Abstract
To study the utility and advantage of CD157 in the paroxysmal nocturnal hemoglobinuria (PNH) screening along with its ability to replace CD24 and CD14. This was a confirmatory study to analyse the role and advantage of CD157 in a single tube five color combination to identify the PNH clones. A serial tenfold dilution experiments was carried out for sensitivity assessment. Reproducibility was checked in the intra-assay and inter-assay experiments. The results obtained with CD157 based assay were compared with the routinely used single tube six color CD24/CD14 based assay. CD157 showed a high degree of sensitivity at the level of 10−4. PNH positive clone sizes were precise with CVs of inter-assay and intra-assay precision analysis for polymorphs/monocytes ranging from 2.94 to 4.31/2.52 to 8.93, and 0.91 to 3.23/1.65 to 5.33%; respectively. The results were similar to those obtained from CD24/CD14 based assay (R2 > 0.993). There was no false positive or false negative result. CD157 was found better in delineating the type II clones. CD157 can be used as a common PNH leucocyte marker with high degree of sensitivity and precision. It can replace CD24 and CD14 from the currently used assays and thus bring down the cost of PNH screening.
Keywords: CD157, Flow cytometry (FCM), Paroxysmal nocturnal hemoglobinuria (PNH)
Introduction
Paroxysmal Nocturnal Hemoglobinuria (PNH) is a clonal hematopoietic stem cell disorder occurring due to somatic mutation of phosphatidylinositol glycan class A (PIGA) gene, present on chromosome ‘X’. This gene is required for the synthesis of a glycosylphosphatidylinositol (GPI) anchor, to which many cell membrane proteins, collectively known as GPI-anchored proteins (GPI-APs) are bound [1, 2]. Among these GPI-APs are the important complement regulatory proteins like CD55 and CD59. Their absence in PNH clones, leads to complement mediated red cell lysis; one of the characteristic features of PNH [3, 4]. Classically, PNH is characterized by features of hemolysis, thrombosis and bone marrow (BM) failure syndromes. These features, however, vary from patient to patient and are also dependent upon the clone size.
Currently, flow cytometry (FCM) is the gold standard diagnostic modality for the detection of GPI-AP deficient red blood cells (RBCs) as well as leucocytes. It utilizes monoclonal antibodies against GPI-APs, which are present on normal cell surface, but are deficient in PNH clones. Some of the commonly targeted antigens for PNH screening are CD55, CD59, CD66b, CD16, CD24 and CD14 [5]. In last few years, fluorescent labelled aerolysin (FLAER) has been recommended as one of the important markers for primary screening of PNH clone by FCM in leucocytes [6]. FLAER is fluorochrome conjugated inactive variant of bacterially derived channel forming protein aerolysin, which binds to GPI moiety on normal cells and leads to cell lysis. Due to the lack of GPI moiety in PNH cells, these cells were found to be resistant towards aerolysin mediated cell lysis [7]. This finding was futher exploited to include FLAER as a marker for GPI deficiency and it is presently a marker of choice for high sensitivity PNH screening. Its binding is less sensitive to the maturational stages of cell, and also, it can be used across different lineages, except RBCs [8–10]. The present study was a confirmatory study on the utility of CD157 as a potent universal PNH marker.
Recent years have witnessed the use of CD157 as a promising PNH marker. It is a GPI linked protein which is expressed both on polymorphs and monocytes. It has been found to have equivalent sensitivity as compared to that of the routinely used 4–6 color analysis [11, 12].
Materials and Methods
Sample
3 mL of Ethylene diamine tetra acetic acid (EDTA) anticoagulated peripheral blood samples were used. They were processed and analysed within 24 h of receipt of sample.
Antibodies
All the antibodies except for FLAER were obtained from BD Biosciences San Jose, CA, USA. FLAER was obtained from Cedarlaine, Ontario, Canada. Antibodies combination (fluporophore, clone) used for routine six color panel was as follows: FLAER (AF488, Cedarlane), CD24 (PE, P67.6) CD15 (PerCPCy5.5, HI98) CD14 (PE-CY7, MOP 9) CD64 (APC,10.1) and CD45 (APCH7, 2D1). The CD157 based five color panel was in following combination: FLAER (AF488, Cedarlane), CD157 (PE, SY/11B5) CD15 (PerCPCy5.5, HI98), CD64 (APC, 10.1) and CD45 (APCH7, 2D1).
