Abstract
Iron deficiency anemia (IDA) continues to be the commonest etiology of anemia in pregnancy. The prevalence of iron deficiency (ID) in pregnant Indian women is amongst the highest in the world. Untreated iron deficiency (ID) has significant adverse feto-maternal consequences. Plethora of investigations are available for diagnosis of IDA, each having specific advantages and disadvantages when used in the pregnancy setting. Therapy for ID includes dietary modification, oral iron supplementation, intravenous iron and blood transfusion. Newer parenteral iron preparations are safe and there is mounting evidence to suggest their use in frontline settings for pregnancy associated IDA in the second and third trimester. Through this review, we suggest an algorithm for diagnosis and treatment of IDA in pregnancy depending on the severity of anemia and period of gestation suited for widespread use in resource limited settings. Also, we recommend ways for increasing public awareness and tackling this health issue including the observance of “National Anemia Awareness and Treatment Day.”
Electronic supplementary material
The online version of this article (10.1007/s12288-018-0949-6) contains supplementary material, which is available to authorized users.
Keywords: Iron deficiency anemia, Pregnancy, India
Introduction
Iron deficiency and its consequences continue to be prevalent in epidemic proportions despite major health reforms over the past century [1]. Although the adverse consequences on maternal and child health are well known, it continues to be sub-optimally managed. The causes of anemia in pregnancy may be numerous, the current article focuses on management of iron deficiency anemia (IDA) in pregnancy. Management of other etiologic causes of anemia are beyond the scope of this review.
Definitions
Anemia is a condition where the red blood cell number or their oxygen-carrying capacity is insufficient to meet physiologic needs, and is conventionally taken as a hemoglobin (Hb) value that is less than two standard deviation (SD) below the median value for healthy matched population by age, sex, altitude, smoking, and pregnancy status [2]. Defining anemia in pregnancy is not straight-forward given the physiologic plasma expansion, the ethnic variations of Hb values and the frequent use of iron supplementation in pregnancy. Center of Disease Control (CDC) defines anemia as pregnancy hemoglobin less than 11 g/dl (Hematocrit;{Hct} < 33%) in the first and third trimester and less than 10.5 g/dl (Hct < 32%) in the second trimester while World Health Organisation (WHO) defines anemia in pregnancy as Hb values less than 11gm/dl [3, 4]. Anemia in postpartum females is defined as Hb less than 10 g/dl by WHO. Table 1 shows WHO classification of severity of anemia in adult females [5].
Table 1.
Hemoglobin levels to diagnose anemia at sea level [5]
| Non Anemia (g/dl) | Anemia (g/dl) | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Non pregnant women(age > 15 years or above) | > = 12 or higher | 11–11.9 | 8–10.9 | Lower than 8 |
| Pregnant women | > = 11 or higher | 10–10.9 | 7–9.9 | < 7 |
Prevalence of IDA in Pregnancy
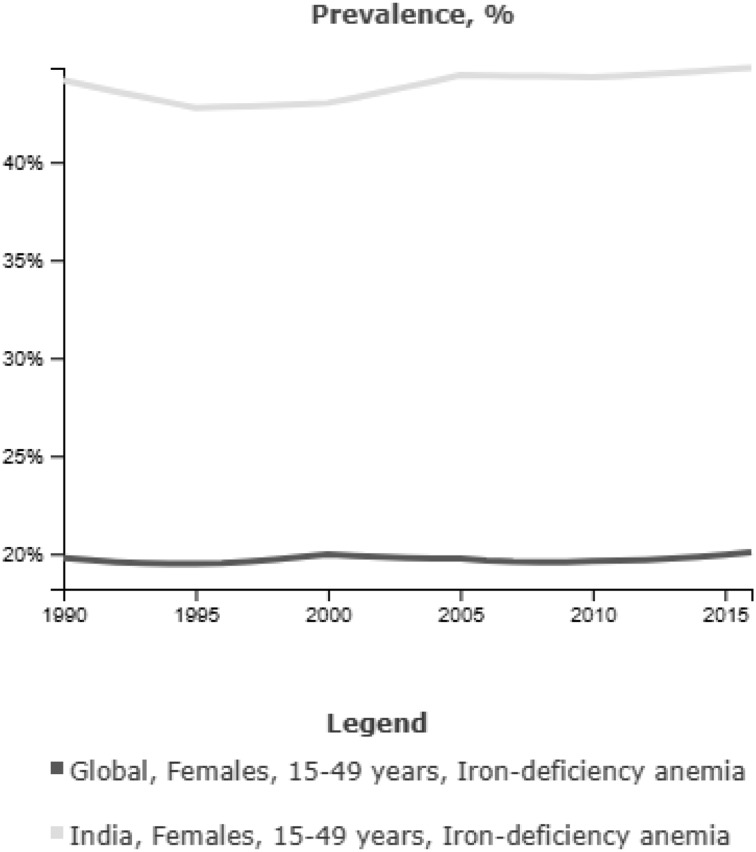
Globally, the commonest cause for anemia in pregnancy is IDA. The Nutrition Impact Model Study, a systematic analysis of 257 population-representative data sources from 107 countries, estimated the global prevalence of anemia in pregnancy as 43% in 1995 and 38% in 2011 with the range varying from 17% in developed and 56.4% in developing countries. Etiology of anemia was attributed to ID in 50% of cases in this study [1]. The prevalence of occult ID in the absence of anemia is estimated to be between 30 and 60% in pregnant women [6]. In a population based study from rural Haryana in 1994–1995,we had found 50% prevalence of anemia among non-pregnant women in the age group of 16–70 years [7]. Twenty years later, the prevalence of anemia still continues to be 53% in non-pregnant women and 50% in pregnant women as per population based surveys of 2016 in our country [8]. As per the Global Nutrition Report 2016, India ranked miserably at 170th in terms of anemia prevalence in women [9] (Figure 1).
Fig. 1.
Global and national trends in IDA in women of reproductive age group [9]
Consequences of IDA in Pregnancy
It is estimated that maternal anemia contributes to 18% of perinatal mortality and 20% of maternal mortality in South Asian countries including India as per a recent meta-analysis [10]. The exact level of maternal hemoglobin that is critical with respect to maternal mortality is not known. According to CHERG, the risk of maternal mortality significantly decreases for every 1 g/dl rise in Hb, however, the association becomes less clear at Hb levels above 8–9 g/dl [11]. With respect to neonatal birth weight, both hemoglobin level > 11 g/dl and < 9 g/dl are associated with 2–3 times increased risk of small for gestational age neonates. The ideal Hb values with respect to prevention of prematurity and LBW lies between 9 to 11.5 g/dl [12]. Table 2 summarizes the consequences of IDA in pregnancy.
