Abstract
Bortezomib began to be used in the treatment of light chain (AL) amyloidosis in recent years. We performed the first meta-analysis of randomized clinical trials and clinical controlled trials to evaluate the effect and safety of bortezomib treatment for AL amyloidosis. We conducted a search (until July 2016) in electronic databases (PubMed databases and the Cochrane Central Register of Controlled Trials bases from the year 2003). There were 205 records we searched and eight studies was included (n = 617 persons). We demonstrated that bortezomib treatment significantly improved overall response rate (ORR), complete response, a cardiac response rate, 2-year overall survival and the risk of neuropathy and reduced overall mortality compared to controls without bortezomib therapy. From the comparison and subgroup analysis of ORR between bortezomib group and no bortezomib group, the patients with bortezomib had a higher ORR, especially patients pretreated with bortezomib before high-dose melphalan followed by autologous stem cell transplant compared to no pretreatment. In addition, patients with bortezomib in standard dosage had significantly higher ORR. According to our results, bortezomib should be used in AL amyloidosis patients to improve response rate and survival rate and future relevant randomized controlled trials require to be performed.
Keywords: Light chain (AL) amyloidosis, Bortezomib treatment, Effect and safety
Introduction
Light chain (AL) amyloidosis is the most common type of amyloidosis, which is caused by a pathological plasma cell clone leading to the deposition of amyloid fibrils. The amyloid fibrils derive from the aggregation of misfolded monoclonal immunoglobulin light chains [1]. The deposition of amyloid fibrils in vital organs, typically the kidney, heart, liver, nerves and soft tissue, can result in progressive organ dysfunction and death [2]. AL amyloidosis treatments are derived from multiple myeloma therapies. The combined treatment of melphalan and prednisone was the first effective regimen performed for AL amyloidosis; however, the responses of the treatment are significantly slow and rarely achieve complete remission [3, 4]. Therefore, the primary goal of therapy is to dominate the disease, reach sustainable remissions and improve quality of life.
Modern therapy regimens (including melphalan, autologous transplantation, lenalidomide, thalidomide, bortezomib) have optimized the treatment outcomes of patients at intermediate or low risk. Bortezomib is a reversible proteasome inhibitor that has demonstrated effective influence on patients with multiple myeloma [5, 6]. Indeed, several studies have shown that bortezomib combined with dexamethasone (BD) is an underlying effective chemotherapy regimen for the treatment of AL amyloidosis [7–10]. However, the data concerning the toxicity and efficacy of BD chemotherapy prior to other therapies and the effects of different treatment schedules and doses of bortezomib are limited for patients with AL amyloidosis. This review will focus on those fields of bortezomib for the treatment of AL amyloidosis.
Bortezomib belongs to reversible proteasome inhibitors that take effect by targeting specific cell receptors, proteins and signalling pathways, or both. Plasma cells of AL amyloidosis patients are discovered that they are especially sensitive to reversible proteasome inhibitors in a pre-clinical model; the accumulation of pre-fibrillar light chains after adding reversible proteasome inhibition to the cellular toxicity [11]. Bortezomib-based opinions have optimized effect on the treatment in AL amyloidosis over the last few years. Data from 33 national centers were combined in another study, reporting on 94 patients receiving bortezomib with or without dexamethasone. Nineteen patients received post-transplant bortezomib combined with dexamethasone, 67% reached a complete response and 60% achieved organ response [7]. In one study, seven of nine AL amyloidosis patients received bortezomib, dexamethasone and cyclophosphamide or lenalidomide/thalidomide. Hematologic response was seen in 89% of patients and organ response was discovered in 78% of patients [12].
Owing to the high response rates and good tolerance of bortezomib combinations regimens, bortezomib-based opinions have been adopted as commonly therapy in AL amyloidosis. Prospective evidence of superiority or better effects of bortezomib treatment for AL amyloidosis compared to other therapies and different treatment schedules and doses of bortezomib, on overall response rate (ORR), complete response (CR), organ responses, overall survival (OS), overall mortality, adverse events (AE) is still lacking. A systematic review is imperative to evaluate the accumulated clinical evidence and effects of bortezomib therapy in AL amyloidosis.
We will evaluate the effect and safety of bortezomib treatment for AL amyloidosis compared to other therapies and different treatment schedules and doses of bortezomib, on overall response rate (ORR), complete response (CR), organ responses, overall survival (OS), overall mortality, adverse events (AE).
Methods
Data Sources and Searches
We searched PubMed databases and the Cochrane Central Register of Controlled Trials bases from the year 2003 (when clinical states of bortezomib for the treatment of AL amyloidosis commenced) to July 2016. Types of studies included randomised controlled trials (RCTs) and clinical controlled trials (CCTs). The search strategy will include disease specific terms: AL amyloidosis, Light chain amyloidosis, amyloidosis (included AL amyloidosis), (using MeSH Terms and/or All Fields where appropriate) and intervention specific terms: proteasome inhibitors, bortezomib, velcade (trade name for bortezomib), (using MeSH Terms and/or All Fields where appropriate).
