Abstract
Fungi are critical organisms for the environment and offer many benefits to modern society through their application in the pharmaceutical, beverage and food industries. In contrast, fungal pathogens are emerging threats to humans, animals, plants and insects with potential to cause devastating mortality, morbidity and economic loss. Outbreaks associated with anthropogenic alterations of the environment, including climate change-related events such as natural disasters, are responsible for human, animal and plant disease. Similarly, fungi and their metabolites also have a negative impact in agriculture, posing a serious threat to our food supplies. Here, we describe the existing knowledge and importance of understanding the relationship of fungi and the environment in the context of human, animal and plant disease. Our goal is to encourage communication between scientists and the general public to create informed awareness about the impact of fungi in their daily lives and their environment.
Keywords: animals, fungi, humans, Infection, natural disasters, plants
Introduction
Fungi are ubiquitous eukaryotic microorganisms found in the environment in association with soil, animals, faeces, water, plant debris or other surfaces. Fungi can be either single-celled microorganisms such as yeasts, or complex multicellular organisms visible to the naked eye, like filamentous moulds (surface moulds) and macroscopic fungi (mushrooms and toadstools). Having a predominant role in organic saprotrophy, fungal decomposition ensures the continuation of biogeochemical nutrient and energy cycles that are fundamental to sustain life. In addition to obtaining nutrients from decomposition of non-living organic matter, fungi can be pathogenic [1], leading to illness in diverse hosts. Thus, the extent of their impact on the environment, economy and human health is considerable.
The widespread domestication of wheat approximately 10 000 years ago participated in the transition of human behaviour from nomadic hunter-gatherer to farmer, resulting in the formation of settlements and eventually leading to increasingly sophisticated societies. The process of fermentation by fungi was instrumental in the production of bread and its trade between ancient civilizations, which remains to the present day. Additionally, the use of yeast fermentation is key for our daily food intake and is used to produce alcohol (e.g. beer, wine and spirits), cheeses, cured meats, soy sauce and vinegars. The economic significance of fungal-derived processes is apparent in the fact that alcohol [2] and cheese [3] alone generate upwards of a trillion US dollars (USD) in total world sales, and the fermentation of soy into various products likely constitutes some of the largest industries in the East. Bread also remains one of the most heavily consumed foods and largest world markets.
In biotechnology and pharmaceutical industries, fungal metabolism is commonly exploited to acquire many antibiotics (e.g. beta [β]-lactams), polysaccharides (e.g. pullulan), vitamins (e.g. riboflavin), lipids (e.g. biofuels), enzymes (e.g. cellulases) and other valuable bioproducts unattainable from other organisms. These bioproducts are instrumental in research and clinical settings and are used extensively in both academic investigation and disease treatment. Most impressively, the advent of the fungal biosynthetic β-lactam class of antibiotics has played a major role in the near doubling of the average human lifespan in the United States (USA) during the 20th century. For example, from 1900 to 2014, the average life expectancy in the USA increased from ~47 to ~79 years [4]. In tandem, from 1900 (797 deaths per 100 000) to 1980 (36 deaths per 100 000), there was a drastic decrease in mortality in the USA attributed to infectious diseases [5]. The clinical introduction of β-lactams in the 20th century is one of a few medical advances that contributed to the reduction of mortality caused by infectious microorganisms [6]. Furthermore, β-lactams are currently the major anti-infective agents in the world, with an estimated market value upwards of 25 billion USD [7]. Similarly, statins (fungal secondary metabolites) have been used for decades to treat coronary artery disease, with natural statins, specifically lovastatin and pravastatin, making up a considerable portion of the estimated 25–30 billion USD worldwide statin sales [8]. Linking biotechnology and food due its extensive use in both industries, citric acid, with a global market value of 2.6 billion and 3.6 billion projections by 2020 [9], is the most important mass-produced organic acid and is mainly made by fermentation using Aspergillus niger, as it is too expensive to isolate from fruits [10]. While not an extensive list, the above-mentioned fungal applications highlight the crucial and beneficial role diverse fungal species have at present for human daily life.
The application of fungi for mass production of foods and pharmaceuticals that benefit daily life is sharply contrasted by their pathogenic devastation on agriculture and infectious disease in humans and animals. In developed countries, consideration for fungal disease is sub-par and mostly associated with superficial infections such as athlete’s foot and genital yeast infections, whereas serious manifestations, such as invasive fungal infections, commonly exist as an afterthought, receiving little attention from the general public and in regards to research funding. An increase in immunocompromised individuals due to the global AIDS pandemic and the use of chemotherapy and immunosuppressive drugs, coupled with the advent of medical interventions such as major surgeries and intravenous therapy, has resulted in the emergence of invasive fungal infections as a serious threat [11, 12]. Similarly, fungi possess a threat to agriculture and closer attention to the potential negative effects on human food supply and other plant products must not be overlooked. Furthermore, fungi are actively affecting the animal kingdom through mass extinctions, leading to unprecedented loss of biodiversity [13].
In this review, we discuss the close relationship of the environment and fungal diseases, especially in the context of climate change and natural disaster, along with the current state of fungal disease in humans, animals and plants. Our aim is to highlight the importance of the urgent initiatives necessary from lawmakers, funding agencies, science educators, health care professionals, agriculture workers and biologists in general to help in improving awareness of the public on the relationship of the environment and fungal disease.
