Abstract
Objectives:
To analyze the effect of celery leaf extract on blood glucose and plasma insulin levels in elderly pre-diabetics.
Methods:
This study was conducted between March and November 2014 at the Faculty of Medicine, Syiah Kuala University, Banda Aceh, Indonesia. A quasi-experimental pretest-posttest with a control group was conducted with elderly pre-diabetic volunteers. The subjects included 16 elderly pre-diabetics older than 60 (6 males and 10 females). The subjects were randomly divided into 2 groups: a control group (placebo-treated) and a treatment group (celery-treated). The treatment consisted of celery leaf extract capsules at the dose of 250 mg, 3 times per day (morning, afternoon and evening), 30 minutes before a meal, for 12 days. Data analysis was performed using the t-test (p<0.05).
Results:
There was a significant decrease in pre-prandial plasma glucose levels (p=0.01) and post-prandial plasma glucose levels (p=0.00), but no significant increase in plasma insulin levels (p=0.15) after celery leaf treatment in elderly pre-diabetics.
Conclusion:
Celery was effective at reducing blood glucose levels, but there was a lack of association between blood glucose levels and plasma insulin levels in elderly pre-diabetics.
Insulin is a hormone that regulates how carbohydrates are metabolized.1 It is produced by the b-cells of pancreas.1 Abnormalities in insulin secretion, insulin action, or both can lead to disturbances in glucose metabolism and occurrences of hyperglycemia.2 Clinically, hyperglycemia is one of the markers of pre-diabetes and diabetes.2,3 Pre-diabetes is a condition involving an increase in blood glucose levels above normal, but it does not meet the criteria for diabetes.3 The prevalence of pre-diabetes in Indonesia is roughly 10% of the total population.3 Chronic hyperglycemia and uncontrolled pre-diabetes are among the causes of diabetes mellitus (DM).4 Diabetes mellitus is a serious public health problem worldwide and is a social and economic burden.4,5 Indonesia is ranked fourth among countries with the largest number of diabetics, after India, China, and the United States.6 Diabetes mellitus is a chronic metabolic disease, a lifetime risk, and a silent killer. It can cause premature death.7-9 Uncontrolled DM can cause co-morbidities and multiple complications. Its complications include retinopathy (blindness), neuropathy, nephropathy (renal disease), ulceration, amputation and cardiovascular disease.2,10 Prevention and treatment of DM is necessary to control the surge in the number of diabetics.11-12 The principles of therapy for diabetic patients consist of lifestyle modification (diet and physical activity) and taking anti-diabetic drugs regularly.7-11 Long-term use of anti-diabetic drugs has an impact on economic costs for DM patients and national health care systems.10 Long-term use of anti-diabetic chemicals also has many side effects and complications, including bone problems, weight gain, and cardiovascular disease.2,13 Therefore, it is necessary to develop new alternative anti-diabetic drugs that have few side effects and are inexpensive. This challenge is still significant worldwide, including in Indonesia. Indonesians have long used medicinal plants, traditionally called “jamu”, to manage various diseases.14-15 Celery (Apium graveolens L.) in Bahasa Indonesian is called “seledri”. Celery is one of the medicinal plants that has potential as an anti-diabetic drug.16 It is one of more than 1200 plants with a hypoglycemic effect.16,17 Celery is low cost and is easily obtained in Indonesia. Therefore, we studied celery for its effects on anti-hyperglycemia in elderly pre-diabetics. A study of the literature revealed that celery had hypoglycemic activity, showing the necessity of conducting research.16,17 We conducted a preliminary study using male rats. The results of that study showed a significant decrease (63.3%; p=0.001) in blood glucose levels after 10 days of treatment with 50 mg/dL dose of celery. The results of this preliminary study formed the basis with which we conducted this study using pre-diabetic subjects.