Study Selection
Two review authors independently inspected the title and the abstracts of retrieved publications, determined if each article was eligible according to inclusion criteria for the review. Any differences and inconsistencies were resolved by discussing with a third review author. If a determination could not be made from the abstract, a full-text of the article was evaluated by two authors to make the final decision according to the inclusion criteria.
Studies enrolling ≤ 5 patients were not included (risk of extreme bias). The types of participants were patients who were 18 years old or older, of any gender or ethnic origin and with any diagnosis of AL amyloidosis. The assessment of organ involvement and diagnosis of AL amyloidosis were according to consensus criteria [13].
Data Extraction and Quality Assessment
Two review authors abstracted the data of eligible studies independently. The extracted data include trial information, trial design, characteristics of interventions, trial participants, trial progress and follow up and outcomes (Table 1). For the quality assessment of the trials, we used Jadad scale to evaluate randomised controlled trials (RCTs) and clinical controlled trials (CCTs). In addition, two review authors independently evaluated intention-to-treat analysis, allocation concealment and blinding. There was an evidence that poor allocation concealment was greatly related to an over-estimation of effect [14]. So, quality classification was defined by: low risk of bias, moderate risk of bias and high risk of bias.
Table 1.
Study characteristics
| Author | Year | Region | Study design | ITT | Jadad scale | N | N of bortezomib | Median age (years) | Outcomes | Allocation concealment |
|---|---|---|---|---|---|---|---|---|---|---|
| Huang e al. | 2014 | China | RCT | Yes | 5 | 56 | 28 | 52.25 | ORR, CR, Organ responses, 2-year OS, 2-year PFS, Overall mortality, AE, Hematologic progression | Low risk bias |
| Kastritis et al. | 2015 | Greece | CCT | Yes | 2 | 85 | 26 | 68 | ORR, CR, Organ responses, 1-year OS, 1-year PFS, 6-month mortality | Low risk bias |
| Palladini G et al. | 2014 | Italy | CCT | Yes | 1 | 174 | 87 | 69 | ORR, CR, Organ responses, 1-year OS Overall mortality, AE | High risk bias |
| Reece et al. | 2014 | Canada, France, Germay, Italy, Spain, and the United States | CCT | Yes | 1 | 70 | 70 | 60.5 | ORR, CR, 4-year OS | High risk bias |
| Venner et al. | 2014 | London | CCT | Yes | 1 | 138 | 69 | 61.85 | ORR, CR, Organ responses, 1-year OS 6-month mortality | High risk bias |
| Scott et al. | 2014 | Portland | CCT | Yes | 1 | 25 | 12 | 60.8 | ORR, CR, Organ responses, 1- and 2-year OS, 1- and 2-year PFS | High risk bias |
| Sayago et al. | 2016 | Spain | CCT | Yes | 1 | 31 | 23 | 61 | ORR, Organ responses, 1-, 2- and 3- years OS, AE | High risk bias |
| Zhao et al. | 2016 | China | CCT | Yes | 1 | 38 | 23 | 53 | Organ responses, Overall mortality, 3- years OS, AE | High risk bias |
ITT intention-to-treat, RCT randomised controlled trials, CCT clinical controlled trials, ORR overall response rate, CR complete response rate, OS overall survival, AE adverse events
Statistical Analysis
We analysed data using an intention-to-treat model and used Risk ratios (RR) and 95% confidence intervals (CI) for all measures of treatment effect. We handled missing data according to the guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). To assess the heterogeneity of the data, we used the Chi2 test at a significance level of P < 0.05. In addition, we performed the I2 statistic to quantify heterogeneity. A value greater than 40% was deemed to substantial heterogeneity (Higgins 2011). We chose fixed-effect models to evaluate the overall effects. To assess potential publication bias, we used inverted funnel plot techniques to describe. We used fixed-effect models throughout and random-effects models when heterogeneity existed. We performed subgroup analyses to indirectly compare the effects of difference of chemotherapy regimen, bortezomib dose and bortezomib schedule for the treatment of AL amyloidosis. We conducted sensitivity analyses using trial quality (low, medium or high risk of bias) on allocation concealment and blinding.
