Abstract
Colorless phenylpropanoid derivatives are known to protect plants from ultraviolet (UV) radiation, but their photoregulation and physiological roles under field conditions have not been investigated in detail. Here we describe a fast method to estimate the degree of UV penetration into photosynthetic tissue, which is based on chlorophyll fluorescence imaging. In Arabidopsis this technique clearly separated the UV-hypersensitive transparent testa (tt) tt5 and tt6 mutants from the wild type (WT) and tt3, tt4, and tt7 mutants. In field-grown soybean (Glycine max), we found significant differences in UV penetration among cultivars with different levels of leaf phenolics, and between plants grown under contrasting levels of solar UV-B. The reduction in UV penetration induced by ambient UV-B had direct implications for DNA integrity in the underlying leaf tissue; thus, the number of cyclobutane pyrimidine dimers caused by a short exposure to solar UV-B was much larger in leaves with high UV transmittance than in leaves pretreated with solar UV-B to increase the content phenylpropanoids. Most of the phenylpropanoid response to solar UV in field-grown soybeans was induced by the UV-B component (λ ≤ 315 nm). Our results indicate that phenolic sunscreens in soybean are highly responsive to the wavelengths that are most affected by variations in ozone levels, and that they play an important role in UV protection in the field.
UV-B radiation (UV-B: 280–315 nm) has several effects on the physiology of terrestrial plants. Reductions in leaf area expansion and, in some cases, biomass accumulation rate have been detected in various species in response to current levels of solar UV-B at low (Searles et al., 1995), intermediate (Ballaré et al., 1996; Mazza et al., 1999), and high latitudes (Rousseaux et al., 1998). Reduced growth may result from direct photochemical damage to key macromolecules such as proteins and nucleic acids, or as an indirect consequence of the increased production of reactive oxygen species in plants exposed to UV-B. The degree of damage caused by UV-B should be strongly dependent on the efficiency of constitutive and UV-induced mechanisms of protection and repair, such as the accumulation of UV-absorbing sunscreens and the activation of antioxidant defenses (Rozema et al., 1997; Jansen et al., 1998).
The sunscreen response has been investigated in some detail (Robberecht and Caldwell, 1978; Tevini et al., 1991; Li et al., 1993; Landry et al., 1995). In higher plants, flavonoids and other phenylpropanoid derivatives (such as sinapate esters) that accumulate in large quantities in the vacuoles of epidermal cells effectively attenuate the UV component of sunlight with minimal effects on the visible region of the spectrum. Genetic blocks in the synthesis of phenolic sunscreens in phenylpropanoid mutants are known to result in increased susceptibility to UV (e.g. Li et al., 1993; Lois and Buchanan, 1994; Stapleton and Walbot, 1994; Landry et al., 1995; Reuber et al., 1996); however, it is not yet clear whether the slight variations in levels of UV-absorbing compounds that are commonly detected among varieties of the same species or between plants subjected to different UV regimes are physiologically significant under field conditions.
In parallel with this lack of information on the functional significance of natural variations in phenylpropanoid levels, there is a knowledge gap regarding the photocontrol of phenylpropanoid accumulation under field conditions. Laboratory studies have demonstrated that the regulation of flavonoid biosynthesis may involve multiple photoreceptors, including the phytochromes, blue-absorbing photoreceptors, and one or more UV photoreceptors (for review, see Beggs and Wellmann, 1994). A clear maximum in quantum effectiveness around 300 nm has been detected in some species, whereas in bean, flavonoid accumulation appears to be triggered most effectively by shorter wavelengths (Beggs and Wellmann, 1994). Knowing the shape of the action spectrum for protective responses is critical to establish the potential for plant acclimation to changes in UV that result from variations in the thickness of the ozone layer, because only the shortest wavelengths are affected by changes in ozone levels (Caldwell et al., 1986; Flint and Caldwell, 1996).
Variations in the UV-filtering capacity of plant tissue can be assessed in many different ways. Commonly used techniques include: measurements of the spectral transmittance of epidermal peels (Robberecht and Caldwell, 1978), direct measurement of the UV levels inside the leaf using fiber-optic microprobes (Day et al., 1992; Cen and Bornman, 1993), detection of UV-induced fluorescence in the yellow-green region of the spectrum (Schmelzer et al., 1988), or quantitation of phenolic compounds in leaf extracts using spectrophotometry, chromatography, and other techniques (see, e.g. Flint et al., 1985; Veit et al., 1996). All of these methods have distinct advantages and limitations; a problem that is common to all of them is that they are time-consuming and therefore have limited application in field studies that require multiple and rapid comparisons among genotypes or among plants subjected to contrasting light treatments.
Chapple et al. (1992) showed that the fah1 mutant of Arabidopsis, which is unable to synthesize UV-absorbing sinapate esters, has a strong chlorophyll fluorescence signal when illuminated with broad-band UV. More recently, Bilger et al. (1997) outlined a method to estimate UV penetration into leaf tissues on the basis of chlorophyll fluorescence determinations obtained with a modified Xe-PAM fluorometer. They convincingly demonstrated that their method can be used to estimate the transmittance of the epidermis in the UV region by comparing the chlorophyll-fluorescence signal obtained with UV irradiation with that induced by blue light. This method has the great advantage of estimating UV penetration without introducing any perturbations to the optical properties of the leaves, and using a natural UV target (chlorophyll) as a reporter of the UV climate within the mesophyll. However, the method cannot be used to obtain simultaneous readings for large numbers of plants, as it would be necessary for comparative field studies or for efficient selection of cultivars or mutants with altered levels of UV-absorbing pigments.