Fungal diseases in humans
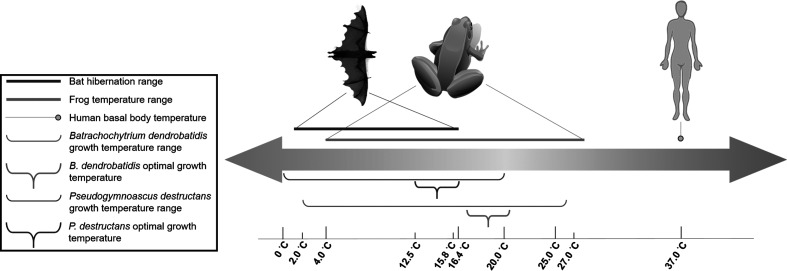
Infection can be defined simply as the acquisition of a microbe by a host, resulting when the microorganism is not eliminated from the host after direct contact between them [14]. Disease can then be an outcome that follows infection [15]; however, human fungal disease is relatively uncommon in healthy individuals. In fact, only several hundred species are known to be pathogenic to humans of an estimated 1.5 million total fungal species on earth, while species pathogenic to plants are estimated to be in in the hundreds of thousands, and insect fungal pathogens number around 1000 [16]. Mycoses can arise from exposure to a large inoculum, especially from opportunistic environmental fungal species such as those found in soils that do not need hosts for growth or survival [17]. Notably, humans display remarkable resilience to fungal diseases except at the extremes of the immune response [14], and this seems to suggest that human characteristics that aid in warding off fungal disease were selected for in evolution. Four criteria have been described as requisites for fungi to infect humans and cause severe disease [18]. Fungi must be able to (1) colonize or penetrate surface barriers such as the mucous membrane and the skin; (2) subsequently lyse and absorb human tissue for its nutritional utilization; (3) resist pressure from innate and adaptive immunity; and (4) grow at human body temperatures (Fig. 1). Relatively high basal body temperature is one necessary characteristic that sufficiently endothermic mammals have evolved to combat fungi that grow at temperatures in hosts’ thermal exclusion zones. Mammals’ high basal temperature surely serves to protect these organisms, including humans, from a large majority of fungi that are unable to grow in the 37 °C range and instead grow optimally in environments of 25–35 °C [11]. Studies on the encapsulated fungus Cryptococcus neoformans pathogenesis in rabbits further corroborate this postulation. In rabbits, whose normal basal temperatures range from 39 to 40 °C, resistance to C. neoformans (grows well at 37 °C and some strains at 40–41 °C) infection has been observed even when the fungus is injected directly into cerebrospinal fluid [19, 20]. The analysis of thermal tolerance of 4802 fungal strains found that most cannot grow at mammalian temperatures, with every 1 °C increase between 30 and 42 °C excluding an additional 6 % of fungal isolates [12]. The utility of fever as a defence mechanism in many animals against microbes further supports these findings [21]. Interestingly, endothermy as a mechanism of protection might have contributed to increased fitness, and consequently the rise in the age of mammals at the Cretaceous–Tertiary (K-T) boundary [11]. During this period, it is possible that there was mass fungal proliferation, due to evidenced large-scale global deforestation [22], which could have provided fungi with rich nutritional sources [11]. The increase in fungi in the environment could then have led to large amounts of aerosolized spores, and consequently large amounts of infectious fungal inoculate being delivered to vulnerable ectotherms [11]. Together, physiologically costly endothermy and adaptive immunity protected mammals, including humans, from most fungal species’ invasion and colonization.
Fig. 1.
A constant and relatively high basal body temperature is an important advantage that humans have evolved to prevent fungal disease. Although bats are mammals and can reach a body temperature of 40 °C while active, during hibernation (range: 1.1–16.4 °C) where the body temperature and metabolism decreases, these animals are susceptible to infection by the psychrophilic fungus Pseudogymnoascus destructans (range: 0–20 °C; optimal: 12.5–15.8 °C), the causative agent of white-nose syndrome. In contrast, frogs cannot regulate their body temperature (range: 4–28 °C), being vulnerable to infection by Batrachochytrium dendrobatidis, the aetiological agent of chytridiomycosis. B. dendrobatidis can grow within a wide temperature range (2–27 °C), with optimal temperatures being in the range 17–21 °C. The similarity to the frog’s body temperature range for growth gives B. dendrobatidis the ability to infect these amphibians.
The emergence of oral candidiasis in the 1950s, which is a disease caused by the commensal Candida albicans, foreshadowed the emergence of serious fungal disease in the future as invasive medical procedures became increasingly ubiquitous. The rise of oral and genital candidiasis became associated with the advent of broad-spectrum antibiotics which reduce the host bacterial flora and promote fungal growth. Further advances in medicine such as intravenous catheters, organ transplantation, aggressive surgeries and the emerging use of intensive care units and cytotoxic chemotherapy all contributed to an increase in fungal incidence [11, 23]. Notably, HIV infection and the AIDS pandemic changed the infectious disease landscape, and individuals with compromised immunity became highly susceptible to fungal infections. In developing nations where there is high prevalence of individuals with AIDS, fungi are a serious threat to this immunocompromised population with Candida spp., Aspergillus spp., Pneumocystis jirovecii and C. neoformans being the most common cause of serious disease. For example, the latest estimates placed the global incidence of cryptococcal meningitis at about 223 000 annual cases, with 73 % or 162 500 of cases in sub-Saharan Africa [24]. Additionally, cryptococcal meningitis is responsible for 15 % of global AIDS-related deaths (~181 000), with 75 % of deaths in sub-Saharan Africa (~135 000) making cryptococcosis the second leading cause of AIDS-related deaths in the continent, just behind tuberculosis [24]. Recent efforts have been undertaken to show the scale of the burden that fungal disease poses to the world. Estimates, spanning 14 countries in the Americas, Europe, Asia and North Africa, show that 1.8 to 3 % of the population of each country is affected by some of the most serious fungal diseases that can cause chronic illness and death [25]. Together, it is estimated that >300 million people are affected by serious mycoses worldwide, and about 1.6 million of these cases result in death annually [25], more than malaria and as many as tuberculosis [15, 26], highlighting the importance of fungal infections.
Fungal dimorphism: an advantage for environmental survival and infection
A fundamental and widespread characteristic of fungi is their ability to switch morphologies throughout their life cycle. Some fungi are considered fully dimorphic because of their ability to switch between hyphae and yeast form depending on environmental conditions, genetic profile or physiological conditions [27]. Often, the major stimulus for a morphologic shift is temperature, and fungi that have this ability are considered thermally dimorphic. Dimorphic fungi can infect humans, animals, plants and insects [27]. For example, the phytopathogenic dimorphic fungus Ophiostoma ulmi caused the first Dutch elm disease epidemic, and a more virulent species now devastates millions of trees in Europe and the USA [28]. Economic losses of 2.5–3.0 million USD are caused by Taphrina deformans contaminating peaches [29]. Blastomyces dermatitidis, Histoplasma capsulatum, Paracoccidioides brasiliensis, Talaromyces marneffei (formerly known as Penicillium marneffei) and Coccidioides immitis/posadasii have all been described as thermally dimorphic and major human pathogens [30].
