihomo-isoprostanes) [79], others correspond to either DNA or protein oxidative damage [80]. Reliable parameters, measured in the biologic fluids (plasma, urine, spinal fluid) and tissues, include relative concentration of isoprostanes (F2-IsoPs, F3-IsoPs), neuroprostanes (F4-NeuroPs), nonprotein-bound iron (NPBI), protein adducts of 4-hydroxynonenal (4-HNE PAs), and advanced oxidation protein products (AOPP), as well as the calculated ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG), which might be considered as either predictive or discriminative indicator of oxidative stress [81–84].
Regional lack or malformation of myelin sheaths corresponding to CNS dys/demyelination unfortunately is not the only one outcome of deficiency of the mature oligodendrocytes. Beyond their major role of ensheathing axons of large and medium caliber [85], oligodendrocytes also were shown to support the nervous cells with energetic substrates like glycogen-derived pyruvate/lactate via the monocarboxylate transporter 1 [86–88] and with trophic factors [89]. The latter include factors promoting neurogenesis and protecting neurons after a temporal imbalance in tissue homeostasis. Thus, deficiency of either mature oligodendrocytes or even their undifferentiated progenitors could negatively influence survival of stressed neurons or those newly born after the insult.
Rescuing Infants from Neonatal Hypoxic-Ischemic Event
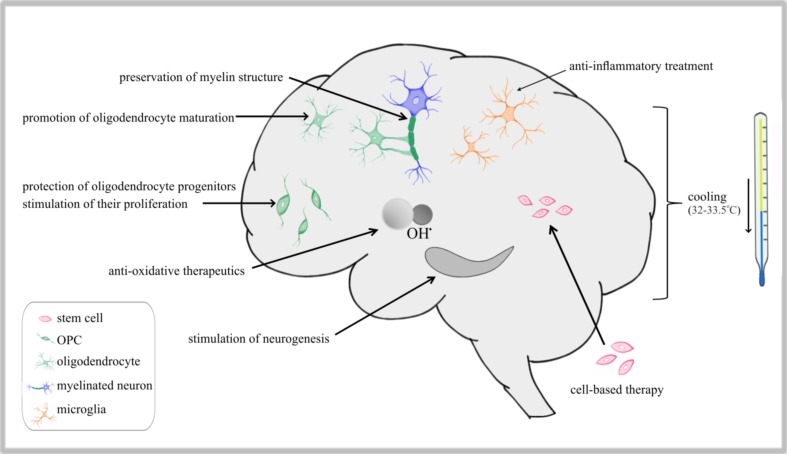
To prevent neurodevelopmental impairments, which are being anticipated as fatal consequences of perinatal asphyxia, different types of remedies have been designed and recommended for clinical implementation (Fig. 4). In clinical practice, the very first treatment applied to the baby who has experienced neonatal hypoxia-ischemia and is unable to breathe without assistance is mechanical ventilation to restore the physiological level of blood oxygenation. To attain this goal, it is also necessary to stabilize blood pressure and to avoid hyperoxygenation which could have severe detrimental effects like bronchopulmonary dysplasia (BPD) and retinopathy of prematurity [90, 91]. Accordingly, the ventilatory support of term infants should be carried out with air and in the case of preterm infants, either air or a low concentration of oxygen (about 30%) should be applied, avoiding large-volume inflations [92, 93]. At the time of or after resuscitation, the body temperature should be maintained between 36.5 and 37.5 °C to ensure infant survival; however, later on mild hypothermia—either selective (head) or systemic (body)—is considered effective therapy preventing the development of neurological dysfunctions. Hypothermia as treatment is recommended for rescuing infants with moderate to severe HIE and is usually based on maintaining body temperature at 33–33.5 °C for 72 h followed by slow and controlled rewarming [94–97]. The published meta-analysis study revealed that mild hypothermia is indeed efficacious in promoting patient survival and in reducing neurological impairments [98, 99].
Fig. 4.
The scheme of targets for therapeutic intervention after perinatal asphyxia. The first and most effective is head cooling applied immediately after the injury, aimed at avoiding/limiting injures resulting from temporal hypoxia-ischemia. Current strategies are based on preventing the development of leukodystrophic disorders (anti-oxidative, neuroprotective are myelin-preserving protocols) and favoring endogenous neuroreparative mechanisms (providing anti-inflammatory therapeutics and trophic support)
It however still remains to be disclosed if hypothermia exerts any influence on protecting the already existing myelin structure and on generation of new compact myelin. On the one hand, it was reported that considerably mild cooling (to 32–33 °C for 48 h) is effective in preventing cell death of oligodendroglial precursors and promoting their differentiation in the in vitro model, as well as contributes to accelerating the rate of their proliferation and increasing the number of myelinated axons in vivo [100, 101]. On the other hand, the delayed hypothermia followed by either slow or rapid rewarming was shown to trigger the apoptosis of myelinating oligodendrocytes in the piglet model of HI encephalopathy [102]. It indicates that very detailed procedures should be elaborated in regard to severity of the asphyxic event, the induction and duration of hypothermia, and the schemes of gradual rewarming, as well as therapeutic window for clinical interventions should be precisely determined to gain the best therapeutic effects.
Nonetheless, the mild hypothermia routinely applied in the case of perinatal asphyxia seemed to at least decrease morbidity among neonates who had experienced a severe hypoxic-ischemic insult. Its beneficial outcomes supposedly would be even better, when hypothermia is combined with other treatments, like melatonin administration, treatment with hydrogen sulfide, or 45–50% argon inhalation to increase brain metabolism, what was tested a piglet model of perinatal asphyxia [103–105].
Pharmacological Treatments to Prevent Developmental Disabilities
For the last two decades, a number of natural biological substances has been included in preclinical studies on animal models, as well as used as treatments in a clinical practice (Table 1). The first group consists of the analogues of physiological compounds, which are known to play important roles in various biological processes. One of them is melatonin (N-acetyl-5-methoxy tryptamine), tested in the context of its utility in preventing neurodevelopmental disabilities. This hormone, which secretion by the pineal gland is regulated by the circadian rhythm (light/dark cycle), is thought to help stabilize basic physiological parameters like body temperature and blood pressure. The expected favorable outcome of melatonin administration is associated however with the reported direct anti-oxidative, anti-apoptotic, and anti-inflammatory effects [106, 107]. The latter is thought to contribute to either the protection of myelin structure and/or promotion of its reconstruction, in spite of the lack of the influence on the reduction of cortical infarct volume resulting from brain injury, as deduced from the study on the rat model [108, 109]. The encouraging data concerning the neuroprotective and immunomodulatory features of melatonin resulted in including this hormone in clinical trials as one of the potential therapeutic means to treat perinatal asphyxia.
Table 1.
