Abstract
Although the clinical features of isocitrate dehydrogenase (IDH) genetic aberrations have been well-characterized in acute myeloid leukemia (AML), definitive information on their prognostic significance is lacking. We aimed to explore the prognostic significance of IDH gene alterations in an Egyptian cohort of adult patients with de novo AML. Diagnostic peripheral blood samples from 51 AML patients were analyzed for the presence of mutations/SNPs in exon 4 of IDH1 and IDH2 genes using polymerase chain reaction amplification followed by direct sequencing. IDH mutational status had no impact on event-free survival (EFS) and overall survival (OS), whereas the presence of IDH1 315C>T SNP was significantly associated with inferior EFS (P = 0.037) and OS (P = 0.034) as compared with wild-type IDH1. IDH1 315C>T SNP but not IDH mutations is associated with unfavorable outcomes, suggesting that AML patients with IDH1 315C>T SNP can represent a new subgroup of patients which allows refined risk stratification.
Keywords: Acute myeloid leukemia, Isocitrate dehydrogenase 1 and 2, Mutations, Single nucleotide polymorphisms, Prognostic markers
Introduction
Acute myeloid leukemia (AML) is a clinically and pathogenetically heterogeneous group of hematopoietic malignancies characterized by maturation arrest and uncontrolled proliferation of early myeloid precursors accompanied by impaired normal hematopoiesis [1].
Conventional and molecular cytogenetics are essential components of risk stratification in the clinical management of AML patients. In addition to structural chromosomal alterations, molecular analysis of mutations in FMS-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD), nucleophosmin 1 (NPM1) and CCAAT/enhancer binding protein alpha (CEBPA) are now broadly accepted in routine clinical practice. Particular, mutations in these three genes allow the stratification of patients with cytogenetically normal AML (CN-AML) into prognostic risk categories. Although the majority of CN-AML patients harbor one or more of the aforementioned mutations, in approximately 15% of the patients, no mutations have been detected, suggesting the existence of hitherto undiscovered genetic alterations that may contribute to further molecular risk stratification of this substantial percentage of patients [2].
A number of recurrently mutated genes have been identified in AML patients and many of the newly identified mutations are in genes that affect the epigenetic regulation of gene expression. Among a wide spectrum of genes, somatic mutations in the isocitrate dehydrogenase 1 (IDH1) and IDH2 have been identified as recurrent genetic aberration in AML [3].
IDH1 and IDH2 are homodimeric enzymes that reversibly convert isocitrate to α-ketoglutarate (α-KG) in the cytosol/peroxisome and mitochondria, respectively, presumably for the purpose of the concomitant reduction of nicotinamide adenine dinucleotide phosphate (NADP+) to nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) [4]. Cancer-associated mutations in the IDH1 and IDH2 genes encoding isocitrate dehydrogenases have been shown to lead to a loss of the enzyme’s ability to catalyze the oxidative decarboxylation of isocitrate to α-KG, and a neo-enzymatic gain of function, the NADPH-dependent reduction of α-KG to the oncometabolite 2-hydroxyglutarate (2-HG) [5, 6], which competitively inhabits α-KG-dependent enzymes that are important for normal DNA methylation [7]. Furthermore, it has been demonstrated that the expression of mutant IDH1/2 proteins leads to global DNA hypermethylation, which contributes to AML pathogenesis through the generation of increased 2-HG levels and impairment of hematopoietic differentiation [8, 9].
Several studies have investigated IDH mutations in different AML patient populations, with an incidence ranging from 15% to 20% [3]. Despite the recent insights into the distinct pathophysiology of IDH mutations, the prognostic significance of IDH mutations in AML is still controversial. While some studies have reported that IDH mutations were found to be associated with poor prognosis [10–13], others have suggested a lack of prognostic significance [14–16]. Previously, a synonymous single nucleotide polymorphism (SNP) located in codon 105 in exon 4 of the IDH1 gene (315C>T: rs11554137) has been identified in AML patients, and has been reported to be associated with poor prognosis [15, 17].
To the best of our knowledge, the prevalence and prognostic value of IDH aberrations in Egyptian patients with AML has not been hitherto reported. The purpose of the present study was to investigate the frequency of genetic alterations in exon 4 of IDH1/2 in an Egyptian cohort of AML patients. Furthermore, the association of IDH genetic aberrations with hematological, cytogenetic and known prognostic molecular markers as well as with clinical outcome was explored.
Patients and Methods
Study Cohort
Fifty-one Egyptian adult patients fulfilling the diagnostic criteria of de novo AML who were referred to the outpatient clinics of the Hematology unit, National Cancer Institute, Cairo University, Cairo, Egypt were enrolled in the present study. A written informed consent was obtained from all the recruited patients prior to inclusion into the study in accordance with the Declaration of Helsinki. The study protocol was approved by the review board of National Cancer Institute, Cairo University. Diagnosis and classification of the patients were based on the French-American-British classification (FAB) criteria [18, 19]. Data for immunophenotyping, cytogenetics and FLT3-ITD status were retrieved from the patients’ medical records. All patients received standard first-line remission induction treatment with a DA-like regimen, which consisted of daunorubicin (DNR, 45 mg/m2/day via intravenous (IV) infusion for 3 days, on days 1–3) and cytarabine (arabinofuranosyl cytidine, ara-C: 100 mg/m2/day by IV infusion for 7 days, on days 1 through 7). All patients received two remission induction courses. After achieving complete remission (CR), patients were given three courses of postremission therapy (consolidation) with high-dose ara-C 3 g/m2 every 12 h by continuous IV infusion over 3 h on days 1, 3 and 5. The three consolidation courses were administered at monthly intervals.
Analysis of NPM1 and CEBPA Gene Aberrations
Prognostically-relevant genes were analyzed for frequently occurring aberrations as previously described. The most common mutation in exon 12 of NPM1 (type A mutation) was tested using allele specific oligonucleotide-PCR [20]. CEBPA mutations were detected using PCR-single strand conformation polymorphism analysis [21].