Methods
Extraction procedure and preparation of celery capsules
The extraction procedure and preparation of the celery capsules were conducted in 3 stages: 1) Preparation of condensed celery: A total of 20 kg of fresh celery leaves were collected from a celery farm in Punge, Banda Aceh, Aceh Province, Indonesia. The fresh celery leaves were washed and dried at room temperature for as long as 3 days. The weight of the celery leaves after drying decreased to 2 kg. The dried celery leaves were mashed with a blender and extraction was accomplished via maceration. The maceration was carried out using as much as 40 liters of ethanol (EtOH) 96%. The dried celery leaves were mixed with ethanol 96% and then mixed well for about 30 minutes and allowed to stand for 24 hours. The maceration was conducted 3 times until the resulting solution became relatively clear and dilute. The macerated celery was filtered using flannel and filter paper to produce a celery filtrate. The celery filtrate was then evaporated using a rotary evaporator at a temperature of 40°C to produce a condensed extract of celery leaves. 2) Preparation of powder and granule celery: A total of 30 grams of the condensed celery leaf extract was added to 5 g of aerosil and mixed until homogeneous. The extract was then dried in an oven at a temperature of 50°C for 30 minutes. This process yielded a celery powder. A total of 15 g of celery powder was mixed with 10 g of Avicel PH 102, 0.15 g of Na benzoate, and 0.9 g of Polyvinylpyrrolidone K-30 (PVP K-30) to produce granules of celery extract. The granules were sieved using a sieve with 1.19 mm in size. The sieve was dried in the oven at 50°C for 30 minutes. The celery extract was tested for water content and weight uniformity using thin layer chromatography. 3) Preparation of the celery capsules. Celery capsules contain as much as 30% (75 mg) fillers, such as lactose, magnesium stearate, and aerosil. A total of 72,000 mg of celery leaf extract were inserted into the capsule (size 00) with a dose of 250 mg/capsule. The total number of capsules was 288. The capsules were stored in clean, dry, air tight, sealed bottles. The capsules were immediately administered and not stored for long periods of time.
Placebo capsules
The control group was treated with placebo capsules (size 00) at a dose of 250 mg containing lactose ± 30% (75 mg), magnesium stearate and aerosil.
Patients
The experimental research was conducted in this study by a quasi-experimental pretest-posttest with a control group. Research subjects were obtained from the elder care facility “Rumoh Seujahtera Geunaseh Sayang” in Ulee Kareng, Banda Aceh, Aceh Province, Indonesia. The total population was 62 elderly individuals (22 males and 40 females). Forty-eight elderly people volunteered to participate. We examined the blood glucose levels of these 48 elderly people and found that 22 of the subjects had hyperglycemia (pre-diabetes). However, 6 of these subjects did not satisfy our inclusion criteria, and the final sample was accordingly 16 people. The research subjects were elderly pre-diabetics with an age of at least 60 (6 males and 10 females).
All participants provided written informed consent to participate in our study. We divided the research subjects into 2 groups: a control group (n=8; 5 females and 3 males) and a treatment group (n=8; 5 females and 3 males). The subjects in each group were selected using a simple random sampling method with a lottery system. The control group was an untreated group (given a placebo). The treatment group was a treated group (given a capsule of celery leaf extracts).
Treatment and laboratory procedures
The treatment was celery extract capsules at a dose of 250 mg, given 3 times a day (morning, afternoon and evening), 30 minutes before meals and for the duration of 12 days. The dose of 250 mg of celery extract was chosen based on the conversion of an animal’s celery dose (a mouse) to a human dose. The calculation was as follows: the dose for mice is 100 mg/kg body weight (BW). The absolute dose is 100 mg/kg BW×0.02 kg=2 mg (the weight of mice is 20 g). Using the conversion factor 0.0026, the required dose for humans is 2 mg×387.9 = 775.8 mg (the dose for humans with an estimated BW of 70 kg). The dose was divided into 3 administration times: morning (250 mg), afternoon (250 mg) and evening (250 mg), and was given 30 minutes before meals (according to the treatment schedule of anti-diabetic drugs).
All of the participants were treated with the celery and the placebo for 12 days. We chose this duration based on the results of our preliminary study with hyperglycemic male rats. That study revealed that the administration of celery with a dose of 50 mg/kg BW daily for 10 days significantly decreased blood glucose levels in hyperglycemic male rats. Measurements of blood glucose levels (pre-prandial plasma glucose levels and post-prandial plasma glucose levels) and insulin were obtained twice, before and after treatment. The glucose oxidase method (GOD-PAP) was used to measure the level of the subjects’ blood glucose. An enzyme-linked immunosorbent assay (ELISA) was performed to measure the level of insulin in the subjects’ plasma.
Ethical clearance
The use of patients and protocols in this research was adapted to the principles of the Declaration of Helsinki. Ethical approval has been obtained from the ethics committee of the Faculty of Medicine, Syiah Kuala University, Banda Aceh, Indonesia (238/KE/FK/2014).