Typically, dimorphic fungi exist in their hyphal form in the environment where they asexually produce infectious spores (conidia) at temperatures of 22–25 °C. In this form, they are able to survive and remain endemic to a region and disperse to other regions over time. After aerosolization, which can be enhanced by soil disruption through human activity or natural disaster, the human host can inhale the infectious conidia into their warm lungs (37 °C) and pulmonary infection can take hold as the thermal switch is made to the yeast form (or spherules for Coccidioides spp). A key defence of endothermic hosts against fungi is their relatively high body temperature (37 °C), which excludes many fungi but not thermally dimorphic fungi [21]. The binding of conidia to immune cells and subsequent phagocytosis and intracellular replication has been described as a mechanism in the switch [31]. Additionally, recent evidence describing the upregulation of genes critical for infectious success during the transition from mould to yeast has begun to emerge [32]. By being able to enter a human host, survive in the warm temperature and evade host immunity, thermally dimorphic fungi are remarkably capable of bypassing many immunological and physical structural barriers and colonizing the host. After localizing in the lungs via inhalation, the infection can cause pneumonia and, if not controlled by the host's immunity, it can disseminate to other organs including the central nervous system.
Natural disasters enhance fungal infection
Disasters have been defined by the World Health Organization (WHO) as a serious disruption of the functioning of a community or a society causing widespread human, material, economic or environmental losses that exceed the ability of the affected community or society to cope using its own resources [33]. In one estimation, the WHO proposes that more than 2.6 billion people have been affected by natural disasters in the last decade alone. Furthermore, yearly there are about 90 000 direct casualties’ and 160 million affected by physical destruction and the biological/social environment during a natural disaster. While the immediate death, suffering and economic tolls during a natural disaster are well established [34], the public health consequences of disasters after natural disasters have taken place are also being increasingly recognized. Notably, the relationship between natural disaster and proliferation of fungal disease is being increasingly investigated as evidence of a possible link between the two becomes more available [35]. It is important to acknowledge that at present, invasive fungal infections outbreaks after natural disasters are uncommon and the potential for post-disaster epidemics can be overestimated despite the lack of supporting evidence [36]. A major disaster happens almost daily somewhere on earth, and estimates indicate that one million thunderstorms, 100 000 floods, tens of thousands of landslides, earthquakes, wildfires, tornadoes, several thousand hurricanes and tropical cyclones, tsunamis and volcanoes occurred between 2005 and 2015 [37]. Therefore, although mycoses do not constitute the majority of adverse health effects post-disaster, the high global incidence of disaster necessitates increased awareness among health care providers and public health officials in disaster zones with known or predicted endemic fungal disease agents.
Regarding disease, endemic agents are the major cause for alarm after a disaster, and increased incidence of fungal infection likely results from an increase in spores that can be displaced from their natural habitat leading to increased concentration in the environment [35]. The propensity for injury during or after natural disaster, coupled with high environmental exposure of persons to soils, water or other debris, typically associated with the disaster, can create the optimal conditions for outbreak of fungal disease, exacerbating morbidity and mortality of otherwise non-life-threatening injuries. Additionally, potential displacement, inadequate sanitary conditions and diminished access to proper health care services can compromise proper wound care by methods such as sterile irrigation, removal of foreign bodies and surgical debridement [38]. Furthermore, most soft tissue infections due to disaster are caused by bacteria [39], and therefore there is a possibility for untimely treatment with proper antifungals during the course of infection – a pressing matter worthy of discussion within clinical settings. A few examples associating fungal diseases with natural disasters are described below:
Tornadoes: Joplin, Missouri
In 2011, a catastrophic tornado rated EF-5 stormed through Joplin, Missouri. The Joplin tornado is recognized as one of the worst natural disasters in recent history on US soil, resulting in over 1000 injured persons and 158 deaths [40]. The physical aftermath was widely covered by conventional and social media; however, a lesser known biological event began to take shape in the following few weeks. Local health officials were notified by a physician of a suspected soft tissue fungal infection in two patients [41]. The patients were 2 of the 13 identified cases with life-threatening mucormycosis after the tornado, 5 of whom eventually died. For 3 of the 5 patients, fungal infection was listed on the death certificate as a primary or contributing cause of death. All 13 patients were hospitalized for injuries sustained during the tornado, and specimens later showed infections with Apophysomyces trapeziformis, Candida spp., Fusarium spp. and Aspergillus spp. as well as Mucor circinelloides. The U.S. Center for Disease Control (CDC) investigation concluded that as the tornado moved through Joplin, fungi from one or more environmental sources were aerosolized and spread throughout the vortex and debris and were inoculated into patients after penetrating trauma injuries [41]. Therefore, the CDC suggested an increased awareness of environmental fungi as a cause of necrotizing soft-tissue infection in patients injured during natural disasters, especially in endemic areas.
While all 13 patients received systemic antifungal therapy for treatment of the disaster-related wounds, 6 received antifungal drugs that were not efficacious against mucormycetes, resulting in the death of 3 of these patients before these fungi were identified [41]. While it is difficult to know whether the deceased patients would have survived if treated with the proper antifungal drugs earlier in the infection, the outcome highlights the importance of health care awareness of the possibility for fungal infection after a natural disaster. Likewise, public health officials should be prepared to react quickly by communicating and posting health advisories of such infections to prevent deaths associated with difficult-to-treat mucormycetes.
Hurricanes and flooding
Hurricanes and other flooding disasters can pose an increased mould threat due to increased humidity that promotes fungal growth, and sufficient evidence has been found for an association between damp indoor spaces, mould and upper/lower respiratory symptoms [42]. This was cause for concern at the time of hurricane Katrina’s landfall due to the potential risks for remediation workers and residents. The aftermath of Katrina was devastating, and the hurricane is considered the costliest natural disaster in US history and one of the five deadliest hurricanes ever to impact the mainland. Eighty per cent of the city sustained heavy floods which remained for weeks, and moist environments remained for months. Less than a month after Katrina’s impact, hurricane Rita’s landfall on Louisiana exacerbated the flooding effects. The CDC and Louisiana Department of Health and Hospitals documented the extent of potential exposure, culminating in a report that estimated mould growth in 46 % of inspected homes and a note that remediation workers did not consistently use appropriate respiratory protection [43]. Predominant fungal species obtained in air samples were Aspergillus spp. and Penicillium spp., and the mean indoor endotoxin levels detected were more than 20 times higher than the average, far exceeding the levels that have been associated with respiratory symptoms [44] and comparable to industrial levels in which decline of pulmonary function has been demonstrated [45]. Others described feeling sick, possibly caused by fungally produced volatile organic compounds (VOCs) [46]. These fungal metabolites might contribute to illness as being associated with the sick building syndrome. Arabidopsis thaliana and Drosophila melanogaster exhibit a range of toxic symptoms after VOC exposure, including neurotoxicity, that might provide insight into neurological health problems associated with damp indoor environments, such as those characteristic of Parkinson’s disease [47, 48]. Interestingly, four cases of coccidioidomycosis were identified in the post-hurricane period [49], with three individuals suffering disseminated mycosis while the other individual’s infection was limited to the respiratory tract. Two patients died due to co-infection with HIV. Due to coccidioidomycosis being non-endemic to New Orleans, public health officials might be aware of using close monitoring of possible non-endemic disease after a natural disaster [49].