Currently used treatments administrated to new born children who experienced perinatal asphyxia
| Biological factor | In vivo function | Natural source |
|---|---|---|
| Docosahexaenoic acid (DHA)—a long-chain omega-3 fatty acid | Sustains membrane fluidity and integrity; contributes to the synaptic functioning; an anti-inflammatory compound | Maternal milk during breast-feeding period; sea fish (like tuna, salmon, herring, sardines) caviar, algae |
| Resveratrol (3,5,4′-trihydroxy-trans-stilbene) | Enhances endogenous anti-oxidative defense | Skin of grapes, blueberries, raspberries, red wine, peanuts, dark chocolate |
| Sodium butyrate | Histone deacetylase inhibitor, regulates gene expression through NF-kappaB cascade; reduces expression of pro-inflammatory cytokines, stimulates neurogenesis; protects oligodendrocytes | Produced from dietary fiber in the gut by endogenous bacteria as the end-product of intestinal microbial fermentation; milk fat (so also in butter and cheese) |
| Erythropoietin (EPO, hematopoietin) | Indispensable for erythropoiesis, enhance angiogenesis, exerts neuroregenerative, anti-inflammatory and anti-apoptotic effects | Hormone produced by interstitial fibroblasts in the kidney |
| Melatonin (N-acetyl-5-methoxy tryptamine) | Direct anti-oxidative, anti-apoptotic and anti-inflammatory effects; protects myelin structure | Hormone secreted predominantly by pineal gland |
| Triiodothyronine (T3) and its prohormone, thyroxine (T4) | Engaged in physiological process of oligodendrocyte maturation; promotes in vivo remyelination | Hormones produced by the thyroid gland |
| Mesenchymal stem cells (MSCs) | Used for cell replacement, provide trophic support to diseased tissue, exert anti-inflammatory and neuroprotective effect | Bone marrow, umbilical cord (cord blood and Wharton’s jelly), adipose tissue, etc. |
Another example of a physiological molecule is erythropoietin (EPO, hematopoietin), a pleiotropic cytokine predominantly produced in kidneys and liver and responsible for erythropoiesis [110]. By binding to its specific membrane receptor EpoR, EPO promotes proliferation and differentiation of erythroid progenitor cell. Importantly, this cytokine expression is upregulated in response to hypoxia [111]. Moreover, EpoR is found on different cell types in brain [112]. EPO was shown to enhance angiogenesis after anoxic event [113–115] and was suggested to have neuroregenerative, anti-inflammatory, and anti-apoptotic effects in the brain [116, 117]. It turned out to be effective in reducing to some extend white matter damage after HI injury in neonatal rats and preterm fetal sheep and in improving behavioral outcomes [118, 119]. Since nowadays EPO is routinely used in clinical practice to avoid or treat neonatal anemia [120], the additional advantages in preventing eventual neurological complications common in preterm neonates might supposedly be expected as well (phase 3 of clinical trials) [121–123].
According to the recently published data, magnesium sulfate was shown to accelerate the differentiation process of rat oligodendrocytes in vitro, although it did not ensure protection for mature cells against hypoxic-ischemic damage [124, 125]. While considering possible strategies aimed at rescuing oligodendrocyte progenitors from cell death and/or promoting their maturation, application of thyroid hormones should be taken into account. Triiodothyronine (T3) and its prohormone, thyroxine (T4), produced by the thyroid gland, are known to be engaged in physiological process of oligodendrocyte maturation and to promote remyelination in vivo [126, 127].
Another group of natural molecules comprising popular nutrients used to diminish fatal consequences of neurorodegenerative processes evoked during the hypoxic-ischemic episode could be distinguished. One of the potential remedies alleviating neurological deficits resulting from the asphyxic event is the docosahexaenoic acid (DHA), which is a long-chain omega-3 fatty acid (long-chain polyunsaturated fatty acids: LCPUFA) and yet the major polyunsaturated fatty acid in the adult mammalian brain. This phospholipid is responsible for membrane organization and integrity, exerts an impact on neurogenesis and neuroplasticity, contributes to modulating signal transduction pathways, participates in the myelination process, and affects the effectiveness of neurotransmission [128–131]. It has also been reported to possess anti-inflammatory properties and to participate in the development of the immune system in childhood [132–135]. Being crucial for a developing brain, DHA can be found in maternal milk during a lactation period (up to 1% of the total fatty acids) [136–138]. Supplementation with DHA was shown to be important for cognitive and visual development early in life and also useful for nervous system functioning during the life span [139–142]. Since LCPUFA, including DHA, can be easily found in seafood and certain plants [98, 143, 144], they have been recommended to be included in everyday, diversified diet. According to the very recent data, treatment with DHA turned out to be effective in the case of rat model of the neonatal hypoxia-ischemia. Accordingly, the beneficial effects of DHA administration seemed to result from both protecting neurons and myelin from destruction in inimical microenvironment and from reducing inflammatory response evoked by the HI episode. It was also shown to be effective in ameliorating cognitive deficits in rodent models [145, 146].
Resveratrol, a natural antioxidant found in grapes skin and red wine, is another example of neuroprotectant acting by enhancing endogenous anti-oxidative defense [147, 148]. Similar to the abovementioned molecules, it was shown to exert some beneficial effects on reducing cognitive impairments in the rodent model of stroke [149].
One of the worth mentioning molecules is also the sodium butyrate (SB), which is produced from dietary fiber in the gut by endogenous bacteria as the end-product of intestinal microbial fermentation. It acts as one of the histone deacetylase inhibitors (HDACs) and thus influences compaction of chromatin and regulates activation of genes engaged in progress of oligodendrocyte differentiation. HDACs may either initiate repression by modulating the acetylation state of nucleosomal histones/transcriptional regulators or directly bind to transcriptional regulators and function as transcriptional co-repressors [150]. SB has been also reported to possess immunomodulatory properties due to reducing the expression of pro-inflammatory cytokines [151–153]. In our recent studies on the rat model of neonatal hypoxia, the administration of SB was shown to exert neuroprotective, neurogenic, and anti-inflammatory effect finally resulting in a significant reduction of brain damage [154]. This hope-rising observation concerned prevention of the HI-induced loss of neuroblasts and oligodendrocyte precursor cells, which is important for the initiation of the compensatory mechanism leading to amelioration of neurological deficiency.