Table 2.
| Antepartum complications | Intrapartum complications | Postpartum complications | Fetal outcome |
|---|---|---|---|
| Increased risk of preterm delivery | Prolonged labor | Postpartum hemorrhage | Low birth weight |
| Premature rupture of membranes | Increased rates of operative delivery and induced labor | Purperal sepsis | Prematurity |
| Preecclampsia | Fetal distress | Lactation failure | Infections |
| Intrauterine Death | Abruption | Pulmonary thromboembolism | Congenital malformation |
| Intercurrent infection | Subinvolution of uterus | Neonatal Anemia | |
| Antepartum hemorrhage | Postpartum depression | Abnormal cognitive development | |
| Congestive Heart Failure | Increased risk of Schizophrenia |
Physiology of Iron Balance During Pregnancy
Table 3 depicts the net iron balance during a normal pregnancy and delivery. As shown, pregnancy costs an approximate 630 mg of extra iron to the mother. In the hierarchy of iron usage the fetus takes the priority, followed by maternal hematocrit while the maternal iron stores are the poor last and are often depleted during the course of pregnancy. The mother indeed requires iron stores for lactation and future pregnancies. To prevent a negative iron balance during pregnancy a mother requires at least 300 mg of iron stores at the time of start of pregnancy if she consumes a diet rich in bioavailable iron, and would require obligatory supplementation if she consumes a suboptimal diet [22]. Majority of iron transfer to the fetus occurs during the second and third trimester. The average daily requirement of iron has been calculated as 0.8 mg/d in the first trimester and increases to 7.5 mg/day in the third trimester. The average daily absorption form a western diet is 1–5 mg/day and average daily absorption form Indian diet varies from 0.8 mg/d to 4.5 mg/d depending on the type of staple used [23].
Table 3.
Approximate iron demands during a normal pregnancy and delivery [22]
| Gross iron demands | |
|---|---|
| Obligatory Iron Loss (0.8mg × 290 days) | 230 mg |
| Increase in red cell mass | 450 mg |
| Newborn baby (birth weight 3.5 kg) | 270 mg |
| Placenta and umbilical cord | 90 mg |
| Blood loss at delivery | 200 mg |
| Total gross | 1240 mg |
| Net Iron demands | |
| Absence of menstruation in pregnancy | − 160 mg |
| Post partum decrease in red cell mass | − 450 mg |
| Total net iron demands | 630 mg |
Diagnosing Iron Deficiency and Iron Deficiency Anemia in Pregnancy
Most guidelines recommend screening for anemia during pregnancy in the first trimester (or at booking) followed by 24–28 weeks and at 36 weeks of gestation [24]. The cut off values defined by WHO/CDC for anemia in pregnancy along with peripheral smear showing normal morphology of RBC with central pallor have often been taken as criteria for defining the physiologic anemia of pregnancy. A deviation from the above parameters should often be treated as pathologic and warrant further testing for the etiology and appropriate management of anemia during pregnancy [25].
History and Clinical Examination
Although known to be less sensitive than laboratory assessment, a history for fatigue, alopecia, pica, restless leg syndrome, pagophagia and a brief examination for pallor, koilonychia, atrophic tongue papillae, glossitis and stomatitis should be undertaken for all pregnant women. Severe cases may manifest with evidence of congestive cardiac failure such as orthopnea, edema, raised Jugular Venous Pulse and pulmonary crepts and would merit urgent treatment [26].
The RBC Traits
RBC indices and morphology characteristics are readily available and are recommended as the first step in the evaluation of pregnancy associated anemia.
Mean Corpuscular Volume (MCV) and Red Cell Distribution Width (RDW)
IDA is characterized by microcytosis, (lowMCV < 80 fl) and hypochromia (Mean Corpuscular Hemoglobin {MCH} < 27 pg) and blood film may confirm characteristic microcytic cells or pencil cells [27].
While IDA is the commonest cause for decreased MCV, low MCV is insensitive and up to 40% of pregnant women with true IDA have normocytic indices. Stimulation of erythropoiesis in pregnancy masks the microcytosis of iron deficiency. Moreover, a low MCV, is not specific, for IDA [28–30].
In settings where detailed biochemical evaluation for iron profile is not feasible, a combination of low MCV accompanied by elevated RDW can be used as a sufficient evidence to start iron therapy. A subsequent marked RDW increase occurring early after the initiation of therapy can be used as a surrogate for confirmation of IDA [30]. Sensitivity and specificity of RDW in the diagnosis of IDA in pregnancy has been reported to be between 72–97 and 82–83% respectively [29, 31].
Newer RBC Parameters
Advanced red blood cell and reticulocyte indices such as percentage hypochromic reticulocytes (%HYPOr), Reticulocyte Hemoglobin content (CHr), and percentage circulating microcytes (%HYPOm) are established markers of iron deficient erythropoiesis and are measurable with modern automated analysers. They have also been proven as earlier markers of response to iron therapy as compared to MCV and can be easily incorporated for diagnosing and monitoring therapy in IDA in pregnancy [32]. Further studies are required for validating the use of these parameters in pregnancy [33].
Table 4 gives the utility and the normal reference ranges for the newer RBC parameters and Table 5 summarizes the interpretation of various iron parameters.
Table 4.
Newer RBC parameters for IDA
| Normal range | Clinical utility | |
|---|---|---|
| %HypoM [32] | < 3.4% | Indicator of IDA |
| %HypoR [32] | < 3.7% | Indicator of IDA |
| CHr [32] | < 25 pg | Measures functional iron available over 3–4 days, early indicator of IDA and response to iron therapy |
| M/H [34] | < 3.7 | Differentiating IDA from Beta Thalassemia trait |
%HypoM %Hypochromic microcytes; %HypoR %Hypochromic reticulocytes; CHr reticulocyte hemoglobin content; M/H Microcytic RBC%/Hypochromic RBC%
Table 5.
Laboratory parameters to diagnose iron status [27]
| ID | IDA | AOCD | ID and AOCD | Normal value (adult female) | |
|---|---|---|---|---|---|
| Serum Iron | ↓ | ↓ | ↓ | ↓ | 10–30 μM/L |
| Tsat% | > 16 | < 16 | N/↓ | N/↓ | > 16 to < 45 |
| Ferritin (μg/dl) | < 30 | < 12 | > 100 | <100 | 20–200 |
| sTFR | ↑ | ↑ | N/↑ | Variable | ↑ |
| sTFR/log ferritin | NA | > 2 | < 1 | > 2 | |
| ZPP | N | ↑ | ↑ | ↑ | ↑ |
| Serum hepcidin | ↓ | ↓ | ↑ | N/↑ | ↑ |
ID iron deficiency; IDA iron deficiency anemia; AOCD anemia of chronic disease; N indicates normal; T sat transferrin saturation; sTFR soluble transferrin receptor ZPP, Zn Protoporphyrin
Serum Ferritin
While bone marrow stainable iron stores is the gold standard for diagnosing IDA, the test is invasive and impractical to use during pregnancy [35]. Serum ferritin is a more sensitive and specific marker for ID than serum iron, transferrin saturation (Tsat), and erythrocyte protoporphyrin values [36]. Currently low serum ferritin values are regarded as the best test for confirmation of iron deficiency in pregnancy [37]. During pregnancy, in women with adequate iron stores, serum ferritin initially rises and later gradually falls to about 50% of pre pregnancy levels by 32 weeks (due to hemodilution), followed by a slight rise in the third trimester [38]. There is considerable debate in serum ferritin thresholds used to define ID in pregnancy. One of the studies suggested a pre pregnancy cut off < 70μg/dl to be predicative of development of IDA in pregnancy [39]. When compared to bone marrow iron stores a threshold of < 12μg/dl is only 25% sensitive to diagnose ID, a cut off of < 30μg/dl is 92% specific and 98% specific in diagnosing ID [40]. As non anemic iron deficiency (NAID) is also known to affect feto-maternal outcome, most obstetricians and hematologists recommend a cut off 30 μg/dl to diagnose and treat ID in pregnancy [41, 42]. However, this higher threshold is bound to increase the socio-economic burden of policy makers in resource limited countries like India.