Earthquakes and sandstorms
Coccidioidomycosis, commonly known as ‘Valley Fever’, ‘San Joaquin Fever’ or ‘Joaquin Valley Fever’, results from inhalation of the causative agents C. immitis and C. posadasii and has been associated with two instances of post-disaster infection. The first instance was in 1994, when a magnitude 6.7 earthquake struck Northridge, California (CA), resulting in 57 deaths and approximately 8700 injuries [50]. The interplay between the original seismic activity, aftershocks, and landslides is strongly implicated in contributing to an increase in aerosolized C. immitis spherules in the environment caused by displacement from their natural habitat and dispersion in the resulting dust clouds [50]. In Ventura County, CA, 203 cases of coccidioidomycosis were identified and a strong association was found between dust-cloud time of exposure and acute illness [50]. A similar airborne outbreak occurred previously in 1977 in the San Joaquin Valley in CA [51]. A dust storm covering an area larger than the state of Maine (87 000 km2) originating near Bakersfield, in which coccidioidomycosis is highly endemic, resulted in high numbers of unusual coccidioidomycosis cases [51]. For instance, 115 valley fever cases were attributed to the dust storm in non-endemic Sacramento. Disseminated mycosis was observed in 16 of those cases. Moreover, 18 coccidioidomycosis cases were reported at a US Navy air station in Kings County [52], 134 cases in Kern county during January and February [53] and many others across other Californian counties.
These outbreaks of fungal infection following natural disasters in areas in which fungal infections are endemic illustrates the need for preventive preparation by healthcare agencies. For instance, in the case of the San Fernando Valley earthquake, fungal infections were not considered a possibility in initial diagnoses, with 93 % of case-patients being treated with >1 antibacterial drug before a mycosis diagnostic [50].
Impact of fungal disease on animal extinction
The loss of biodiversity is universally recognized as being negatively impactful to the human condition, and fungal pathogens in animals are a prominent driving force in biodiversity homogenization. Analyses on the role of pathogenic fungi in the loss of biodiversity have shown that animals suffer the greatest insults from fungal disease, with 91 % of recent fungal-related extirpations and extinctions occurring in animals rather than in plants [13]. Here, we describe the animal species of major concern affected by fungal disease in recent times resulting in significant loss of animals.
White nose syndrome in bats
A driver of biodiversity homogenization, white nose syndrome (WNS) is an emerging disease of North American bats [54], caused by the psychrophilic fungi Pseudogymnoascus destructans [55], responsible for drastic mortality in these mammals. Regional extirpations and extinctions due to WNS are expected [56, 57]. In 2012, estimates by the U.S. Fish and Wildlife Service placed the total North American bat death toll at over 6 million, and mortality rates reach up to 100 % at many hibernacula sites [58]. Since its discovery, P. destructans has rapidly spread over two thousand kilometres from northeastern USA and as of 2017, one decade after its initial discovery, P. destructans is confirmed in 33 states and 5 Canadian provinces [59]. Infected bats develop visible fungal growth on the nose, ears or wings (hairless skin) during hibernation when body temperatures lower sufficiently during winter torpor for fungal growth (Fig. 1) [60]. Physiological changes associated with increased bat arousal to normothermia from torpor might result in compromising energy expenditure and ultimately death [61–63]. Weight loss, dehydration and electrolyte imbalance have also been described as possible factors in mortality [64]. More than half of the 47 bat species living in the USA and Canada rely on winter torpor and therefore serve as potential hosts to this infection [59]. So far, nine species are confirmed to have WNS and six additional species are confirmed carriers of P. destructans without WNS diagnostic symptoms [59]. Of these, one species is classified as threatened and two endangered species are at risk of extinction. This dramatic decline is worrisome, and bats’ important role as primary predators of insects and keystone species for cave ecosystems necessitates an increase of attention towards this emerging problem [65, 66]. For example, the value of bats in suppressing pest insects in continental USA was approximated at 3.7 billion USD/year [67]. However, this estimate was the lowest extreme with the highest extreme being 53 billion USD/year, and the real value is likely approximately 23 billion USD/year [67].
Chytridiomycosis in amphibians
Batrachochytrium dendrobatidis, responsible for the emerging chytridiomycosis in amphibians, is a devastating fungal agent responsible for perhaps the greatest disease-caused loss of biodiversity in recorded history [68]. B. dendrobatidis’ growth temperature ranges (2–27 °C) are similar to the internal temperature ranges of frogs (4–28 °C) [69], making these amphibians vulnerable to infection (Fig. 1). Chytridiomycosis is characterized by cutaneous infection of amphibian keratinized tissues, and infection typically leads to hyperkeratosis or thickening of the outer keratin skin layer, compromising the osmotic regulation necessary for amphibian respiration, hydration, electrolyte transport and thermoregulation [68]. Due to the increased susceptibility of electrolyte uptake skin patches to B. dendrobatidis infection, chytridiomycosis progression typically precedes cardiac arrest [70]. Catastrophic population declines and extinction events are occurring worldwide due to this fungus [68, 71]. B. dendrobatidis is found on all continents inhabited by amphibians and, because of extremely low species specificity, every continent bears these declines, with Australia, Central America and North America taking the brunt of the pathogen insults [72]. As of 2013, 516 of 1240 (42 %) amphibian species tested are known to have been infected with B. dendrobatidis and in the past three decades, B. dendrobatidis has caused the catastrophic decline or extinction of up to 200 species of frogs [72]. Spread of B. dendrobatidis is usually in the form of a rampant wave front and in many cases, upon arrival of the pathogen in an area, amphibian populations are infected and/or collapse in less than a year. For instance, eight populations of frogs in Sierra Nevada showing low prevalence and intensity of infection in initial samples suddenly increased to nearly 100 % in each population just 50 days later [73]. In Central America, B. dendrobatidis advanced southeast of Costa Rica causing tremendous mortality among amphibians, with 50 % of local species eradicated within four to six months [74]. Similar episodes of population crashes have been reported in Brazil [75], Panama [76] and Australia [77].