Keeping in mind the diversified and detrimental effects of perinatal asphyxia, new treatments are intensely searched for and preclinically tested in context of their eventual adverse effects and the desired effectiveness in preventing neurodevelopmental disorders. Pharmacological interventions are difficult in vulnerable newborns, since they might interfere with the intense developmental processes. Among proposed new treatments, administration of the free radical scavengers and anti-oxidative compounds seem to confer beneficiary effects in terms of enhancing natural, endogenous anti-oxidative defense, which is inefficient in early human development [78]. Catalpol, an iridoid glycoside extracted from Rehmannia root, has been shown to protect pre-myelinating oligodendrocytes through ERK1/2 signaling pathway by suppressing Ca2+ influx, reducing mitochondrial damage and inhibiting ROS overproduction [155]. Neuroprotective effects have been also achieved by administration of allopurinol (inhibitor of xanthine oxidase, the enzyme engaged in generation of superoxide particle), vitamin E (α-tocopherol and β-tocopherol) with vitamin C (ascorbic acid) together counteracting lipid peroxidation, as well as deferoxamine, which is chelating agent for free iron (highly concentrated in oligodendrocytes) [156, 157]. Nonetheless, the new effective therapies preventing the harmful effects of oxidative stress and promoting reparative processes are needed to be developed and preclinically tested to provide safe and efficient treatment options for newborns.
Cell Transplantation as a Neuroreparative Strategy
A growing list of evidence from preclinical studies indicates that cell transplantation is an effective treatment option applied to prevent the symptoms of neurodegenerative processes which develop as a consequence of cell death or alteration in their differentiation and biological functions. Directly replenishing the nervous tissue with cells which are depleted in a result of the insult seems to be one of the main advantages of cell-based therapies. The mesenchymal stem cells obtained from various sources might be used either for direct engraftment with aim of their in vivo differentiation or for generation of neural progenitors. One of the most promising sources of mesenchymal stem cells is umbilical cord (cord blood and Wharton’s jelly) [158, 159]. In our very recent studies, human cord blood-derived cells were shown to be relatively easily differentiated into oligodendroglia-biased progenitors by application of the serum-free protocol based of using analogues of physiological molecules (PDGF-AA, T3, and extracellular matrix components like laminin and fibronectin) [160]. The obtained progenitors might in vivo give rise to oligodendrocyte with myelinating potential and/or to favorably act by modifying the local tissue microenvironment by secreting trophic factors and anti-inflammatory cytokines [89, 161].
Promoting neurological recovery via indirect bystander actions seems to be one of the main advantages of using cells with stem cell/progenitor characteristics [162, 163]. Accordingly, transplantation of the mesenchymal stem cells was shown to contribute to the reduction of the lesion volume and protection of white matter and consequently to improve the motor function [164–166]. Thus, the cell-based therapies offer few advantages: a direct supplementation of the traumatized tissue with the exogenous cells, protection against further brain damage, promotion of the neuroregeneration, and improvement of the behavioral functions.
Clinical Perspective
When concluding on the therapeutic strategies for preventing leukodystrophic disorders resulting from perinatal asphyxia, it should be taken into consideration that neurons and glial cells are functionally interdependent. During development, oligodendrocyte maturation and myelinogenesis is guided by the external stimuli, including signals provided by differentiating neurons [167–169]. While some of them regulate the differentiation process (PDGF-A, neuregulin: NGR, etc.), others play a role in matching oligodendrocytes to the axonal surface (cell adhesion molecule L1, the polysialylated neuronal cell adhesion molecule: PSA-NCAM; Jagged 1). Similarly, neuron survival and proficient functioning strongly depends on oligodendrocytes and compact myelin sheath [170–173]. Keeping the above in mind, both the compounds protecting either neurons or oligodendrocytes, as well as those preserving the myelin structure, might turn out to be effective in preventing white matter damage and subsequent neurodevelopmental disorders. On the one hand, the common feature of the enumerated compounds is their relative fine tolerance by very young organisms, as deduced from very rare reports on side effects evoked by natural molecules administration. On the other hand, however, their efficacy of improving the overall well-being of human neonates, who experienced perinatal hypoxia, is still very limited. It results from a wide range of the injuries evoked by HI, the course of pregnancy (like potential chronic hypoxia, hemorrhages), the procedures initiated in response to the insult and time of their implementation, very limited number of clinical trials, and confined abilities of the already existing compounds to cure severe trauma. The solution for increasing the efficiency of successful treatment would be to foster preclinical studies on animal models with new substances leading to detailed description of mechanisms of their action and their eventual side effects on both molecular and systemic levels. The other one could be combining the already known treatments with the aim of increasing their beneficial effects, like for instance administration of neuroprotectants (sodium butyrate, melatonin, DHA) with cell therapies promoting endogenous repair. As deduced from the available data concerning the outcomes of the applied therapies (which turned out to be ineffective in some cases), in spite there is a growing list of the treatments available, the clinical protocol should be every time chosen individually for the given case of perinatal asphyxia event.
Acknowledgements
This study was financially supported by NCN (National Science Centre, Poland) grant no. 2014/15/B/NZ4/01875. Authors express their gratitude to Professor Malgorzata Frontczak-Baniewicz, who performed detailed ultrastructural studies at Electron Microscopy Platform (Mossakowski Medical Research Centre, Polish Academy of Sciences).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr. 2007;166(7):645–654. doi: 10.1007/s00431-007-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Ment LR, Hirtz D, Hüppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8(11):1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee AC, Cousens S, Wall SN, Niermeyer S, Darmstadt GL, Carlo WA, Keenan WJ, Bhutta ZA, Gill C, Lawn JE. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health Suppl. 2001;3:S12. doi: 10.1186/1471-2458-11-S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pammi M, Dempsey EM, Ryan CA, Barrington KJ. Newborn resuscitation training programmes reduce early neonatal mortality. Neonatology. 2016;110:210–224. doi: 10.1159/000443875. [DOI] [PubMed] [Google Scholar]