Analysis of IDH1 and IDH2 Gene Alterations
Genomic DNA was extracted from mononuclear cells obtained from 51 AML patients using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) as per the manufacturer’s instructions. DNA fragments spanning exons 4 of IDH1 and IDH2, previously identified as hot spots for mutations in these genes [22], were amplified by PCR. Primer sequences for PCR were as follows: for IDH1, forward 5′- TGTGTTGAGATGGACGCCTATTTG-3′ and reverse 5′- TGCCACCAACGACCAAGTCA-3′; for IDH2, forward 5′-GGGGTTCAAATTCTGGTTGA-3′ and reverse 5′- CTAGGCGAGGAGCTCCAGT-3′ as previously described [23]. Both PCRs were performed in a 25 µL volume, containing 15 mM Tris–HCl + 50 mM KCl (Gene Amp 10X PCR Gold Buffer), 1.5 mM MgCl2 (Gene Amp 25 mM MgCl2), 200 µM of each dNTP (Gene Amp 10 mM dNTP Blend), 1 U Taq DNA polymerase (AmpliTaq Gold 5 U/µL DNA polymerase) (Applied Biosystems, Foster City, CA, USA), 0.5 µM each of forward and reverse primers (Sigma-Aldrich, St. Louis, MO, USA) and 100 ng of DNA. The thermal cycling conditions were as follows: initial denaturation at 94 °C for 3 min followed by 35 amplification cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 1 min followed by a final extension step at 72 °C for 10 min. The PCR products were electrophoresed on a 2% agarose gel (Applied Biosystems/Ambion, Austin, USA) to verify the specificity of the products. The PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. The purified amplicons were directly sequenced in both directions with forward and reverse primers using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) in conjunction with GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. Sequence data were analyzed using the Sequencing Analysis Software version 5.3.1 (Applied Biosystems, Foster City, CA, USA). The sequences were compared to the wild-type IDH1 and IDH2 cDNA. All aberrations were verified by sequencing non-amplified genomic DNA.
Statistical Analyses
Categorical variables were reported as the number of cases (percentage) and compared using the Pearson’s Chi square (χ2) test or Fisher’s exact test as appropriate. Continuous variables were expressed as mean ± standard deviation (SD) if normally distributed and compared using the independent Student’s t test or one-way analysis of variance (ANOVA) as appropriate. In contrast, continuous variables were expressed as median (range or interquartile range, IQR: 25th quartile to 75th quartile or minimum–maximum as appropriate) if non-normally distributed and compared using the non-parametric Mann–Whitney U test or Kruskal–Wallis test as appropriate. Univariate and multivariate logistic regression models were used to identify independent prognostic factors influencing CR, EFS and OS. The probabilities of EFS and OS were estimated using the Kaplan–Meier method and were compared among subsets of patients using the log-rank test. For all statistical analyses, the P values were two-sided, and a P value of <0.05 was deemed statistically significant. Data statistical analyses were performed using the statistical package for the social sciences (SPSS Statistics for Windows, Version 20.0; IBM Corp., Armonk, NY, USA). Genotypes and alleles frequency was estimated. Genotype frequencies were compared with the frequencies expected by the Hardy–Weinberg equilibrium (HWE) using a χ2 goodness of fit test. All genetic analyses were performed using Haploview version 3.32 (Broad Institute, Cambridge, MA, USA) [24].
Results
Frequency of IDH Gene Alterations
In the overall cohort of 51 AML patients, IDH mutations were restricted to IDH1 with 2 types of IDH1 mutations have been identified in 4 patients. In contrast, no mutations affecting IDH2 were detected in the entire cohort (Table 1). All patients with a mutated IDH1 retained a wild-type allele, indicating heterozygosity of the mutant allele. Moreover, no patient concurrently had both c.C394T (p.R132C) and c.G395A (p.R132H) mutations, suggesting that these mutations are mutually exclusive.
Table 1.
Clinical characteristics and outcomes of AML patients with wild-type or mutant-type IDH1
| Characteristic | All cases (n = 51) n(%) | IDH1 mutation status | IDH1 mutation type | ||||
|---|---|---|---|---|---|---|---|
| IDH1WT (n = 47, 92.2%) n(%) | IDH1MT (n = 4, 7.8%) n(%) | P value | C394T (R132C) (n = 1, 25%) n(%) | G395A (R132H) (n = 3, 75%) n(%) | P value | ||
| Age | 37.8 ± 12.4 | 37.5 ± 12.5 | 41.3 ± 11.2 | 0.563 | 50 | 38.3 ± 11.7 | 1.000 |
| <40 | 27 (52.9) | 25 (53.2) | 2 (50) | 0.649 | 0 (0) | 2 (66.7) | 0.500 |
| ≥40 | 24 (47.1) | 22 (50.0) | 2 (50) | 1 (100) | 1 (33.3) | ||
| Sex, n(%) | |||||||
| Male | 23 (45.1) | 23 (48.9) | 0 (0) | 0.082 | 0 (0) | 0 (0) | 1.000 |
| Female | 28 (54.9) | 24 (51.1) | 4 (100) | 1 (100) | 3 (100) | ||
| Male/female ratio | 0.82 | 0.96 | 0.0 | 0.0 | 0.0 | ||
| WBC (× 109/L) | 34 (7.4–84.7) | 29.8 (7.2–93.8) | 62.8 (29.2–72.8) | 0.445 | 55 | 70.62 (20.5–73.5) | 1.000 |
