Abstract
The work describes a novel approach for sustained photobiological production of H2 gas via the reversible hydrogenase pathway in the green alga Chlamydomonas reinhardtii. This single-organism, two-stage H2 production method circumvents the severe O2 sensitivity of the reversible hydrogenase by temporally separating photosynthetic O2 evolution and carbon accumulation (stage 1) from the consumption of cellular metabolites and concomitant H2 production (stage 2). A transition from stage 1 to stage 2 was effected upon S deprivation of the culture, which reversibly inactivated photosystem II (PSII) and O2 evolution. Under these conditions, oxidative respiration by the cells in the light depleted O2 and caused anaerobiosis in the culture, which was necessary and sufficient for the induction of the reversible hydrogenase. Subsequently, sustained cellular H2 gas production was observed in the light but not in the dark. The mechanism of H2 production entailed protein consumption and electron transport from endogenous substrate to the cytochrome b6-f and PSI complexes in the chloroplast thylakoids. Light absorption by PSI was required for H2 evolution, suggesting that photoreduction of ferredoxin is followed by electron donation to the reversible hydrogenase. The latter catalyzes the reduction of protons to molecular H2 in the chloroplast stroma.
Interactions between molecular H2 and living matter are widespread in nature, and are facilitated by a diverse group of enzymes collectively known as “hydrogenases” (Adams, 1990; Albracht, 1994). Pathways of H2 metabolism vary widely among different prokaryotic and eukaryotic organisms (Hallenbeck and Benemann, 1979; Weaver et al., 1980; Hall et al., 1995; Appel and Schulz, 1998; Boichenko et al., 1999). H2 reactions can generally be divided into those that utilize the reducing power of H2 to drive metabolic processes (H2 consumption) and those that generate molecular H2. In the first category, many photosynthetic and non-photosynthetic organisms can grow by using H2 as the source of reductant (Weaver et al., 1980). In the second category, reduction of protons by hydrogenase (Voordouw and Brenner, 1985; Voordouw et al., 1989; Meyer and Gagnon, 1991; Peters et al., 1998) forms H2 gas, which serves to dissipate excess “electron pressure” within a cell. For example, anaerobic fermentative bacteria partially degrade organic C substrates to generate ATP. In the absence of an efficient electron sink (lack of O2), some of these organisms use protons as a terminal electron acceptor, thus releasing H2 and permitting additional degradative steps in their metabolic pathways (Schlegel and Schneider, 1978; Aoyama et al., 1997). Under low partial pressures of molecular N2, cyanobacterial heterocysts use reductant supplied in the form of sugars by vegetative cells and the enzyme nitrogenase to generate H2 from protons (Benemann and Weare, 1974; Hall et al., 1995).
In eukaryotic algae, photosynthetic H2 evolution has been detected transiently upon illumination (Gaffron and Rubin, 1942), but only after a period of dark, anaerobic incubation of the culture that “induces” the cell's ability to photoproduce H2 (Roessler and Lien, 1984; Happe et al., 1994; Ghirardi et al., 1997). Photosynthetic H2 evolution is accentuated under conditions of limiting CO2, suggesting that the hydrogenase pathway operates in competition with the CO2 fixation pathway in the consumption of chloroplast reductant (Kessler, 1973, 1974, 1976). Moreover, electron transport via the hydrogenase pathway is coupled to photosynthetic phosphorylation in the thylakoid membrane (Arnon et al., 1961), thus generating ATP, which is essential for the maintenance and repair functions of the cell (Melis, 1991).
Currently, photobiological production of H2 by eukaryotic algae is of interest because it holds the promise of generating a renewable fuel from nature's most plentiful resources, light and water. Green algae in particular can utilize the energy of sunlight in photosynthesis to extract electrons from water molecules on the oxidizing side of photosystem II (PSII). The potential energy of these electrons is increased, first at PSII and subsequently at photosystem I (PSI), in sequential light-driven reactions. Thus, electrons released upon the oxidation of water (Em7, +820 mV) are eventually transported to the Fe-S protein ferredoxin (Em7, −450 mV) on the reducing side of PSI. The so-called “reversible hydrogenase” in the stroma of the algal chloroplast (see below) accepts electrons from reduced ferredoxin and efficiently donates them to 2H+ to generate one H2 molecule:
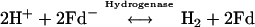 |
1 |
Since the Em7 for H2 oxidation is −420 mV and that for ferredoxin is −450 mV, it is thought that the equilibrium constant of the above reaction could be close to 1, and so the term “reversible” was assigned to the function of this hydrogenase.