Here we outline a fast and sensitive technique to detect small differences in UV penetration to the mesophyll that is based on chlorophyll fluorescence imaging. We have employed this technique with field-grown soybean (Glycine max) crops to test the hypothesis that UV-induced phenolic sunscreens provide effective protection to solar UV-B, and to investigate the spectral sensitivity of the phenylpropanoid response induced by solar radiation under natural field conditions.
MATERIALS AND METHODS
Plant Material, Experimental Design, and UV Dosimetry
All the experiments were carried out in the experimental fields of IFEVA (34° 35′ S; 58° 29′ W), Buenos Aires. Soybean (Glycine max) seeds were planted in rows to large, replicated field plots. The distance between rows was 15 cm; plant density was 60 m−2. The plots were watered as needed and weeds were controlled manually.
The soybean data presented in this paper are from two field experiments. One of them involved eight different soybean genotypes of maturity groups (MG) III to VIII grown under two UV-B levels (UV-B attenuation experiment); in the second experiment we grew a single soybean line (cv Williams) under filters that transmitted different wavelengths of the solar UV spectrum (spectral response experiment).
UV-B Attenuation Experiment
Crops of the cv Williams (MG III), cv Nidera A4423RG, cv Dekalb CX458 (MG IV), cv Nidera A5634RG and A5308 (MG V), cv Nidera A6445RG (MG VI), cv Charata-76 (MG VII), and cv A8000RG (MG VIII) were allowed to emerge and grow in the field under 3- × 4.2-m aluminum frames covered with either clear polyester films (Mylar-D, DuPont, Wilmington, DE; 0.1 mm thick), which virtually cut off all UV radiation below 310 nm (−UV-B treatment), or “Stretch” films (Bemis Co. Minneapolis; 0.025 mm thick), which had very high transmittance over the whole UV waveband (+UV-B treatment). The planting date was November 13, 1998, and there were five true replicates (independent plots) of each genotype × UV-B treatment combination. The filters were raised periodically to maintain them approximately 5 cm above the upper leaf layer; on each individual plot the filter was changed one or two times during the course of the growing season because the plastics tended to deteriorate and accumulate dust. The level of UV-B attenuation at the center of the −UV-B plots (measured with a broad-band UV-B detector SUD/240/W attached to a IL-1700 research radiometer; International Light, Newburyport, MA; peak spectral response at 290 nm; half-bandwidth = 20 nm) was found to be consistently greater than 95%. All the leaves used for analysis were collected from plants grown near the center of the plots.
Spectral Response
Crops of the cv Williams (MG III) were allowed to emerge in the field under 1.2- × 1.2-m aluminum frames covered with either Aclar (type 22-A, Allied Signal, Pottsville, PA; 0.04 mm thick) films (full UV treatment), clear polyester (Mylar-D) films (filtered out the UV-B component of sunlight), 5-mm-thick window glass sheets (filtered out the short-wave UV-A and all the UV-B), or 3-mm-thick Lexan (General Electric, Fairfield, CT) sheets (removed nearly all the UV-B and UV-A). Sowing date was February 11, 1998, and there were four true replicates of each spectral treatment. The filters were raised periodically to maintain them approximately 5 cm above the upper leaf layer. Aclar and Mylar films were replaced at least once during the course of the growing season. Spectral measurements at canopy level were obtained at midday using an double-monochromator spectroradiometer (IL-1700, International Light). The radiometer was calibrated against an standard lamp (OL-40, Optronic, Orlando, FL) in the short-wavelength range and a model 1800 calibrator (LI-COR, Lincoln, NE) for λ ≥ 320 nm. Wavelength accuracy was checked using a germicidal UV-C lamp.
Data on ambient UV and photosynthetic photon flux density (PPFD) received over the field site during the days in which we measured short-term effects of solar UV-B on DNA damage were downloaded from a GUV-511 multiband radiometer (Biospherical Instruments, San Diego) run by the INGEBI (Consejo Nacional de Investigaciones Científicas y Técnicas) in the city of Buenos Aires (http://uvarg.dna.uba.ar/site1.htm). In the UV spectral range the instrument acquires data at four fixed wavelengths (305, 320, 340, and 380 nm) every minute; irradiance levels at 305 nm are used here as a descriptor of the amount of UV-B received by the plants.
Arabidopsis plants used as a control in some experiments were grown in a controlled environment under continuous light from fluorescent tubes (approximately 100 μmol m−2 s−1; 25°C). The original seeds of the Landsberg erecta ecotype and the transparent testa mutants with altered phenylpropanoid metabolism (tt3-1, tt4-1, tt5-1, tt6-1, and tt7-1) were obtained from the Arabidopsis Biological Resource Center (Columbus, OH).
Measurements of Leaf Phenolics, Chlorophyll Content, and Fluorescence
For all the determinations involving field-grown soybean, leaf samples were collected at solar noon on sunny days only from plants grown at the center of the plots.
Crude Extracts
For spectrophotometric determination of phenolic contents we sampled four leaves (each from a different plant) per plot (youngest fully expanded leaf). Each sample (one 0.5-cm diameter leaf disc) was placed in 1.4 mL of 99:1 methanol:HCl and allowed to extract for 48 h at −20°C. Absorbance of the extracts was read at 305 nm for determinations of total UV-absorbing compounds. At the time of sampling the plants were 2 months old and the canopies intercepted more than 80% of the incident PPFD (average of all cultivars and treatments).