- 7.Te Pas AB, Sobotka K, Hooper SB. Novel approaches to neonatal resuscitation and the impact on birth asphyxia. Clin Perinatol. 2016;43(3):455–467. doi: 10.1016/j.clp.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 8.de Haan M, Wyatt JS, Roth S, Vargha-Khadem F, Gadian D, Mishkin M. Brain and cognitive-behavioural development after asphyxia at term birth. Dev Sci. 2006;9(4):350–358. doi: 10.1111/j.1467-7687.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- 9.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 10.Marret S, Marchand-Martin L, Picaud JC, Hascoët JM, Arnaud C, Rozé JC, Truffert P, Larroque B, Kaminski M, Ancel PY. EPIPAGE study group brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One. 2013;8(5):e62683. doi: 10.1371/journal.pone.0062683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton BL, Huang A, Dukala D, Soliven B, Popko B (2017) Neonatal hypoxia results in peripheral nerve abnormalities. Am J Pathol [DOI] [PMC free article] [PubMed]
- 12.Budday S, Steinmann P, Kuhl E. Physical biology of human brain development. Front Cell Neurosci. 2015;9:257. doi: 10.3389/fncel.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S, Kaiser M. Developmental time windows for axon growth influence neuronal network topology. Biol Cybern. 2015;109(2):275–286. doi: 10.1007/s00422-014-0641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Nardelli J. Cellular and molecular introduction to brain development. Neurobiol Dis. 2016;92(Pt A):3–17. doi: 10.1016/j.nbd.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat Neurosci. 2005;8(2):242–252. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- 16.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62(9):1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 17.von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol. 2016;524(18):3865–3895. doi: 10.1002/cne.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakhti M, Aggarwal S, Simons M. Myelin architecture: zippering membranes tightly together. Cell Mol Life Sci. 2014;71(7):1265–1277. doi: 10.1007/s00018-013-1492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mobius W, Nave KA, Werner HB (2016) Electron microscopy of myelin: structure preservation by high-pressure freezing. Brain Res. doi:10.1016/j.brainres.2016.02.027 [DOI] [PubMed]
- 20.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 21.Dean JM, Moravec MD, Grafe M, Abend N, Ren J, Gong X, et al. Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev Neurosci. 2011;33(3–4):251–260. doi: 10.1159/000327242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barateiro A, Fernandes A. Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta. 2014;1843(9):1917–1929. doi: 10.1016/j.bbamcr.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Farrer RG, Quarles RH. GT3 and its O-acetylated derivative are the principal A2B5-reactive gangliosides in cultured O2A lineage cells and are down-regulated along with O-acetyl GD3 during differentiation to oligodendrocytes. J Neurosci Res. 1999;57(3):371–380. doi: 10.1002/(SICI)1097-4547(19990801)57:3<371::AID-JNR9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24.Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61(5):471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Binamé F, Sakry D, Dimou L, Jolivel V, Trotter J. NG2 regulates directional migration of oligodendrocyte precursor cells via Rho GTPases and polarity complex proteins. J Neurosci. 2013;33(26):10858–10874. doi: 10.1523/JNEUROSCI.5010-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- 28.Kuhlmann T, Miron V, Cui Q, Wegner C, Antel J, Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(Pt 7):1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 29.Islam MS, Tatsumi K, Okuda H, Shiosaka S, Wanaka A. Olig2-expressing progenitor cells preferentially differentiate into oligodendrocytes in cuprizone-induced demyelinated lesions. Neurochem Int. 2009;54(3–4):192–198. doi: 10.1016/j.neuint.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Niu J, Mei F, Wang L, Liu S, Tian Y, Mo W, Li H, Lu QR, Xiao L. Phosphorylated olig1 localizes to the cytosol of oligodendrocytes and promotes membrane expansion and maturation. Glia. 2012;60(9):1427–1436. doi: 10.1002/glia.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai J, Bercury KK, Jin W, Macklin WB. Olig1 acetylation and nuclear export mediate oligodendrocyte development. J Neurosci. 2015;35(48):15875–15893. doi: 10.1523/JNEUROSCI.0882-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13(1):62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- 33.Hermann A, Brandt MD, Loewenbrück KF, Storch A. “Silenced” polydendrocytes: a new cell type within the oligodendrocyte progenitor cell population? Cell Tissue Res. 2010;340(1):45–50. doi: 10.1007/s00441-010-0940-5. [DOI] [PubMed] [Google Scholar]
- 34.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24(1):39–47. doi: 10.1016/S0166-2236(00)01691-X. [DOI] [PubMed] [Google Scholar]
- 35.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24(2):476–488. doi: 10.1016/S1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 36.Geha S, Pallud J, Junier MP, Devaux B, Leonard N, Chassoux F, Chneiweiss H, Daumas-Duport C, Varlet P. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol. 2010;20(2):399–411. doi: 10.1111/j.1750-3639.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon C, Götz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59(6):869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- 38.Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- 39.Bansal R, Warrington AE, Gard A, Ranscht B, Pfeiffer S. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989;24:548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(SICI)1097-4547(19970301)47:5<455::AID-JNR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 41.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21(4):1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunner C, Lassmann H, Waehneldt TV, Matthieu JM, Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2′, 3′-cyclic nucleotide 3′-phosphodiesterase in the CNS of adult rats. J Neurochem. 1989;52:296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x. [DOI] [PubMed] [Google Scholar]
- 43.Sprinkle TJ. 3′-cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol. 1989;4(3):235–301. [PubMed] [Google Scholar]
- 44.Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-K. [DOI] [PubMed] [Google Scholar]
- 45.McAlarney T, Ogino M, Apostolski S, Latov N. Specificity and cross-reactivity of anti-galactocerebroside antibodies. Immunol Investig. 1995;24(4):595–606. doi: 10.3109/08820139509066860. [DOI] [PubMed] [Google Scholar]