| <25 | 24 (47.1) | 23 (48.9) | 1 (25) | 0.351 | 0 (0) | 1 (33.3) | 0.750 |
| ≥25 | 27 (52.9) | 24 (51.1) | 3 (75) | 1 (100) | 2 (66.7) | ||
| Hb (g/dL) | 7.2 ± 1.8 | 7.2 ± 1.8 | 7.5 ± 1.0 | 0.696 | 6.7 | 7.8 ± 1.0 | 0.434 |
| <8 | 35 (68.6) | 32 (68.1) | 3 (75) | 0.629 | 1 (100) | 2 (66.7) | 0.750 |
| ≥8 | 16 (31.4) | 15 (31.9) | 1 (25) | 0 (0) | 1 (33.3) | ||
| Plt (× 109/L) | 31 (20–56) | 32 (20–57) | 27.0 (18.5–31.7) | 0.466 | 17.0 | 31 (23–32) | 0.500 |
| <30 | 25 (49) | 23 (48.9) | 2 (50) | 0.680 | 1 (100) | 1 (33.3) | 0.500 |
| ≥30 | 26 (51) | 24 (51.1) | 2 (50) | 0 (0) | 2 (66.7) | ||
| BM blasts (%) | 62 (34–80) | 62 (34–79) | 67 (28–91) | 0.800 | 91.0 | 43 (23–91) | 0.500 |
| <60 | 24 (47.1) | 22 (46.8) | 2 (50) | 0.649 | 0 (0) | 2 (66.7) | 1.000 |
| ≥60 | 27 (52.9) | 25 (53.2) | 2 (50) | 1 (100) | 1 (33.3) | ||
| FAB subtype, n(%) | |||||||
| M0 | 2 (3.9) | 2 (4.3) | 0 (0.0) | 0.229 | 0 (0.0) | 0 (0.0) | 0.500 |
| M1 | 13 (25.5) | 11 (23.4) | 2 (50.0) | 1 (100) | 1 (33.3) | ||
| M2 | 18 (35.3) | 16 (34.0) | 2 (50.0) | 0 (0) | 2 (66.7) | ||
| M4 | 18 (35.3) | 18 (38.3) | 0.0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Karyotype | |||||||
| Normal | 48 (94.1) | 44 (93.6) | 4 (100) | 0.779 | 1 (100) | 3 (100) | 1.000 |
| t(8;21) | 3 (5.9) | 3 (6.4) | 0 (0) | 0 (0) | 0 (0) | ||
| Cytogenetic risk group | |||||||
| Favorable | 3 (5.9) | 3 (6.4) | 0 (0) | 0.779 | 0 (0) | 0 (0) | 1.000 |
| Intermediate | 48 (94.1) | 44 (93.6) | 4 (100) | 1 (100) | 3 (100) | ||
| Immunophenotype | |||||||
| CD4 | 18 (35.3) | 18 (38.3) | 0 (0) | 0.164 | 1 (100) | 3 (100) | 1.000 |
| CD7 | 2 (3.9) | 2 (4.3) | 0 (0) | 0.848 | 1 (100) | 3 (100) | 1.000 |
| CD13 | 51 (100.0) | 47 (100.0) | 4 (100) | 1.000 | 1 (100) | 3 (100) | 1.000 |
| CD14 | 18 (35.3) | 18 (38.3) | 0 (0) | 0.164 | 1 (100) | 3 (100) | 1.000 |
| CD15 | 36 (70.6) | 34 (72.3) | 2 (50) | 0.336 | 0 (0.0) | 2 (66.7) | 0.500 |
| CD33 | 49 (96.1) | 45 (95.8) | 4 (100) | 0.848 | 1 (100) | 3 (100) | 1.000 |
| CD34 | 20 (39.2) | 18 (38.3) | 2 (50) | 0.514 | 1 (100) | 1 (33.3) | 0.500 |
| CD117 | 40 (78.4) | 36 (76.6) | 4 (100) | 0.366 | 1 (100) | 3 (100) | 1.000 |
| HLA-DR | 51 (100.0) | 47 (100.0) | 4 (100) | 1.000 | 1 (100) | 3 (100) | 1.000 |
| FLT3-ITD | |||||||
| Present | 11 (21.6) | 10 (21.3) | 1 (25) | 0.634 | 0 (0.0) | 1 (33.3) | 0.750 |
| Absent | 40 (78.4) | 37 (78.7) | 3 (75) | 1 (100) | 2 (66.7) | ||
| CR | |||||||
| Yes | 27 (52.9) | 25 (53.2) | 2 (50) | 0.649 | 0 (0.0) | 2 (66.7) | 0.500 |
| No | 24 (47.1) | 22 (46.8) | 2 (50) | 1 (100) | 1 (33.3) | ||
| Event-free survival (EFS, months) | 0.69 (0.44–4.44) | 0.68 (0.44–4.44) | 0.76 (0.43–3.74) | 0.933 | 0.72 | 43 (23–91) | 0.500 |
| Yes | 7 (13.7) | 7(14.9) | 0 (0) | 0534 | 0 (0.0) | 0 (0.0) | 1.000 |
| No | 44 (86.3) | 40 (85.1) | 4 (100) | 1 (100) | 3 (100) | ||
| Overall survival (OS, months) | 1.12 (0.79–5.39) | 1.18 (0.72–5.39) | 0.97 (0.87–4.9) | 0.826 | 1.02 | 0.79 (0.33–4.73) | 0.500 |
| Alive | 18 (35.3) | 17 (36.2) | 1 (25) | 0.557 | 0 (0.0) | 1 (33.3) | 0.750 |
| Dead | 33 (64.7) | 30 (63.8) | 3 (75) | 1 (100) | 2 (66.7) | ||
Risk status: favorable-risk: t(8;21); Intermediate-risk: normal
Qualitative data are represented as the number of cases (%), whereas quantitative data are represented as mean ± SD (range, minimum–maximum) if normally distributed or as median (range or interquartile range, IQR: 25th quartile to 75th quartile) if non-normally distributed
The nucleotide sequence variations and amino acid changes are designated according to the recommendations of the Human Genome Variation Society (http://www.hgvs.org/mutnomen/). IDH1 nucleotide numbering uses the first base of the translation start codon as nucleotide _1 on the basis of National Center for Biotechnology Information sequence NM_005896.2
BM bone marrow, C cysteine, CD cluster of differentiation, CR complete remission, EFS event-free survival, FAB French-American-British, FLT3 FMS-like tyrosine kinase-3, H histidine, Hb hemoglobin, IDH isocitrate dehydrogenase, ITD internal tandem duplication, MT mutant-type, OS overall survival, Plt platelets, R arginine, WBC white blood cells, WT wild-type
The c.315C>T synonymous (p.G105G) IDH1 SNP (rs11554137) was detected in 6 patients (11.8%) (Table 2). All patients with the c.315C>T SNP were heterozygous for the minor allele (T allele). The observed genotype frequencies of c.315C>T SNP did not deviate significantly from HWE expectations (P = 0.655). Two patients (3.9%) had IDH2 SNPs, including one patient carried the intronic c.535-40G>A IDH2 SNP (rs142033117) and another patient carried the synonymous c.429G>C (p.L143L) IDH2 SNP (rs144712130) (Table 2). The patient with the c.535-40G>A SNP and the patient with the c.429G>C SNP were heterozygous for the minor allele (A allele and C allele, respectively). Similarly, the distribution of the observed genotypes of the two IDH2 SNPs was not significantly different from the expected distribution according to HWE (P = 0.944).
Table 2.