Results
Results of the Search
Study Design and Description
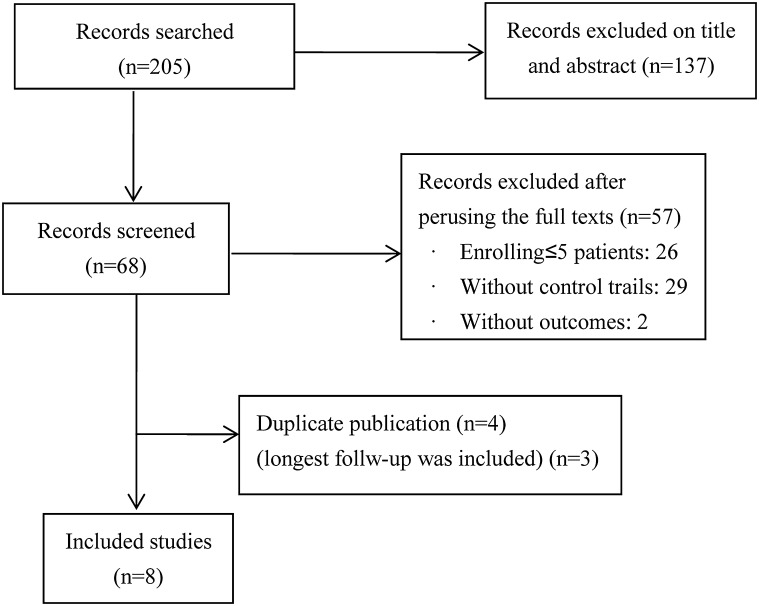
There were 205 records we searched. Sixty-eight of them were relevant based title or abstract. Fifty-seven studies were excluded after perusing the full texts. The reasons were as follows: twenty-six studies enrolling ≤ 5 patients; twenty-nine without control trials; two without outcomes. Four were double published [8, 15–17]. We chose the article had longest follow-up [15]. Eight articles met inclusion criteria [15, 18–24] (Fig. 1).
Fig. 1.
Flow diagram of the articles assessed for inclusion in meta-analysis, 2003–2016. There were 205 records we searched. Sixty-eight of them were relevant based title or abstract. Fifty-seven studies were excluded after perusing the full texts. The reasons were as follows: twenty-six studies enrolling ≤ 5 patients; twenty-nine without control trials; two without outcomes. Four were double published
Among five publications, there was only one RCT [21]. Overall study quality was greatly reduced. 7 were controlled clinical trials [15, 18–20, 22–24]. Seven of the eight eligible trials included patients with newly diagnosed AL amyloidosis [18–24]. One included patients with relapsed or refractory [15].
Induction Treatment
Induction treatment included following ways: (1) Bortezomib versus no bortezomib (with same background therapy in each trial): bortezomib and dexamethasone followed by autologous stem cell transplantation (BD + HDM/SCT) versus autologous stem cell transplantation(HDM/SCT) [21, 22]; bortezomib with melphalan and dexamethasone (BMD) versus melphalan and dexamethasone (MD) [20]; bortezomib with dexamethasone (BD), bortezomib-melphalan with dexamethasone (BMD) and bortezomib-cyclophosphamide with dexamethasone (BCD) versus melphalan and dexamethasone or prednisone (MD), cyclophosphamide and dexamethasone or prednisone (CD) [23] (2) Bortezomib versus other agent(s): cyclophosphamide, bortezomib and dexamethasone (BCD) versus cyclophosphamide, thalidomide and dexamethasone (TCD) [18]; bortezomib with dexamethasone (B) versus lenalidomide (L) [19]; bortezomib with dexamethasone (BD) versus autologous stem cell transplantation(HDM/SCT) [24] (3) Bortezomib with dexamethasone comparisons of different schedules [15, 19].
Interventions
Dose four of the eight eligible trials the dose of bortezomib was 1.3 mg/m2 [19–21, 23, 24]; two was included 1.0, 1.3 and 1.5 mg/m2 [18, 22]; one was 1.6 or 1.3 mg/m2 [15].
Schedule three of the five eligible trials on the schedule of bortezomib was 8 cycles [15, 18, 19]; one was 2 cycles [21]; one was 6 cycles [20]. Three of the five eligible trials on the schedule of bortezomib was twice-weekly [18, 20, 21]; two was once-weekly or twice-weekly [15, 19].
Risk of Bias in Included Studies
We chose Jadad scale to assess RCTs and CCTs quality. Seven CCTs studies were not randomized and blinded [15, 18–20, 22–24]. Generation of randomization sequence did not describe in the only one RCT [21]. Allocation concealment was assessed as low risk bias in the two studies [19, 21], and the others assessed as high risk bias [15, 18–20, 22–24]. Only one RCT was blinded [21]. Intention-to-treat analysis was performed in all trials.
Effects of Interventions
Bortezomib Versus no Bortezomib
Overall Response Rate (ORR)
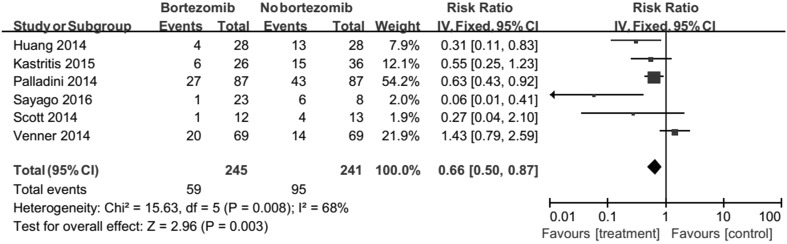
There were 186 among the 245 and 146 among the 241 responded to therapy, corresponding to an RR of 0.66 (95% CI 0.50–0.87, Fig. 2). Results revealed that bortezomib significantly caused more patients to respond to treatment. But, there was significant heterogeneity between the two trials was detected (Chi2 = 15.63, df = 5, P = 0.008).