Cell Preparation and Staining Protocol
A Stain-Lyse-Wash protocol was used for all the cases, except for those where absolute neutrophil count (ANC) was less than 0.5 × 109/L. Briefly, 100–300 µL of well mixed blood, depending on the TLC, was taken into 12 × 75 mm BD falcon tubes. To it pre-titrated antibody cocktails were added and incubated in dark for 20 min, followed by washing with phosphate buffered saline (PBS). It was followed by RBC lysis using the commercial FACS Lyse ® and washing with PBS and the final suspension in 0.5 mL of paraformaldehyde (PFA). For samples with very low ANC, bulk lysis with 1 mL of blood was done, followed by staining of the cell pellet.
Acquisition and Analysis
Samples were acquired on a BD FACS Canto II platform using FACS Diva version 8 software. A standard to high sensitivity analysis was done, where for every case a minimum 1.0 lac polymorphs were acquired and analysed. A minimum of 50 PNH positive events were acquired for each positive case. The PNH clone sizes were taken up to two decimals points.
Sensitivity Assessment of the Assay Using Serial Dilutions
A fresh PNH positive sample with large clone size was serially diluted in a tenfold dilution till 1:10,000, with a normal peripheral blood. These were then stained with the five color CD157 cocktail. The proportions of PNH granulocytes/monocytes were noted and correlated with the dilution.
Obtaining the Background Limit of PNH Phenotypes in Normal Sample
The proportion of gated leukocytes (neutrophils and monocytes) with PNH phenotype was determined on 10 normal samples.
Intra-assay and Inter-assay Precision Analysis
Representative samples (n = 6) were taken from the four different clone size groups namely: very small (<1%), small (1–10%), medium (11–50%) and large (>50%), as defined in our previous published study [13]. For intra-assay precision, samples were acquired thrice from the same stained tube and the coefficient of variation (CV) was calculated for each of monocytes and granulocytes. For inter assay variability the same representative samples were processed and stained thrice in separate tubes and were analysed.
Comparison with the Predicate 6 Colour Analysis
A total of 40 samples were analysed using both CD157 based five color and CD24/CD14 based six color approach. This included 30 PNH positive and 10 negative samples.
Statistical Analysis
The results for inter and intra assay variability were analysed as mean, standard deviation (SD) and CV. Comparison between the single tube 5 colour and 6 colour analysis was done by Wilcoxon signed rank test for paired samples significant at level less than 0.05, linear regression analysis and Pearson’s correlation coefficient. All these statistical calculations were done using IBM SPSS version 21 software.
Results
We first titrated the CD157-PE antibody from BD biosciences to optimise the staining and best separation between the negative and positive populations. This was done both with a normal sample, where lymphocytes served as internal negative control as well as PNH positive samples where the PNH clone served as negative population. In both these situations, 3.5 µL of antibodies showed very good separation between negative and positive population and had the best signal to noise ratio. Subsequently, this volume was used for further experiments.
The tenfold dilution experiment showed that CD157 based assay was pretty sensitive till 1:10,000 dilutions (0.01%) (Fig. 1). For background PNH clone assessment in the normal sample, a mean of 180,000 granulocytes (range 100,000–288,000) and 32,000 monocytes (range 9500–41,500) were analysed. Only two of these samples showed presence of PNH positive polymorphs in a very low proportion. One case showed three PNH positive events out of ~220,000 CD15 gated polymorphs (0.0014%); while the other case showed only one PNH positive event out of 150,000 CD15 gated events (0.0007%). These samples showed only one PNH positive event out of 32,000 CD64 gated monocytes (0.0031%) and no PNH positive monocytes respectively.
Fig. 1.
Dilution experiments showing the sensitivity of CD157 based assay in detection of PNH positive clones
Representative samples over a variable clone sizes (range 0.11–83.82%) were analysed and the PNH clone sizes obtained were found precise. There was not much variation in the clone sizes obtained from these assay. The CVs of inter assay precision for granulocytes/monocytes ranged from 2.94 to 4.31/2.52 to 8.93%, respectively. The corresponding values for intra assay precision analysis were 0.91 to 3.23/1.65 to 5.33%; respectively (Table 1).
Table 1.