Possible causes of chytridiomycosis and WNS emergence
There is evidence to suggest that B. dendrobatidis and P. destructans were introduced to the endemic areas by transport that is anthropogenic in nature [78]. During the emergence of chytridiomycosis as a serious global fungal threat to biodiversity, it was unclear whether B. dendrobatidis was globally endemic, increased in pathogenicity or introduced into the affected areas. The evidence suggests that B. dendrobatidis strains isolated globally have low levels of genetic diversity; fitting the profile of a recently expanded pathogen [79]. Humans might have contributed to that expansion, and studies confirming trade as responsible for cases of chytridiomycosis emergence lend credence to that postulation [80]. Likewise, B. dendrobatidis can also be observed in pet stores, zoos and museums [78], further suggesting that trade played a role in its distribution. In contrast, the emergence of P. destructans as a pathogen of bats is still unclear. There is speculation that human transport resulted in the introduction of the fungus to susceptible bats in North America because bats in Europe are known to carry P. destructans asymptomatically [81]. P. destructans genotyping data suggest host-independent spread of a single clone and that recent introduction of the pathogen is likely responsible for its rapid spread [82]. This possibility is also supported by the fact that the first reported case of WNS occurred in a touristic cave in New York, a state of considerable transit of people from all over the world [81]. However, this is just circumstantial evidence, and more thorough genetic analyses studies are needed to elucidate the WNS emergence with certainty.
Fungal disease as a threat to global food supply
Of the many different threats fungi pose, the infection of plants seems to be the most recognized possibly due to economic interest and people’s dependence on agriculture for food supply. The threat fungi pose to food security is a major concern given that fungi cause disease in rice, wheat, maize and potato crops which constitute most of global food consumption. For example, rice alone has been estimated to constitute half of the total global caloric intake [83] and rice blast, caused by Magnaporthe oryzae, is the most destructive disease in cultivated rice [84]. Since fungi is responsible for 70–80 % of all plant diseases, a recently generated ‘top ten list’ of the most scientifically and economically important fungal pathogens, M. oryzae overwhelmingly ranked 1st for its catastrophic potential to devastate global food supplies [85]. Other plant diseases such as soybean rust (Phakopsora pachyrhizi), wheat stem rust (Puccinia graminis), maize corn smut (Ustilago maydis), and potato late blight (Phytophtora infestans), contribute to the loss of 125 million tons of these crops (enough to feed 600 million people) and cause $60 billion USD of global cost [13]. Furthermore, while highly unlikely, it is estimated that concurrent epidemics in 5 of the top food crops would result in the loss of about 900 million tons of food leading to cataclysmic global famine with starvation in 2.7 billion people [13]. Nevertheless, fungi have previously compromised food security seriously. For example, in the 1840’s, the oomycete P. infestans caused the Irish potato famine resulting in the death of a million people due to starvation and displaced another million leading to a 20 % decrease in the population of Ireland [86]. This event was not only a humanitarian tragedy, but it also significantly affected the course of European political and economic history. In India, the Bengal famine of 1943 caused by Cochliobolus miyabeanus resulted in the deaths of 2–3 million people due to the population dependence on rice as a primary source of nutrition [87]. In the U.S., Cochliobolus heterostrophus also caused the 1971–1972 southern corn leaf blight epidemic, resulting in severe crop failure [88].
Fungi can also produce mycotoxins, a variety of secondary metabolites, including some that are toxic for humans and animals [89, 90]. These mycotoxins can contaminate food directly due to mold growth at many stages of production and cultivation in the field or in storage. There are a few species of fungi capable of producing mycotoxins including: Penicillium expansum (apple juice), Fusarium graminearum (cereal products), and A. flavus/Aspergillus parasiticus (maize, wheat, rice, nuts and figs) [91]. Illness and disease possibly caused by the metabolites of these organisms ranges from diarrhea and vomiting to premature puberty in girls, cervical cancer, primary hepatocellular carcinoma, cirrhosis and Reye’s syndrome [91]. The estimated amount of crops contaminated with mycotoxins is considerable and about 25 % and outbreaks of mycotoxicosis associated to consumption have been reported in humans and animals [92]. Climate change is also implicated in modulation of fungal growth and mycotoxin production. In fact, prolonged dry weather has been associated with an increase in myco-aflatoxins in Europe [93]. Plants themselves, such as corn, are also more susceptible to mycotoxins under stress [94, 95]. Mycotoxins have been described as a more important chronic food safety factor than synthetic food contaminants, food additives, pesticide residues, and plant toxins by experts in the risk assessment field [96]. As investigation into mycotoxins and their effects continue, the true scale of the risk to human and animal health will surely be elucidated.
Conclusion
The threats fungi pose to public health and the environment are substantial. In humans, fungal diseases have been emerging in the past century and the global epidemic of HIV along with advances in medicine serve to exacerbate their mortality and morbidity. Moreover, instances of fungal disease outbreak after natural disasters are clearly documented and reports continuously caution public health officials and clinicians to strive for better preparedness in regard to endemic fungal pathogens that may arise from these fortuitous events. This subject is particularly relevant now, as a concerning number of natural disasters due to climate change have recently occurred generating media attention and public awareness. Landfalls by hurricanes Harvey and Irma in Houston, TX, and Maria in Puerto Rico as well as the earthquake in central Mexico all occurred in the span of less than a month in 2017 and while there is still a lack of reports of mycoses following these events, the possibility should not be overlooked. Fungal disease is also a major player in the environment, as it is a driving force behind biodiversity homogenization, causing millions of bat deaths in only a decade and the greatest recorded disease-caused extinction as observed in amphibians. Likewise, fungi are pathogens of plants and some species are producers of mycotoxins, constantly threatening agriculture and food supply. Therefore, it is important to understand at the molecular level how fungi adapt to climate change or to non-endemic environments. Finally, it is necessary to create awareness among the public on the infectious potential of fungi in order to reduce the morbidity and mortality associated to environmental disturbances as well as preserving our flora and wildlife.
