Abstract
Significant reduction in morbidity and mortality have been documented in patients with sickle cell disease (HbSS) by most of the studies using hydroxyurea at a dose of 25–35 mg/kg/day or maximum tolerated dose. But toxicities, need for frequent monitoring, compliance and cost are important hurdles particularly in Indian set up. We undertook this study to find out the efficacy, safety compliance rate of low fixed dose of hydroxyurea (10 mg/kg/day) in patients presenting to our hospital and its impact on clinical profile and laboratory parameters. A cohort of 128 (82 males, 46 females) confirmed HbSS cases (each >18 years age, vaso-occlusive crisis >2/years and/ or rate of transfusion 1–2 units/month) with no disease related end organ damage were assessed prospectively between 2013 and 2016. They were started on 10 mg/kg/day hydroxyurea along with other supportive care and followed up monthly for 1 year. Clinical and laboratory parameters before and after therapy were reviewed and compared. In 92% of cases presenting with repeated vaso-occlusive crisis, VOC disappeared completely during follow up and in 8% we found significant reduction in severity as well as frequency of attacks (p < 0.01). Again in 87%, no further transfusion was required during follow up and in 13%, it further reduced the rate of transfusion (p < 0.01). The median time of response for VOC was 3 months and in transfusion requirement was 5 months. There was also significant reduction in S.Billirubin, S.LDH, disease related complications and rate of hospitalisation with significant improvement in Hb, MCV, and MCH. There is insignificant increase in HbF with median (1.5–2.4)% and in 5 cases >5%. We did not find any remarkable adverse effect of the drug during the study period. Low fixed dose hydroxyurea (10 mg/kg/day) is beneficial in reducing the vaso-occlusive crisis and transfusion requirement in adult HbSS Patients (Arab-Indian Haplotype). It is safe, suitable and is a effective mode of treatment in resource poor setting like India.
Keywords: Adults, Hydroxyurea, Low fixed dose, Sickle cell disease
Introduction
Patients with SCD are associated with multiple morbidities and premature mortalities. Pathophysiologically it is characterised by recurrent erythrocyte sickling with subsequent vaso-occlusive complications, chronic intra and extra vascular hemolysis, progressive endothelial dysfunction. Repeated painful episodes, acute chest syndrome (ACS), splenic sequestration, cerebrovascular events, serious infections, persistent anaemia, multi organ involvement, aseptic necrosis, leg ulcers are the major clinical manifestations.
Hydroxyurea (HU) is an approved pharmacological therapy for SCD and has proven its efficacy both in reducing the morbidity and mortality. Such evidences documented from various studies have used higher doses of hydroxyurea (15–35 mg/kg/day) [1–4]. More over therapy with maximal tolerated dose of hydroxyurea requires frequent monitoring of haematological and clinical parameters albeit its high cost and low compliance. But in a resource poor setting like India, it is not pragmatic to follow up patients monthly. In two recent studies it has been documented the efficacy of low fixed dose hydroxyurea (10 mg/kg/day) in reducing VOC and transfusion requirements in SCD requiring less frequent monitoring with better compliance and overall survival [5, 6]. Since 3 years, all the SCD patients are receiving HU free in a government sponsored program. Patients are dispensed with this drug for a period of 1–3 month after a periodic evaluation in the tertiary care centres including our institution.
The aim of this study is to assess the effectiveness and safety of low fixed dose of hydroxyurea (10 mg/k/day) on VOC, transfusion need, clinical and other laboratory parameters in adult HbSS patients.