- 46.Snaidero N, Velte C, Myllykoski M, Raasakka A, Ignatev A, Werner HB, Erwig MS, Mobius W, Kursula P, Nave KA, Simons M. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 2017;18(2):314–323. doi: 10.1016/j.celrep.2016.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salzer JL, Holmes WP, Colman DR. The amino acid sequences of the myelin-associated glycoproteins: homology to the superimmunoglobulin gene superfamily. J Cell Biol. 1987;104:957–965. doi: 10.1083/jcb.104.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popot JL, Pham-Dinh D, Dautigny A. Major myelin proteolipid: the 4-alpha-helix topology. J Membr Biol. 1991;120:233–246.12. doi: 10.1007/BF01868534. [DOI] [PubMed] [Google Scholar]
- 49.Weimbs T, Stoffel W. Topology of CNS myelin proteolipid protein: evidence for the nonenzymatic glycosylation of extracytoplasmic domains in normal and diabetic animals. Biochemistry. 1994;33:10408–10415. doi: 10.1021/bi00200a023. [DOI] [PubMed] [Google Scholar]
- 50.Han H, Myllykoski M, Ruskamo S, Wang C, Kursula P. Myelin-specific proteins: a structurally diverse group of membrane-interacting molecules. Biofactors. 2013;39(3):233–241. doi: 10.1002/biof.1076. [DOI] [PubMed] [Google Scholar]
- 51.Inouye H, Kirschner DA (2015) Evolution of myelin ultrastructure and the major structural myelin proteins. Brain Res. doi:10.1016/j.brainres.2015.%2010.037 [DOI] [PubMed]
- 52.Vassall KA, Bamm VV, Harauz G. MyelStones: the executive roles of myelin basic protein in myelin assembly and destabilization in multiple sclerosis. Biochem J. 2015;472(1):17–32. doi: 10.1042/BJ20150710. [DOI] [PubMed] [Google Scholar]
- 53.Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E, Rowitch DH. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;58(2):383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skoff RP, Bessert DA, Barks JD, Song D, Cerghet M, Silverstein FS. Hypoxic-ischemic injury results in acute disruption of myelin gene expression and death of oligodendroglial precursors in neonatal mice. Int J Dev Neurosci. 2001;19(2):197–208. doi: 10.1016/S0736-5748(00)00075-7. [DOI] [PubMed] [Google Scholar]
- 55.Rothstein RP, Levison SW. Gray matter oligodendrocyte progenitors and neurons die caspase-3 mediated deaths subsequent to mild perinatal hypoxic/ischemic insults. Dev Neurosci. 2005;27(2–4):149–159. doi: 10.1159/000085987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63(4):520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71(1):93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaidi AU, Bessert DA, Ong JE, Xu H, Barks JD, Silverstein FS, Skoff RP. New oligodendrocytes are generated after neonatal hypoxic-ischemic brain injury in rodents. Glia. 2004;46(4):380–390. doi: 10.1002/glia.20013. [DOI] [PubMed] [Google Scholar]
- 59.Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128(Pt 5):1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- 60.Morell P, Quarles R. Basic neurochemistry. 6th. Philadelphia: Lippincott-Raven; 1995. Myelin formation, structure and biochemistry. [Google Scholar]
- 61.Schmitt S, Castelvetri LC, Simons M. Metabolism and functions of lipids in myelin. Biochim Biophys Acta. 2015;1851(8):999–1005. doi: 10.1016/j.bbalip.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Susuki K. Myelin: a specialized membrane for cell communication. Nat Educ. 2010;3(9):59. [Google Scholar]
- 63.Miron VE, Kuhlmann T, Antel JP. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta. 2011;1812(2):184–193. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29(14):4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pham L-DD, Hayakawa K, Seo JH, Nguyen M-N, Som AT, Lee BJ, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maki T, Maeda M, Uemura M, Lo EK, Terasaki Y, Liang AC, Shindo A, Choi YK, Taguchi A, Matsuyama T, Takahashi R, Ihara M, Arai K. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci Lett. 2015;15(597):164–169. doi: 10.1016/j.neulet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang L, Shen F, Degos V, Schonemann M, Pleasure SJ, Mellon SH, Young WL, Su H. Oligogenesis and oligodendrocyte progenitor maturation vary in different brain regions and partially correlate with local angiogenesis after ischemic stroke. Transl Stroke Res. 2011;2(3):366–375. doi: 10.1007/s12975-011-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koutsakis C, Kazanis I. How necessary is the vasculature in the life of neural stem and progenitor cells? Evidence from evolution, development and the adult nervous system. Front Cell Neurosci. 2016;10:35. doi: 10.3389/fncel.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seo JH, Maki T, Maeda M, Miyamoto N, Liang AC, Hayakawa K, Pham LD, Suwa F, Taguchi A, Matsuyama T, Ihara M, Kim KW, Lo EH, Arai K. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS One. 2014;9(7):e103174. doi: 10.1371/journal.pone.0103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delcour M, Russier M, Amin M, Baud O, Paban V, Barbe MF, Coq JO. Impact of prenatal ischemia on behavior, cognitive abilities and neuroanatomy in adult rats with white matter damage. Behav Brain Res. 2012;232(1):233–244. doi: 10.1016/j.bbr.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 71.Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, Rutherford MA, Cowan FM. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012;61(5):799–807. doi: 10.1016/j.jpeds.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 72.Baud O, Li J, Zhang Y, Neve RL, Volpe JJ. Rosenberg PA nitric oxide-induced cell death in developing oligodendrocytes is associated with mitochondrial dysfunction and apoptosis-inducing factor translocation. Eur J Neurosci. 2004;20(7):1713–1726. doi: 10.1111/j.1460-9568.2004.03616.x. [DOI] [PubMed] [Google Scholar]
- 73.Martinvalet D, Zhu P, Lieberman J. Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity. 2005;22(3):355–370. doi: 10.1016/j.immuni.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 74.di Penta A, Moreno B, Reix S, Fernandez-Diez B, Villanueva M, Errea O, Escala N, Vandenbroeck K, Comella JX, Villoslada P. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One. 2013;8(2):e54722. doi: 10.1371/journal.pone.0054722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med. 2005;39:823–831. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87(14):3076–3087. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baud O, Haynes R, Wang H, Folkerth RD, Li J, Volpe J, Rosenberg PA. Developmental up-regulation of MnSOD in rat oligodendrocytes confers protection against oxidative injury. Eur J Neurosci. 2004;19:2669–2681. doi: 10.1111/j.0953-816X.2004.03396.x. [DOI] [PubMed] [Google Scholar]
- 78.Folkerth R, Haynes R, Borenstein NS, Volpe JJ, Kinney HC. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 2004;63:990–999. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- 79.VanRollins M, Woltjer RL, Yin H, Morrow JD, Montine TJ. F2-dihomo-isoprostanes arise from free radical attack on adrenic acid. J Lipid Res. 2008;49(5):995–1005. doi: 10.1194/jlr.M700503-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tonni G, Leoncini S, Signorini C, Ciccoli L, De Felice C. Pathology of perinatal brain damage: background and oxidative stress markers. Arch Gynecol Obstet. 2014;290(1):13–20. doi: 10.1007/s00404-014-3208-6. [DOI] [PubMed] [Google Scholar]