Fig. 2.
Forest plot of comparison: bortezomib versus no bortezomib, outcome: overall response rate (ORR). There were 186 among the 245 and 146 among the 241 responded to therapy, corresponding to an RR of 0.66 (95% CI 0.50–0.87). Results revealed that bortezomib significantly caused more patients to respond to treatment. But, there was significant heterogeneity between the two trials was detected (Chi2 = 15.63, df = 5, P = 0.008)
Complete Response (CR)
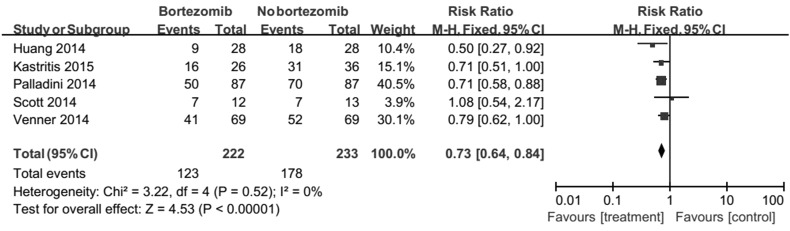
Patients treated with bortezomib had a greatly better CR compared to without bortezomib (RR 0.73, 95% CI 0.64–0.84) (Fig. 3). No heterogeneity was detected (Chi2 = 3.22, df = 4, P = 0.52).
Fig. 3.
Forest plot of comparison: bortezomib versus no bortezomib, outcome: complete response (CR). Patients treated with bortezomib had a greatly better CR compared to without bortezomib (RR 0.73, 95% CI 0.64–0.84). No heterogeneity was detected (Chi2 = 3.22, df = 4, P = 0.52)
Organ Responses: A Renal Response
The RR was 0.95 (95% CI 0.80–1.13). There was no sufficient evidence to prove that bortezomib could improve renal response of AL amyloidosis.
Organ Response: A Cardiac Response
Sixty-seven among the 178 and 33 among the 149 had a cardiac response. The RR of 0.83 (95% CI 0.73–0.95). Results indicated that patients treated with bortezomib had a significantly better cardiac response compared to without bortezomib. But, there was significant heterogeneity between the two trials was detected (Chi2 = 18.06, df = 5, P = 0.003).
1-year Overall Survival (OS)
There was no evidence that bortezomib could increase 1-year OS of AL amyloidosis (RR 0.85, 95% CI 0.66–1.10). No heterogeneity was found (Chi2 = 7.26, df = 4, P = 0.12).
2-year Overall Survival (OS)
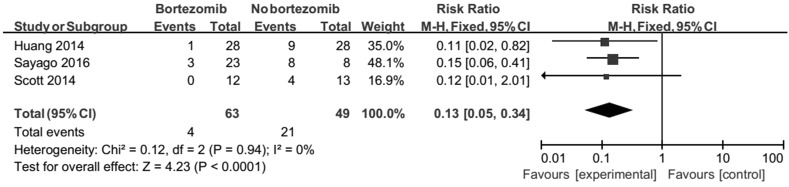
There was a sufficient evidence that bortezomib could increase 2-year OS of AL amyloidosis (RR 0.13, 95% CI 0.05–0.34). No heterogeneity was detected (Chi2 = 0.12, df = 2, P = 0.94, Fig. 4).
Fig. 4.
Forest plot of comparison: bortezomib versus no bortezomib, outcome: 2-year Overall survival (OS). There was a sufficient evidence that bortezomib could increase 2-year OS of AL amyloidosis (RR 0.13, 95% CI 0.05–0.34). No heterogeneity was detected (Chi2 = 0.12, df = 2, P = 0.94)
Overall Mortality
There were 42 deaths among 138 patients with bortezomib, and 58 deaths among 130 patients without bortezomib. The RR is 0.69 (95% CI 0.51–0.94). Results suggested that bortezomib reduce death rate significantly. No heterogeneity was found (Chi2 = 1.31, df = 2, P = 0.52).
Adverse Events (AE): Neuropathy
From two trials (292 patients) the RR was 7.64 (95% CI 1.87–31.3). Results revealed that bortezomib caused more risk for neuropathy significantly.
Adverse Events (AE): Renal Toxicity
From two studies 6 out of 138 patients in the bortezomib group and 8 out of 130 in the no bortezomib group occurred renal toxicity, with a RR of 0.71 (95% CI 0.28–1.81). There was no sufficient evidence that bortezomib could result in less risk for renal toxicity.
Bortezomib Weekly Versus Twice-Weekly
Overall Response Rate (ORR)
Results displayed that there was no proof of the improvement of ORR with bortezomib once-weekly or twice-weekly (RR 0.99, 95% CI 0.49–1.98). No heterogeneity was found (Chi2 = 0.08, df = 1, P = 0.78).
Complete Response (CR)
From two studies, 13 out of 44 patients in the bortezomib twice-weekly group and 10 out of 28 in the bortezomib once-weekly group received CR, with a RR of 1.07 (95% CI 0.75–1.53). There was no sufficient evidence to show that bortezomib once-weekly could improve CR. No heterogeneity was detected between two trials (Chi2 = 0.94, df = 1, P = 0.33).