Diagnosing ID in the Setting of Inflammation
In the setting of inflammation (e.g. post-operative state) or infection during pregnancy, serum ferritin may be falsely elevated concomitant with CRP levels [43]. Serum ferritin levels also exhibit a post-partum rise consequent to inflammation and are no longer representative of iron status [44]. There is no gold standard test that diagnoses ID in the setting of inflammation. A low Tsat < 16% in the setting of low ferritin (empirically set at < 100μg/dl) or the ratio between soluble transferrin receptor (sTFR) and log ferritin levels, may be used in this setting [26, 45]. As the sTFR levels are not influenced by the post partum inflammatory state, they can be used in diagnosing IDA in early puerperal phase or when there is unexplained anemia with high CRP levels [46].
Indications of Serum Ferritin Testing
Routine screening with serum ferritin in patients with pregnancy associated anemia is not yet proven to be a cost-effective strategy to diagnose IDA [47]. Table 6 summarizes indications of serum ferritin testing for pregnancy associated anemia in resource constraint settings.
Table 6.
| 1. Prior to starting iron therapy in therapeutic doses in patients with known hemoglobinopathy |
| 2. When an alternative etiology of microcytic anemia is being considered (chronic inflammation, lead toxicity, sideroblastic anemia) |
| 3. Suboptimal response to oral iron when compliance is doubtful |
| 4. In non-anemic women at increased risk of iron depletion, such as those with previous anaemia, multiple pregnancy, teenage pregnancy, pregnancy with high risk of bleeding, consecutive pregnancies with less than a year’s interval |
| 5. After 8 weeks of therapeutic iron therapy when non anemia iron deficiency is being treated (i.e. serum ferritin < 30 ug/dl without anemia) |
| 6. Preferably prior to parenteral iron therapy to confirm iron deficiency |
Other Iron Parameters
Serum iron shows diurnal variation and is affected by dietary influences [48]. Pregnancy itself increases total iron binding capacity (TIBC) even in the absence of IDA. Thus serum iron and TIBC are unreliable markers to be used in pregnancy [30]. Tsat values also fluctuate with diurnal variation and nutritional status and are therefore unreliable [49]. It is recommended that samples for measurement of serum iron, Tsat and TIBC be drawn after an overnight fast which may be frequently impracticable in pregnancy. The role of serum hepcidin for diagnosing pregnancy associated ID is an area of active research [50]. Serum Zinc Protoporphyrin (ZPP) increases with ID. Its levels are not influenced by the pregnancy associated plasma dilution and are less affected by inflammation and infection. However the test is rarely available at most centers [51]. Table 5 describes the differential diagnosis of IDA and other microcytic anemias in pregnancy.
Diagnosing the Etiology of IDA in Pregnancy
A history of pre-pregnancy menorrhagia, pre-pregnancy Hb, frequent child births, passage of worms and gastrointestinal blood loss should be taken in pregnant patients presenting with anemia. As the etiology of IDA in pregnancy often reflects a mismatch between the supply and demand of iron, we do not recommend stool testing for occult blood, worm infestation, GI endoscopies or screening for celiac disease unless specifically indicated [52].
Preventing IDA in Pregnancy
Iron Prophylaxis and Controversies
Effective communication to all pregnant women about diet and nutrition is an integral part of preventing anemia in pregnancy. However, as the extra demand of iron is often unmet by a routine diet, regular iron supplementation is recommended by most experts during pregnancy. Recommendations for supplementation of iron vary from region to region, the CDC recommends that all pregnant women begin a 30 mg per day iron supplement at the first prenatal visit [53]. WHO suggests 30–60 mg per day of elemental iron for all pregnant women whereas British guidelines do not recommend any routine iron supplementation in pregnancy [35, 54]. In populations with an anemia prevalence among pregnant women of less than 20% WHO recommends, intermittent oral iron and folic acid supplementation with 120 mg of elemental iron and 2.8 mg of folic acid once weekly for pregnant women to improve maternal and neonatal outcomes [35, 54]. The equivalent of 60 mg of elemental iron is 300 mg ferrous sulfate heptahydrate, 180 mg ferrous fumarate or 500 mg of ferrous gluconate [55]. In a recent Cochrane review that analysed feto-maternal outcome in women receiving intermittent versus daily iron supplementation, authors concluded that women receiving intermittent iron (80–300 mg of elemental iron per week) had similar risk of anemia at term, premature delivery, low birth weight with fewer side effects and lesser risk of high Hb concentration at term, although the quality of evidence was graded as low to very low [56]. Accordingly, WHO added a context specific recommendation for once weekly intermittent oral iron supplementation when daily iron is not acceptable due to gastrointestinal side effects [54]. Before commencing the intermittent regime, accurate measurements of maternal Hb are required to confirm the absence of anemia and if a woman is diagnosed with anemia she should receive 120 mg of elemental iron and 0.4 mg of folic daily till anemia is corrected. Thereafter she can continue with standard daily regime or intermittent regime as per tolerability [54]. National iron plus initiative recommends iron folic acid [IFA] supplementation of 100 mg elemental iron and 500 μg of folic acid every day for at least 100 days starting after the first trimester at 14–16 weeks of gestation for all non-anemic pregnant women followed by the same dose for 100 days postpartum [57].
Dietary Recommendations
The Recommended Dietary Allowance (RDA) of iron in third trimester is 30 mg/day. The average iron density in an average Indian diet is 8.5 mg/1000 KCal and the average iron absorption from a rice based and wheat based Indian diet in pregnancy is 13.3 and 5.3% respectively [22, 23]. Elaborate recommendations on increasing iron intake in diet during pregnancy are available [35, 54]. Dietary modifications are cheap and culturally acceptable. However, considering the Indian food practices, dietary modifications are likely to be inadequate and most pregnant women require supplementary iron. Supplement 1 gives the list of drugs and dietary products that interfere with iron absorption. Smart phone applications can guide women on their day to day iron needs, iron content of various foods and help them monitor their dietary iron intake. We encourage the development and use of such applications adapted for Indian settings.