Chlorophyll Fluorescence Imaging
The intensity of chlorophyll fluorescence in the red region of the spectrum induced by UV radiation (RFUV) was measured for intact leaves and leaf discs. A modified Fluor-S MultiImager (Bio-Rad, Hercules, CA) was used to induce and quantify the fluorescent signal. RFUV induction was obtained with the original UV epi-illuminator of the apparatus. The UV lamps are located approximately 30 cm above the leaf samples and provide diffuse, broad-band UV radiation (λmax = 302 nm; range 290–365 nm). To evaluate the fluorescent signal induced specifically by UV-B (λ ≤ 315 nm) (RFUVB), we subtracted from RFUV the fluorescence excited by the UV-A component of the light source, which was determined after placing a 100-μm-thick clear polyester film between the sample and the broad band UV source. Detection of the fluorescent signals was achieved with the sensitive CCD chip of the imager operating in high sensitivity mode. The camera was fitted with a 620-nm long pass filter and an RG695 filter to cut-off visible light produced by yellow-green fluorescence (Lichtenthaler and Miehé, 1997). Preliminary experiments with this configuration showed that RFUV was undetectable in metal mirrors used as controls and extremely low in plant tissues devoid of chlorophyll (such as the margins of spider-plant [Chlorophytum elatum {Ait.} R.Br.] leaves) (Ballaré et al., 1999). Because variations among genotypes or UV treatments in the red fluorescence signal could be caused by variations in chlorophyll levels or in the functioning of the photosynthetic apparatus, we used the intensity of the fluorescence signal induced by blue light (RFB) as a control (for discussion, see Bilger et al., 1997). RFB was induced using a portable halogen lamp covered with a 449-nm interference filter (Schott, Mainz, Germany; irradiance at sample level = 1.15 μmol m−2 s−1). For quantitative determinations of RFUV, RFUVB, and RFB in soybean samples we used four leaf discs per plot (0.5 cm in diameter; youngest fully expanded leaf; each leaf from a different plant). The RF signal was generally more intense when the leaves were excited through the lower epidermis; therefore, all the RF values reported here for soybean are derived from images taken with the leaves positioned in the fluorometer with their abaxial surface up. Entire leaves were used in the case of Arabidopsis, and RF was induced through the upper epidermis. The discs or the leaves were placed on a bed of blotting paper saturated with tap water; quantification of the fluorescence intensity signals was achieved using the volume procedure of the Multi-Analyst/PC v. 1.1 software, which was run on 0.3-cm-diameter circles selected at the center of each leaf disc. In all cases the illumination and signal integration time was 30 s.
Chlorophyll Determinations
Chlorophyll was extracted with N,N-dimethylformamide (four 0.5-cm diameter leaf discs in 1.6 mL); absorbance was read at 647 and 664 nm. The contents of total chlorophyll and of chlorophyll a and b were calculated according to Inskeep and Bloom (1985).
DNA Damage Analysis
Quantitation of DNA Damage Levels
For DNA damage analysis in soybean we used the central leaflet of the youngest fully expanded leaf available at the time of sampling (four leaves per plot, each from a different plant; five true replicates per UV-B treatment). The samples were collected around midday and immediately frozen at −80°C. DNA extraction was carried out under dim orange light, essentially as described by Doyle and Doyle (1987) using a CTAB-based procedure modified by the use of PVP to eliminate polyphenols during DNA purification. DNA was quantified with ethidium bromide (Gallagher, 1994) using commercial herring DNA (Sigma, St.Louis) as a standard and a Peltier-cooled CCD camera/imager system (Fluor-S MultiImager, Bio-Rad) for fluorescence detection according to the manufacturer's directions. These measurements were not affected by the protein contamination levels present in our DNA samples. DNA damage was assayed by determination of cyclobutane pyrimidine dimers (CPDs) using a method adapted from Stapleton et al. (1993). In brief, DNA samples (2 μg) in TE buffer were denaturated and immobilized on a positively-charged nylon-blotting-membrane (Zeta-Probe, Bio-Rad); CPDs were detected using the TDM-2 monoclonal antibody (gift of Dr. Toshio Mori, Nara Medical University, Japan). The method is based on the detection of primary-bound antibody by alkaline phosphatase-conjugated secondary antibody (Bio-Rad) using a chemiluminescent substrate (CSPD, Tropix, Bedford, MA). Chemiluminescence was detected with autoradiographic film (Kodak X-Omat, Eastman Kodak, Rochester, NJ) or with a cooled CCD camera (Fluor-S MultiImager). Commercial DNA from herring (10 ng μL−1 in TE buffer [pH = 8] + 10 mm NaCl) was irradiated with 0, 1.6, and 4.0 J m−2 of 254-nm UV-C to serve as damage standards in all the blots. To create these standards, the DNA solutions (1 mL) were exposed in flat cuvettes (the optical path through the solution was <1 mm) in order to obtain a uniform exposure. The UV-C dose was determined with a calibrated IL-1700 double-monochromator spectroradiometer (International Light). One unit of DNA damage is defined as the amount of CPD induced by 1 J m−2 of 254-nm radiation in 1 ng of purified herring DNA.
Repair of DNA Damage
In some experiments we used visible light to lower the CPD level in leaf samples. Detached leaves were exposed with their petioles immersed in water to 200 μmol m−2 s−1 PPFD of white light provided by fluorescent bulbs in a growth chamber; air temperature was 25°C.
Statistical Analyses
Statistical analyses were performed using PROC GLM and PROC REG in the SAS version 6.12 package (SAS Institute, Cary, NC); appropriate transformations of the primary data were used when needed to meet the assumptions of the analysis of variance. In the field studies with soybeans the analyses are based on data from five (UV-B attenuation experiment) or four (spectral response experiment) independent field plots for each UV treatment.