Clinical characteristics and outcomes of AML patients with or without IDH1/2 SNPs
| Characteristic | All cases (n = 51) n(%) | IDH SNP status | IDH1 SNP status | IDH2 SNP status | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IDH SNP− (n = 43, 84.3%) n(%) | IDH SNP+ (n = 8, 15.7%) n(%) | P value | IDH1 SNP− (n = 45, 88.2%) n(%) | IDH1 SNP+ (n = 6, 11.8%) n(%) | P value | IDH2 SNP − (n = 49, 96.1%) n(%) | IDH2 SNP + (n = 2, 3.9%) n(%) | P value | ||
| Age | 37.8 ± 12.4 | 38.5 ± 12.5 | 33.9 ± 10.9 | 0.333 | 38.5 ± 12.2 | 32.2 ± 12.3 | 0.575 | 37.7 ± 12.5 | 39.0 ± 2.8 | 0.888 |
| <40 | 27 (52.9) | 22 (51.2) | 5 (62.5) | 0.422 | 23 (51.1) | 4 (66.7) | 26 (53.1) | 1 (50) | ||
| ≥40 | 24 (47.1) | 21 (48.8) | 5 (37.5) | 22 (49.8) | 2 (33.3) | 0.393 | 23 (46.9) | 1 (50) | 0.725 | |
| Sex, n(%) | ||||||||||
| Male | 23 (45.1) | 19 (44.2) | 4 (50) | 0.529 | 21 (46.7) | 2 (33.3) | 0.434 | 21 (42.9) | 2 (100) | 0.198 |
| Female | 28 (54.9) | 24 (55.8) | 4 (50) | 24 (53.3) | 4 (66.7) | 28 (57.1) | 0 (0) | |||
| Male/female ratio | 0.82 | 0.79 | 1.0 | 0.75 | – | |||||
| WBC (× 109/L) | 34 (7.4–84.7) | 39.0 (5.0–93.8) | 29.0 (17.2–58.5) | 0.829 | 39.0 (6.1–89.3) | 61.8 (14.3–103.2) | 0.853 | 34 (7.3–89.3) | 39.2 (24.6–53.8) | 0.941 |
| <25 | 24 (47.1) | 20 (46.5) | 4 (50) | 0.578 | 21 (46.7) | 3 (50) | 0.607 | 23 (46.9) | 1 (50) | 0.725 |
| ≥25 | 27 (52.9) | 23 (53.5) | 4 (50) | 24 (53.3) | 3 (50) | 26 (53.1) | 1 (50) | |||
| Hb (g/dL) | 7.2 ± 1.8 | 7.2 ± 1.7 | 7.5 ± 2.4 | 0.685 | 7.2 ± 1.8 | 7.2 ± 2.0 | 0.850 | 7.2 ± 1.7 | 8.4 ± 4.2 | 0.359 |
| <8 | 35 (68.6) | 30 (69.8) | 5 (62.5) | 0.488 | 31 (68.9) | 4 (66.7) | 0.621 | 34 (69.4) | 1 (50) | 0.533 |
| ≥8 | 16 (31.4) | 13 (30.2) | 3 (37.5) | 14 (31.1) | 2 (33.3) | 15 (30.6) | 1 (50) | |||
| Plt (× 109/L) | 31 (20–56) | 32 (20–60) | 20.5 (17.2–34.5) | 0.112 | 32.0 (20.0–58.5) | 20.0 (10.8–35.5) | 0.082 | 31 (19–56.5) | 29.5 (21–38) | 0.941 |
| <30 | 25 (49) | 20 (48.8) | 5 (62.5) | 0.329 | 21 (46.7) | 4 (66.7) | 0.315 | 24 (49) | 1 (50) | 0.745 |
| ≥30 | 26 (51) | 23 (51.2) | 3 (37.5) | 24 (53.3) | 2 (33.3) | 25 (51) | 1 (50) | |||
| BM blasts (%) | 62 (34–80) | 62 (30–76) | 71.5 (52–89) | 0.254 | 62 (32–77) | 72 (45.7–93.3) | 0.294 | 62 (32–80) | 65.5 (52–79) | 0.692 |
| <60 | 24 (47.1) | 21 (48.7) | 3 (37.5) | 0.422 | 22 (49.8) | 2 (33.3) | 0.393 | 23 (46.9) | 1 (50) | 0.725 |
| ≥60 | 27 (52.9) | 22 (51.3) | 5 (37.5) | 23 (51.1) | 4 (66.7) | 26 (53.1) | 1 (50) | |||
| FAB subtype, n(%) | ||||||||||
| M0 | 2 (3.9) | 2 (4.7) | 0 (0.0) | 0.677 | 2 (4.4) | 0 (0.0) | 0.607 | 2 (4.1) | 0 (0.0) | 0.699 |
| M1 | 13 (25.5) | 11 (25.6) | 2 (25.0) | 11 (24.4) | 2 (33.3) | 13 (26.5) | 0 (0.0) | |||
| M2 | 18 (35.3) | 14 (32.6) | 4 (50.0) | 15 (33.4) | 3 (50.0) | 17 (34.7) | 1 (50.0) | |||
| M4 | 18 (35.3) | 16 (37.2) | 2 (25.0) | 17 (37.8) | 1 (16.7) | 17 (34.7) | 1 (50.0) | |||
| Karyotype, n(%) | ||||||||||
| Normal | 48 (94.1) | 40 (93.0) | 8 (100) | 0.593 | 42 (93.3) | 6 (100) | 0.681 | 46 (93.9) | 2 (100) | 0.885 |
| t(8;21) | 3 (5.9) | 3 (7.0) | 0 (0) | 3 (6.7) | 0 (0) | 3 (6.1) | 0 (0) | |||
| Cytogenetic risk group, n(%) | ||||||||||
| Favorable | 3 (5.9) | 3 (7.0) | 0 (0) | 0.593 | 3 (6.7) | 0 (0) | 0.681 | 3 (6.1) | 0 (0) | 0.885 |
| Intermediate | 48 (94.1) | 40 (93) | 8 (100) | 38 (93.3) | 6 (100) | 46 (93.9) | 2 (100) | |||
| Immunophenotype, n(%) | ||||||||||
| CD4 | 18 (35.3) | 16 (37.2) | 2 (25.0) | 0.409 | 17 (37.8) | 1 (16.7) | 0.299 | 17 (34.7) | 1 (50) | 0.586 |
| CD7 | 2 (3.9) | 2 (4.7) | 0 (0.0) | 0.708 | 2 (4.4) | 0 (0) | 0.776 | 2 (4.1) | 0 (0) | 0.922 |
| CD13 | 51 (100.0) | 43 (100.0) | 8 (100) | 1.000 | 45 (100) | 6 (100) | 1.000 | 49 (100) | 2 (100) | 1.000 |
| CD14 | 18 (35.3) | 16 (37.2) | 2 (25.0) | 0.113 | 17 (37.8) | 1 (16.7) | 0.229 | 17 (41) | 1 (10) | 0.062 |
| CD15 | 36 (70.6) | 30 (69.8) | 6 (75.0) | 0.565 | 32 (71.1) | 4 (66.7) | 0.776 | 34 (69.4) | 2 (100) | 0.494 |
| CD33 | 49 (96.1) | 41 (95.3) | 8 (10.00) | 0.708 | 43 (95.6) | 6 (100) | 0.562 | 47 (95.9) | 2 (100) | 0.922 |