The concept of direct biophotolysis (Benemann et al., 1973; Bishop et al., 1977; McBride et al., 1977; Weaver et al., 1980; Greenbaum, 1982, 1988; Miura, 1995) envisions light-driven simultaneous O2 evolution on the oxidizing side of PSII and H2 production on the reducing side of PSI, with a maximum H2:O2 (mol/mol) ratio of 2:1. In practice, this potential has not as yet materialized under ambient conditions because the reversible hydrogenase is extremely O2 sensitive and is promptly deactivated at <2% O2 partial pressure (Ghirardi et al., 1997). An alternative approach to photoproducing H2 is based on the concept of indirect biophotolysis, in which metabolite accumulation acts as an intermediary step between photosynthetic H2O oxidation and H2 production. In this approach, the two reactions, O2 evolution and H2 production, are spatially and/or temporally separated from each other (Benemann, 1996). The present work describes sustainable photosynthetic production of H2 in a two-stage indirect biophotolysis process in which O2 and H2 production are temporally separated. This process of H2 production was operated continuously for several days.
MATERIALS AND METHODS
Growth of the Algae
Chlamydomonas reinhardtii strain C137 (mt+) was grown photoheterotrophically in a Tris-acetate-phosphate medium, pH 7.0. Liquid cultures, bubbled with 3% CO2 in air, were grown at 25°C in flat bottles (3–5-cm optical path length) upon stirring and under continuous cool-white fluorescence illumination at approximately 200 μmol of photons m−2 s−1. Culture density was measured by cell counting with the improved Neubauer ultraplane hemacytometer and an BH-2 light microscope (Olympus, Tokyo) operated at a magnification of 200×. Cells were grown to the late logarithmic phase (about 3–6 × 106 cells/mL). After they reached this density, cells were suspended in the absence of S and incubated under continuous illumination for up to 150 h.
O2 Exchange and H2 Evolution Measurements
At the University of California (Berkeley), O2 exchange activity of the cultures was measured at 25°C with a Clark-type O2 electrode illuminated with a slide projector lamp. Yellow actinic excitation of saturating intensity was provided by a CS 3–69 cut-off filter (Corning, Corning, NY). A 5-mL aliquot of the culture was supplemented with 100 μL of 0.5 m NaHCO3, pH 7.4 (Melis et al., 1999). Measurements were taken with the O2 electrode, beginning with the registration of dark respiration in the cell suspension and followed by measurement of the light-saturated rate of O2 evolution. The rate of each process was recorded for about 5 min. At the National Renewable Energy Laboratory (Golden, CO), O2 and H2 evolution activities were measured with two different Clark-type electrodes, each poised for optimal measurement. Calibration of the electrodes was done as previously described (Seibert et al., 1998). Saturating actinic illumination of about 1,300 μmol photons m−2 s−1 was provided by a high-intensity actinic source (model 170-D, Nolan-Jenner) filtered through a 1% (w/v) CuSO4 solution. Samples for H2 evolution measurements were transferred from the culture bottle with Ar-flushed gas-tight syringes into the Ar-flushed Clark-type electrode chamber. The chamber was then bubbled with Ar for approximately 3 min to remove H2 dissolved into the growth medium. The H2 concentration signal from the electrode was amplified with an in-line amplifier (model 1201, Ithaco, Ithaca, NY) modified with a custom-built current-to-voltage converter, and analyzed with a data acquisition system (DT31-EZ A/D, Data Translation, Marlboro, MA) using customized DTVee software. Photosynthetic O2 evolution and oxidative respiration rates were measured as described above.
Gas Collection Measurements
Culture bottles (Schott or Roux type) were fitted with a number 25 thread (Ace, Vineland, NJ) and smaller side ports for liquid sampling. A threaded glass stopper with capillaries for gas sampling was fitted with an O-ring (Viton, DuPont-Dow Elastomers L.L.C., Wilmington, DE) and used to seal the reactor. Threaded side-arm and gas-sampling ports were sealed with rubber-laminated Teflon septa. Teflon tubing (HPLC, Aminco, Lake Forest, CA), attached to one of the gas ports, was used to conduct gas evolved by the algae in the culture bottles to an upside-down graduated cylinder filled with water. The gas collection tubing was detached from the culture bottle during liquid and gas sampling to avoid disturbance of gas volume readings in the graduated cylinder.