Methods
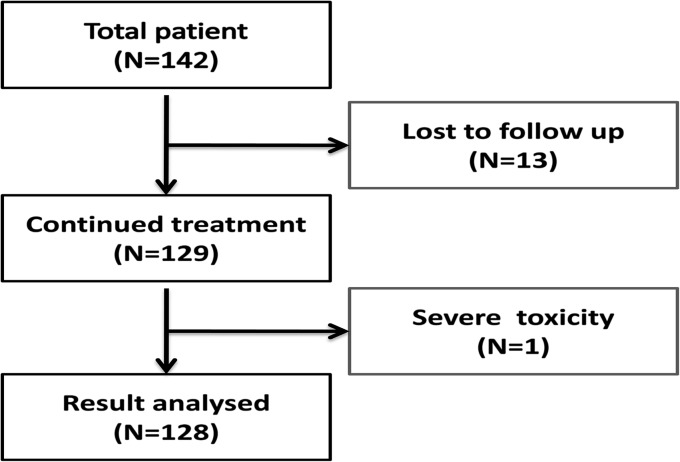
A prospective study was carried out in our department of clinical Haematology, SCB Medical College and Hospital, Cuttack over 3 years (2013–2016). Out of 142 patients enrolled initially for the study, 13 patients lost to follow up and one developed severe toxicity and excluded from the study. One hundred twenty eight patients (82 males and 46 females) with SCD (HbSS) were included in the study (Fig. 1).
Fig. 1.
Study population flow chart
Confirmed HbSS cases with age ≥18 years, >2 attacks of vaso-occlusive crisis per year and/or rate of transfusion 1–2 units/month were included in the study. Subjects with pregnancy, human immunodeficiency virus infection or medications that could potentially enhance HU toxicity, serum creatinine above the upper limit of normal for age and serum alanine aminotransferase (ALT) more than twice the upper limit of normal for age at the time of entry were excluded from the study.
All patients were started on fixed low dose HU (10 mg/kg/day) along with other supportive measures. As hydroxyurea is available in India as one strength (500 mg capsule), in some cases the dose of HU per day was altered on regular basis so as to ensure the equivalent dose of 10 mg/kg/day in all cases. All the patients were advised to take folic acid (5 mg/day) and ensure adequate fluid intake. Information regarding clinical response and laboratory findings at the start of HU and for each visit for 1 year was collected. A clinical proforma was used for follow up which was done every month. Laboratory findings including hemoglobin level, mean corpuscular volume (MCV), fetal hemoglobin (HbF) level, white blood cell count and platelet count, serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total serum bilirubin (direct, indirect) were documented at the initiation of HU therapy and during each follow up. In addition, other data collected included side effects, consanguinity, age and gender. Frequency of vasoocclusive crises and blood transfusions in previous year, other baseline clinical and laboratory parameters (for Hb, HbF, MCV, MCH, total leucocyte count, total platelet count S.Billirubin, S.LDH) were compared with values after 12 months of therapy with low fixed dose hydroxyurea (10 m/kg/day). Patient compliance was measured by checking the number of remaining capsules of HU during follow up visits.
During the follow up, the drug was stopped temporarily if ALT was elevated above two times the upper limit of normal for age and serum creatinine was elevated above the upper limit of the normal, when the Hb was <6 g/dl, absolute neutrophil count was <2000/μl or platelet count was <80,000/μl. The abnormal parameters were closely followed for recovery. If recovery occurred, the drug was restarted at the same dose (10 mg/kg/day) with close monitoring for reappearance of side effects.
All data was processed manually and by using the Statistical Package for Social Sciences program version 20. Paired ‘t’ test was used to compare the baseline findings with those after HU therapy at 12 months. A p < 0.05 was considered statistically significant.
Results
Out of 142 patients enrolled initially for the study, 13 patients lost to follow up and one developed severe toxicity (nephropathy) and excluded from the study. One hundred twenty eight patients (82 males and 46 females) with SCA were included in the study. In our study 82 cases (64%) presented with repeated VOC, 17 cases (13%) with transfusion dependency and 29 cases (23%) with both the above features. All cases were started on hydroxyurea at low dose (10 mg/kg/day) in a way as mentioned in the previous section.