| CD34 | 20 (39.2) | 18 (41.9) | 2 (25.0) | 0.315 | 18 (40.0) | 2 (33.3) | 0.613 | 20 (40.8) | 0 (0) | 0.365 |
| CD117 | 40 (78.4) | 34 (79.0) | 6 (75.0) | 0.557 | 35 (77.8) | 5 (83.3) | 0.615 | 39 (79.6) | 1 (50) | 0.388 |
| HLA-DR | 51 (100.0) | 43 (100.0) | 8 (100.0) | 1.000 | 45 (100) | 6 (100) | 1.000 | 49 (100) | 2 (100) | 1.000 |
| FLT3-ITD, n(%) | ||||||||||
| Present | 11 (21.6) | 9 (20.9) | 2 (25.0) | 0.557 | 9 (20.0) | 2 (33.3) | 0.385 | 11 (22.4) | 0 (0) | 0.612 |
| Absent | 40 (78.4) | 34 (79.1) | 6 (75.0) | 36 (80.0) | 4 (66.7) | 38 (77.6) | 2 (100) | |||
| CR, n(%) | ||||||||||
| Yes | 27 (52.9) | 24 (55.8) | 3 (37.5) | 0.285 | 25 (55.6) | 4 (33.3) | 0.278 | 26 (53.1) | 1 (50) | 0.725 |
| No | 24 (47.1) | 19 (44.2) | 5 (62.5) | 20 (44.4) | 6 (66.7) | 23 (46.9) | 1 (50) | |||
| Event-free survival (EFS, months) | 0.69 (0.44–4.44) | 0.79 (0.49–4.44) | 0.49 (0.38–3.46) | 0.186 | 0.79 (0.49–4.4) | 0.44 (0.34–2.37) | 0.120 | 0.69 (0.43–4.37) | 2.47 (0.49–4.44) | 0.904 |
| Yes | 7 (13.7) | 5 (11.6) | 2 (25.0) | 0.300 | 5 (11.1) | 2 (33.3) | 0.186 | 7 (14.3) | 0 (0) | 0.742 |
| No | 44 (86.3) | 38 (88.4) | 6 (75.0) | 40 (88.9) | 4 (66.7) | 42 (85.7) | 2 (100) | |||
| Overall survival (OS, months) | 1.12 (0.79–5.39) | 1.22 (0.86–6.2) | 0.81 (0.61–4.33) | 0.213 | 1.2 (0.84–5.8) | 0.77 (0.57–3.5) | 0.179 | 1.12 (0.81–5.8) | 3.06 (0.72–5.39) | 0.941 |
| Alive | 18 (35.3) | 18 (41.9) | 0 (0.0) | 0.022* | 18 (40.0) | 0 (0.0) | 0.061 | 18 (36.7) | 0 (0) | 0.414 |
| Dead | 33 (64.7) | 25 (58.1) | 8 (100.0) | 27 (60.0) | 6 (100.0) | 31 (63.3) | 2 (100) | |||
Risk status: favorable-risk: t(8;21); Intermediate-risk: normal
Qualitative data are represented as the number of cases (%), whereas quantitative data are represented as mean ± SD (range, minimum–maximum) if normally distributed or as median (range or interquartile range, IQR: 25th quartile to 75th quartile) if non-normally distributed
The nucleotide sequence variations and amino acid changes are designated according to the recommendations of the Human Genome Variation Society (http://www.hgvs.org/mutnomen/). IDH1 nucleotide numbering uses the first base of the translation start codon as nucleotide _1 on the basis of National Center for Biotechnology Information sequence NM_005896.2
BM bone marrow, CD cluster of differentiation, CR complete remission, EFS event-free survival, FAB French-American-British, FLT3 FMS-like tyrosine kinase-3, Hb hemoglobin, IDH isocitrate dehydrogenase, ITD internal tandem duplication, OS Overall survival, Plt platelets, SNP single nucleotide polymorphism, WBC white blood cells
* indicates a statistical significant difference
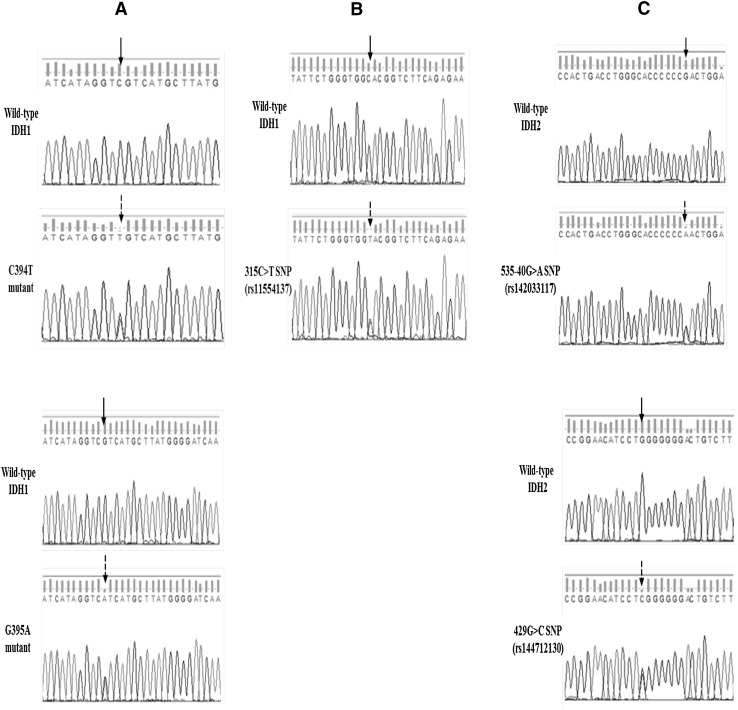
We observed no association between the IDH SNP and the incidence of IDH mutations: Of the four patients with mutated IDH1, none harboring neither IDH1 SNPs nor IDH2 SNPs. Representative direct sequencing chromatograms of the identified IDH aberrations are shown in Fig. 1.
Fig. 1.
Direct sequencing chromatograms of IDH1 mutations and IDH1/2 SNPs. a Representative chromatograms of the C394T and G395A IDH1 mutations. b Representative chromatograms of the 315C>T (rs11554137) IDH1 SNP. c Representative chromatograms of the 535-40G > A (rs142033117) and 429G > C (rs144712130) IDH2 SNPs. The solid arrows indicate the position of the wild-type nucleotide, whereas the dotted arrows indicate the position of the mutated nucleotide
Association of IDH Gene Alterations with Pre-treatment Characteristics and Clinical Outcome
Patients with IDH mutations/SNPs showed no significant differences in demographic and clinical features as compared to those without IDH mutations (Table 1) or SNPs (Table 2).
The distribution of IDH mutations and SNPs didn’t differ significantly among the different FAB subtypes, indicating that there was no preference of IDH mutations/SNPs among the different FAB subtypes. Interestingly, all patients carrying IDH mutations/SNPs had a normal karyotype. Furthermore, no significant association was observed between IDH mutations/SNPs and FLT3-ITD mutations.