Statistical analysis
The statistical analysis was conducted using a test of homogeneity of variances according to a Levene statistic (p<0.05), a test of normality using the Kolmogorov-Smirnov test (p<0.05), and a paired samples t-test (p<0.05). The results of the statistical analysis indicated that the data were homogeneous and normally distributed. The paired sample t-test analysis was used to determine differences in blood glucose levels and plasma insulin levels between the placebo and the celery group.
Results
The characteristics of the research subjects, such as their ages, weights, and blood pressures did not significantly differ between the placebo group and the celery group (p<0.05), as shown in Table 1.
Table 1.
Physical characteristics of the subject of research.
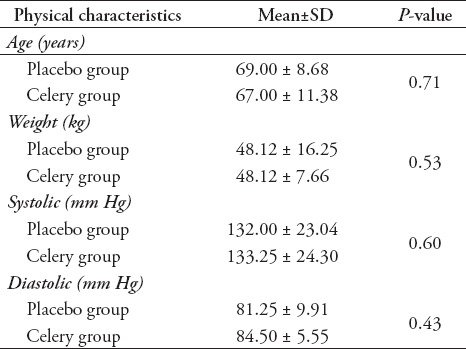
Figure 1 also shows that there was a difference in the untreated plasma insulin levels in the placebo and celery groups. The difference was caused by the fact that there were some patients who had very high levels of blood glucose in the celery group despite their not being aware of this situation. However, because of the limited number of patients in our cohort, we did not exclude elderly with very high blood sugar from this study. There was a different response to therapy in both groups. The results showed that plasma insulin levels decreased after therapy in the placebo group, whereas the opposite occurred in the celery group. The results of this study showed that plasma insulin levels slightly increase (1.16 µg; 0.08%) after treatment in the celery group. There was a significant decrease in insulin level after treatment (21.67 µg; 7.8%) in the placebo group, as shown in Figure 1.
Figure 1.

Description of plasma insulin levels before and after treatment in both groups.
Figure 2 shows that the levels of pre-prandial blood glucose increased after treatment in the placebo group. This effect was different from that observed in the celery group. Pre-prandial blood glucose levels decreased by 9.8% after celery therapy in the celery group. The results of this study indicate that celery can lower pre-prandial blood glucose levels in elderly patients with pre-diabetes.
Figure 2.

The decreasing of pre-prandial blood glucose levels in both groups.
Figure 3 shows an increase in post-prandial blood glucose levels after therapy in the placebo group. Patients in the celery group exhibited the opposite effect. Post-prandial blood glucose levels decreased by 19.5% after treatment in the celery group. These results indicate that celery can lower pre-prandial blood glucose and may also decrease post-prandial blood glucose levels in elderly people with pre-diabetes. The results also show that celery therapy has a better effect on decreased post-prandial blood glucose levels compared with decreased pre-prandial blood glucose levels in elderly pre-diabetics. The results of this study indicate that celery consumption as a pre-diabetic therapy in the elderly should be coupled with the provision of anti-diabetic drugs that have the effect of decreasing pre-prandial blood glucose levels.
Figure 3.

The reduction of postprandial blood glucose levels in both groups.
The results of homogeneity tests (p<0.05) of insulin and blood glucose levels before treatment (pretest) revealed that the data were homogenous. A Kolmogorov-Smirnov normality test demonstrated that the pretest data of insulin and blood glucose levels were normally distributed (p<0.05). A paired samples t-test (p<0.05) was used to determine the effect of treatment on insulin and blood glucose levels in placebo and celery group. The results of the paired samples t-test (p<0.05) are listed in Table 2.
Table 2.
Effects of treated on insulin, glucose pre-prandial and glucose postprandial levels in placebo and celery groups.

Table 2 shows that the mean values of pre-prandial glucose (p=0.01) and post-prandial glucose were significantly (p=0.00) decreased after treatment with capsules of celery leaf extract. The results showed that there was no significant (p=0.15) increase in insulin levels after treatment in celery and control group. The results of this study indicate that celery decreased pre-prandial blood glucose levels and post-prandial blood glucose levels but only slightly increased plasma insulin levels among elderly pre-diabetics. These findings may be due to several factors, namely: 1) The celery extract used was a pure extract rather than a synthesis of the chemical components of celery with anti-diabetic activity, such as flavonoids (kaempferol, quercetin, triterpenes and luteolin), 2) The duration of celery therapy, which needs to be considered for long-term administration, 3) Subjects were elderly people.