Role of Deworming
WHO recommends routine deworming using single dose Albendazole (400 mg) or mebendazole (500 mg) in all pregnant patients after first trimester in areas where both (1) baseline prevalence of hookworm/Trichuris Trichura infection is > 20% and (2) prevalence of anemia in pregnant women is > 40% and is therefore applicable to India. Infected women in non endemic areas should receive anti-helmenthic treatment on a case to case basis. The safety of anti-helmenthic agents in pregnancy have not been unequivocally established however deworming is advocated in regions where benefits outweigh the risks [54].
Treatment of Anemia
A recent Cochrane review failed to show clear benefit of treating IDA in pregnancy despite concrete evidence of ID on adverse fetomaternal outcome [18]. In concordance with most international guidelines we recommend treating IDA in pregnancy [35, 54].
Management as per the Trimester/Postpartum State
The mode of repletion of iron stores in IDA of pregnancy is guided by the severity of anemia, the stage of pregnancy, obstetric risks of hemorrhage (e.g. premature labor, placenta praevia) and non-obstetric maternal comorbidities (hemoglobinopathy, chronic disease etc.). The flowchart depicts our approach to managing IDA in pregnancy depending on the stage and response (Figure 2).
Fig. 2.
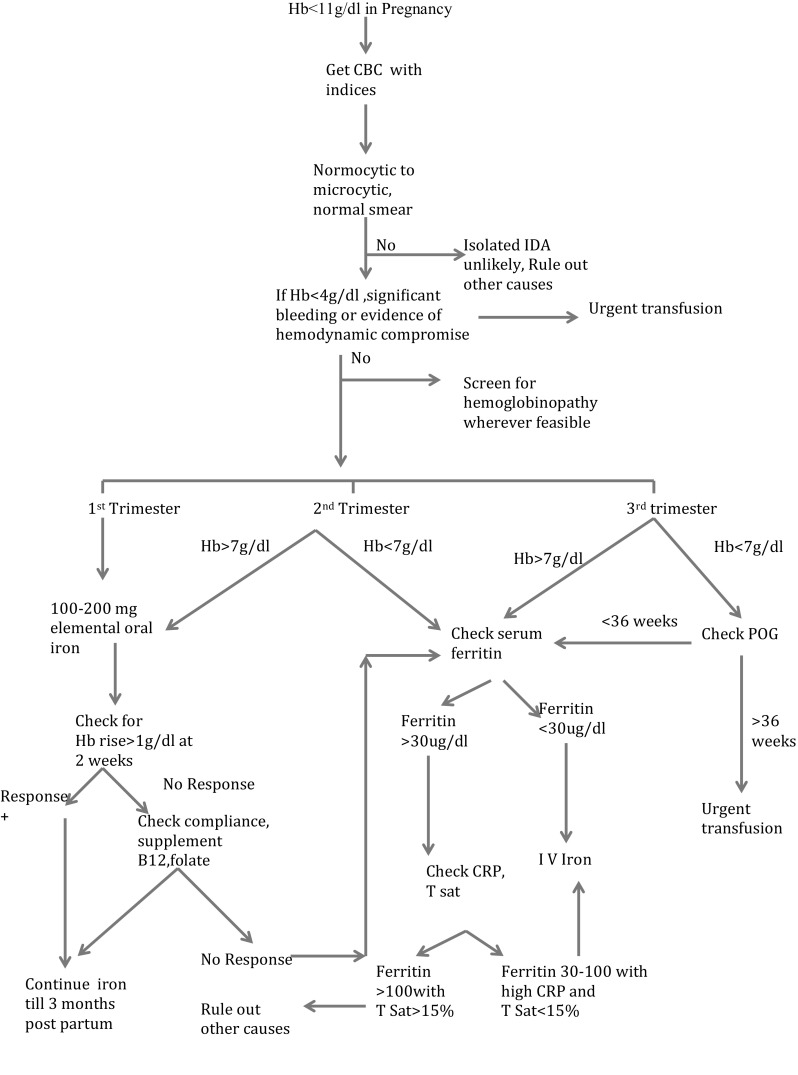
Approach to IDA in pregnancy. Hb hemoglobin; IDA iron deficiency anemia; CRP C reactive protein; POG period of gestation; TSat transferrin saturation; IV intravenous
Oral Iron
Dependent on the type of preparation only 1–8% of iron is absorbed from the available oral iron preparations. There are considerable controversies regarding the optimal frequency, dose and type of oral iron preparation to be used. The absorption of oral iron increases with increasing doses of oral iron only up to 160 mg/day [37]. Accordingly, recommended dose of elemental iron for treating IDA in pregnancy is between 100 and 200 mg/day in the British guidelines and 120 mg/d in the WHO guidelines [35, 54]. Increasing dose beyond this dose leads to increased gastrointestinal side effects without improving the efficacy. Recent data on hepcidin kinetics comparing once daily with twice daily and thrice daily iron administration show little added benefit of twice or thrice daily dosing and suggest an equivalent iron absorption even with alternate day iron dosing [58, 59]. Considering the increased frequency of GI side effects in pregnancy [60] these data become relevant and require further investigation in pregnancy setting. Multiple studies comparing various oral preparations do not show conclusive evidence of superiority of one iron preparation over the other. We recommend avoiding the use of enteric coated and delayed release preparations as they have proven poor bioavailability [61]. All pregnant women should be instructed to take oral iron empty stomach or 1 h after meals for better absorption preferably with vitamin C rich product such as orange juice or guava [62]. Supplement 2 enlists the available oral iron preparations for use in pregnancy.
Management of Gastrointestinal (GI) Side Effects of Oral Iron
In general Ferric salts (III) have a superior GI tolerability than Ferrous (II) salts at the cost of reduced iron absorption [63]. Gastrointestinal side effects (nausea, constipation, diarrhea, indigestion, and metallic taste) are reported in 70% of pregnant patients owing to the progesterone induced decreased GI motility and effect of gravid uterus. Twenty percent of women stop iron irrespective of iron preparation. Few patients adhere to the prescribed duration of 3–6 months [64, 65]. Measures such as reducing the frequency, content of oral iron and changing it to an alternative preparation or taking the iron with meals may be employed to reduce GI side effects [35].
Parenteral Iron Therapy
Intramuscular (IM) Iron
The Ministry of Health and Family Welfare (MoHFW) guidelines for treatment of IDA in pregnancy continue to recommend IM iron following a test dose as a cost-effective treatment for moderate anemia in pregnancy [57]. However the intramuscular route has essentially been replaced by intravenous route because of the inconvenience of painful injection, dark discoloration of the skin, and the risk of myalgias, arthritis, hypersensitivity, lymphadenopathy at most centers [66]. Moreover, there is increased risk of development of sarcoma at the site of injection in treated animals [27]. Low molecular weight iron dextran is the only preparation which can be recommended for intra muscular use in primary care settings with a Z technique if resuscitation facilities are available [57].