Precision analysis using inter-assay and intra-assay variability testing
| Sample no. | Intra-assay precision analysis | Inter- assay precision analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monocytes | Polymorphs | Monocytes | Polymorphs | |||||||||
| Clone size | Mean | CV (%) | Clone size | Mean | CV (%) | Clone size | Mean | CV (%) | Clone size | Mean | CV (%) | |
| 1. | 0.11 | 0.11 | 5.33 | 0.22 | 0.21 | 2.63 | 0.11 | 0.12 | 8.93 | 0.22 | 0.23 | 4.31 |
| 0.11 | 0.22 | 0.13 | 0.23 | |||||||||
| 0.12 | 0.21 | 0.12 | 0.24 | |||||||||
| 2 | 3.65 | 3.54 | 2.52 | 3.59 | 3.54 | 1.40 | 3.65 | 3.66 | 3.28 | 3.59 | 3.56 | 3.78 |
| 3.50 | 3.54 | 3.55 | 3.67 | |||||||||
| 3.49 | 3.49 | 3.79 | 3.43 | |||||||||
| 3. | 8.44 | 8.49 | 1.96 | 12.79 | 12.51 | 2.07 | 8.44 | 8.51 | 2.52 | 12.79 | 12.23 | 4.01 |
| 8.36 | 12.28 | 8.76 | 11.88 | |||||||||
| 8.68 | 12.45 | 8.35 | 12.01 | |||||||||
| 4. | 14.62 | 14.12 | 3.77 | 11.94 | 11.54 | 3.23 | 14.62 | 13.72 | 6.52 | 11.94 | 11.79 | 3.56 |
| 14.2 | 11.48 | 13.71 | 11.32 | |||||||||
| 13.56 | 11.20 | 12.83 | 12.12 | |||||||||
| 5. | 24.62 | 24.02 | 1.65 | 29.10 | 29.17 | 1.48 | 24.62 | 23.82 | 3.16 | 29.10 | 28.14 | 3.41 |
| 24.17 | 29.64 | 23.72 | 28.14 | |||||||||
| 23.82 | 28.78 | 23.12 | 27.18 | |||||||||
| 6. | 85.62 | 84.11 | 2.09 | 88.12 | 88.06 | 0.91 | 85.62 | 83.84 | 2.85 | 88.12 | 85.51 | 2.94 |
| 84.54 | 87.24 | 81.12 | 83.1 | |||||||||
| 82.18 | 88.83 | 84.8 | 85.32 | |||||||||
The bold figures are minimum and maximum values for each coloum
Comparison between the CD157 based five color and the predicate six color tube in determining the clone size over a wide range (0.11–98.7%) showed a good correlation for both granulocytes and monocytes (Fig. 2). There was no false negative or positive result.
Fig. 2.
Correlation of PNH clone sizes as obtained from a CD157 based 5 colour assay and CD24 (granulocytes)/CD14 (monocytes) based 6 colour assay
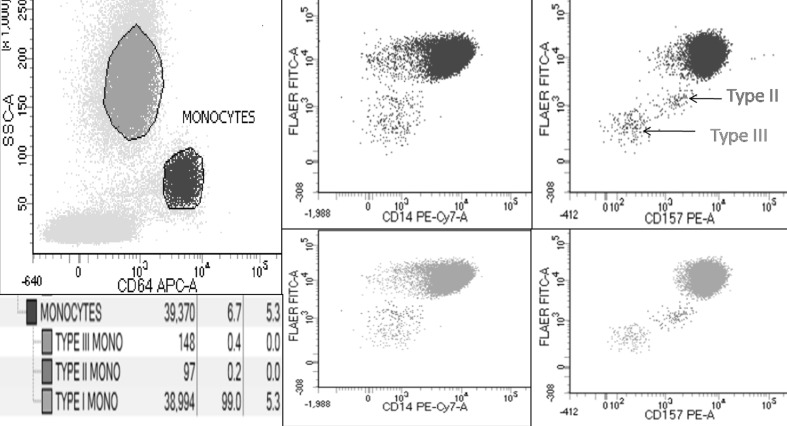
CD157 was found to have a much better separation between the normal population and PNH clone in comparison to CD24/CD14. It was also able to separate out the type II population from the type III population (Fig. 3). Type II clones were noted in two of the thirty cases using CD157. Although, presence of type II clones in one of these cases could be identified using CD24/CD14, the discrimination was not crisp. Though there was no reduction in the cost of technical time, there was a reduction of 10–15% antibody cost using single tube five color assay as compared to that of the predicate six color analysis.
Fig. 3.