Discussion
The results of this study show that after treatment with celery, elderly pre-diabetics exhibited a significant decrease in pre-prandial and post-prandial blood glucose levels. However, there was no significant increase in plasma insulin levels. These results may be influenced by the fact that celery works at lowering blood glucose levels by affecting the absorption of glucose in the intestine, not by stimulating the production of insulin by the pancreas.16-18 Other possible explanations pertain to the duration of celery leaf extract treatment and the fact that the subjects were not specifically type 1 DM patients (Insulin-Dependent Diabetes Mellitus/IDDM) or type 2 DM (Noninsulin-Dependent Diabetes Mellitus/NIDDM). Type 1 diabetes is caused by damage to the pancreas, which leads the pancreas to little or no insulin.17-19 The use of elderly subjects is also one of the factors that may have influenced the results of this study. The elderly exhibit a decrease in almost all physiological functions, including the function of the pancreas.17-19 A manifestation of reduced glucose tolerance in the elderly is an increase in post-prandial glucose levels. Decreased relative insulin secretion and peripheral insulin resistance cause glucose intolerance.17-19
Another factor of this study was its sole reliance on celery leaf without a comparison with celery seed. Celery seed extract can lead to decreased blood glucose levels and increased serum insulin levels in diabetic rats.20 Celery seeds contain the flavonoids apigenin, luteolin, and phenolics.21-25 Apigenin inhibits the aldose reductase enzyme.20,21 This enzyme is a key enzyme in the polyol pathway (sorbitol-aldose-reductase pathway).21 The polyol pathway is a process that converts glucose to sorbitol.20,21 Increased levels of sorbitol in diabetic patients will lead to complications such as cataracts, retinopathy, and neuropathy.21 Therefore, celery can be used as an anti-diabetic and to prevent diabetic complications.26
Celery seeds possess anti-diabetic properties, and they stimulate increased insulin secretion by pancreatic beta cells as well as decreased gluconeogenesis in the liver.22,26,27 Histology test results have shown that celery seeds can improve the integrity of pancreatic beta cells.22,27 Celery also contains steroids, flavonoids (apigenin, apiin, isoquercitrin), alkaloids, carbohydrates, Vitamin A, Vitamin C, and glycosides.23-25 Celery leaves contain phenols, apigenin, luteolin, chrysoeriol 7-glucosides, furanocomarin, psoralen, bergapten, xanthotoxin, isopimpinellin, and phthalide.25, 26 Celery functions as an antioxidant, and flavonoids are one of the antioxidants contained in celery.26 Flavonoids play a role in neutralizing free radicals and preventing damage to pancreatic beta cells.14,24-26 Flavonoids control the absorption of glucose in the intestine, carbohydrate digestion, and glucose uptake with the regulation of the cell-signaling AMP-activated protein kinase pathways. They also improve glucose uptake in the skeletal muscle cells.25-28 Celery has some potent hypoglycemic agents, such as essential oils, phenolics, triterpenes, and flavonoids (kaempferol, quercetin, triterpenes and luteolin), which possess anti-diabetic activity.29 Flavonoids act as anti-diabetics by reducing apoptosis, increasing pancreatic beta cell proliferation, stimulating insulin secretion, controlling glucose metabolism in the liver and decreasing hyperglycemia.29 They also play a role in decreasing insulin resistance, decreasing inflammation in adiposity cells, and inhibiting oxidative stress in skeletal muscles.30 Kaempferol acts as an anti-diabetic due to its action in pancreatic beta cell protection, a function associated with type 2 diabetes.27 In rats, kaempferol has been reported to reduce hyperglycemia and to increase glucose uptake through the PI3K and protein kinase C (PKC) pathways in muscle.28 Oral therapy of kaempferol has been found to decrease fasting blood glucose and HbA1c and to increase insulin resistance.28