RESULTS AND DISCUSSION
Solar UV-B Increases the Concentration of UV-Absorbing Compounds
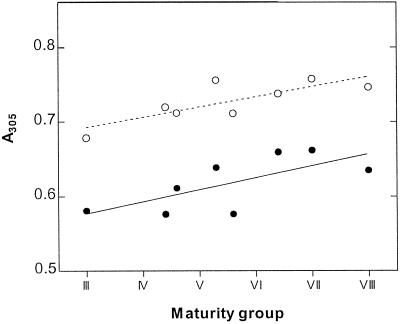
To study the impact of solar UV-B on the levels of phenolic sunscreens we grew eight commercial soybean lines in large (3- × 4.2-m) field plots that were covered with either UV-B-transparent or UV-B-opaque clear plastic films. In all the genotypes tested the content of UV-absorbing compounds in crude alcoholic extracts (A305) increased in response to exposure to solar UV-B (Fig. 1). There were also some differences among genotypes in A305, with the tropical varieties (higher MG) showing greater A305 values. In general, the differences between UV-B treatments were larger than the differences among genotypes. Significant effects of ambient UV-B on A305 of leaf extracts were reported by Robberecht and Caldwell (1986) for Rumex spp., and by Ballaré et al. (1999) for several species of annual plants. Other workers have reported increased levels of extractable phenolics in response to artificial UV-B supplementation treatments in field-grown soybean crops (Mirecki and Teramura, 1984).
Figure 1.
Effects of solar UV-B on the concentration of UV-absorbing compounds (per unit leaf area) in the leaves of eight soybean cultivars arranged in order of increasing maturity group (MG); from MG III to VIII: Williams, A4423RG, CX458, A5634RG, A5308, A6445RG, Charata-76, and A8000RG. Notice that for the Dekalb and Nidera varieties we used the MG information provided by the breeders, which is indicated by the first two digits in the alpha-numeric cultivar name (e.g. Nidera A5308 belongs to maturity group 5.3). Samples were taken from the central leaflet of the youngest fully-expanded trifoliate at midday on January 12, 1999 (2 months after crop seeding). Each datum point is the average of five independent field plots. ○, +UV-B (r2 = 0.64; P = 0.02); ●, −UV-B (r2 = 0.49; P = 0.05). The UV-B effect is significant at P < 0.0001.
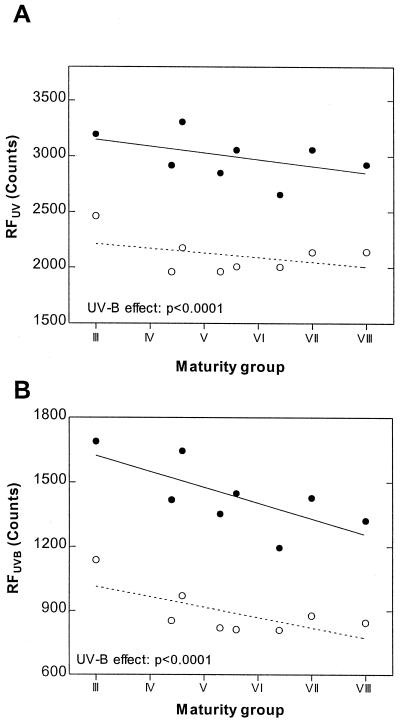
Leaves from plants grown under near ambient UV-B levels emitted markedly less red fluorescence under broadband UV radiation (RFUV) than leaves from plants grown under filters that excluded the UV-B component (−UV-B treatment) (Fig. 2). The effect of solar UV-B on RFUV was highly significant (P < 0.0001) and it was similar in all the cultivars tested (nonsignificant genotype × treatment interaction) (Fig. 3A). The differences between UV-B treatments in chlorophyll fluorescence were even more marked when we measured the RF signal induced specifically by the UV-B component of the radiation emitted by the excitation source (RFUVB) (Fig. 3B). Both RFUV and RFUVB tended to drop with maturity group—i.e. the tropical genotypes tended to be less fluorescent than the temperate cultivars (Fig. 3).
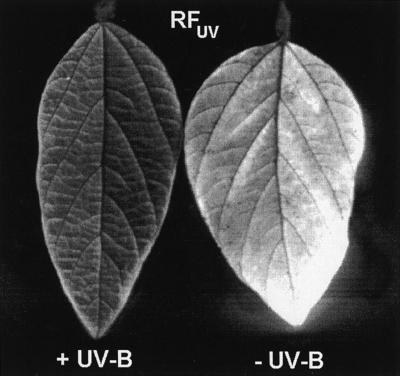
Figure 2.
Effects of solar UV-B radiation on chlorophyll fluorescence induced by UV radiation (RFUV). The figure shows representative fluorescence images of soybean leaves (abaxial surface; central leaflet of the youngest fully expanded leaves; cv Williams) exposed to the indicated UV treatments in the field (darker tones indicate less fluorescence).
Figure 3.
Effects of solar UV-B radiation on the intensity of chlorophyll fluorescence induced by UV (RFUV) and UV-B (RFUVB) in leaves of eight soybean lines arranged in order of increasing maturity group (MG); from MG III to VIII: Williams, A4423RG, CX458, A5634RG, A5308, A6445RG, Charata-76, and A8000RG. Notice that for the Dekalb and Nidera varieties we used the MG information provided by the breeders, which is indicated by the first two digits in the alpha-numeric cultivar name (e.g. Nidera A5308 belongs to maturity group 5.3). Samples were taken from the central leaflet of the youngest fully-expanded trifoliate (abaxial surface) at midday on January 12, 1999 (2 months after crop seeding). Each datum point is the average of five independent field plots. The slope of the RFUVB/MG relationship is significant at P = 0.04 (average of the two UV-B treatments). Notice that because the geometry of fluorescence excitation in this experiment was slightly different from the one used to produce the data reported in Figures 7 and 8, the absolute values RFUV values cannot be directly compared. ○, +UV-B; ●, −UV-B; in both panels the UV-B effect is significant at P < 0.0001.