CD157 being better in delineation of type II and type III clones
Discussion
Screening for paroxysmal nocturnal hemoglobinuria clones is an important laboratory tool, with its utility ranging from diagnosis of Coombs negative haemolytic anemia, thrombosis at unusual sites to detection of PNH clones in bone marrow failure syndromes like aplastic anemia and myelodysplastic syndrome. FLAER based flow cytometry assays are the current ‘gold standard’ for the detection of PNH clones. Among other GPI anchored proteins which are targeted for PNH screening, CD24 and CD14 are the most widely used ones. CD157 has recently been proved to be a promising universal marker which can be used both for polymorph and monocyte screening. CD157 or BST 1, which stands for bone marrow stromal cell antigen 1 (BST 1) is a single-chain GPI-anchored protein that facilitates pre B cell growth. It is widely expressed on various cell types: B cell progenitors before Ig gene rearrangements, T progenitors, granulocytes and monocytes, to name a few. It is a bio-functional ectoenzyme with cyclase and hydrolase activities, and is structurally and functionally similar to CD38.
In the present study, laboratory validation of CD157 was performed; and a five color panel comprising of FLAER, CD157, CD15, CD64 and CD45 was compared with routinely used single tube six color panel which consisted of FLAER, CD24, CD15, CD14, CD64 and CD45.
The background PNH positive population was almost negligible in healthy control samples of our cohort. In few of the samples where PNH positive events could be identified, the proportion of was very low, ranging from 0.0007 to 0.0031% of the gated polymorphs or monocytes. Sutherland et al. [11] also reported a similar results, where after analysing a median of 160,000 polymorphs from the healthy donors, they observed only 0–2 events with a PNH positive phenotype.
CD157 based PNH screening was found to be very precise in both inter-assay as well as intra-assay variability testing. Six representative samples analyzed over a wide range of clone sizes (0.1–98.9%) showed an acceptable CVs. Similar results were also demonstrated by Marinov et al. who analysed three representative sample from three different clone size groups viz big (>20%), small to intermediate (1–20%) and minor (<1%) [12]. The CVs for intra assay precision analysis for polymorphs/monocytes in a similar five color experiment were 0.9–3.0/0.7–8.1%. Corresponding figures for inter assay precision analysis were 0.1–3.7/0.7–4.8%. Similar to their observation we also noted a higher variation in the samples with the lower clone sizes.
CD157 was found to be better than CD24 and CD14 in separating the PNH negative and positive populations. CD157 provided cleaner results, especially the cluster of normal (type I) monocytes. Additionally, there was a better delineation of type II PNH clones compared to CD24/CD14 based assay. Similar findings have been noted in other studied [11, 14]. Though, the clinical or biological significance of this delineation is currently undiscerned, it may highlight more accurate binding of CD157 molecule vis-á-vis density of GPI anchored proteins on PNH clones. The issue of FLAER positivity and CD157 negativity highlighted by Marinov et al. [12] as well as with some other researchers (verbal communication) was not observed in our any of our cases. It can be presumed to be because of the presence of eosinophils (CD157 negative) overlapping with gated neutrophils. This can be demonstrated by back gating the FLAER positive CD157 negative population, which usually falls in the high SSC region and bright CD45 region, on SSC-CD45 plot. Samples with eosinophilia particularly demonstrated this phenomenon, which could perhaps be solved on stringent gating of neutrophils and awareness of this phenomenon. Additionally, eosinophils could also serve as an internal negative control for CD157 and in fact help in deciding quadrants.
Comparison of five color panel with six color was performed using linear regression analysis and Wilcoxon sign rank test showed highly comparable results, with r2 > 0.995. Our results are in unison with those reported by other authors who studied the utility of CD157 in PNH screening as a single tube five color assay [11, 12, 15].
There was no reduction in the cost of technical time with CD157 usage in our scenario, as we were routinely using the single tube panel. However, the cost of antibody could be reduced to ~10–15%. In laboratories, where the practice is to use two tube four color combinations, addition of CD157 in a single tube five color analysis will definitely lead to a whopping reduction in the investigation cost. This will be a significant advantage in developing countries like India, where the cost of investigation is a rate limiting step in the management of patients.
In conclusion, our results confirmed the utility of CD157 as a common marker for PNH screening. The results obtained were precise and were comparable to our routinely used six color single tube panel. The new CD157 based five color single tube combination is ready to replace CD24 and CD14 from the single tube six color combination, and thus will bring down the cost of PNH screening by flow cytometry.