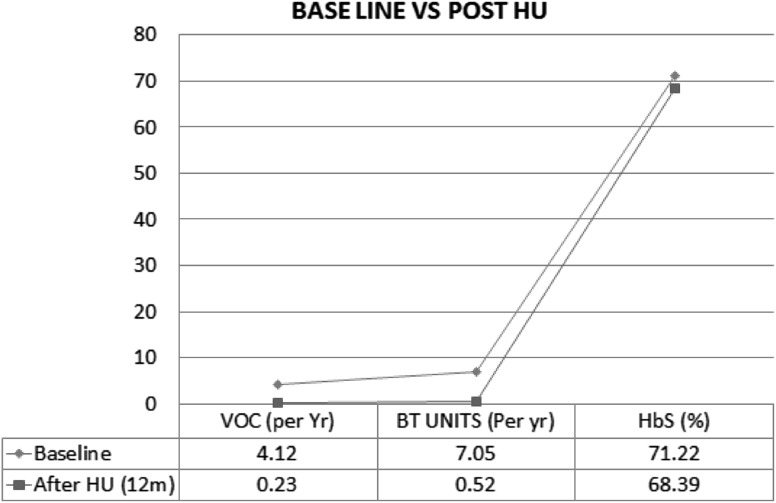
Important information at baseline and after completion of 12 month of HU therapy are depicted in Table 1. Out of 111 cases (82 cases with only VOC plus 29 cases with both VOC and transfusion dependency) presenting with repeated VOC, it disappeared completely in 102 cases (92%) and in rest 9 cases (8%) there was significant reduction in severity and frequency of attacks after 12 months of hydroxyurea therapy. The VOC was reduced to occasional 1/year which was mild and was managed with symptomatic therapy without hospitalisation.
Table 1.
Haematological and clinical parameters at baseline and after 12 months of HU therapy of the study cohort
| Observation Total study population (n) = 128 | |||
|---|---|---|---|
| Parameters | Baseline (±SD) | POST HU (±SD) (10 mg/kg/day) | p value |
| Vaso-occlusive crisis (per year) £n1 = 111(86.7%) |
4.12 ± 0.9 | 0.23 ± 0.92 | <0.001 |
| Rate of blood transfusion (per year) £n2 = 46(35.93%) |
7.05 ± 1.07 | 0.52 ± 1.27 | <0.001 |
| Hb (g/dl)£ | 8.42 ± 1.14 | 9.78 ± 1.13 | <0.001 |
| MCV (fl) | 76.20 ± 8.20 | 90.78 ± 6.59 | <0.001 |
| MCH (pg) | 24.32 ± 2.69 | 26.24 ± 2.40 | <0.001 |
| TLC × 1000/mm3 | 12,283.48 ± 1307.34 | 8469.50 ± 1307.34 | <0.001 |
| Platelet count × 1000/mm3 | 242.48 ± 72.19 | 185.30 ± 52.89 | <0.001 |
| HbS (%) | 71.22 ± 5.01 | 68.39 ± 4.86 | <0.001 |
| HbF (%) | 18.06 ± 7.26 | 21.66 ± 6.77 | >0.05 |
| S.BILLIRUBIN(T) (mg/dl) | 2.19 ± 0.56 | 1.89 ± 0.54 | <0.001 |
| S.LDH (U/l) | 925.96 ± 135.54 | 742.02 ± 133.86 | <0.001 |
£n1, n2 are total no of cases presenting with repeated VOC and transfusion dependency respectively as per inclusion criteria
Out of 46 cases (17 cases with only transfusion dependency plus 29 cases with both transfusion dependency and VOC) requiring repeated transfusions, 40 cases (87%) required no further transfusion and in 6 cases (13%), it further reduced the rate of transfusion significantly to the extent of occasional need (1–2 units in 6 month) after 12 month of HU therapy. There was no significant adverse effect of HU noted in our study population.
Discussion
This present prospective study using low dose of hydroxyurea in adult Indian sickle cell patients (HbSS) proved the safety and efficacy of such protocol. It also validates the success of state govt. scheme of free distribution of HU to all the HbSS patients. This may suggest continuing such free scheme models by various state governments for SCD (HbSS) patients in future.
Many long term studies have clearly demonstrated the efficacy of HU in African children with SCA [7]. There is only one study in India documenting the efficacy of low fixed dose HU (10 mg/kg/day) on adults showing its effectiveness on recurrent VOC and reducing transfusion requirements apart from other clinical and haematological effects. They found 92.2 and 95% reduction in VOC and rate of transfusion respectively in adults compared to baseline [5]. Few studies from outside India have also documented the efficacy of low dose HU in SCD patients [8, 9]. So we undertook a study on a large Indian adult population to document efficacy of low fixed dose hydroxyurea (10 mg/kg/day).