Do the Differences in RFUV and RFUVB Reflect Differences in the Content of UV-Absorbing Phenolic Sunscreens?
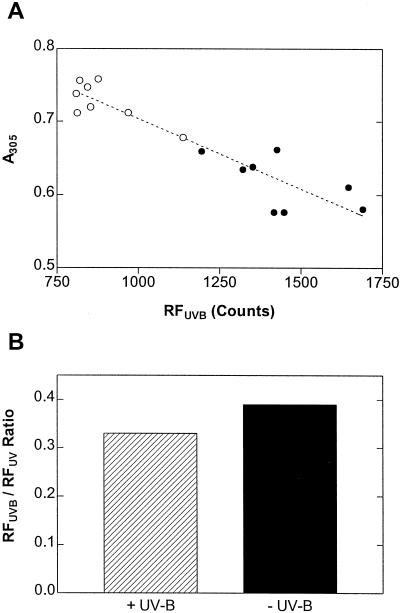
In the soybean data set we found a highly significant, inverse correlation between A305 and RFUVB (Fig. 4A), providing circumstantial evidence for the hypothesis that the variation in UV-B-induced chlorophyll fluorescence was indeed caused by variation in the content of UV-absorbing phenolics. Furthermore, we found that the RFUVB:RFUV ratio was lower in leaves from the +UV-B treatment than in leaves from −UV-B plants (Fig. 4B). This result suggests that the exposure to solar UV-B in the field preferentially induced the accumulation of compounds whose filtering action is particularly effective in the more damaging (UV-B) region of the solar spectrum.
Figure 4.
Relationship between the content of extractable UV-absorbing leaf phenolics (A305) and UV-B-excited chlorophyll fluorescence (RFUVB) in eight soybean genotypes exposed to two contrasting levels of solar UV-B in the field (original data in Figs. 1 and 3B). ○, +UV-B; ●, −UV-B. r2 = 0.86; significance of the slope: P < 0.0001. B, Effects of exposure to solar UV-B on the RFUVB:RFUV ratio. P = 0.0001 (n = 576).
To further investigate the connection between chlorophyll fluorescence and leaf phenolics we used a two-way approach. First we took advantage of Arabidopsis mutants that are specifically deficient in certain phenylpropanoid derivatives (Koornneef, 1981; Li et al., 1993), and we compared their RFUV signals with those obtained in leaves of WT plants grown under the same environmental conditions. Second, in soybean leaves, we measured the intensity of chlorophyll fluorescence induced by blue light (RFB); the purpose of these measurements was to assess the intensity of the fluorescent signal using wavelengths that are not affected by colorless UV-absorbing phenolics. It is important to point out that the soybean varieties used in our experiments have very low levels of anthocyanins. When present in large quantities these pigments can seriously complicate the estimation of UV penetration from RFUV/RFB data (Dr. Paul W. Barnes, S.W. Texas State University, personal communication), because they effectively absorb in the blue-green region of the spectrum.
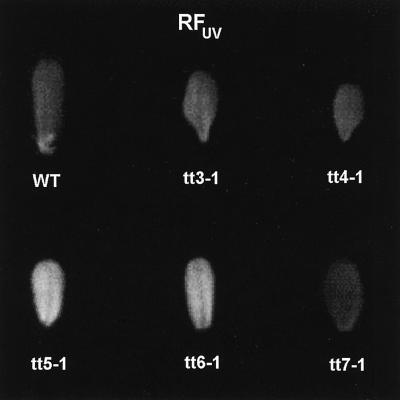
The Arabidopsis experiments showed that RFUV was much more intense in leaves of the tt5 and tt6 mutants than in the leaves of WT, tt3, tt4, and tt7 plants (Fig. 5). Li et al. (1993) reported that the tt5 (deficient in chalcone isomerase) and tt6 (deficient in flavonoid synthase) mutants fail to accumulate the major extractable leaf flavonols and display reduced quantities of sinapate esters. The same study also demonstrated that tt5 is extremely sensitive to even mild doses of UV-B (the sensitivity of tt6 was not tested). In contrast, tt4 (a chalcone synthase mutant) fails to accumulate flavonols, but shows higher levels of sinapic acid esters than the WT and has a UV-B-sensitive phenotype only at high UV-B doses (Li et al., 1993). tt3 and tt7 have mutations that affect anthocyanin metabolism and presumably have levels of UV-absorbing flavonols and sinapate esters that are not lower than those in WT plants. Thus, our RFUV results (Fig. 5) are consistent with the information derived from biochemical and physiological studies with the tt mutants, and demonstrate that a phenylpropanoid deficiency known to produce severe UV-B sensitivity can be readily detected with RFUV measurements.
Figure 5.
Fluorescence images obtained using individual leaves of WT and transparent testa mutants of Arabidopsis (adaxial surface).
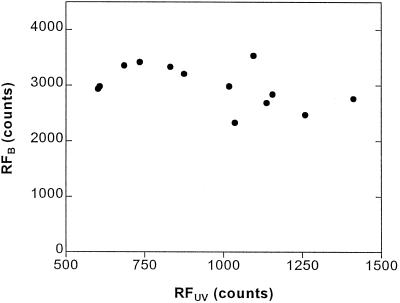
In soybean leaves, peeling off the epidermis (which proved to be very difficult in the varieties used in our experiments) greatly increased the RFUV signal (not shown). We also found that samples that differed by a factor of approximately 3 in RFUV presented very similar values of RFB (Fig. 6), and treatment or genotype effects on chlorophyll content were not detected (P ≥ 0.20; not shown). These results are consistent with the observations of Bilger et al. (1997) and suggest that the variations in RFUVB among cultivars or UV-B treatments (Figs. 2 and 3) do not reflect differences among leaves in chlorophyll levels or PSII functioning.