Acknowledgements
The authors would like to thanks Mr Kaushal Kumar, Technical staff, Flow cytometry Lab, SGPGI, Lucknow.
Compliance with Ethical Standards
Conflict of interest
None.
Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was taken from all the individuals participants included in this study.
References
- 1.Nagarajan S, Brodsky RA, Young NS, Medof ME. Genetic defects underlying paroxysmal nocturnal hemoglobinuria that arises out of aplastic anemia. Blood. 1995;86:4656–4661. [PubMed] [Google Scholar]
- 2.Takahashi M, Takeda J, Hirose S, Hyman R, Inoue N, Miyata T, et al. Deficient biosynthesis of N-acetylglucosaminyl-phosphatidylinositol, the first intermediate of glycosyl phosphatidylinositol anchor biosynthesis, in cell lines established from patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1993;177:517–521. doi: 10.1084/jem.177.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144:3478–3483. [PubMed] [Google Scholar]
- 5.Borowitz MJ, Craig FE, DiGiuseppe J, Illingworth AJ, Rosse W, Sutherland R, et al. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by Flow cytometry. Cytom B. 2010;78B:211–230. doi: 10.1002/cyto.b.20525. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland DR, Kuek N, Davidson J, Barth D, Chang H, Yeo E, et al. Diagnosing PNH with FLAER and multiparameter flow cytometry. Cytom B. 2007;72B:167–177. doi: 10.1002/cyto.b.20151. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky RA, Mukhina GL, Nelson KL, Lawrence TS, Jones RJ, Buckley JT. Resistance of paroxysmal nocturnal hemoglobinuria cells to the glycosylphosphatidylinositol-binding toxin aerolysin. Blood. 1999;93:1749–1756. [PubMed] [Google Scholar]
- 8.Hernandez-Campo PM, Almeida J, Sanchez ML, Malvezzi M, Orfao A. Normal patterns of expression of glycosylphosphatidylinositolanchored proteins on different subsets of peripheral blood cells: aframe of reference for the diagnosis of paroxysmal nocturnal hemoglobinuria. Cytom B. 2006;70B:71–81. doi: 10.1002/cyto.b.20087. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Campo PM, Almeida J, Matarraz S, de Santiago M, Sánchez ML, Orfao A. Quantitative analysis of the expression of glycosylphosphatidylinositol- anchored proteins during the maturation of different hematopoietic cell compartments of normal bone marrow. Cytom B. 2007;72:34–42. doi: 10.1002/cyto.b.20143. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky RA, Mukhina GL, Li S, Nelson KL, Chiurazzi PL, Buckley JT, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114:459–466. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland DR, Acton E, Keeney M, Davis BH, Illingworth A. Use of CD157 in FLAER based assay for high sensitivity PNH Granulocyte and PNH monocyte detection. Cytom B. 2014;86B:44–55. doi: 10.1002/cytob.21111. [DOI] [PubMed] [Google Scholar]
- 12.Marinov I, Kohutova M, Tkakova V, Pesek A, Cermak J, Cetkovsky P. Clinical relevance of CD157 for rapid and cost effective simultaneous evaluation of PNH granulocyte and monocytes by low cytometry. Int J Lab Hematol. 2015;37:231–237. doi: 10.1111/ijlh.12271. [DOI] [PubMed] [Google Scholar]
- 13.Rahman K, Gupta R, Yadav G, Husein N, Singh MK, Nityanand S. Fluorescent Aerolysin (FLAER)-based paroxysmal nocturnal hemoglobinuria (PNH) screening: a single center experience from India. Int. J Lab Hematol. 2017;39:261–271. doi: 10.1111/ijlh.12619. [DOI] [PubMed] [Google Scholar]
- 14.Correia RP, Bento LC, Bortolucci ACP, Alexandre AM, Vaz ADC, Schimidel D, Pedro EdC, Perin F, Nozawa Mendes CEA, Barroso RdS, Bacal NS. Technical advances in flow cytometry based diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria. Einstein. 2016;14(3):366–373. doi: 10.1590/S1679-45082016AO3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galtseva IV, Fidarova ZT, Mikhailova EA, Borisov VI, Troitskaia VV, Parovichnikova EN, Savchenko VG. A new approach of PNH clones detection. Blood. 2014;124:5149. [Google Scholar]