- 81.Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ., 2nd Isoprostane generation and function. Chem Rev. 2011;11(10):5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Longini M, Belvisi E, Proietti F, Bazzini F, Buonocore G, Perrone S. Oxidative stress biomarkers: establishment of reference values for isoprostanes, AOPP, and NPBI in cord blood. Mediat Inflamm. 2017;2017:1758432. doi: 10.1155/2017/1758432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garcia-Flores LA, Medina S, Martínez-Hernández P, Oger C, Galano JM, Durand T, Casas-Pina T, Ferreres F, Gil-Izquierdo Á. Snapshot situation of oxidative degradation of the nervous system, kidney, and adrenal glands biomarkers-neuroprostane and dihomo-isoprostanes-urinary biomarkers from infancy to elderly adults. Redox Biol. 2017;11:586–591. doi: 10.1016/j.redox.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Back SA, Luo NL, Mallinson RA, O’Malley JP, Wallen LD, Frei B, Morrow JD, Petito CK, Roberts CT, Jr, Murdoch GH, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58:108–120. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 85.Simons M, Trajkovic K. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J Cell Biochem. 2006;119:4381–4389. doi: 10.1242/jcs.03242. [DOI] [PubMed] [Google Scholar]
- 86.Morrison BM, Lee Y, Rothstein JD. Oligodendroglia: metabolic supporters of axons. Trends Cell Biol. 2013;23(12):644–651. doi: 10.1016/j.tcb.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saab AS, Tzvetanova ID, Nave KA. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr Opin Neurobiol. 2013;23(6):1065–1072. doi: 10.1016/j.conb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 88.Baltan S. Can lactate serve as an energy substrate for axons in good times and in bad, in sickness and in health? Metab Brain Dis. 2015;30(1):25–30. doi: 10.1007/s11011-014-9595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sypecka J, Sarnowska A. The neuroprotective effect exerted by oligodendroglial progenitors on ischemically impaired hippocampal cells. Mol Neurobiol. 2014;49(2):685–701. doi: 10.1007/s12035-013-8549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bizzarro MJ, Li FY, Katz K, Shabanova V, Ehrenkranz RA, Bhandari V. Temporal quantification of oxygen saturation ranges: an effort to reduce hyperoxia in the neonatal intensive care unit. J Perinatol. 2014;34(1):33–38. doi: 10.1038/jp.2013.122. [DOI] [PubMed] [Google Scholar]
- 91.Sola A, Golombek SG, Montes Bueno MT, Lemus-Varela L, Zuluaga C, Domínguez F, Baquero H, Young Sarmiento AE, Natta D, Rodriguez Perez JM, Deulofeut R, Quiroga A, Flores GL, Morgues M, Pérez AG, Van Overmeire B, van Bel F. Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014;103(10):1009–1018. doi: 10.1111/apa.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vali P, Mathew B, Lakshminrusimha S (2015) Neonatal resuscitation: evolving strategies. Matern Health Neonatol Perinatol 1 [DOI] [PMC free article] [PubMed]
- 93.Wyllie J, Bruinenberg J, Roehr CC, Rüdiger M, Trevisanuto D, Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015: Section 7. Resuscitation and support of transition of babies at birth. Resuscitation. 2015;95:249–263. doi: 10.1016/j.resuscitation.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 94.Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28(9):1353–1365. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Verklan MT. The chilling details: hypoxic-ischemic encephalopathy. J Perinat Neonatal Nurs. 2009;23(1):59–68. doi: 10.1097/01.JPN.0000346221.48202.7e. [DOI] [PubMed] [Google Scholar]
- 96.Cooper DJ. Induced hypothermia for neonatal hypoxic-ischemic encephalopathy: pathophysiology, current treatment, and nursing considerations. Neonatal Netw. 2011;30(1):29–35. doi: 10.1891/0730-0832.30.1.29. [DOI] [PubMed] [Google Scholar]
- 97.Silveira RC, Procianoy RS. Hypothermia therapy for newborns with hypoxic ischemic encephalopathy. J Pediatr. 2015;91(6 Suppl 1):S78–S83. doi: 10.1016/j.jped.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Doughman SD, Krupanidhi S, Sanjeevi CB. Omega-3 fatty acids for nutrition and medicine: considering microalgae oil as a vegetarian source of EPA and DHA. Curr Diabetes Rev. 2007;3(3):198–203. doi: 10.2174/157339907781368968. [DOI] [PubMed] [Google Scholar]
- 99.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD. TOBY study group. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2):140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 100.Xiong M, Li J, Ma SM, Yang Y, Zhou WH. Effects of hypothermia on oligodendrocyte precursor cell proliferation, differentiation and maturation following hypoxia ischemia in vivo and in vitro. Exp Neurol. 2013;247:720–729. doi: 10.1016/j.expneurol.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 101.Ichinose M, Kamei Y, Iriyama T, Imada S, Seyama T, Toshimitsu M, Asou H, Yamamoto M, Fujii T. Hypothermia attenuates apoptosis and protects contact between myelin basic protein-expressing oligodendroglial-lineage cells and neurons against hypoxia-ischemia. J Neurosci Res. 2014;92(10):1270–1285. doi: 10.1002/jnr.23418. [DOI] [PubMed] [Google Scholar]
- 102.Wang B, Armstrong JS, Reyes M, Kulikowicz E, Lee JH, Spicer D, Bhalala U, Yang ZJ, Koehler RC, Martin LJ, Lee JK. White matter apoptosis is increased by delayed hypothermia and rewarming in a neonatal piglet model of hypoxic ischemic encephalopathy. Neuroscience. 2016;316:296–310. doi: 10.1016/j.neuroscience.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Robertson NJ, Faulkner S, Fleiss B, Bainbridge A, Andorka C, Price D, Powell E, Lecky-Thompson L, Thei L, Chandrasekaran M, Hristova M, Cady EB, Gressens P, Golay X, Raivich G. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136(Pt 1):90–105. doi: 10.1093/brain/aws285. [DOI] [PubMed] [Google Scholar]
- 104.Broad KD, Fierens I, Fleiss B, Rocha-Ferreira E, Ezzati M, Hassell J, Alonso-Alconada D, Bainbridge A, Kawano G, Ma D, Tachtsidis I, Gressens P, Golay X, Sanders RD, Robertson NJ. Inhaled 45-50% argon augments hypothermic brain protection in a piglet model of perinatal asphyxia. Neurobiol Dis. 2016;87:29–38. doi: 10.1016/j.nbd.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dai HB, Xu MM, Lv J, Ji XJ, Zhu SH, Ma RM, Miao XL, Duan ML. Mild hypothermia combined with hydrogen sulfide treatment during resuscitation reduces hippocampal neuron apoptosis via NR2A, NR2B, and PI3K-Akt signaling in a rat model of cerebral ischemia-reperfusion injury. Mol Neurobiol. 2016;53(7):4865–4873. doi: 10.1007/s12035-015-9391-z. [DOI] [PubMed] [Google Scholar]
- 106.Biran V, Phan Duy A, Decobert F, Bednarek N, Alberti C, Baud O. Is melatonin ready to be used in preterm infants as a neuroprotectant? Dev Med Child Neurol. 2014;56(8):717–723. doi: 10.1111/dmcn.12415. [DOI] [PubMed] [Google Scholar]
- 107.Joshi N, Biswas J, Nath C, Singh S. Promising role of melatonin as neuroprotectant in neurodegenerative pathology. Mol Neurobiol. 2015;52(1):330–340. doi: 10.1007/s12035-014-8865-8. [DOI] [PubMed] [Google Scholar]
- 108.Villapol S, Fau S, Renolleau S, Biran V, Charriaut-Marlangue C, Baud O. Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke. Pediatr Res. 2011;69(1):51–55. doi: 10.1203/PDR.0b013e3181fcb40b. [DOI] [PubMed] [Google Scholar]