Figure 6.
Relationship between the fluorescent signals induced by UV (RFUV) and blue light (RFB) in a group of soybean samples that differed greatly in their RFUV values.
Collectively, the Arabidopsis and soybean data (Figs. 5 and 6) provide strong support for the idea that the decrease in RFUV induced by solar UV-B (Figs. 2 and 3) represents a specific decrease in the UV transmittance of the epidermal tissue, which is caused by the accumulation of colorless phenylpropanoid derivatives (Fig. 4). In field-grown soybeans, the differences in RFUVB between UV-B treatments were particularly obvious in the abaxial images; the upper surface images generally showed very low RFUVB values and only small differences between treatments and among cultivars (not shown). However, since more than 50% of the global UV-B is diffuse radiation at low-elevation, temperate latitudes (e.g. Caldwell, 1971), variations among leaves in phenylpropanoid accumulation in the lower epidermis are likely to be physiologically significant. This issue is addressed in the experiments reported below.
Are the Variations in UV Penetration Physiologically Significant?
We addressed this question using measurements of DNA damage to gauge the degree of cellular perturbation induced by solar radiation in Arabidopsis and soybean leaves.
In field-grown soybean plants, leaf tissue harvested around noon on sunny days had measurable levels of CPDs. A sizable fraction of the total DNA damage was caused by the UV-B component of sunlight, as indicated by the highly significant difference in damage density between +UV-B and −UV-B plots (Fig. 7A, Soybean cv A8000 RG). This result is consistent with those reported for field-grown plants of Datura ferox and barley (Ballaré et al., 1996; Mazza et al., 1999).
Figure 7.
Effects of solar UV-B radiation on CPD density in leaf DNA and the impact of constitutive and UV-B-induced variations in sunscreen levels. A, Effect of solar UV-B on CPD density in DNA extracted from soybean leaves harvested at noon on January 12, 1999 (2 months after sowing; youngest fully expanded leaf, cv A8000 RG; UV-B [305 nm] at sampling time approximately 9 μW cm−2 nm−1). The digital image shows representative slots; the difference between treatments in CPD density was significant at P < 0.0001; n = 5 independent plots per treatment. B, Effect of a 150-min exposure to midday sunlight on CPD density in DNA extracted from WT and tt5 Arabidopsis plants. Before the exposure, the plants were grown in a growth chamber under 100 μmol m−2 s−1 PPFD. The experiment was carried out on March 30, 1999; the average UV-B irradiance (305 channel) during the course of the experiment was 5 μW cm−2 nm−1. Representative images of chlorophyll fluorescence excited by UV-B are given for WT and tt5 leaves. C, Protective function of UV-B-induced sunscreens in field-grown soybean (cv A8000 RG). The youngest fully expanded leaves of +UV-B and −UV-B plots were harvested on March 24, 1999, and placed in flower pots with their petioles kept under water. The leaves (three replicate leaf groups per treatment) were sampled for CPD and RFUVB determinations (abaxial surface), and then placed under fluorescent light (200 μmol m−2 s−1 PPFD) to drive DNA photorepair. After 150 min the leaves were placed outdoors and exposed to direct sunlight for 45 min (average UV-B irradiance [305 nm] during the exposure = 5 μW cm−2 nm−1). At the end of this exposure (15:15 h) the leaves were sampled again for CPD and RFUVB determinations. In all panels, one unit of damage is the CPD level induced by 1 J m−2 of 254-nm radiation in 1 ng of purified herring DNA (see “Materials and Methods”). Non-irradiated herring DNA gave no signal in the blots. Pretreatments: ○, +UV-B; ●, −UV-B.
We wanted to know whether the large, constitutive differences in tissue transmittance to UV between tt5 and WT Arabidopsis (Fig. 5) or the more subtle differences resulting from UV-B-induced accumulation phenolics in a given soybean cultivar (Figs. 2 and 3) had functional significance in terms of influencing the DNA damage level under field conditions. In Arabidopsis, leaves of the highly fluorescent tt5 plants accumulated much more CPDs after a short exposure to sunlight than their WT counterparts exposed to the same experimental conditions (Fig. 7B). To test the impact of the difference in sunscreen levels induced by solar UV-B in soybeans, we collected leaves from the −UV-B and +UV-B treatments, allowed them to lower the DNA damage burden under photorepairing light, and measured the amount of CPDs that accumulated after a 45-min pulse of sunlight in the field.
Initially, the plants from the +UV-B treatment had greater levels of CPDs and less RFUVB (more UV-absorbing phenolics) than the plants from the −UV-B treatment, as expected (Fig. 7C). White light was effective in lowering the CPD burden in plants of both pretreatments (Fig. 7C, period under “Cool WL”). When the photorepaired leaves were taken back to the field and exposed to solar light (trying to maintain their natural angle of display), the ones from the −UV-B pretreatment accumulated significantly more CPD than leaves from the +UV-B group (Fig. 7C). Since obvious differences in photorepair capacity between +UV-B and −UV-B leaves were not evident under our experimental conditions, these results suggest that the UV-B-induced accumulation of phenolics effectively protected soybean DNA from the damaging action of present-day levels of solar UV-B.
Which UV Wavelengths Induce Sunscreen Responses in the Field?