Intravenous iron
Intravenous (IV) iron combines the advantages of complete bioavailability with fewer GI side effects and faster recovery of Hb than oral iron. However the increased risk of oxidant damage, increased cost and small but finite risk of hypersensitivity reaction limit the widespread use of IV iron [67]. The odds ratio/overall risk (OR) of reported total absolute rates of adverse life-threatening events with parenteral iron is 38 per million doses, predominantly with high molecular weight iron dextran [68]. Thus, while the use of high-molecular iron dextran is no longer justified, numerous other iron preparations have been proven to be safe in pregnancy. One of the previous disadvantage of IV iron was the requirement of multiple infusions. This has been circumvented by the newer preparations like iron-isomaltoside and iron carboxymaltose which allow larger infusion doses of elemental iron to be administered over a short period of time [37, 70]. Supplement 3 summarizes the various IV iron preparations available in Indian market, with their infusion time and dose (Table 7).
Table 7.
| Indications | Contraindications |
|---|---|
| 1. Failure to oral iron therapy | 1. Lack of facilities for resuscitation |
| 2. Non-compliance or intolerance to oral iron | 2. Known history of anaphylaxis or reactions to parenteral iron |
| 3. Late second or third trimester with moderate to severe IDA | 3. Gestation period < 12 weeks |
| 4. Malabsorption (e.g. Bowerl-resection/Celiac disease) | 4. Known state of iron overload |
| 5. Bleeding diathesis when hemorrhage is likely to continue | |
| 6. In combination with recombinant erythropoietin patients with pregnancy and chronic disease | |
| 7. Moderate to severe post partum anemia when compliance to oral iron and follow up to health care facility is doubtful |
IDA iron deficiency anemia
We recommend that parenteral iron should always be administered once ID is confirmed using serum ferritin or other specific investigations, after an informed consent at a center where resuscitation facilities are available. Vitals should be checked periodically during and at the end of infusion by a physician, nurse or trained mid wife. A test dose is required only for LMW Iron dextran while other parenteral iron preparations do not require test dosing. Patient should be explained about the transient side effects of IV iron supplementation include nausea, vomiting, pruritus, headache, and flushing; myalgia, arthralgia, and back and chest pain that usually resolve within 48 h of infusion [27].
Ganzoni's equation is the standard formula used for calculating the dose of par enteral iron in pregnancy is [69]:
In practice the dose can be titrated according to the available iron stores, history of ongoing blood loss and hemoglobin response.
Assessing Response to iron
Increase in reticulocyte Hb content (CHr) is the earliest marker of response as early as 3 days.It requires validation in pregnancy and is not widely available currently [33]. A rise in hemoglobin by 1 g/dl is expected at the end of 2 weeks and by 2 g/dl by the end of 4 weeks in the absence of other micronutrient deficiencies and ongoing blood loss for patients on oral iron [35, 37]. A suboptimal rise is an indication for checking compliance, reconfirmation of diagnosis and consideration for parenteral iron therapy. Once the Hb is in normal range, 100–200 mg/day of iron should continue for at least 3 months and at least 6 weeks postpartum to replenish the stores and 60–100 mg/d oral iron should continue for at least 3–6 months postpartum [26].
Postpartum Anemia
Postpartum anemia is associated with poor quality of life (QOL) and increased rates of depression in women [70]. Routine postpartum prophylaxis by WHO recommended doses of 60 mg/d for three months or MoHFW doses of 100 mg/d to non-anemic women for 6 months has been shown to be cost effective in decreasing the rates of anemia in our country. It should be reinforced at the time of discharge from health care facility to the lactating mother [57]. Hemoglobin should be checked in all women where estimated blood loss is > 500 ml within 48 h of delivery or in women known to have antepartum anemia or having symptoms of anemia [71]. While mildly symptomatic women can be treated with therapeutic oral iron at the dose of 100–200 mg/d for next 3 months, IV iron can be considered in moderate anemia [72]. In case of severe anemia or evidence of hemodynamic comprise patients should receive transfusion prior to discharge from health facility. Wherever feasible, a follow up CBC with serum ferritin should be considered before discontinuation of iron therapy at 3 to 6 months.
Role of Erythropoietin
Recombinant human erythropoietin (RhuEPO) has been shown to be safe and effective for the rapid correction of severe peripartum anemia in conjunction with IV iron, particularly in cases with antepartum and postpartum hemorrhage and patients with rare blood groups. However currently there is insufficient evidence for routine use of EPO in pregnancy except in cases with renal disease [73].
Role of Transfusion
Transfusion in pregnancy carries additional risks of RBC allo-immunization, volume overload and fetal hemolytic disease. The current AABB and the RCOG guidelines suggest a threshold of Hb < 7 g/dl for transfusion and a threshold of < 8 g/dl in patients with pre-existing cardiovascular disease [74, 75]. However in obstetrics, the decision of transfusion should be individualized depending on available alternatives of oral and parenteral iron, present and future risk of hemorrhage, comorbidities like DIC, thrombocytopenia, acuteness of fall in Hb and cardiovascular status. Partial exchange transfusion has not been shown to be superior to transfusion under diuretic cover for patients who present with severe anemia and congestive cardiac failure at term [76]. We do not recommend the routine use of exchange transfusion except for patients with sickle cell disease. Table 8 summarizes the indications for transfusion in case of ID.
Table 8.
| Antepartum period | Intrapartum period | Post partum period |
|---|---|---|
| 1. Pregnancy < 36 weeks a. Hb < 4 g/dL with or without signs of cardiac failure or hypoxia b. 5–7 g/dL with presence of impending heart failure,hemodynamic instability or acute hemorrhage 2. Pregnancy > 36 weeks a. Hb < 7 g/dL even without signs of cardiac failure or hypoxia b. Severe anemia with decompensation or acute hemorrhage with decompensation c. Hemoglobinopathy/Bone marrow failure syndromes or malignancy |
a. Hb < 7 g/dL[in labor] [Decision of blood transfusion depends on medical history or symptoms] b. Severe anemia with decompensation or acute hemorrhage with decompensation |
a. Anemia with signs of shock/acute hemorrhage with signs of hemodynamic instability b. Hb < 7gm %:Decision of transfusion depends on medical history or symptoms |
Management of Labor in Patients with Anemia
Increasing use of regional anaesthesia, use of upright position during delivery, manual removal of placenta and episiotomy lead to heavier blood loss even in modern obstetrics [12]. All patients with anemia should preferably undergo delivery where facility of blood transfusion and intra venous access are available. Cross matched blood units should be kept reserved in patients with moderate to severe anemia undergoing delivery. In all anemic patients with pregnancy active management of third stage of labor with the use of syntocin or misoprostol is effective in reducing the blood loss and should be practiced [79].