Quercetin plays a role in lowering plasma glucose levels in Alloxan-induced diabetic rats.29 Quercetin has been identified not only to contribute to GLUT-4 mRNA translocation to cell membranes in adipocytes and skeletal muscle cells but also to upregulate GLUT-4 mRNA levels.29 Quercetin and naringenin play a role in protecting beta cells from cytokine toxicity via the Phosphatidylinositol 3- kinase (PI3K) pathway. Quercetin has been found to reduce blood glucose levels through the Glucose Transporter 4 (GLUT-4) as well as through glycogenolysis and gluconeogenesis in the liver and to increase glucose uptake in the liver and to stimulate insulin secretion by pancreatic b-cells.30 Apigenin and luteolin have potential as Sodium-glucose Cotransporter-2 (SGLT-2) inhibitors in neuropathic diabetes.30 Research has shown that there is a significant decrease in blood glucose levels in apigenin-treated diabetic rats.27 Flavonoids play a role in the up-regulation of Glucose Transporter-1 (GLUT-1) expression levels and are useful for the treatment of type-2 DM (T2DM).31 Phenolics are known to increase glucose uptake and Glucose Transporter-4 (GLUT-4) expression.31
Apigenin acts as an anti-hyperglycemic agent.30 Diabetic rats treated with apigenin showed improvement in hyperglycemia levels and antioxidant status.32 Alloxan-induced diabetic rats showed a decrease in glucose levels and the ability to repair pancreatic beta cells after apigenin treatment.32 Luteolin has been investigated for its potential to increase insulin action and to stimulate GLUT-4 activity in diabetic rats, as well as to increase antioxidants in diabetic nephropathy. This antioxidant increases insulin secretion via the NF-kB and iNOS-NO signaling pathway.32
The literature review and the results of this study have shown that DM pathogenesis and diabetic complications are associated with oxidative stress.33 The formation and accumulation of free radicals in DM patients occur due to increased glucose oxidation, non-enzymatic glycation of proteins, and oxidative degradation of proteins.33 Abnormalities of free radicals and chronic declines in endogenous antioxidants result in damage to cell organelles and oxidative enzymes.33 That study showed that celery can act as an exogenous antioxidant in elderly pre-diabetics.31 Based on our results, the fact that this study did not exhibit an increase in plasma insulin levels was probably also caused by the method of treatment with a pure extract of celery leaf, which contains a variety of active substances. The use of a flavonoid extract of celery leaf is likely to have had different effects on plasma insulin levels.
Study limitations
A limitation of this study was its small sample size. It was therefore very weak as a clinical trial study. In addition, celery leaf extract was administered as a pure extract. The study should have been conducted using a comparison of the roots, stems, and seeds of the celery plant. This study was preliminary and is the basis for our continued research. The study can be expanded to adult diabetic patients, using a specific extract of celery such as flavonoids or other active chemical components of celery.
In conclusion, celery leaf extract reduced pre-prandial blood glucose levels and post-prandial blood glucose levels, but it slightly increased plasma insulin levels in elderly pre-diabetics. These findings indicate a lack of association between blood glucose levels and insulin plasma in elderly pre-diabetics.
Footnotes
References
- 1.Joshi SR, Parikh RM, Das AK. Insulin history, biochemistry, physiology and pharmacology. J Assoc Physicians India. 2007;55:19–25. [PubMed] [Google Scholar]
- 2.Mane K, Chaluvaraju K, Niranjan M, Zaranappa T, Manjuthej T. Review of insulin and its analogues in diabetes mellitus. J Basic Clin Pharm. 2012;3:283–293. doi: 10.4103/0976-0105.103822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soewondo P, Pramono LA. Prevalence, characteristics, and predictors of pre-diabetes in Indonesia. Med J Indones. 2011;20:283–294. [Google Scholar]
- 4.Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin?A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14:933–946. doi: 10.4158/EP.14.7.933. [DOI] [PubMed] [Google Scholar]
- 5.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Global report on diabetes. Geneva (CH): WHO Library Cataloguing-in-Publication Data; 2016. pp. 1–88. From: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf . [Google Scholar]
- 7.Fabiola A, John A, Seweng A, Khaing NE, Tai ES. Prevalence of Diabetes Mellitus among sub-urban pulation in Makassar, Indonesia [IJSR] International Journal of Science and Research. 2016;5:2014–2017. [Google Scholar]