There were no significant differences in CR rates between IDH-mutated and unmutated patients (Table 1) or between patients with and without IDH SNPs (Table 2). In addition, there were no significant differences in the distribution of IDH mutations/SNPs between patients with events and those without events. Likewise, the distribution of IDH mutations was not significantly different between surviving and non- surviving patients (Table 1). In contrast, the distribution of IDH SNPs differed significantly between surviving and non- surviving patients (Table 2).
Prognostic Impact of IDH Gene Alterations
As shown in Table 3, univariate analysis showed that only the percentage of BM blasts had a significant impact on CR and OS. Furthermore, only WBC count had a significant impact on EFS. The significant variables were selected as prognostic variables, and multivariate analysis was performed stepwise. Consequently, high BM blasts percentage (≥60%) was identified as independent prognostic predictor for lower CR rate and shorter OS after adjusting for other variables. Interestingly, high WBC count (≥25 × 109/L) tended to be independently associated with shorter EFS when considering other factors.
Table 3.
Univariate and multivariate analyses of prognostic factors for CR, EFS and OS in 51 patients with de novo AML
| Variable | CR | EFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI (Lower–Upper) | P value | OR | 95% CI (Lower–Upper) | P value | OR | 95% CI (Lower–Upper) |
P
value |
|
| Univariate logistic regression analysis | |||||||||
| Gender (Male vs. Female) | 0.297 | (0.06–1.40) | 0.125 | 0.13 | 0.01–1.31 | 0.084 | 0.987 | 0.23–4.26 | 0.986 |
| Age (years) <40 versus ≥40 | 0.280 | (0.07–1.14) | 0.076 | 1.71 | 0.24–11.96 | 0.589 | 2.16 | 0.53–8.82 | 0.284 |
| WBC (×109/L) <25 versus ≥25 | 0.706 | (0.20–2.50) | 0.588 | 0.08 | 0.01–0.86 | 0.037* | 1.71 | 0.48–6.15 | 0.411 |
| Hb (g/dL) <8 versus ≥8 | 0.760 | (0.19–3.11) | 0.703 | 1.30 | 0.18–9.21 | 0.796 | 0.50 | 0.12–2.09 | 0.341 |
| Plt (×109/L) <30 versus ≥30 | 0.534 | (0.15–1.92) | 0.336 | 1.12 | 0.16–7.94 | 0.910 | 1.48 | 0.41–5.41 | 0.553 |
| BM blasts (%) <60 versus ≥60 | 0.108 | (0.02–0.53) | 0.006* | 0.35 | 0.04–2.89 | 0.331 | 6.48 | 1.46–28.75 | 0.014* |
| Constant | 80.916 | 0.019 | 7.52 | 0.338 | 0.44 | 0.606 | |||
| Multivariate logistic regression analysis | |||||||||
| WBC (×109/L) <25 versus ≥25 | 0.12 | 0.01–1.04 | 0.054 | ||||||
| BM blasts (%) <60 versus ≥60 | 0.24 | (0.08–0.79) | 0.018* | 5.2 | 1.48–18.33 | 0.01* | |||
| Constant | 2.43 | 0.048 | 0.33 | 0.020 | 0.85 | 0.683 | |||
Risk status: favorable-risk: t(8;21); Intermediate-risk: normal
CR is defined as bone marrow cellularity of at least 20% with maturation in all cell lineages, fewer than 5% bone marrow blast cells, no Auer rods, recovery of neutrophils ≥1.5 × 109/L and platelets >100 × 109/L in peripheral blood, as well as no evidence for circulating blasts and/or extramedullary leukemia, all of which had persisted for at least 1 month. Relapse is defined as reoccurrence of more than 5% of leukemic blasts in bone marrow, reappearance of circulating blasts or the development of extramedullary leukemia. OS is defined as the time elapsed from the date of diagnosis to the date of last follow-up or death from any cause. EFS is defined as the time interval between the date of diagnosis and the date of CR induction failure, relapse, secondary malignancy or death from any cause, whichever occurred first
BM bone marrow, CR complete remission, EFS event-free survival, Hb hemoglobin, ITD internal tandem duplication, OR odds ratio, OS overall survival, 95% CI 95% confidence interval, Plt platelets, WBC white blood cells
* indicates a statistical significant difference
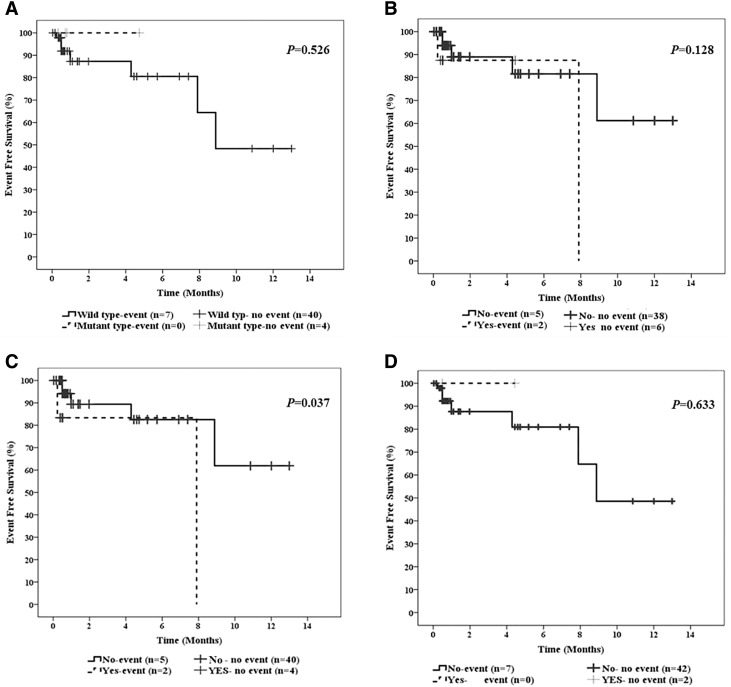
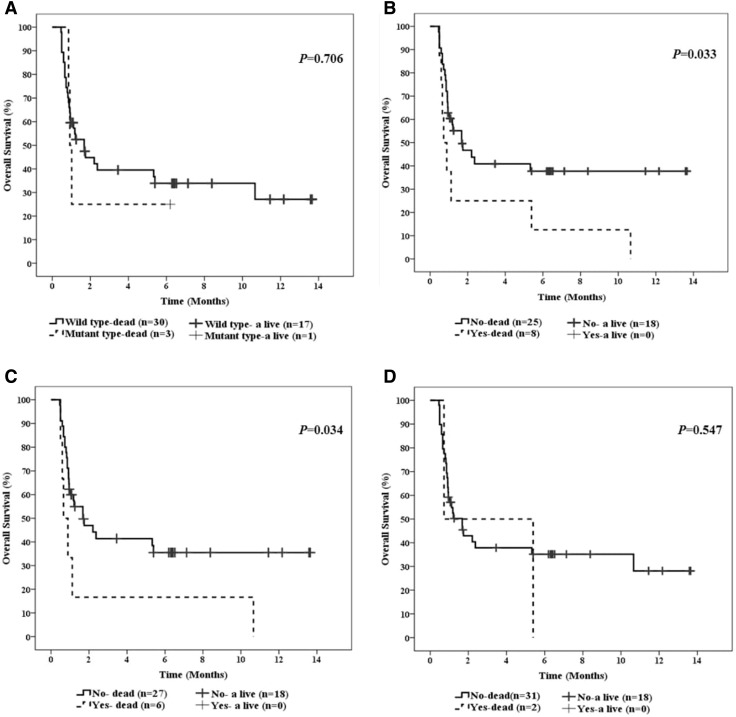
Kaplan–Meier survival analyses were carried out to estimate the distribution of EFS (Fig. 2) and OS (Fig. 3). There was no significant difference in terms of EFS between patients with and without IDH mutations (Fig. 2a). Similarly, no significant difference regarding EFS between patients with and without IDH SNPs, neither in the entire group (Fig. 2b) nor in the subgroup of IDH2 SNPs (Fig. 2d). In contrast, patients with the c.315C>T IDH1 SNP had poorer EFS as compared to those with wild-type IDH1 (Fig. 2c). OS didn’t differ significantly between patients with and without IDH mutations (Fig. 3a). In contrast, patients with IDH SNPs showed inferior OS as compared to those with wild-type IDH (Fig. 3b). Furthermore, patients with the c.315C>T IDH1 SNP were significantly associated with inferior OS as compared to those with wild-type IDH1 (Fig. 3c). On the other hand, OS was not significantly different between patients with and without IDH2 SNPs (Fig. 3D). However, the power of this analysis is limited by the relatively small number of patients carrying IDH2 SNPs.