Conclusions and Future Vision
IDA in pregnancy is readily manageable yet an unmet health demand. The management strategy is dependent upon the period of gestation and severity of anemia. Widespread implementation of preventive and therapeutic strategies is still lacking in our country. Organization of awareness camps, patient group meetings and the use of social media can spread awareness of this public health issue. We strongly advocate observation of “National Anemia Awareness and Treatment Day” with countrywide participation of health care personnel to target the vulnerable groups specially the pregnant women and teenagers of the country. The day can be used as a platform not only to disseminate knowledge regarding the consequences of anemia on feto-maternal and adolescent health but also to deliver cheap and easy treatment options available for the same. We are hopeful that using a pragmatic approach that combines the recent scientific knowledge with an empathetic attitude of medical fraternity to tackle this problem, the country can achieve the WHO 2025 global nutrition target of fifty percent reduction in prevalence of anemia in reproductive age group [9].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not involve any study with participation with human participants or animals by the authors.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12288-018-0949-6) contains supplementary material, which is available to authorized users.
References
- 1.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107(5):1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC (1989) CDC criteria for anemia in children and childbearing-aged women. MMWR Morbidity and mortality weekly report. 138(22):400–404 [PubMed]
- 4.WHO (2001) Iron deficiency anemia: assessment, prevention and control. WHO/NHD/01.3, Geneva. World Health Organization, Switzerland
- 5.WHO (2011) VMNIS. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System, WHO, Geneva, World Health Organisation, Switerzerland
- 6.Daru J, Cooper NA, Khan KS. Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy. Acta Obstet Gynecol Scand. 2016;95(3):270–279. doi: 10.1111/aogs.12812. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra P, Kumari S, Kumar R, et al. Prevalence of anemia in adult rural population of North India. J Assoc Phys India. 2004;52:18–20. [PubMed] [Google Scholar]
- 8.Ministry of Health and Family Welfare (2015–2016) Govt. of India, National Family Health Survey (NFHS-4), State Fact Sheet. Mumbai:International Institute for Population Sciences
- 9.Haddad L, Hawkes C, Udomkesmalee E, et al. Global Nutrition Report 2016: From Promise to Impact: Ending Malnutrition by 2030. Washington: International Food Policy Research Institute; 2016. [Google Scholar]
- 10.Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495–504. doi: 10.3945/ajcn.115.107896. [DOI] [PubMed] [Google Scholar]
- 11.Murray-Kolb L (2012) Maternal mortality, child mortality, perinatal mortality, child cognition, and estimates of prevalence of anemia due to iron deficiency. CHERG
- 12.Breymann C. Iron deficiency anemia in pregnancy. Semin Hematol. 2015;52(4):339–347. doi: 10.1053/j.seminhematol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Milman N. Anemia–still a major health problem in many parts of the world! Ann Hematol. 2011;90(4):369–377. doi: 10.1007/s00277-010-1144-5. [DOI] [PubMed] [Google Scholar]
- 14.Geng F, Mai X, Zhan J, et al. Impact of fetal-neonatal iron deficiency on recognition memory at 2 months of age. J Pediatr. 2015;167(6):1226–1232. doi: 10.1016/j.jpeds.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Congdon EL, Westerlund A, Algarin CR, et al. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatr. 2012;160(6):1027–1033. doi: 10.1016/j.jpeds.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milman N, Agger AO, Nielsen OJ. Iron status markers and serum erythropoietin in 120 mothers and newborn infants. Effect of iron supplementation in normal pregnancy. Acta Obstet Gynecol Scand. 1994;73(3):200–204. doi: 10.3109/00016349409023439. [DOI] [PubMed] [Google Scholar]
- 17.Arnold DL, Williams MA, Miller RS, et al. Iron deficiency anemia, cigarette smoking and risk of abruptio placentae. J Obstetr Gynaecol Res. 2009;35(3):446–452. doi: 10.1111/j.1447-0756.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 18.Reveiz L, Gyte GM, Cuervo LG, et al. Treatments for iron-deficiency anaemia in pregnancy. The Cochrane database of systematic reviews. 2011;2(10):3094. doi: 10.1002/14651858.CD003094.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Goshtasebi A, Alizadeh M, Gandevani SB. Association between maternal anaemia and postpartum depression in an Urban sample of pregnant women in Iran. J Health Popul Nutr. 2013;31(3):398–402. doi: 10.3329/jhpn.v31i3.16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gambling L, Lang C, McArdle HJ. Fetal regulation of iron transport during pregnancy. Am J Clin Nutr. 2011;94(6 Suppl):1903s–1907s. doi: 10.3945/ajcn.110.000885. [DOI] [PubMed] [Google Scholar]
- 21.Insel BJ, Schaefer CA, McKeague IW, et al. Maternal iron deficiency and the risk of Schizophrenia in offspring. Arch Gen Psychiatry. 2008;65(10):1136–1144. doi: 10.1001/archpsyc.65.10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 Suppl):257s–264s. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- 23.Rammohan A, Awofeso N, Robitaille M-C (2011) Addressing female iron-deficiency anaemia in india: is vegetarianism the major obstacle? ISRN Public Health 2012
- 24.McDonagh M, Cantor A, Bougatsos C et al (2015) U.S. preventive services task force evidence syntheses, formerly systematic evidence reviews. routine iron supplementation and screening for iron deficiency anemia in pregnant women: a systematic review to update the US preventive services task force recommendation. Agency for Healthcare Research and Quality (US), Rockville [PubMed]
- 25.Sharma JB, Shankar M. Anaemia in pregnancy. J Int Med Sci Acad. 2010;23:253–60. [Google Scholar]
- 26.Camaschella C. Iron-deficiency anemia. New Engl J Med. 2015;372(19):1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 27.Camaschella C. Iron deficiency: new insights into diagnosis and treatment. Hematol Am Soc Hematol Educ Program. 2015;2015:8–13. doi: 10.1182/asheducation-2015.1.8. [DOI] [PubMed] [Google Scholar]
- 28.Bermejo F, García-López S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol WJG. 2009;15(37):4638–4643. doi: 10.3748/wjg.15.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sultana GS, Haque SA, Sultana T, et al. Value of red cell distribution width (RDW) and RBC indices in the detection of iron deficiency anemia. Mymensingh Med. 2013;22:370–376. [PubMed] [Google Scholar]