- 8.Mihardja L, Delima Manz HS, Ghani L, Soegondo S. Prevalence and determinants of diabetes mellitus and impaired glucose tolerance in Indonesia (a part of basic health research/Riskesdas) Acta Med Indones. 2009;41:169–174. [PubMed] [Google Scholar]
- 9.Soewondo P, Ferrario A, Tahapary DL. Challenges in diabetes management in Indonesia: a literature review. Global Health. 2013;9:1–17. doi: 10.1186/1744-8603-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 12.Cheng D. Prevalence, predisposition and prevention of type II Diabetes. Nutr Metab. 2005;2:1–12. doi: 10.1186/1743-7075-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Wang J, Chan P. Treating type 2 Diabetes Mellitus with traditional chinese and indian medicinal herbs. Evid Based Complement Alternat Med. 2013;2013:343594. doi: 10.1155/2013/343594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aditama TY. Jamu dan kesehatan [Internet] 2nd ed. Jakarta, Indonesia: Lembaga Penerbit Badan Penelitian dan Pengembangan Kesehatan (LPB); 2015. p. 20. [Google Scholar]
- 15.[Traditional herbal medicine] Warta Ekspor. 2014;5:1–20. Indonesian. [Google Scholar]
- 16.Yaser AJ, Muneer A, Abdelhafid B, Daoudi CS A LH. Chemical and phytochemical analysis of some diabetic plants in Yemen. International research journal of pharmacy. 2013;4:72–76. [Google Scholar]
- 17.Roberto J, Almeida S, Souza GR. Glucose Tolerance [Internet] India: Intech publisher; 2012. Chapter 11: Medicinal Plants and Natural Compounds from the Genus Morus (oraceae) with Hypoglycemic Activity: A Review. [Google Scholar]
- 18.Choate CJ. Modern medicine and traditional Chinese medicine Diabetes Mellitus. J Chin Med. 1998:1. [Google Scholar]
- 19.Scheen AJ. Diabetes mellitus in the elderly: insulin resistance and/or impaired insulin secretion? Diabetes Metab. 2005;31:S27. doi: 10.1016/s1262-3636(05)73649-1. [DOI] [PubMed] [Google Scholar]
- 20.Chentli F, Azzoug S, Mahgoun S. Diabetes mellitus in elderly. Indian J Endocrinol Metab. 2015;19:744–752. doi: 10.4103/2230-8210.167553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niaz K, Gull S, Zia MA. Antihyperglycemic/hypoglycemic effect of celery seeds (ajwain/ajmod) in streptozotocin induced diabetic rats. Journal of Rawalpindi Medical College. 2013;17:134–137. [Google Scholar]
- 23.Tang WH, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87. doi: 10.3389/fphar.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Sa'aidi JA, Al-shihmani BA. Anti-hyperglycaemic and pancreatic regenerative effect of n-butanol extract of celery (Apium graveolens) seed in STZ- induced diabetic male rats. Research in Pharmaceutical Biotechnology. 2013;4:24–29. [Google Scholar]
- 25.Kooti W, Ali-akbari S, Asadi-samani M, Ghadery H, Ashtary-larky D. A review on medicinal plant of Apium graveolens. 2014;1:48–59. [Google Scholar]
- 26.Barnes J, Anderson LA. Herbal medicines. 3rd edition. Book. USA: Pharmaceutical Press; 2007. p. 146. [Google Scholar]
- 27.Al-Sa'aidi JAA, Alrodhan MNA, Ismael AK. Antioxidant activity of n-butanol extract of celery (Apium graveolens) seed in streptozotocin-induced diabetic male rats. Research in Pharmaceutical Biotechnology. 2012;4:24–29. [Google Scholar]
- 28.Ebrahimi E, Shirali S, Afrisham R. Effect and mechanism of herbal ingredients in improving diabetes mellitus complications. Jundishapur J Nat Pharm Prod. 2016:1–9. [Google Scholar]
- 29.Gutierrez RM, Juarez VA, Sauceda JV, Sosa IA. In vitro and in vivo antidiabetic and antiglycation properties of apium graveolens in type 1 and 2 diabetic rats. Int J Pharmacol. 2014;10:368–379. [Google Scholar]
- 30.Kawser Hossain M, Abdal Dayem A, Han J, Yin Y, Kim K, Kumar Saha S, et al. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci. 2016;17:569. doi: 10.3390/ijms17040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajiaghaalipour F, Khalilpourfarshbafi M, Arya A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int J Biol Sci. 2015;11:508–524. doi: 10.7150/ijbs.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab (Lond) 2015;12:60. doi: 10.1186/s12986-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukačínová A, Mojžiš J, Beňačka R, Keller J, Maguth T, Kurila P, et al. Preventive effects of flavonoids on alloxan-induced Diabetes Mellitus in rats. Acta Vet Brno. 2008;77:175–182. [Google Scholar]