Subgroup Analysis (on ORR)
Therapy in control group there were the same background treatment between experimental and control in four trials [20–23], including bortezomib and dexamethasone followed by autologous stem cell transplantation (BD + HDM/SCT) versus autologous stem cell transplantation(HDM/SCT) [21, 22]; control of the other two trials was other agent(s) [18, 19, 24]. Under the same treatment background, the patients with bortezomib had a higher ORR, especially patients pretreated with bortezomib before HDM/SCT compared to no pretreatment (RR 0.62, 95% CI 0.48–0.82). Results indicate that other agent(s) in control group relate to heterogeneity. There was no sufficient evidence to show that bortezomib could improve ORR compared to other agent(s) treatment (thalidomide, lenalidomide or HDM/SCT) (Fig. 5).
Fig. 5.
Forest plot of comparison: bortezomib versus no bortezomib, outcome: subgroup analysis (on ORR): type of control. Under the same treatment background, the patients with bortezomib had a higher ORR, especially patients pretreated with bortezomib before HDM/SCT compared to no pretreatment (RR 0.62, 95% CI 0.48–0.82). Results indicate that other agent(s) in control group relate to heterogeneity
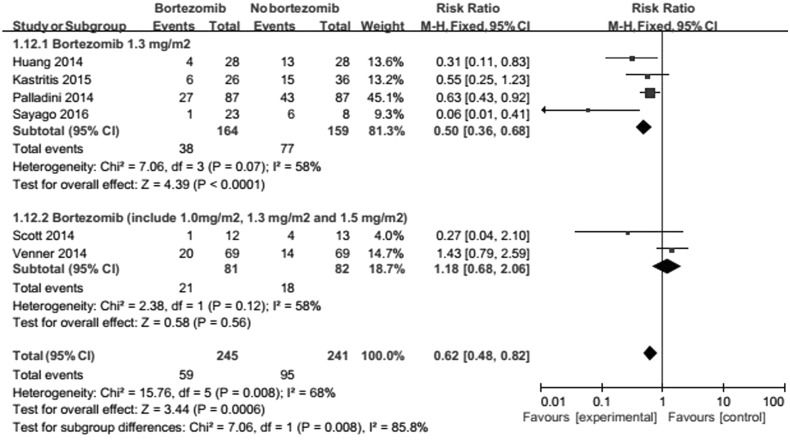
Bortezomib dose the dose of bortezomib treatment included 1.0, 1.3 and 1.5 mg/m2 in two studies [18, 22]; the other trials were standard dosage (1.3 mg/m2) [19–21, 23]. Results suggest that the difference dose of bortezomib treatment is relevant to heterogeneity (Fig. 6). The difference dose influence the beneficial effect of bortezomib on ORR.
Fig. 6.
Forest plot of comparison: bortezomib versus no bortezomib, outcome: subgroup analysis (on ORR): bortezomib dosage. The difference dose of bortezomib treatment is relevant to heterogeneity. The difference influences the beneficial effect of bortezomib on ORR
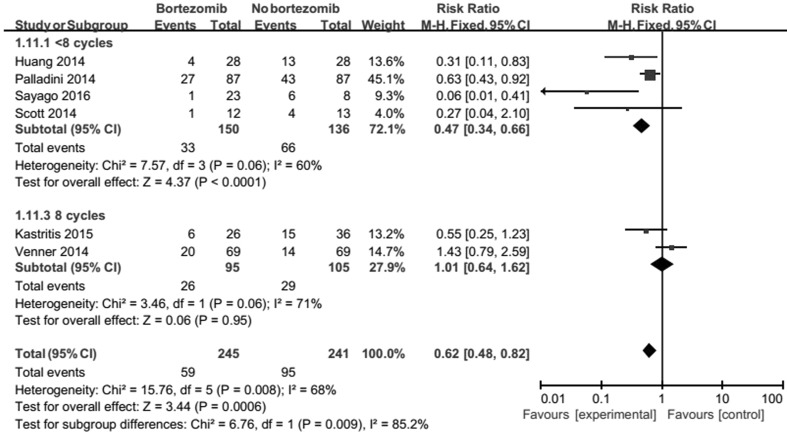
Bortezomib schedule outcomes show that the therapy schedule of bortezomib do not change heterogeneity (Fig. 7).
Fig. 7.