- 30.Van den Broek NR, Letsky EA, White SA, et al. Iron status in pregnant women: which measurements are valid? Br J Haematol. 1998;103(3):817–824. doi: 10.1046/j.1365-2141.1998.01035.x. [DOI] [PubMed] [Google Scholar]
- 31.Tiwari M, Kotwal J, Kotwal A, et al. Correlation of haemoglobin and red cell indices with serum ferritin in Indian women in second and third trimester of pregnancy. Medical Journal Armed Forces India. 2013;69:31–36. doi: 10.1016/j.mjafi.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ervasti M, Kotisaari S, Heinonen S, et al. Use of advanced red blood cell and reticulocyte indices improves the accuracy in diagnosing iron deficiency in pregnant women at term. Eur J Haematol. 2007;79(6):539–545. doi: 10.1111/j.1600-0609.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- 33.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 34.Saleem M, Qureshi TZ, Anwar M, et al. Evaluation of M/H ratio for screening of beta thalassaemia trait. J Pak Med Assoc. 1995;45:84–86. [PubMed] [Google Scholar]
- 35.Pavord S, Myers B, Robinson S, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2012;156(5):588–600. doi: 10.1111/j.1365-2141.2011.09012.x. [DOI] [PubMed] [Google Scholar]
- 36.Romslo I, Haram K, Sagen N, et al. Iron requirement in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. Br J Obstet Gynaecol. 1983;90:101–7. doi: 10.1111/j.1471-0528.1983.tb08891.x. [DOI] [PubMed] [Google Scholar]
- 37.Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin, and folate. Blood. 2017;129(8):940–949. doi: 10.1182/blood-2016-08-672246. [DOI] [PubMed] [Google Scholar]
- 38.Volpi E, De Grandis T, Alba E, et al. Variations in ferritin levels in blood during physiological pregnancy. Minerva Ginecol. 1991;43(9):387–391. [PubMed] [Google Scholar]
- 39.Milman N, Agger AO, Nielsen OJ. Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan Med Bull. 1991;38(6):471–476. [PubMed] [Google Scholar]
- 40.Mast AE, Blinder MA, Gronowski AM, et al. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44(1):45–51. [PubMed] [Google Scholar]
- 41.Ayub R, Tariq N, Adil MM, et al. Efficacy and safety of total dose infusion of low molecular weight iron dextran in the treatment of iron deficiency anemia during pregnancy. J Coll Phys Surg-Pak. 2008;18:424–427. [PubMed] [Google Scholar]
- 42.Daru J, Moores R, Dodds J, et al. Non-anaemic iron deficiency in pregnancy: the views of health service users and health care professionals. Transfusion Medicine. 2015;25:27–32. doi: 10.1111/tme.12184. [DOI] [PubMed] [Google Scholar]
- 43.Lee AI, Okam MM. Anemia in pregnancy. Hematol/Oncol Clin North Am. 2011;25(2):241–259. doi: 10.1016/j.hoc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Krafft A, Huch R, Breymann C. Impact of parturition on iron status in nonanaemic iron deficiency. Eur J Clin Invest. 2003;33(10):919–923. doi: 10.1046/j.1365-2362.2003.01244.x. [DOI] [PubMed] [Google Scholar]
- 45.Weiss G, Goodnough LT. Anemia of chronic disease. New Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 46.Choi JW, Im MW, Pai SH. Serum transferrin receptor concentrations during normal pregnancy. Clin Chem. 2000;46(5):725–727. [PubMed] [Google Scholar]
- 47.Nosratnejad S, Barfar E, Hosseini H, et al. Cost-effectiveness of anemia screening in vulnerable groups: a systematic review. Int J Prev Med. 2014;5(7):813–819. [PMC free article] [PubMed] [Google Scholar]
- 48.Tietz NW, Rinker AD, Morrison SR. When is a serum iron really a serum iron? the status of serum iron measurements. Clin Chem. 1994;40(4):546–551. [PubMed] [Google Scholar]
- 49.Adams PC, Reboussin DM, Press RD, et al. Biological variability of transferrin saturation and unsaturated iron-binding capacity. Am J Med. 2007;120(11):999.e1-7. doi: 10.1016/j.amjmed.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bah A, Wegmuller R, Cerami C, et al. A double blind randomised controlled trial comparing standard dose of iron supplementation for pregnant women with two screen-and-treat approaches using hepcidin as a biomarker for ready and safe to receive iron. BMC Pregnancy Childbirth. 2016;16(1):157. doi: 10.1186/s12884-016-0934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodlin RC. Role of ferritin supported in diagnosis of anemias of pregnancy. Am J Obstet Gynecol. 1989;161(1):258–259. doi: 10.1016/0002-9378(89)90284-6. [DOI] [PubMed] [Google Scholar]
- 52.Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014;123(3):326–333. doi: 10.1182/blood-2013-10-512624. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (1998) Recommendations to prevent and control iron deficiency in the United States.MMWR Recomm Rep Morb Mortal Week Rep Recomm Rep 47(Rr-3):1–29 [PubMed]
- 54.World Health Organization (2016) WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, World Health Organization, Switzerland [PubMed]
- 55.World Health Organization (2011) Logic model for micronutrient interventions in public health: vitamin and mineral nutrition information system. Geneva, World Health Organization, Switzerland
- 56.Peña-Rosas JP, De-Regil LM, Dowswell T, et al. Intermittent oral iron supplementation during pregnancy (Review) The Cochrane Database of Systematic Reviews. 2012;7:CD009997-CD. doi: 10.1002/14651858.CD009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapil U, Singh Bhadoria A. National iron-plus initiative guidelines for control of iron deficiency anaemia in India, 2013. The National Medical Journal of India. 2014;27:27–9. [PubMed] [Google Scholar]
- 58.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981–1989. doi: 10.1182/blood-2015-05-642223. [DOI] [PubMed] [Google Scholar]
- 59.Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4(11):524–533. doi: 10.1016/S2352-3026(17)30182-5. [DOI] [PubMed] [Google Scholar]
- 60.Ugwu EO, Olibe AO, Obi SN, et al. Determinants of compliance to iron supplementation among pregnant women in Enugu, Southeastern Nigeria. Niger J Clin Pract. 2014;17(5):608–612. doi: 10.4103/1119-3077.141427. [DOI] [PubMed] [Google Scholar]
- 61.Roth DE, Pezzack B, Al Mahmud A, et al. Bioavailability of enteric-coated microencapsulated calcium during pregnancy: a randomized crossover trial in Bangladesh. Am J Clin Nutr. 2014;100(6):1587–1595. doi: 10.3945/ajcn.114.090621. [DOI] [PubMed] [Google Scholar]
- 62.Lloyd C. Medical disorders associated with pregnancy: Myles Textbook for Midwives. 15. Edinburgh: Churchill Livingstone; 2009. pp. 361–396. [Google Scholar]
- 63.Ortiz R, Toblli JE, Romero JD, et al. Efficacy and safety of oral iron (III) polymaltose complex versus ferrous sulfate in pregnant women with iron-deficiency anemia: a multicenter, randomized, controlled study. J Matern-Fetal Neonatal Med. 2011;24(11):1347–1352. doi: 10.3109/14767058.2011.599080. [DOI] [PubMed] [Google Scholar]