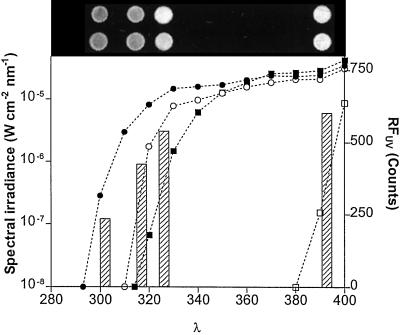
Having determined that RFUV and RFUVB can be used to detect biologically meaningful variations in levels of UV-absorbing sunscreens (Fig. 7), we wanted to find out which wavelengths in the solar spectrum are most effective in inducing changes in RFUV. We addressed this question using large cut-off filters placed above entire soybean canopies exposed to solar radiation in the field.
Our results (Fig. 8) indicate that most of the effect of solar UV on the accumulation of UV-absorbing sunscreens can be attributed to the UV-B component (λ ≤ 315 nm). Exposure to long-wave UV-A (λ ≥ 325 nm) alone did not result in significant RFUV changes compared with the no-UV (Lexan) treatment. Moreover, even the shortest wavelengths of the UV-A spectral region had only a small effect on the accumulation of UV-absorbing compounds. Therefore, the activity spectrum for the induction of UV-absorbing sunscreens in soybean appears to have a sharp increase in quantum efficiency below 325 nm, resembling the generalized plant action spectrum (Caldwell, 1971), which assumes very little activity in the UV-A region. Our findings have parallels with the laboratory studies of Beggs and Wellmann (1994), which showed that isoflavonoid responses in bean are triggered most effectively by short-wave UV-B radiation, presumably in response to DNA damage. Regardless of the mechanism, the results in Figure 8 indicate that the accumulation of UV sunscreens in soybeans can be very plastic in response to variations in the light environment that result from changes in the thickness of the ozone layer, which only affect the shortest wavelengths of the solar UV spectrum.
Figure 8.
Effects of filters that cut off different portions of the solar UV spectrum on the accumulation of UV-absorbing compounds in field-grown soybean plants of the cv Williams. The curves represent representative measurements of the spectral irradiance under the different filters obtained on March 26, 1998. The bars represent the average RFUV values for each of the radiation treatments; fluorescence images of representative samples are shown on top of each bar (darker tones indicate less fluorescence). Notice that each RFUV bar is positioned at the wavelength in which the spectral irradiance for the relevant treatment is ≈1% of the spectral irradiance at 400 nm. All samples were taken from the central leaflet of the youngest fully expanded leaf on March 20, 1998; each bar is the average of four true replicates (independent field plots). ●, Aclar filter; ○, Mylar filter; ▪, glass filter; □, Lexan filter.
CONCLUSIONS
The evidence presented in this paper shows that chlorophyll fluorescence imaging can be used to detect variations in the degree of UV penetration to the mesophyll in leaves of field-grown soybean plants. The method is sensitive enough to capture subtle differences in UV penetration between plants of the same species and to detect changes in UV-absorbing compounds induced by exposure to solar UV-B radiation. Our results suggest that these relatively subtle variations in UV penetration are functionally significant: measurements of DNA damage show that the UV-B component of sunlight induced greater perturbations in the cells of those leaves that scored as more UV transparent in the fluorescence determinations. We also determined that, under field conditions, most of the sunscreen response induced by solar UV in soybean can be attributed to the UV-B component. Collectively, our results suggest that in vivo measurements of UV penetration can be extremely useful in physiological and genetic studies of the biochemistry of plant acclimation to solar UV-B radiation.
ACKNOWLEDGMENTS
We thank Dr. Toshio Mori for the antibodies used for the detection of CPDs, Drs. Rodolfo Sánchez and Paul Barnes for stimulating discussions, Drs. Luis Orce and Alex Paladini for allowing on-line access to the GUV-511 data, Mariela Szwarcberg-Bracchitta and Ana Zima for help with some of the experiments and many useful discussions, and two anonymous reviewers for thoughtful comments on an earlier version of this manuscript. Andrés Arakelian, Pedro Gundel, and María Irianni provided excellent technical assistance. Adriana Kantolic and Patricia Giménez helped with the set-up and coordination of the field component of this study. We thank Nidera S.A., the INTA Marcos Juárez, the U.S. Department of Agriculture-Agricultural Research Service Soybean Germplasm Collection, and the Arabidopsis Biological Resource Center for the provision of the plant materials used in this project.
Footnotes
This research was supported by grants from the Secretariat of Science and Technology (Agencia Nacional de Promoción Cientifica y Tecnológica, BID OC–AR 802 PID no. 394 and PICT no. 00342).