Fig. 2.
Impact of IDH1 mutations and IDH1/2 SNPs on event-free survival (EFS) in the entire series of AML patients. a EFS of AML patients harboring wild-type or mutated IDH. b EFS of AML patients stratified by IDH SNPs status. c EFS of AML patients with or without IDH1 SNPs. d EFS of AML patients with or without IDH2 SNPs. The log-rank test P value is indicated per Kaplan–Meier survival curve
Fig. 3.
Impact of IDH1 mutations and IDH1/2 SNPs on overall survival (OS) in the entire series of AML patients. a OS of AML patients harboring wild-type or mutated IDH. b OS of AML patients stratified by IDH SNPs status. c OS of AML patients with or without IDH1 SNPs. d OS of AML patients with or without IDH2 SNPs. The log-rank test P value is indicated per Kaplan–Meier survival curve
Discussion
To date, analysis of cytogenetic aberrations at diagnosis provides the most important prognostic information of AML. In addition, the identification of FLT3- ITD, NPM1 and CEBPA mutations and their incorporation into prognostic models was shown to improve risk stratification, especially in the large group of patients with CN-AML [2].
Although these genes have become clinically established prognostic markers in CN-AML, there is still a large group of intermediate risk patients without FLT3-ITD, NPM1 and CEBPA mutations or other reliable prognostic markers, highlighting the need for additional markers that could explain the differential outcome in this heterogeneous patient group [25].
Mutations in the genes encoding, IDH1 and IDH2, are among the most commonly occurring mutations found in AML. Although several studies have reported on the incidence and prognosis of IDH mutations in patients with AML, available results are complicated by the fact that a number of studies (1) examined the clinical relevance of only 1 or 2 mutations in IDH1 and IDH2, (2) have included a limited number of additional genetic alterations beyond IDH1/2 in the analysis, and/or (3) have studied patients receiving different therapeutic modalities [26]. In the present study, we investigated the incidence and prognostic impact of IDH aberrations in an Egyptian cohort of AML patients.
Multiple studies have evaluated IDH mutations in different patient populations, with an incidence of 6–16% for IDH1 and 8–19% for IDH2 [3]. In our study cohort, similar to the frequency of IDH1 mutations in previously published reports, 7.8% of patients had IDH1 mutations. Interestingly, we did not find any mutations affecting IDH2 gene. This finding appears to be in line with a previous study by Mardis et al., who did not detect IDH2 mutations in 188 patients with AML [3].
The frequency discrepancies among various reports may be explained by the variable inclusion criteria of the study samples, the variable sensitivity of the detection assays, the selective inclusion or exclusion of certain IDH aberrations, or the ethnic variability. In addition, most of the previous studies have focused on patients with the normal karyotype of AML, so data from patients belonging to other cytogenetic risk groups are scarce [27].
In the present study, all detected IDH1 mutations were heterozygous, consistent with the retention of the wild-type allele, a finding which is in agreement with previous reports [5, 6].
In the current study, we identified the 315C>T IDH1 SNP in 11.8% of patients, a frequency which was similar to that previously reported by Wagner et al. (12%) in a cohort of CN-AML patients [15]. In contrast to the 315C>T IDH1 SNP, IDH2 SNPs were exceedingly rare, with only 3.9% of the patients harboring IDH2 SNPs. To the best of our knowledge, there were no previous reports documenting the frequencies of the IDH2 SNPs in patients with AML worldwide and our study is the first to report IDH2 SNPs in a cohort of AML patients. Notably, although the 315C>T IDH1 SNP is located very close to IDH1 codon 132, we observed no correlation between this SNP and the overall occurrence of IDH1 codon 132 mutations.
It has been previously reported that AML patients with IDH mutations shared several common clinical characteristics, such as manifestation at older age or higher Plt count at diagnosis [11]. However, in the present study, IDH mutations were nearly evenly distributed between the two age groups (<40 vs. ≥40 years) and were not significantly associated with higher Plt count. Consistent with a previous observation [28], in our cohort, IDH mutated cases were found to be frequently females rather than males. Contrary to previous studies [3, 28], which indicated that IDH1 mutations were significantly associated with FAB AML-M1, our study suggested that the distribution of IDH1 mutations didn’t differ significantly among the different FAB subtypes.
The prognostic impact of IDH mutations in AML has been intensively studied but remains debatable, and it varies considerably among the different mutation types detected, and also by the presence of concurrent mutations in other clinically relevant genes. Although some studies have suggested a worse prognostic effect in CN-AML patients [10–13], others have not found any prognostic effect [14–16], which is in accordance with our findings. In our cohort of patients, we did not demonstrate any significant association of IDH mutations with EFS or OS, although it should be stressed that this finding is certainly limited by the small sample size as well as the low number of IDH-mutated patients. Although it seems counter-intuitive to see differences between the different studies, it has been suggested that differences in sizes of patient cohorts analyzed, varying inclusion criteria, age, and treatment administered might contribute to these discrepancies among studies.