- 64.Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117383. doi: 10.1371/journal.pone.0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beard JL. Effectiveness and strategies of iron supplementation during pregnancy. Am J Clin Nutr. 2000;71(5 Suppl):1288s–1294s. doi: 10.1093/ajcn/71.5.1288s. [DOI] [PubMed] [Google Scholar]
- 66.Silverstein SB, Rodgers GM. Parenteral iron therapy options. Am J Hematol. 2004;76(1):74–78. doi: 10.1002/ajh.20056. [DOI] [PubMed] [Google Scholar]
- 67.Aronoff GR. Safety of intravenous iron in clinical practice: implications for anemia management protocols. J Am Soc Nephrol JASN. 2004;15(Suppl 2):S99–S106. doi: 10.1097/01.ASN.0000143815.15433.87. [DOI] [PubMed] [Google Scholar]
- 68.Chertow GM, Mason PD, Vaage-Nilsen O, et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21(2):378–382. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 69.Koch TA, Myers J, Goodnough LT. Intravenous iron therapy in patients with iron deficiency anemia: dosing considerations. Anemia. 2015;2015:763576. doi: 10.1155/2015/763576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atkinson LS, Baxley EG. Postpartum fatigue. Am Fam Physician. 1994;50(1):113–118. [PubMed] [Google Scholar]
- 71.Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol. 2011;90(11):1247–1253. doi: 10.1007/s00277-011-1279-z. [DOI] [PubMed] [Google Scholar]
- 72.Westad S, Backe B, Salvesen KA, et al. A 12-week randomised study comparing intravenous iron sucrose versus oral ferrous sulphate for treatment of postpartum anemia. Acta Obstet Gynecol Scand. 2008;87(9):916–923. doi: 10.1080/00016340802317802. [DOI] [PubMed] [Google Scholar]
- 73.Sienas L, Wong T, Collins R, Smith J. Contemporary uses of erythropoietin in pregnancy: a literature review. Obstet Gynecol Surv. 2013;68(8):594–602. doi: 10.1097/OGX.0b013e3182a2d51c. [DOI] [PubMed] [Google Scholar]
- 74.RCOG (2015) Blood transfusion in obstetrics. RCOG green-top guideline 47. Royal College of Obstetricians and Gynaecologists, London
- 75.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316(19):2025–2035. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 76.Salhan S, Tripathi V, Singh R, et al. Evaluation of hematological parameters in partial exchange and packed cell transfusion in treatment of severe anemia in pregnancy. Anemia. 2012;2012:7. doi: 10.1155/2012/608658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kriplani A, Sharma A, Radhika A.G., et al (2016) FOGSI general recommendations for clinical practice
- 78.IDA Good clinical practice recommendations for iron deficiency anemia in pregnancy (IDA) in pregnancy in India. J Obstetr Gynaecol India. 2011;61(5):569–571. [Google Scholar]
- 79.Rogers J, Wood J, McCandlish R, Ayers S, et al. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial 1998. Lancet. 1998;351:693–9. doi: 10.1016/S0140-6736(97)09409-9. [DOI] [PubMed] [Google Scholar]
- 80.Souza AId, Batista Filho M, Bresani CC et al (2009) Adherence and side effects of three ferrous sulfate treatment regimens on anemic pregnant women in clinical trials. Cadernos de Saúde Pública. 25:1225-1233 [DOI] [PubMed]
- 81.Froessler B, Cocchiaro C, Saadat-Gilani K, et al. Intravenous iron sucrose versus oral iron ferrous sulfate for antenatal and postpartum iron deficiency anemia: a randomized trial. J Matern-fetal Neonatal Med. 2013;26(7):654–659. doi: 10.3109/14767058.2012.746299. [DOI] [PubMed] [Google Scholar]
- 82.Smita S, Sukhija S, Renu T, et al. Pregnancy induced iron deficiency and the evaluation and comparison of the efficacy and safety of ferrous fumarate and carbonyl iron in its treatment. J Obstet Gynecol India. 2009;59(6):552–562. [Google Scholar]
- 83.Melamed N, Ben-Haroush A, Kaplan B, et al. Iron supplementation in pregnancy–does the preparation matter? Arch Gynecol Obstet. 2007;276(6):601–604. doi: 10.1007/s00404-007-0388-3. [DOI] [PubMed] [Google Scholar]
- 84.Panchal PJ, Desai MK (2014) Comparison of efficacy, safety and cost of therapy with oral ferrous ascorbate and ferrous sulphate in patients with iron deficiency anemia. J Drug Discov Ther 2(20)
- 85.Gogineni S, Vemulapalli P. Comparative study of parenteral iron sucrose vs oral ferrous ascorbate for prophylactic iron therapy in pregnancy. IOSR-JDMS. 2015;14(12):95–97. [Google Scholar]
- 86.Fuchs K, Peyser M, Peretz H. Ferrous calcium citrate in the treatment of anemia in pregnancy. Harefuah. 1962;62:88–89. [PubMed] [Google Scholar]
- 87.Holmes JM. Ferrous calcium citrate in pregnancy anaemia. The Practitioner. 1071;1957(179):295–296. [PubMed] [Google Scholar]
- 88.Rajadhyaksha G, Shahani S, Pawar D. Evaluation of efficacy and tolerability of iron polymaltose complex tablets in iron deficiency anaemia during pregnancy. JAMA India. 2000;3:53–55. [Google Scholar]
- 89.Hasan S, Hashim B, Sultana A. Iron therapy in iron deficiency anemia in Pregnancy: intravenous iron sucrose versus oral iron hydroxide polymaltose complex in anemia. Ann Abbasi Shaheed Hosp Karachi Med Dent Coll Dec. 2003;8(2):435–440. [Google Scholar]
- 90.Singhal SR, Kadian V, Singh S, et al. Comparison of various oral iron salts in the treatment of iron deficiency anemia in pregnancy. Indian J Obstetr Gynaecol Res. 2015;2(3):155–158. [Google Scholar]
- 91.Milman N, Jønsson L, Dyre P, et al. Ferrous bisglycinate 25 mg iron is as effective as ferrous sulfate 50 mg iron in the prophylaxis of iron deficiency and anemia during pregnancy in a randomized trial. J Perinat Med. 2014;42(2):197–206. doi: 10.1515/jpm-2013-0153. [DOI] [PubMed] [Google Scholar]
- 92.Morales-Borges R. Anemia in pregnancy and parenteral iron therapy. J Blood Disord Transf. 2013;4(171):2. [Google Scholar]
- 93.Al RA, Unlubilgin E, Kandemir O, Yalvac S, Cakir L, Haberal A. Intravenous versus oral iron for treatment of anemia in pregnancy: a randomized trial. Obstet Gynecol. 2005;106(6):1335–1340. doi: 10.1097/01.AOG.0000185260.82466.b4. [DOI] [PubMed] [Google Scholar]
- 94.Myers B, Myers O, Moore J. Comparative efficacy and safety of intravenous ferric carboxymaltose (Ferinject) and iron(III) hydroxide dextran (Cosmofer) in pregnancy. Obstetric Med. 2012;5(3):105–107. doi: 10.1258/om.2012.110095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schneider J, Krafft A, Manconi M, Hubner A, Baumann C, Werth E, et al. Open-label study of the efficacy and safety of intravenous ferric carboxymaltose in pregnant women with restless legs syndrome. Sleep Med. 2015;16(11):1342–1347. doi: 10.1016/j.sleep.2015.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.