LITERATURE CITED
- Ballaré CL, Scopel AL, Mazza CA. Effects of solar UV-B radiation on terrestrial ecosystems: case studies from southern South America. In: Rozema J, editor. Stratospheric Ozone Depletion: The Effects of Enhanced UV-B Radiation. Leiden, The Netherlands: Backhuys; 1999. pp. 293–311. [Google Scholar]
- Ballaré CL, Scopel AL, Stapleton AL, Yanovsky MJ. Solar ultraviolet-B radiation affects seedling emergence, DNA integrity, plant morphology, growth rate, and attractiveness to herbivore insects in Datura ferox. Plant Physiol. 1996;112:161–170. doi: 10.1104/pp.112.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs CJ, Wellmann E. Photocontrol of flavonoid biosynthesis. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 733–751. [Google Scholar]
- Bilger W, Veit M, Schreiber L, Schreiber U. Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol Plant. 1997;101:754–763. [Google Scholar]
- Caldwell MM. Solar UV irradiation and the growth and development of higher plants. In: Giese AC, editor. Photophysiology. Vol. 6. New York: Academic Press; 1971. pp. 131–177. [Google Scholar]
- Caldwell MM, Camp LB, Warner CW, Flint SD. Action spectra and their key role in assessing the biological consequences of solar UV-B radiation change. In: Worrest RC, Caldwell MM, editors. Stratospheric Ozone Reduction, Solar Ultraviolet Radiation and Plant Life. Heidelberg: Springer Verlag; 1986. pp. 87–111. [Google Scholar]
- Cen Y-P, Bornman JF. The effect of exposure to enhanced UV-B radiation on the penetration of monochromatic and polychromatic UV-B radiation in leaves of Brassica napus. Physiol Plant. 1993;87:249–255. [Google Scholar]
- Chapple CCS, Vogt T, Ellis BE, Somerville CR. An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell. 1992;4:1413–1424. doi: 10.1105/tpc.4.11.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Vogelmann TC, DeLucia EH. Are some plant life forms more effective than others in screening out ultraviolet-B radiation? Oecologia. 1992;92:513–519. doi: 10.1007/BF00317843. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- Flint SD, Caldwell MM. Scaling plant ultraviolet spectral responses from laboratory action spectra to field spectral weighting factors. J Plant Physiol. 1996;148:107–114. [Google Scholar]
- Flint SD, Jordan PW, Caldwell MM. Plant protective response to enhanced UV-B radiation under field conditions: leaf optical properties and photosynthesis. Photochem Photobiol. 1985;41:95–99. [Google Scholar]
- Gallagher SR. Quantitation of DNA and RNA with absorption and fluorescence spectroscopy. In: Ausubel DM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. 4, Supplement 28. New York: John Wiley & Sons; 1994. , Appendix A, p 3D. [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficient of chlorophyll a and b in N,N-dimetylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AK, Gaba V, Greenberg BM. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998;3:131–135. [Google Scholar]
- Koornneef M. The complex syndrome of TTG mutants. Arabidopsis Inf Serv. 1981;18:45–51. [Google Scholar]
- Landry LG, Chapple CCS, Last RL. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 1995;109:1159–1166. doi: 10.1104/pp.109.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell. 1993;5:171–179. doi: 10.1105/tpc.5.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Miehé JA. Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci. 1997;2:316–320. [Google Scholar]
- Lois R, Buchanan BB. Severe sensitivity to ultraviolet radiation in Arabidopsis mutant deficient in flavonoid accumulation. II. Mechanisms of UV-resistence in Arabidopsis. Planta. 1994;194:504–509. [Google Scholar]
- Mazza CA, Battista D, Zima AM, Szwarcberg-Bracchitta M, Giordano CV, Acevedo A, Scopel AL, Ballaré CL. The effects of solar UV-B radiation on the growth and yield of barley are accompanied by increased DNA damage and antioxidant responses. Plant Cell Environ. 1999;22:61–70. [Google Scholar]
- Mirecki RM, Teramura AH. Effects of ultraviolet-B irradiance in soybean. V. Dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiol. 1984;74:475–480. doi: 10.1104/pp.74.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber S, Bornman JF, Weissenböck G. A flavonoid mutant of barley (Hordeum vulgare L.) exhibits increased sensitivity to UV-B radiation in the primary leaf. Plant Cell Environ. 1996;19:593–601. [Google Scholar]
- Robberecht R, Caldwell MM. Leaf epidermal transmittance of ultraviolet radiation and its implications for plant sensitivity to ultraviolet-radiation induced injury. Oecologia. 1978;32:277–287. doi: 10.1007/BF00345107. [DOI] [PubMed] [Google Scholar]
- Robberecht R, Caldwell MM. Leaf UV optical properties of Rumex patientia L. and Rumex obtusifolius L. in regard to a protective mechanism against solar UV-B radiation. In: Worrest RC, Caldwell MM, editors. Stratospheric Ozone Reduction, Solar Ultraviolet Radiation and Plant Life. Heidelberg: Springer Verlag; 1986. pp. 252–259. [Google Scholar]
- Rousseaux MC, Ballaré CL, Scopel AL, Searles PS, Caldwell MM. Solar ultraviolet-B radiation affects plant-insect interactions in a natural ecosystem of Tierra del Fuego (southern Argentina) Oecologia. 1998;116:528–535. doi: 10.1007/s004420050618. [DOI] [PubMed] [Google Scholar]
- Rozema J, van de Staaij J, Björn LO, Caldwell MM. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol. 1997;12:22–28. doi: 10.1016/s0169-5347(96)10062-8. [DOI] [PubMed] [Google Scholar]
- Schmelzer E, Jahnen W, Hahlbrock K. In situ localization of light-induced chalcone synthase m RNA, chalcone synthase, and flavonoid products in epidermal cells of parsley leaves. Proc Natl Acad Sci USA. 1988;85:2989–2993. doi: 10.1073/pnas.85.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles PS, Caldwell MM, Winter K. The response of five tropical species to solar ultraviolet-B radiation. Am J Bot. 1995;82:445–453. [Google Scholar]
- Stapleton AE, Mori T, Walbot V. A simple and sensitive antibody-based method to measure UV-induced DNA damage in Zea mays. Plant Mol Biol Rep. 1993;11:230–236. [Google Scholar]
- Stapleton AE, Walbot V. Flavonoids can protect maize DNA from the induction of ultraviolet radiation damage. Plant Physiol. 1994;105:881–889. doi: 10.1104/pp.105.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevini M, Braun J, Fieser G. The protective function of the epidermal layer of rye seedlings against ultraviolet-B radiation. Photochem Photobiol. 1991;53:329–333. [Google Scholar]
- Veit M, Bilger W, Mühlbauer T, Brummet W, Winter K. Diurnal changes in flavonoids. J Plant Physiol. 1996;148:478–482. [Google Scholar]