Forest plot of comparison: bortezomib versus no bortezomib, outcome: subgroup analysis (on ORR): type of bortezomib schedules. The therapy schedule of bortezomib do not change heterogeneity
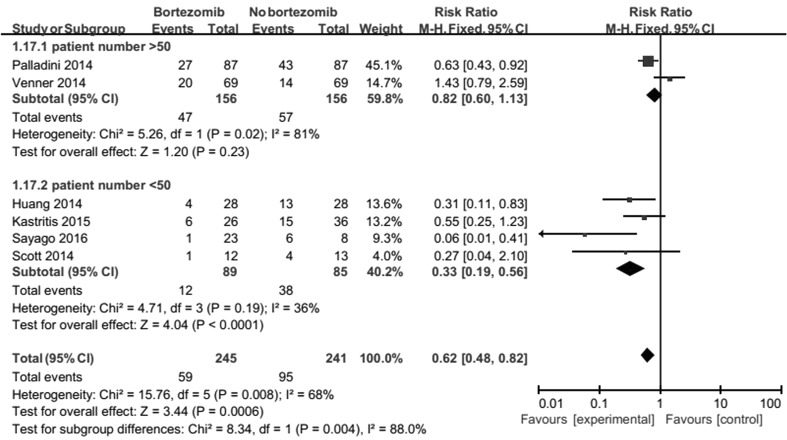
Patient number results suggest that number of patient in the group of bortezomib treatment with < 50 is relevant to heterogeneity (Fig. 8). This may be related to the lack of a large number of randomized controlled studies.
Fig. 8.
Forest plot of comparison: bortezomib versus no bortezomib, outcome: subgroup analysis (on ORR): type of patient number. The number of patient in the group of bortezomib treatment with < 50 is relevant to heterogeneity
There was not enough information to analyse ORR according to age, sex, grade of AL amyloidosis, schedule of treatment and performance status of patients.
Sensitivity Analysis (on ORR)
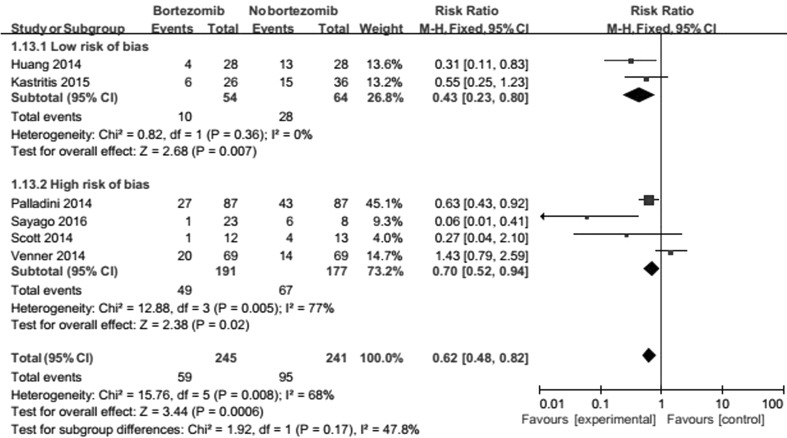
Allocation concealment one trial was assessed as low risk bias [19, 21], and the others assessed as high-risk bias. Outcomes revealed that quality of allocation concealment had an effect on of heterogeneity of ORR (Fig. 9).
Fig. 9.
Forest plot of comparison: bortezomib versus no bortezomib, outcome: sensitivity analysis (on ORR): study quality. Quality of allocation concealment had an effect on of heterogeneity of ORR
Publication Bias
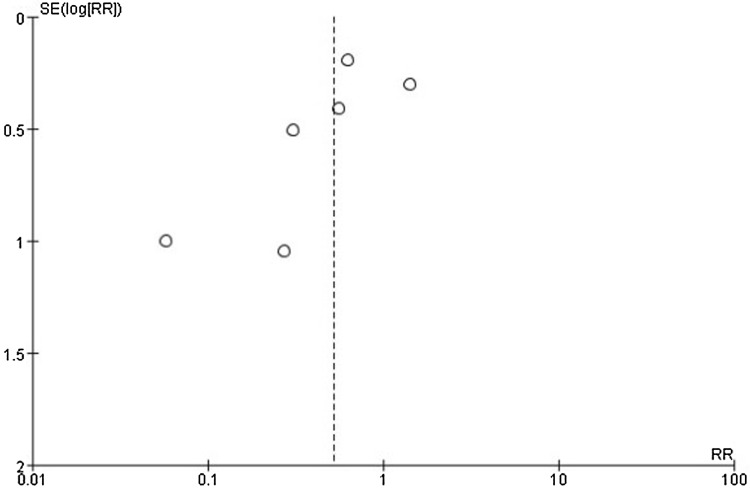
Publication bias was assessed utilizing a funnel plot of the ORR of bortezomib versus no bortezomib (Fig. 10). Only six studies were eligible to assess including a total of 486 patients. There was no clear symmetry in the plot. Even though the funnel plot revealed the existence of publication bias, a quantitative or detailed assessment was not possible owing to the small number of trials.
Fig. 10.
Funnel plot of comparison: bortezomib versus no bortezomib, outcome: overall response rate (ORR). Only six studies were eligible to assess including a total of 486 patients. There was no clear symmetry in the plot. Even though the funnel plot revealed the existence of publication bias, a quantitative or detailed assessment was not possible owing to the small number of trials
Discussion
The objective of this analysis is to assess the effect and safety of bortezomib therapy for AL amyloidosis. Our systematic review and meta-analysis demonstrated that bortezomib treatment improves overall response rate (ORR), complete response (CR), a cardiac response rate, survival rate and the risk of neuropathy compared to controls without bortezomib therapy.