Sickle cell disease is an inherited blood disorder. It is non sex linked with no apparent sex predilection. But in our study there were more number of male registered patients than females (82 males, 46 females). This may be due to gender bias still prevalent in our country. In our society, family and parents are more concerned with boys and are given more importance particularly in rural setup and other backward areas. Males are brought to health care system more frequently than females. Two similar studies in India too demonstrated similar male predominance attributed to gender bias [5, 6]. Our study had 9 cases (7.03%) born out of consanguinity. This is due to the local practice where families prefer to marry close relatives without premarital screening for inherited diseases.
We started low fixed dose of hydroxyurea (10 mg/k/day) and followed patients monthly for 1 year for hematological and clinical parameters. There was significant reduction in the severity and frequency of VOC as well as rate of transfusion. The baseline haemoglobin, MCV and MCH improved significantly with significant reduction in HbS, TLC, TPC, Serum total billirubin, LDH (signifying reduction in intravascular hemolysis). The low baseline MCV (76.20 ± 8.20 fl) in our study population may be attributed to concomitant iron deficiency very much prevalent in our community specially in the rural population. The median time of response for VOC and transfusion were 3 and 5 months respectively. Over all there was reduction in rate of hospitalisation and length of hospital stay after HU therapy. In our study we didn’t find any correlation between baseline HbF and painful crisis due to selection of subjects with severe manifestations irrespective of baseline HbF levels.
Though maximal tolerated dose (MTD) of HU may cause greater effects on clinical and laboratory parameters compared to low fixed dose, but it requires frequent monitoring. In our population with rural predominance, it is not possible to follow up patients regularly. MTD of HU too increases cost of the treatment and also creates economic burden on the family and on health care system. But low fixed dose requires less frequent monitoring with proven clinical and laboratory benefits. Low fixed dose HU has also better compliance due to once daily dosing, low cost, lower toxicity and better safety profile apart from clinical benefit [10]. This may be particularly valuable in regions with limited resources for health care.
The beneficial effect of low fixed dose of HU has been reported in this study without significant rise of HbF (baseline vs. post 1 year low fixed dose HU therapy) may be due to high baseline values in our haplotype. This effect of low fixed dose hyroxyurea can be attributed to its pleotropic effects apart from HbF induction [11]. It also reduces red cell endothelial interaction [12], improves red cell hydration [13], deformability [14] and rheology, altered expression of cell surface markers, releases of nitric oxide [15, 16].
Low fixed dose HU is very safe. In our study there were no significant adverse effects or long term toxicity noted among the participants. Out of 128 cases, four cases developed hepatic dysfunction, two developed renal dysfunction, three patients developed neutropenia (ANC < 2000/µl), two patients developed thrombocytopenia (<80,000/µl), two developed bicytopenia (Table 2). We discontinued hydroxyurea in all the above cases and restarted again after normalisation of above parameters.
Table 2.
Drug toxicity
| No. of patients | ||
|---|---|---|
| Temporary | Permanent | |
| Abnormal liver function | 4 | |
| Abnormal kidney functions | 2 | 1 |
| Neutropenia (ANC < 2000/μl) | 3 | |
| Thrombocytopenia (<80,000/μl) | 2 | |
| Bicytopenia | 2 | |
| Malignancy | 0 | |
All the above dysfunctions were transient only. These patients tolerated low fixed dose of hydroxyurea later after restarting with close monitoring during follow up without any further toxicity. However one patient in our study developed nephrotoxicity with persistently elevated s.creatinine and was excluded from the study. This may be attributed to the disease process itself rather than effect of hydroxyurea. Other similar type of studies too has documented long term safety of low fixed dose HU both in children and adult population [5].
Conclusion
Low fixed dose Hydroxyurea (10 mg/kg/day) is safe, requires less frequent monitoring, easy to administer, economical, well tolerated with better compliance without significant adverse effects. it is beneficial in reducing the vaso-occlusive crisis and transfusion requirement in Adult HbSS Patients (Arab-Indian Haplotype) (Fig. 2).
Fig. 2.
Impact of HU (10 mg/kg/day) on frequency of VOC and blood transfusion of the study population
Acknowledgements
We would like to thank all the patients and the family members, the data collectors and the laboratory technicians who participated in the study.
Compliance with Ethical Standards
Conflict of Interest
All the authors have equal contribution for this study.