Determination of the Concentrations of CO2 and H2
A gas chromatograph (model 3760, Varian, Palo Alto, CA) with data analysis software (Star 4.0, Varian) was used to determine the levels of CO2 and H2 in the headspace of the reactor. A molecular sieve column (MS-5A, Supelco, Bellefonte, PA) with Ar as the carrier gas was used to separate O2, N2, and H2. A Porapak Q column (Supelco) with He as the carrier gas was used to assay for CO2. Signals were generated by the instrument's thermal conductivity detector. Dissolved CO2 was driven into the gas phase by injection of the liquid sample into 2 n hydrochloric acid in an Ar-flushed, septum-capped vial. The signals were calibrated by injection of known amounts of O2, N2, H2, and CO2.
Thylakoid Membrane Isolation and Analysis
Cells were harvested by centrifugation at 3,000g for 3 min at 4°C. Pellets were diluted with sonication buffer containing 100 mm Tris-HCl (pH 6.8), 10 mm NaCl, 1 mm p-aminobenzamidine-2HCl, 1 mm 6-aminocaproic acid, 10 mm EDTA, and 100 μm phenylmethylsulfonyl fluoride. Cells were disrupted by sonication for 2 min in a sonifier (Cell Disruptor 200, Branson, Danbury, CT) operated in the pulsed mode with a 50% duty cycle and an output power setting of 5. Unbroken cells and other large cell fragments were removed by centrifugation at 3,000g for 3 min at 4°C. The supernatant was then centrifuged at 75,000g for 30 min at 4°C. The chlorophyll (Chl) a + b content of the samples was measured in 80% (v/v) acetone by the method of Arnon (1949).
Spectrophotometric Measurements
The amplitude of the light minus dark absorbance difference measurements at 700 and 320 nm was employed for the direct quantitation of P700 and QA in the C. reinhardtii cultures (Melis, 1989, 1991). These measurements provided estimates of the concentration of functional PSI and PSII reaction centers, respectively, in the samples at various times following S deprivation. The amplitude of the hydroquinone-reduced minus ferricyanide-oxidized absorbance difference measurement at 554 nm, with isosbestic points at 544 and 560 nm, was employed in the quantitation of cytochrome f. Thylakoid membrane purification and preparation for these measurements were described previously (Melis et al., 1996).
Acetate, Starch, and Protein Quantitations
The level of acetate was measured in the supernatant of the culture following centrifugation of the algal cells at 1,000g for 2 min. A fully integrated HPLC (model 1050, Hewlett-Packard, Palo Alto, CA) with an ion-exchange column (Aminex HPX-87H, Bio-Rad, Hercules, CA) and UV detector was used for these measurements. H2SO4 (4 mm) served as the mobile phase to separate organic acids. The output signals were analyzed with Chemstation software (Hewlett-Packard). Starch determinations were performed according to the method of Gfeller and Gibbs (1984) using amyloglucosidase (Sigma, St. Louis) to convert starch from methanol-solubilized cells to Glc. The concentration of Glc was then determined using a D-Glc test kit (Boehringer Mannheim/Roche, Basel). This test depends upon two enzymatic reactions, the phosphorylation of Glc to Glc-6-P by hexokinase, and subsequent reduction of NAD+ to NADH by Glc-6-P. The amount of NADH accumulated was measured spectrophotometrically by determining the absorption change at 340 nm. Protein quantitation was implemented according to the method of Lowry et al. (1951).
RESULTS
Sustained Photobiological Production of H2 Gas in C. reinhardtii
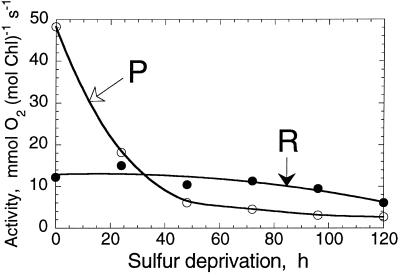
When C. reinhardtii cultures are deprived of inorganic S, the light-saturated rates of O2 evolution and CO2 fixation decline significantly within 24 h in the light, without a proportional loss of chloroplast or thylakoid membrane electron transport components (Davies et al., 1994; Yildiz et al., 1994). Analysis indicated that such loss in electron transport activity is due to the conversion of PSII centers from the QB-reducing to a QB-non-reducing form (Wykoff et al., 1998). The effect of inorganic S deprivation on photosynthesis and cellular respiration over a longer period of time (0–120 h) is shown in Figure 1. The activity of photosynthesis, measured from the light-saturated rate of O2 evolution in C. reinhardtii (Fig. 1, P), declined bi-exponentially from 48 mmol O2 mol−1 Chl s−1 at t = 0 h to less than 3 mmol O2 mol−1 Chl s−1 at t = 120 h. Cellular respiration, measured from the rate of O2 consumption in the dark (Fig. 1, R), remained fairly constant at approximately 13 mmol O2 mol−1 Chl s−1 over the 0- to 70-h period and declined slightly thereafter. It is important to note that the absolute activity of photosynthesis decreased below the level of respiration in C. reinhardtii after about 24 to 30 h of S deprivation.