From the comparison and subgroup analysis of ORR between bortezomib group and no bortezomib group, the patients with bortezomib had a higher ORR, especially patients pretreated with bortezomib before HDM/SCT compared to no pretreatment. But, there was no sufficient evidence to indicate that bortezomib could improve ORR compared to thalidomide, lenalidomide or HDM/SCT on account of small sample sizes. In addition, the difference of bortezomib dose affected heterogeneity of bortezomib on ORR for AL amyloidosis. Patients with bortezomib in standard dosage had significantly higher ORR. Future studies require to be practiced.
When we compared the complete response rate and a cardiac response rate, we discovered a considerable benefit of bortezomib treatment. However, there was no enough evidence to see that bortezomib had an impact on a renal response rate and risk of renal toxicity for AL amyloidosis, either because there was no difference effectively or owing to small trials number.
There were 42 deaths among 138 patients with bortezomib, and 58 deaths among 130 patients without bortezomib. We concluded that bortezomib treatment significantly reduced overall mortality and increased survival rate. However, our review showed that whether bortezomib was used to therapy AL amyloidosis did not impact on the 1-year overall survival.
There are several limitations that the eight included trials vary in their treatments. The chemotherapy regimen of their therapy differed among each trial. But, seven of the eight eligible trials included patients with newly diagnosed AL amyloidosis. One included patients with relapsed or refractory. Besides, we added the effect of therapy types, dose and schedule cycles on the heterogeneity of overall response rate. In addition, Kastritis E et al. indicated that higher doses of dexamethasone and addition of cyclophosphamide do not seem to have a profound effect on efficacy and survival [25]. This suggests that the changes of supportive care do not necessarily affect the efficacy of bortezomib therapy for amyloidosis. The beneficial effect on the improvement of response rate due to bortezomib treatment for AL amyloidosis retained to be detected irrespective of the different chemotherapy regimen. The analysis about effects of dose and schedule on bortezomib treatment is not enough because of small included trials. The optimal dose and schedule of bortezomib therapy in AL amyloidosis is still unclear.
From sensitivity analysis on ORR, we found quality of allocation concealment had an effect on of heterogeneity of ORR. There are one RCT and seven CCTs in our included publication. Because of only one RCT, overall study quality of our review is significantly reduced. Because seven CCTs are not randomized and blinded, high risk bias existed in our review. Currently, there is no enough RCTs to evaluate the efficacy and safety of bortezomib for the treatment of AL amyloidosis. Future randomized controlled trials to address this aspect require to be performed.
Conclusions
Bortezomib for the treatment of AL amyloidosis improve response rate (ORR, CR, a cardiac response rate), survival rate and the risk of neuropathy, but results on inhibiting adverse events (renal toxicity) require be confirmed by additional studies with larger sample size.
The effect on progression-free survival, treatment-related death, treatment-free interval and health-related quality of life of bortezomib for the treatment of AL amyloidosis should be further explored. There is no enough RCTs to evaluate the efficacy of bortezomib for the treatment of AL amyloidosis. Future randomized controlled trials to address this aspect require to be performed.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Higgins 2011 Higgins JPT, Altman DG, Sterne JAC. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2008. Available from www.cochrane-handbook.org.
Acknowledgements
We wish to acknowledge the Health Statistics for Tianjin Medical University. This work was supported by the National Natural Science Foundation of China (Grant nos. 81570106, 814000888, 81600093), the anticancer major special project of Tianjin (Grant nos. 12ZCDZSY18000), the Tianjin Municipal Natural Science Foundation (Grant nos. 14JCYBJC25400, 15JCYBJC24300), Tianjin Health and Family Planning Commision (Grant nos. 15KG150). Fengjuan Jiang received Postgraduate Innovation Fund of ‘13th Five-Year comprehensive investment’ award from Tianjin Medical University (YJSCX201717).
Author contribution statement
Concept (RF) and design of study (FJ). Acquisition of data (FJ, JC, HL), analysis and interpretation of data (FJ, JC, LL,WL). Revision and intellectual contribution (FJ, JC, LL, HL, RF). All authors approved the final version. Drafting the article or revising it critically for important intellectual (FJ, RF, LL,WL). Final approval of the version to be published (WL, RF, LL).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studides with human participants or animals performed by any of the authors.