- 109.Alonso-Alconada D, Alvarez A, Lacalle J, Hilario E. Histological study of the protective effect of melatonin on neural cells after neonatal hypoxia-ischemia. Histol Histopathol. 2012;27(6):771–783. doi: 10.14670/HH-27.771. [DOI] [PubMed] [Google Scholar]
- 110.Jelkmann W. Regulation of erythropoietin production. J Physiol. 2011;589(Pt 6):1251–1258. doi: 10.1113/jphysiol.2010.195057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol. 2006;33(10):968–979. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- 112.Ott C, Martens H, Hassouna I, Oliveira B, Erck C, Zafeiriou MP, Peteri UK, Hesse D et al (2015) Widespread expression of erythropoietin receptor in brain and its induction by injury. Mol Med. doi:10.2119/molmed.2015.00192 [DOI] [PMC free article] [PubMed]
- 113.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 114.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 115.Yan F, Zhang M, Meng Y, Li H, Yu L, Fu X, Tang Y, Jiang C. Erythropoietin improves hypoxic-ischemic encephalopathy in neonatal rats after short-term anoxia by enhancing angiogenesis. Brain Res. 2016;1651:104–113. doi: 10.1016/j.brainres.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 116.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31:403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fan X, van Bel F, van der Kooij MA, Heijnen CJ, Groenendaal F. Hypothermia and erythropoietin for neuroprotection after neonatal brain damage. Pediatr Res. 2013;73:18–23. doi: 10.1038/pr.2012.139. [DOI] [PubMed] [Google Scholar]
- 118.Larpthaveesarp A, Georgevits M, Ferriero DM, Gonzalez FF. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol Dis. 2016;3:57–63. doi: 10.1016/j.nbd.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ren Q, Zhang XF, Yang JY. Erythropoietin reduces white matter damage in two-day-old rats exposed to hypoxic/ischemia injury. Neurol Res. 2016;8:1–7. doi: 10.1080/01616412.2016.1242451. [DOI] [PubMed] [Google Scholar]
- 120.Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2014;23(4):CD004868. doi: 10.1002/14651858.CD004868.pub4. [DOI] [PubMed] [Google Scholar]
- 121.Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, Chang T, Durand DJ, Song D, Bonifacio SL, Gonzalez FF, Glass HC, Juul SE. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130(4):683–691. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol Perinatol. 2015;1:27. doi: 10.1186/s40748-015-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoeber D, Sifringer M, van de Looij Y, Herz J, Sizonenko SV, Kempe K, Serdar M, Palasz J, Hadamitzky M, Endesfelder S, Fandrey J, Felderhoff-Müser U, Bendix I. Erythropoietin restores long-term neurocognitive function involving mechanisms of neuronal plasticity in a model of hyperoxia-induced preterm brain injury. Oxidative Med Cell Longev. 2016;2016:9247493. doi: 10.1155/2016/9247493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anabrees J. Magnesium sulphate for newborns with HIE; synopsis of evidence from a systematic review. J Clin Neonatol. 2013;2(3):114–116. doi: 10.4103/2249-4847.119989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Itoh K, Maki T, Shindo A, Egawa N, Liang AC, Itoh N, Lo EH, Lok J, Arai K. Magnesium sulfate protects oligodendrocyte lineage cells in a rat cell-culture model of hypoxic-ischemic injury. Neurosci Res. 2016;106:66–69. doi: 10.1016/j.neures.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fernández M, Baldassarro VA, Sivilia S, Giardino L, Calzà L. Inflammation severely alters thyroid hormone signaling in the central nervous system during experimental allergic encephalomyelitis in rat: direct impact on OPCs differentiation failure. Glia. 2016;64(9):1573–1589. doi: 10.1002/glia.23025. [DOI] [PubMed] [Google Scholar]
- 127.Lee JY, Petratos S. Thyroid hormone signaling in oligodendrocytes: from extracellular transport to intracellular signal. Mol Neurobiol. 2016;53(9):6568–6583. doi: 10.1007/s12035-016-0013-1. [DOI] [PubMed] [Google Scholar]
- 128.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 129.Guesnet P, Alessandri JM. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS)—implications for dietary recommendations. Biochimie. 2011;93(1):7–12. doi: 10.1016/j.biochi.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 130.Crupi R, Marino A, Cuzzocrea S. n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem. 2013;20(24):2953–63.1. doi: 10.2174/09298673113209990140. [DOI] [PubMed] [Google Scholar]
- 131.Weiser MJ, Butt CM, Mohajeri MH. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. 2016;8(2):99. doi: 10.3390/nu8020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Si TL, Liu Q, Ren YF, Li H, Xu XY, Li EH, Pan SY, Zhang JL, Wang KX. Enhanced anti-inflammatory effects of DHA and quercetin in lipopolysaccharide-induced RAW264.7 macrophages by inhibiting NF-κB and MAPK activation. Mol Med Rep. 2016;14(1):499–508. doi: 10.3892/mmr.2016.5259. [DOI] [PubMed] [Google Scholar]
- 133.Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, Ma DW, Serhan CN, Bazinet RP. Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem. 2013;127(3):378–393. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heras-Sandoval D, Pedraza-Chaverri J, Pérez-Rojas JM. Role of docosahexaenoic acid in the modulation of glial cells in Alzheimer’s disease. J Neuroinflammation. 2016;13(1):61. doi: 10.1186/s12974-016-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Richard C, Lewis ED, Field CJ. Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant’s immune system early in life. Appl Physiol Nutr Metab. 2016;41(5):461–475. doi: 10.1139/apnm-2015-0660. [DOI] [PubMed] [Google Scholar]
- 136.Gibson RA, Makrides M. Long-chain polyunsaturated fatty acids in breast milk: are they essential? Adv Exp Med Biol. 2001;501:375–383. doi: 10.1007/978-1-4615-1371-1_46. [DOI] [PubMed] [Google Scholar]
- 137.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85(6):1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 138.Moukarze S, Dyer RA, Keller BO, Elango R, Innis SM (2016) Human milk plasmalogens are highly enriched in long-chain PUFAs. J Nutr [DOI] [PubMed]
- 139.Auestad N, Scott DT, Janowsky JS, Jacobsen C, Carroll RE, Montalto MB, Halter R, Qiu W, Jacobs JR, Connor WE, Connor SL, Taylor JA, Neuringer M, Fitzgerald KM, Hall RT. Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics. 2003;112(3 Pt 1):e177–e183. doi: 10.1542/peds.112.3.e177. [DOI] [PubMed] [Google Scholar]
- 140.Cederholm T, Salem N, Jr, Palmblad J. ω-3 fatty acids in the prevention of cognitive decline in humans. Adv Nutr. 2013;4(6):672–676. doi: 10.3945/an.113.004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gould JF, Smithers LG, Makrides M. The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97(3):531–544. doi: 10.3945/ajcn.112.045781. [DOI] [PubMed] [Google Scholar]
- 142.Joffre C, Nadjar A, Lebbadi M, Calon F, Laye S. n-3 LCPUFA improves cognition: the young, the old and the sick. Prostaglandins Leukot Essent Fat Acids. 2014;91(1–2):1–20. doi: 10.1016/j.plefa.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 143.Howe PR, Downing JA, Grenyer BF, Grigonis-Deane EM, Bryden WL. Tuna fishmeal as a source of DHA for n-3 PUFA enrichment of pork, chicken, and eggs. Lipids. 2002;37(11):1067–1076. doi: 10.1007/s11745-002-1002-3. [DOI] [PubMed] [Google Scholar]