Funding information
H. H. was supported by National Institute of General Medical Sciences (NIGMS) RISE training grant R25 GM069621-15. L. R. M. was partially supported by the NIGMS of the US National Institutes of Health (NIH) under award number 1R15GM117501-01A1. L. R. M. is partially funded and has an appointment in the Infectious Diseases and Immunology cluster of the Border Biomedical Research Center (BBRC; National Institute on Minority Health and Health Disparities award number 2G12MD007592), UTEP’s Research Centers in Minority Institutions Program. L. R. M. serves as a Leshner Leadership Institute Public Engagement Fellow in infectious diseases with the American Association for the Advancement of Science.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CA, California; CDC, U.S. Center for Disease Control; K-T, cretaceous-tertiary; USD, US dollars; VOCs, volatile organic compounds; WHO, World Health Organization; WNS, white nose syndrome.
References
- 1.Walsh TJ, Groll AH. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl Infect Dis. 1999;1:247–261. doi: 10.1034/j.1399-3062.1999.010404.x. [DOI] [PubMed] [Google Scholar]
- 2.Alcoholic Beverages Market Expected to Reach $1,594 Billion, Globally, by 2022 – Allied Market Research. 2017. www.prnewswire.com/news-releases/alcoholic-beverages-market-expected-to-reach-1594-billion-globally-by-2022---allied-market-research-618354513.html
- 3.Conick H. Global cheese market to exceed $100 billion by 2019. 2017. www.dairyreporter.com/Article/2016/01/19/Global-cheese-market-to-exceed-100bn-by-2019
- 4.NCHS – Death rates and life expectancy at birth. https://data.cdc.gov/NCHS/NCHS-Death-rates-and-life-expectancy-at-birth/w9j2-ggv5
- 5.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Demain AL, Elander RP. The beta-lactam antibiotics: past, present, and future. Antonie van Leeuwenhoek. 1999;75:5–19. doi: 10.1023/A:1001738823146. [DOI] [PubMed] [Google Scholar]
- 7.Thakuria B, Lahon K. The beta lactam antibiotics as an empirical therapy in a developing country: an update on their current status and recommendations to counter the resistance against them. J Clin Diagn Res. 2013;7:1207–1214. doi: 10.7860/JCDR/2013/5239.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis JP. More than a billion people taking statins?: Potential implications of the new cardiovascular guidelines. JAMA. 2014;311:463–464. doi: 10.1001/jama.2013.284657. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Li H, Zhu L, Tan F, Li Y, et al. High-efficient production of citric acid by Aspergillus niger from high concentration of substrate based on the staged-addition glucoamylase strategy. Bioprocess Biosyst Eng. 2017;40:891–899. doi: 10.1007/s00449-017-1753-7. [DOI] [PubMed] [Google Scholar]
- 10.Soccol CR, Vandenberghe LPS, Rodrigues C, Pandey A. New perspectives for citric acid production and application. Food Technol Biotech. 2006;44:141–149. [Google Scholar]
- 11.Casadevall A. Fungal virulence, vertebrate endothermy, and dinosaur extinction: is there a connection? Fungal Genet Biol. 2005;42:98–106. doi: 10.1016/j.fgb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Robert VA, Casadevall A. Vertebrate endothermy restricts most fungi as potential pathogens. J Infect Dis. 2009;200:1623–1626. doi: 10.1086/644642. [DOI] [PubMed] [Google Scholar]
- 13.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casadevall A, Pirofski LA. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67:3703–3713. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien HE, Parrent JL, Jackson JA, Moncalvo JM, Vilgalys R. Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microbiol. 2005;71:5544–5550. doi: 10.1128/AEM.71.9.5544-5550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casadevall A, Pirofski LA. Accidental virulence, cryptic pathogenesis, martians, lost hosts, and the pathogenicity of environmental microbes. Eukaryot Cell. 2007;6:2169–2174. doi: 10.1128/EC.00308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler JR, Hube B, Puccia R, Casadevall A, Perfect JR. Fungi that infect humans. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.FUNK-0014-2016. [DOI] [PubMed] [Google Scholar]
- 19.Martinez LR, Garcia-Rivera J, Casadevall A. Cryptococcus neoformans var. neoformans (serotype D) strains are more susceptible to heat than C. neoformans var. grubii (serotype A) strains. J Clin Microbiol. 2001;39:3365–3367. doi: 10.1128/JCM.39.9.3365-3367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfect JR, Lang SD, Durack DT. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980;101:177–194. [PMC free article] [PubMed] [Google Scholar]
- 21.Casadevall A. Thermal restriction as an antimicrobial function of fever. PLoS Pathog. 2016;12:e1005577. doi: 10.1371/journal.ppat.1005577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vajda V, Raine JI, Hollis CJ. Indication of global deforestation at the Cretaceous-Tertiary boundary by New Zealand fern spike. Science. 2001;294:1700–1702. doi: 10.1126/science.1064706. [DOI] [PubMed] [Google Scholar]
- 23.Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67:1567–1601. doi: 10.2165/00003495-200767110-00004. [DOI] [PubMed] [Google Scholar]
- 24.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The burden of fungal disease: new evidence to show the scale of the problem across the globe. 2017. www.life-worldwide.org/media-centre/article/the-burden-of-fungal-disease-new-evidence-to-show-the-scale-of-the-problem
- 26.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 27.Gauthier GM. Dimorphism in fungal pathogens of mammals, plants, and insects. PLoS Pathog. 2015;11:e1004608. doi: 10.1371/journal.ppat.1004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naruzawa ES, Bernier L. Control of yeast-mycelium dimorphism in vitro in Dutch elm disease fungi by manipulation of specific external stimuli. Fungal Biol. 2014;118:872–884. doi: 10.1016/j.funbio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Cissé OH, Almeida JM, Fonseca A, Kumar AA, Salojärvi J, et al. Genome sequencing of the plant pathogen Taphrina deformans, the causal agent of peach leaf curl. MBio. 2013;4:e00055-13. doi: 10.1128/mBio.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gauthier GM. Fungal dimorphism and virulence: molecular mechanisms for temperature adaptation, immune evasion, and in vivo survival. Mediators Inflamm. 2017;2017:1–8. doi: 10.1155/2017/8491383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inglis DO, Voorhies M, Hocking Murray DR, Sil A. Comparative transcriptomics of infectious spores from the fungal pathogen Histoplasma capsulatum reveals a core set of transcripts that specify infectious and pathogenic states. Eukaryot Cell. 2013;12:828–852. doi: 10.1128/EC.00069-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyce KJ, Schreider L, Kirszenblat L, Andrianopoulos A. The two-component histidine kinases DrkA and SlnA are required for in vivo growth in the human pathogen Penicillium marneffei. Mol Microbiol. 2011;82:1164–1184. doi: 10.1111/j.1365-2958.2011.07878.x. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization WHO definitions: emergencies. 2017. www.who.int/hac/about/definitions/en/