Several lines of evidence suggested that the 315C>T IDH1 SNP was associated with poor prognosis in AML [15, 17]. In line with this finding, we have demonstrated in our cohort of patients that the presence of the 315C>T IDH1 SNP could identify a subset of patients with particularly poor prognosis in terms of both EFS and OS.
Potential mechanisms by which a “silent” SNP may alter gene function include alterations in mRNA stability, folding, and splicing, differences in tRNA selection, or binding of non-coding RNAs [29]. The biological consequences of the silent 315C>T IDH1 SNP remains to be investigated in AML. One speculative explanation is the alteration of the kinetics of protein translation, which may be affected by the frequency of usage of a particular codon. The 315C>T IDH1 SNP represents a GGC>GGT transversion, resulting in the replacement of a common (GGC) codon by a rare (GGT) codon encoding glycine. The replacement of a commonly used codon with a rarely used one via a synonymous SNP may slow down the rate of protein translation, resulting in altered protein folding and, ultimately, decreased protein function. In support of this possibility, the C3435T SNP in the multidrug resistance 1 (MDR1) gene, which also encodes a GGC>GGT transversion, generates a protein with altered structure and function [30].
In conclusion, our findings further strengthen previous data suggesting that IDH mutations is a recurrent event in AML patients. Although IDH mutations did not confer an adverse outcome in our cohort of AML patients, further studies in larger cohorts with a less heterogeneous genetic background may be warranted to provide further insight into their association with the clinical outcome of AML patients. Our preliminary results suggest that the synonymous 315C>T IDH1 SNP is associated with a negative prognostic impact in patients with CN-AML. Our findings clearly provide a solid rationale to include the molecular testing of 315C>T IDH1 SNP in future prospective studies with larger patient numbers from various ethnic backgrounds to confirm the prognostic significance of this SNP.
Author Contributions
Study conception and design: M.A.M.A. and R.H.; Patients recruitment and clinical monitoring: M.M.A.A.; Conducting the mutations analysis: M.A.M.A., E.K.A. and R.H.; acquisition of data: M.M.A.A. and R.H.; Analysis and interpretation of data: M.A.M.A.; Conceptualization and drafting of the manuscript: M.A.M.A.; Critical revision of the manuscript: M.A.M.A., E.K.A., M.M.A.A. and R.H.; All authors have approved the final version of the submitted manuscript. All authors have contributed significantly to this study, and all of them are in agreement with the content of the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the National Cancer Institute research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.European LeukemiaNet. Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 3.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonardi R, Subramanian C, Jackowski S, Rock CO. Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J Biol Chem. 2012;287:14615–14620. doi: 10.1074/jbc.C112.353946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network. Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boissel N, Nibourel O, Renneville A, Gardin C, Reman O, Contentin N, et al. Prognostic impact of isocitrate dehydrogenase enzyme isoforms 1 and 2 mutations in acute myeloid leukemia: a study by the Acute Leukemia French Association group. J Clin Oncol. 2010;28:3717–3723. doi: 10.1200/JCO.2010.28.2285. [DOI] [PubMed] [Google Scholar]
- 11.Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrózek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a cancer and leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi S, Iwanaga E, Tokunaga K, Nanri T, Shimomura T, Suzushima H, et al. IDH1 and IDH2 mutations confer an adverse effect in patients with acute myeloid leukemia lacking the NPM1 mutation. Eur J Haematol. 2014;92:471–477. doi: 10.1111/ejh.12271. [DOI] [PubMed] [Google Scholar]
- 13.Virijevic M, Karan-Djurasevic T, Marjanovic I, Tosic N, Mitrovic M, Djunic I, et al. Somatic mutations of isocitrate dehydrogenases 1 and 2 are prognostic and follow-up markers in patients with acute myeloid leukaemia with normal karyotype. Radiol Oncol. 2016;50:385–393. doi: 10.1515/raon-2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thol F, Damm F, Wagner K, Gohring G, Schlegelberger B, Hoelzer D, et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood. 2010;116:614–616. doi: 10.1182/blood-2010-03-272146. [DOI] [PubMed] [Google Scholar]
- 15.Wagner K, Damm F, Göhring G, Görlich K, Heuser M, Schäfer I, et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol. 2010;28:2356–2364. doi: 10.1200/JCO.2009.27.6899. [DOI] [PubMed] [Google Scholar]
- 16.DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90:732–736. doi: 10.1002/ajh.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho PA, Kopecky KJ, Alonzo TA, Gerbing RB, Miller KL, Kuhn J, et al. Prognostic implications of the IDH1 synonymous SNP rs11554137 in pediatric and adult AML: a report from the Children’s Oncology Group and SWOG. Blood. 2011;118:4561–4566. doi: 10.1182/blood-2011-04-348888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia: a report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–625. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 19.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias French-American-British (FAB) co-operative group. Br J Haematol. 1976;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 20.Ottone T, Ammatuna E, Lavorgna S, Noguera NI, Buccisano F, Venditti A, et al. An allele-specific RT-PCR assay to detect type A mutation of the nucleophosmin-1 gene in acute myeloid leukemia. J Mol Diagn. 2008;10:212–216. doi: 10.2353/jmoldx.2008.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiesmeier J, Czwalinna A, Müller-Tidow C, Krauter J, Serve H, Heil G, et al. Evidence for allelic evolution of C/EBPalpha mutations in acute myeloid leukaemia. Br J Haematol. 2003;123:413–419. doi: 10.1046/j.1365-2141.2003.04618.x. [DOI] [PubMed] [Google Scholar]
- 22.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thol F, Weissinger EM, Krauter J, Wagner K, Damm F, Wichmann M, et al. IDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosis. Haematologica. 2010;95:1668–1674. doi: 10.3324/haematol.2010.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 25.Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88:318–327. doi: 10.1002/ajh.23404. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Wahab O, Levine RL. Mutations in epigenetic modifiers in the pathogenesis and therapy of acute myeloid leukemia. Blood. 2013;121:3563–3572. doi: 10.1182/blood-2013-01-451781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnittger S, Haferlach C, Ulke M, Alpermann T, Kern W, Haferlach T. IDH1 mutations are detected in 6.6% of 1414 AML patients and are associated with intermediate risk karyotype and unfavorable prognosis in adults younger than 60 years and unmutated NPM1 status. Blood. 2010;116:5486–5496. doi: 10.1182/blood-2010-02-267955. [DOI] [PubMed] [Google Scholar]
- 29.Komar AA. Silent SNPs: impact on gene function and phenotype. Pharmacogenomics. 2007;8:1075–1080. doi: 10.2217/14622416.8.8.1075. [DOI] [PubMed] [Google Scholar]
- 30.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]