- 144.Baker EJ, Miles EA, Burdge GC, Yaqoob P, Calder PC. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog Lipid Res. 2016;64:30–56. doi: 10.1016/j.plipres.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 145.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS One. 2011;6(1):e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arteaga O, Revuelta M, Urigüen L, Martínez-Millán L, Hilario E, Álvarez A (2016) Docosahexaenoic acid reduces cerebral damage and ameliorates long-term cognitive impairments caused by neonatal hypoxia-ischemia in rats. Mol Neurobiol 2016 [DOI] [PubMed]
- 147.Singh N, Agrawal M, Doré S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem Neurosci. 2013;4(8):1151–1162. doi: 10.1021/cn400094w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lopez MS, Dempsey RJ, Vemuganti R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem Int. 2015;89:75–82. doi: 10.1016/j.neuint.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arteaga O, Revuelta M, Urigüen L, Álvarez A, Montalvo H, Hilario E. Pretreatment with resveratrol prevents neuronal injury and cognitive deficits induced by perinatal hypoxia-ischemia in rats. PLoS One. 2015;10(11):e0142424. doi: 10.1371/journal.pone.0142424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu Y, Casaccia P, Lu QR. Shaping the oligodendrocyte identity by epigenetic control. Epigenetics. 2010;5(2):124–128. doi: 10.4161/epi.5.2.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321(3):892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- 152.Patnala R, Arumugam TV, Gupta N, Dheen ST (2016) HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol Neurobiol 2016. doi:10.1007/s12035-016-0149-z [DOI] [PubMed]
- 153.Xu J, Chen X, Yu S, Su Y, Zhu W. Effects of early intervention with sodium butyrate on gut microbiota and the expression of inflammatory cytokines in neonatal piglets. PLoS One. 2016;11(9):e0162461. doi: 10.1371/journal.pone.0162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ziemka-Nalecz M, Jaworska J, Sypecka J, Polowy R, Filipkowski RK, Zalewska T (2016) Sodium butyrate, a histone deacetylase inhibitor, exhibits neuroprotective/neurogenic effects in a rat model of neonatal hypoxia-ischemia. Mol Neurobiol. doi:10.1007/s12035-016-0049-2 [DOI] [PMC free article] [PubMed]
- 155.Cai Q, Ma T, Li C, Tian Y, Li H (2016) Catalpol protects pre-myelinating oligodendrocytes against ischemia-induced oxidative injury through ERK1/2 signaling pathway. Int J Biol Sci 12(12):1415–1426 [DOI] [PMC free article] [PubMed]
- 156.Papazisis G, Pourzitaki C, Sardeli C, Lallas A, Amaniti E, Kouvelas D (2008) Deferoxamine decreases the excitatory amino acid levels and improves the histological outcome in the hippocampus of neonatal rats after hypoxia-ischemia. Pharmacol Res 57(1):73–78 [DOI] [PubMed]
- 157.Tataranno ML, Perrone S, Longini M, Buonocore G. (2015) New antioxidant drugs for neonatal brain injury. Oxid Med Cell Longev 2015:108251. [DOI] [PMC free article] [PubMed]
- 158.Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2014;164(5):973–979.e1. doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sypecka J, Sarnowska A. Mesenchymal cells of umbilical cord and umbilical cord blood as a source of human oligodendrocyte progenitors. Life Sci. 2015;139:24–29. doi: 10.1016/j.lfs.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 160.Sypecka J, Ziemka-Nalecz M, Dragun-Szymczak P, Zalewska T. A simple, xeno-free method for oligodendrocyte generation from human neural stem cells derived from umbilical cord: engagement of gelatinases in cell commitment and differentiation. J Tissue Eng Regen Med. 2017;11(5):1442–1455. doi: 10.1002/term.2042. [DOI] [PubMed] [Google Scholar]
- 161.Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;30(18):6236–6246. doi: 10.1523/JNEUROSCI.0567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Donega V, van Velthoven CT, Nijboer CH, Kavelaars A, Heijnen CJ. The endogenous regenerative capacity of the damaged newborn brain: boosting neurogenesis with mesenchymal stem cell treatment. J Cereb Blood Flow Metab. 2013;33(5):625–634. doi: 10.1038/jcbfm.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Doeppner TR, Hermann DM. Stem cell-based treatments against stroke: observations from human proof-of-concept studies and considerations regarding clinical applicability. Front Cell Neurosci. 2014;8:357. doi: 10.3389/fncel.2014.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bae SH, Kong TH, Lee HS, Kim KS, Hong KS, Chopp M, Kang MS, Moon J. Long-lasting paracrine effects of human cord blood cells on damaged neocortex in an animal model of cerebral palsy. Cell Transplant. 2012;21(11):2497–2515. doi: 10.3727/096368912X640457. [DOI] [PubMed] [Google Scholar]
- 165.Zhu LH, Bai X, Zhang N, Wang SY, Li W, Jiang L. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014;1563:13–21. doi: 10.1016/j.brainres.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 166.van Velthoven CT, Dzietko M, Wendland MF, Derugin N, Faustino J, Heijnen CJ, Ferriero DM, Vexler ZS (2016) Mesenchymal stem cells attenuate MRI-identifiable injury, protect white matter, and improve long-term functional outcomes after neonatal focal stroke in rats. J Neurosci Res. doi:10.1002/jnr.23954 [DOI] [PMC free article] [PubMed]
- 167.Trajkovic K, Dhaunchak AS, Goncalves JT, Wenzel D, Schneider A, Bunt G, Nave KA, Simons M. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J Cell Biol. 2006;172(6):937–948. doi: 10.1083/jcb.200509022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Piaton G, Gould RM, Lubetzki C. Axon-oligodendrocyte interactions during developmental myelination, demyelination and repair. J Neurochem. 2010;114(5):1243–1260. doi: 10.1111/j.1471-4159.2010.06831.x. [DOI] [PubMed] [Google Scholar]
- 169.Bhatt A, Fan LW, Pang Y. Strategies for myelin regeneration: lessons learned from development. Neural Regen Res. 2014;9(14):1347–1350. doi: 10.4103/1673-5374.137586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ghosh A, Manrique-Hoyos N, Voigt A, Schulz J, Kreutzfeldt M, Merkler D, Simons M (2011) Targeted ablation of oligodendrocytes triggers axonal damage. PLoS One 6(7) [DOI] [PMC free article] [PubMed]
- 171.Oluich LJ, Stratton JA, Xing YL, Ng SW, Cate HS, Sah P, Windels F, Kilpatrick TJ, Merson TD. Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination. J Neurosci. 2012;32(24):8317–8330. doi: 10.1523/JNEUROSCI.1053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.de Hoz L, Simons M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. BioEssays. 2015;37(1):60–69. doi: 10.1002/bies.201400127. [DOI] [PubMed] [Google Scholar]
- 173.Makarewicz D, Słomka M, Danysz W, Łazarewicz JW. Effects of mGluR5 positive and negative allosteric modulators on brain damage evoked by hypoxia-ischemia in neonatal rats. Folia Neuropathol. 2015;53(4):301–308. doi: 10.5114/fn.2015.56544. [DOI] [PubMed] [Google Scholar]