References
- 1.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 2.Comenzo RL. Amyloidosis. Curr Treat Options Oncol. 2006;7:225–236. doi: 10.1007/s11864-006-0015-8. [DOI] [PubMed] [Google Scholar]
- 3.Gertz MA, Lacy MQ, Lust JA, et al. Prospective randomized trial of melphalan and prednisone versus vincristine, carmustine, melphalan, cyclophosphamide, and prednisone in the treatment of primary systemic amyloidosis. J Clin Oncol. 1999;17:262–267. doi: 10.1200/JCO.1999.17.1.262. [DOI] [PubMed] [Google Scholar]
- 4.Skinner M, Anderson J, Simms R, et al. Treatment of 100 patients with primary amyloidosis: a randomized trial of melphalan, prednisone, and colchicine versus colchicine only. Am J Med. 1996;100:290–298. doi: 10.1016/S0002-9343(97)89487-9. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 6.San MJ, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 7.Kastritis E, Wechalekar AD, Dimopoulos MA, et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 2010;28:1031–1037. doi: 10.1200/JCO.2009.23.8220. [DOI] [PubMed] [Google Scholar]
- 8.Reece DE, Sanchorawala V, Hegenbart U, et al. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study. Blood. 2009;114:1489–1497. doi: 10.1182/blood-2009-02-203398. [DOI] [PubMed] [Google Scholar]
- 9.Kastritis E, Anagnostopoulos A, Roussou M, et al. Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica. 2007;92:1351–1358. doi: 10.3324/haematol.11325. [DOI] [PubMed] [Google Scholar]
- 10.Wechalekar AD, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD. Efficacy of bortezomib in systemic AL amyloidosis with relapsed/refractory clonal disease. Haematologica. 2008;93:295–298. doi: 10.3324/haematol.11627. [DOI] [PubMed] [Google Scholar]
- 11.Sitia R, Palladini G, Merlini G. Bortezomib in the treatment of AL amyloidosis: targeted therapy? Haematologica. 2007;92:1302–1307. doi: 10.3324/haematol.12136. [DOI] [PubMed] [Google Scholar]
- 12.Chari A, Barley K, Jagannath S, Osman K. Safety and efficacy of triplet regimens in newly diagnosed light chain amyloidosis. Clin Lymphoma Myeloma Leuk. 2013;13:55–61. doi: 10.1016/j.clml.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 14.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.1995.03520290060030. [DOI] [PubMed] [Google Scholar]
- 15.Reece DE, Hegenbart U, Sanchorawala V, et al. Long-term follow-up from a phase 1/2 study of single-agent bortezomib in relapsed systemic AL amyloidosis. Blood. 2014;124:2498–2506. doi: 10.1182/blood-2014-04-568329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reece DE, Hegenbart U, Sanchorawala V, et al. Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118:865–873. doi: 10.1182/blood-2011-02-334227. [DOI] [PubMed] [Google Scholar]
- 17.Dubrey SW, Reece DE, Sanchorawala V, et al. Bortezomib in a phase 1 trial for patients with relapsed AL amyloidosis: cardiac responses and overall effects. QJM. 2011;104:957–970. doi: 10.1093/qjmed/hcr105. [DOI] [PubMed] [Google Scholar]
- 18.Venner CP, Gillmore JD, Sachchithanantham S, et al. A matched comparison of cyclophosphamide, bortezomib and dexamethasone (CVD) versus risk-adapted cyclophosphamide, thalidomide and dexamethasone (CTD) in AL amyloidosis. Leukemia. 2014;28:2304–2310. doi: 10.1038/leu.2014.218. [DOI] [PubMed] [Google Scholar]
- 19.Kastritis E, Roussou M, Gavriatopoulou M, et al. Long-term outcomes of primary systemic light chain (AL) amyloidosis in patients treated upfront with bortezomib or lenalidomide and the importance of risk adapted strategies. Am J Hematol. 2015;90:E60–E65. doi: 10.1002/ajh.23936. [DOI] [PubMed] [Google Scholar]
- 20.Palladini G, Milani P, Foli A, et al. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia. 2014;28:2311–2316. doi: 10.1038/leu.2014.227. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Wang Q, Chen W, et al. Induction therapy with bortezomib and dexamethasone followed by autologous stem cell transplantation versus autologous stem cell transplantation alone in the treatment of renal AL amyloidosis: a randomized controlled trial. BMC Med. 2014;12:2. doi: 10.1186/1741-7015-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott EC, Heitner SB, Dibb W, et al. Induction bortezomib in AL amyloidosis followed by high dose melphalan and autologous stem cell transplantation: a single institution retrospective study. Clin Lymphoma Myeloma Leukemia. 2014;14:424–430. doi: 10.1016/j.clml.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Sayago I, Krsnik I, Gómez-Bueno M, et al. Analysis of diagnostic and therapeutic strategies in advanced cardiac light-chain amyloidosis. J Heart Lung Transplant. 2016;35:995–1002. doi: 10.1016/j.healun.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q, Wang L, Song P, et al. Comparison analysis of outcomes in primary light chain amyloidosis patients treated by auto peripheral blood stem cell transplantation or bortezomib plus dexamethasone. Zhonghua Xue Ye Xue Za Zhi. 2016;37:283–287. doi: 10.3760/cma.j.issn.0253-2727.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kastritis E, Gavriatopoulou M, Roussou M, et al. Addition of cyclophosphamide and higher doses of dexamethasone do not improve outcomes of patients with AL amyloidosis treated with bortezomib. Blood Cancer J. 2017;7:e570. doi: 10.1038/bcj.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]