Informed Consent
Informed consent was obtained from all the participants included under the study.
Ethical Standard
Institutional Ethical Clearance has been done for this study as per the protocol. All the procedures perform in this study were in accordance with the Ethical Standard of the Institutional Ethical Committee and with the 1964 Helsinki declaration and its later amendments or comparable Ethical standard.
References
- 1.Charache S, Terrin ML, Moore RD, et al. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 2.Gilmore A, Cho G, Howard J, et al. North West London Haemoglobinopathy Registry Group. Feasibility and benefit of hydroxycarbamide as a long-term treatment for sickle cell disease patients: results from the North West London Sickle Cell Disease Registry. Am J Hematol. 2011;86(11):958–961. doi: 10.1002/ajh.22146. [DOI] [PubMed] [Google Scholar]
- 3.Lobo CL, Pinto JF, Nascimento EM, Moura PG, Cardoso GP, Hankins JS. The effect of hydroxcarbamide therapy on survival of children with sickle cell disease. Br J Haematol. 2013;161(6):852–860. doi: 10.1111/bjh.12323. [DOI] [PubMed] [Google Scholar]
- 4.Ware RE, Helms RW, SWiTCH Investigators Stroke with transfusions changing to hydroxyurea (SWiTCH) Blood. 2012;119(17):3925–3932. doi: 10.1182/blood-2011-11-392340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel DK, Mashon RS, Patel S, Das BS, Purohit P, Bishwal SC. Low dose hydroxyurea is effective in reducing the incidence of painful crisis and frequency of blood transfusion in sickle cell anemia patients from eastern India. Hemoglobin. 2012;36:409–420. doi: 10.3109/03630269.2012.709897. [DOI] [PubMed] [Google Scholar]
- 6.Jain DL, Apte M, Colah R, Sarathi V, Desai S, Gokhale A, et al. Efficacy of fixed low dose hydroxyurea in Indian children with sickle cell anemia: a single centre experience. Indian Pediatr. 2013;50(10):929–933. doi: 10.1007/s13312-013-0264-0. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman SA, Schultz WH, Davis JS, PickensCV MortierNA, Howard TA, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 8.Svarch E, Machín S, Nieves RM, Mancia de Reyes AG, Navarrete M, Rodríguez H. Hydroxyurea treatment in children with sickle cell anemia in Central America and the Caribbean countries. Pediatr Blood Cancer. 2006;47:111–112. doi: 10.1002/pbc.20823. [DOI] [PubMed] [Google Scholar]
- 9.Al-Nood HA, Al-Khawlani MM, Al-Akwa A. Fetalhemoglobin response to hydroxyurea in Yemeni sicklecell disease patients. Hemoglobin. 2011;35:13–21. doi: 10.3109/03630269.2011.551748. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan JM, Steward S, Howard TA, Mortier NA, Kimble AC, Aygun B, et al. Hydroxycarbamide alters erythroid gene expression in children with sickle cell anaemia. Br J Haematol. 2012;157(2):240–248. doi: 10.1111/j.1365-2141.2012.09061.x. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg MA, Brugnara C, Dover GJ, Schapira L, Charache S, Bunn HF. Treatment of sickle cell anemia with hydroxyurea and erythropoietin. N Engl J Med. 1990;323:366–372. doi: 10.1056/NEJM199008093230602. [DOI] [PubMed] [Google Scholar]
- 12.Adragna NC, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood. 1994;83:553–560. [PubMed] [Google Scholar]
- 13.Orringer EP, Blythe DSB, Johnson AE, Phillips G, Jr, Dover GJ, Parker JC. Effects of hydroxyurea on hemoglobin F and water content in the red blood cells of dogs and of patients with sickle cell anemia. Blood. 1991;78:212–216. [PubMed] [Google Scholar]
- 14.Ballas SK, Dover GJ, Charache S. The effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol. 1989;32:104–111. doi: 10.1002/ajh.2830320206. [DOI] [PubMed] [Google Scholar]
- 15.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King SB. Nitric oxide production from Hydroxyurea. Free Radic Biol Med. 2004;37(6):737–744. doi: 10.1016/j.freeradbiomed.2004.02.073. [DOI] [PubMed] [Google Scholar]