- 34.Noji EK. Public health issues in disasters. Crit Care Med. 2005;33:S29–S33. doi: 10.1097/01.CCM.0000151064.98207.9C. [DOI] [PubMed] [Google Scholar]
- 35.Benedict K, Park BJ. Invasive fungal infections after natural disasters. Emerg Infect Dis. 2014;20:349–355. doi: 10.3201/eid2003.131230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson JT, Gayer M, Connolly MA. Epidemics after natural disasters. Emerg Infect Dis. 2007;13:1–5. doi: 10.3201/eid1301.060779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parente M, Tofani M, de Santis R, Esposito G, Santilli V, et al. The role of the occupational therapist in disaster areas: systematic review. Occup Ther Int. 2017;2017:1–8. doi: 10.1155/2017/6474761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivers LC, Ryan ET. Infectious diseases of severe weather-related and flood-related natural disasters. Curr Opin Infect Dis. 2006;19:408–414. doi: 10.1097/01.qco.0000244044.85393.9e. [DOI] [PubMed] [Google Scholar]
- 39.Bandino JP, Hang A, Norton SA. The infectious and noninfectious dermatological consequences of flooding: a field manual for the responding provider. Am J Clin Dermatol. 2015;16:399–424. doi: 10.1007/s40257-015-0138-4. [DOI] [PubMed] [Google Scholar]
- 40.Luo J, Cong Z, Liang D. Number of warning information sources and decision making during tornadoes. Am J Prev Med. 2015;48:334–337. doi: 10.1016/j.amepre.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214–2225. doi: 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 42.Bennett JW. Silver linings: a personal memoir about Hurricane Katrina and fungal volatiles. Front Microbiol. 2015;6:206. doi: 10.3389/fmicb.2015.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwab KJ, Gibson KE, Williams DL, Kulbicki KM, Lo CP, et al. Microbial and chemical assessment of regions within New Orleans, LA impacted by Hurricane Katrina. Environ Sci Technol. 2007;41:2401–2406. doi: 10.1021/es062916x. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds SJ, Black DW, Borin SS, Breuer G, Burmeister LF, et al. Indoor environmental quality in six commercial office buildings in the midwest United States. Appl Occup Environ Hyg. 2001;16:1065–1077. doi: 10.1080/104732201753214170. [DOI] [PubMed] [Google Scholar]
- 45.Douwes J, Pearce N, Heederik D. Does environmental endotoxin exposure prevent asthma? Thorax. 2002;57:86–90. doi: 10.1136/thorax.57.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett JW. The fungi that ate my house. Science. 2015;349:1018. doi: 10.1126/science.349.6251.1018. [DOI] [PubMed] [Google Scholar]
- 47.Inamdar AA, Masurekar P, Bennett JW. Neurotoxicity of fungal volatile organic compounds in Drosophila melanogaster. Toxicol Sci. 2010;117:418–426. doi: 10.1093/toxsci/kfq222. [DOI] [PubMed] [Google Scholar]
- 48.Empting LD. Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure. Toxicol Ind Health. 2009;25:577–581. doi: 10.1177/0748233709348393. [DOI] [PubMed] [Google Scholar]
- 49.Schieffelin JS, Torrellas M, Lartchenko S, Gill F, Garcia-Diaz J, et al. How natural disasters change natural patterns: coccidioidomycosis imported to New Orleans. J La State Med Soc. 2013;165:145–149. [PubMed] [Google Scholar]
- 50.Schneider E, Hajjeh RA, Spiegel RA, Jibson RW, Harp EL, et al. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA. 1997;277:904–908. doi: 10.1001/jama.1997.03540350054033. [DOI] [PubMed] [Google Scholar]
- 51.Flynn NM, Hoeprich PD, Kawachi MM, Lee KK, Lawrence RM, et al. An unusual outbreak of windborne coccidioidomycosis. N Engl J Med. 1979;301:358–361. doi: 10.1056/NEJM197908163010705. [DOI] [PubMed] [Google Scholar]
- 52.Williams PL, Sable DL, Mendez P, Smyth LT. Symptomatic coccidioidomycosis following a severe natural dust storm. An outbreak at the Naval Air Station, Lemoore, Calif. Chest. 1979;76:566–570. doi: 10.1378/chest.76.5.566. [DOI] [PubMed] [Google Scholar]
- 53.Pappagianis D, Einstein H. Tempest from Tehachapi takes toll or Coccidioides conveyed aloft and afar. West J Med. 1978;129:527–530. [PMC free article] [PubMed] [Google Scholar]
- 54.Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, et al. Bat white-nose syndrome: an emerging fungal pathogen? Science. 2009;323:227. doi: 10.1126/science.1163874. [DOI] [PubMed] [Google Scholar]
- 55.Lorch JM, Meteyer CU, Behr MJ, Boyles JG, Cryan PM, et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature. 2011;480:376–378. doi: 10.1038/nature10590. [DOI] [PubMed] [Google Scholar]
- 56.Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, et al. An emerging disease causes regional population collapse of a common North American bat species. Science. 2010;329:679–682. doi: 10.1126/science.1188594. [DOI] [PubMed] [Google Scholar]
- 57.Hoyt JR, Langwig KE, Sun K, Lu G, Parise KL, et al. Host persistence or extinction from emerging infectious disease: insights from white-nose syndrome in endemic and invading regions. Proc Biol Sci. 2016;283:20152861. doi: 10.1098/rspb.2015.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.U.S. Fish and Wildlife Service North American bat death toll exceeds 5.5 million from white-nose syndrome. 2012. www.batcon.org/pdfs/USFWS_WNS_Mortality_2012_NR_FINAL.pdf
- 59.U.S. Fish and Wildlife Service White-nose syndrome. www.whitenosesyndrome.org/
- 60.Verant ML, Boyles JG, Waldrep W, Wibbelt G, Blehert DS. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS One. 2012;7:e46280. doi: 10.1371/journal.pone.0046280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson JS, Reeder DM, McMichael JW, Meierhofer MB, Stern DW, et al. Host, pathogen, and environmental characteristics predict white-nose syndrome mortality in captive little brown myotis (Myotis lucifugus) PLoS One. 2014;9:e112502. doi: 10.1371/journal.pone.0112502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reeder DM, Frank CL, Turner GG, Meteyer CU, Kurta A, et al. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS One. 2012;7:e38920. doi: 10.1371/journal.pone.0038920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, et al. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc Natl Acad Sci USA. 2012;109:6999–7003. doi: 10.1073/pnas.1200374109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verant ML, Meteyer CU, Speakman JR, Cryan PM, Lorch JM, et al. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 2014;14:10. doi: 10.1186/s12899-014-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalka MB, Smith AR, Kalko EK. Bats limit arthropods and herbivory in a tropical forest. Science. 2008;320:71. doi: 10.1126/science.1153352. [DOI] [PubMed] [Google Scholar]
- 66.Williams-Guillén K, Perfecto I, Vandermeer J. Bats limit insects in a neotropical agroforestry system. Science. 2008;320:70. doi: 10.1126/science.1152944. [DOI] [PubMed] [Google Scholar]
- 67.Boyles JG, Cryan PM, McCracken GF, Kunz TH. Conservation. Economic importance of bats in agriculture. Science. 2011;332:41–42. doi: 10.1126/science.1201366. [DOI] [PubMed] [Google Scholar]
- 68.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knapp RA, Briggs CJ, Smith TC, Maurer JR. Nowhere to hide: impact of a temperature-sensitive amphibian pathogen along an elevation gradient in the temperate zone. Ecosphere. 2011;2:art93. doi: 10.1890/ES11-00028.1. [DOI] [Google Scholar]
- 70.Voyles J, Young S, Berger L, Campbell C, Voyles WF, et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326:582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- 71.Schoegel Lm HJ, Berger L, Speare R, McDonald K, Daszak P. The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? J Herpetol. 2006;3:35–40. [Google Scholar]
- 72.Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, et al. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS One. 2013;8:e56802. doi: 10.1371/journal.pone.0056802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heyer WR, Rand AS, da Cruz CAG, Peixoto OL. Decimations, extinctions, and colonizations of frog populations in southeast Brazil and their evolutionary implications. Biotropica. 1988;20:230–235. doi: 10.2307/2388238. [DOI] [Google Scholar]
- 76.Lips KR. Mass mortality and population declines of anurans at an upland Site in Western Panama. Conservation Biology. 1999;13:117–125. doi: 10.1046/j.1523-1739.1999.97185.x. [DOI] [Google Scholar]
- 77.Laurance WF, McDonald KR, Speare R. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conservation Biology. 1996;10:406–413. doi: 10.1046/j.1523-1739.1996.10020406.x. [DOI] [Google Scholar]
- 78.Eskew EA, Todd BD. Parallels in amphibian and bat declines from pathogenic fungi. Emerg Infect Dis. 2013;19:379–385. doi: 10.3201/eid1903.120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kilpatrick AM, Briggs CJ, Daszak P. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol Evol. 2010;25:109–118. doi: 10.1016/j.tree.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Farrer RA, Weinert LA, Bielby J, Garner TW, Balloux F, et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc Natl Acad Sci USA. 2011;108:18732–18736. doi: 10.1073/pnas.1111915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wibbelt G, Kurth A, Hellmann D, Weishaar M, Barlow A, et al. White-nose syndrome fungus (Geomyces destructans) in bats, Europe. Emerg Infect Dis. 2010;16:1237–1243. doi: 10.3201/eid1608.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren P, Haman KH, Last LA, Rajkumar SS, Keel MK, et al. Clonal spread of Geomyces destructans among bats, midwestern and southern United States. Emerg Infect Dis. 2012;18:883–885. doi: 10.3201/eid1805.111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khush GS. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol. 2005;59:1–6. doi: 10.1007/s11103-005-2159-5. [DOI] [PubMed] [Google Scholar]
- 84.Ou SH. Pathogen variability and host-resistance in rice blast disease. Annu Rev Phytopathol. 1980;18:167–187. [Google Scholar]
- 85.Dean R, van Kan JA, Pretorius ZA, Hammond-Kosack KE, di Pietro A, et al. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fry WE, Goodwin SB. Resurgence of the Irish potato famine fungus. BioScience. 1997;47:363–371. doi: 10.2307/1313151. [DOI] [Google Scholar]
- 87.Padmanabhan SY. The great Bengal famine. Annu Rev Phytopathol. 1973;11:11–24. doi: 10.1146/annurev.py.11.090173.000303. [DOI] [Google Scholar]
- 88.Ullstrup AJ. The impacts of the southern corn leaf blight epidemics of 1970-1971. Annu Rev Phytopathol. 1972;10:37–50. doi: 10.1146/annurev.py.10.090172.000345. [DOI] [Google Scholar]
- 89.Peraica M, Radić B, Lucić A, Pavlović M. Toxic effects of mycotoxins in humans. Bull World Health Organ. 1999;77:754–766. [PMC free article] [PubMed] [Google Scholar]
- 90.Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101–134. doi: 10.1016/S0300-483X(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 91.Reddy KRN, Salleh B, Saad B, Abbas HK, Abel CA, et al. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010;29:3–26. doi: 10.3109/15569541003598553. [DOI] [Google Scholar]
- 92.Fink-Gremmels J. Mycotoxins: their implications for human and animal health. Vet Q. 1999;21:115–120. doi: 10.1080/01652176.1999.9695005. [DOI] [PubMed] [Google Scholar]
- 93.Miraglia M, Marvin HJ, Kleter GA, Battilani P, Brera C, et al. Climate change and food safety: an emerging issue with special focus on Europe. Food Chem Toxicol. 2009;47:1009–1021. doi: 10.1016/j.fct.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 94.Kebede H, Abbas HK, Fisher DK, Bellaloui N. Relationship between aflatoxin contamination and physiological responses of corn plants under drought and heat stress. Toxins. 2012;4:1385–1403. doi: 10.3390/toxins4111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bircan C, Barringer SA, Ulken U, Pehlivan R. Aflatoxin levels in dried figs, nuts and paprika for export from Turkey. Int J Food Sci Tech. 2008;43:1492–1498. doi: 10.1111/j.1365-2621.2008.01726.x. [DOI] [Google Scholar]
- 96.Kuiper-Goodman T. Food safety: mycotoxins and phycotoxins in perspective. In: Miraglia M, van Egmond H, Brera C, Gilbert J, editors. Mycotoxins and Phycotoxins – Developments in Chemistry, Toxicology and Food Safety. Fort Collins, CO: Alaken; 1998. pp. 25–48. (editors) [Google Scholar]