Figure 1.
Absolute activity of oxygenic photosynthesis (P) and oxidative respiration (R) in C. reinhardtii cells suspended in a medium devoid of S. Incubation under S-deprived conditions started at 0 h. Cells were suspended in the presence of 10 mm NaHCO3, pH 7.6. The rate of cellular respiration was recorded in the dark, followed by a measurement of the rate of light-saturated photosynthesis. Rates of photosynthesis were corrected for the rate of dark respiration.
We reasoned that, sometime after about 24 to 30 h of S deprivation, a sealed C. reinhardtii culture would quickly become anaerobic in the light, due to the significantly greater rate of respiration than photosynthesis of the cells. This was indeed confirmed by measurements with a Clark-type O2 electrode (results not shown). It was of particular interest, therefore, to test whether the hydrogenase activity of the cells could be induced and sustained under these conditions. As shown below, anaerobiosis (but not darkness) is necessary and sufficient for induction of the reversible hydrogenase and for light-induced H2 production in C. reinhardtii.
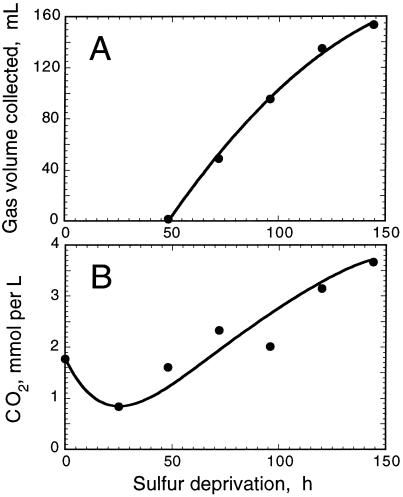
Figure 2 shows the results of such measurements, conducted at the National Renewable Energy Laboratory, with a S-deprived culture of C. reinhardtii. In this experiment, a 1-L culture of algae at a cell density of about 6 × 106 cells/mL was incubated in S-deprived medium under continuous illumination. The flask was sealed 42 h after S deprivation, when the rate of photosynthetic O2 evolution was determined to be equal to or less than the rate of respiration. H2 evolution activity measured with a Clark-type H2 electrode (Seibert et al., 1998) was detected in aliquots taken from the culture at t > 42 h (results not shown). Thus, S deprivation itself does not appear to exert a negative effect on the induction of the reversible hydrogenase. H2 gas accumulation was determined by measuring the amount of water that was displaced in an inverted graduated cylinder (Fig. 2A). The rate of gas accumulation was constant at approximately 2 mL h−1 (equivalent to 1.2 mmol H2 mol−1 Chl s−1) for up to about 120 h and slightly declined thereafter. Gas chromatographic analysis revealed that the composition of gases in the headspace of the culture bottle at 150 h was about 87% (v/v) H2, 1% (v/v) CO2, with the remainder being N2 and traces of O2.
Figure 2.
A, H2 gas volume accumulated by displacement of water in an inverted graduated cylinder as a function of cell incubation time in the absence of S. B, Quantitation of dissolved CO2 produced in tandem with H2 by S-deprived C. reinhardtii. The culture was sealed at about 42 h after suspension of the cells in a S-free medium. Values correspond to 1 L of culture.
In addition to H2, algal anaerobic photofermentations are expected to produce CO2 and small amounts of formate and ethanol (Gfeller and Gibbs, 1984). Figure 2B shows that the amount of dissolved CO2 (about 1.8 mmol per L) declined during the 0- to 30-h period and subsequently increased during the 50- to 150-h period from about 1.25 to about 3.7 mmol of CO2 L−1 culture. From the results of Figure 2 we estimated a H2:CO2 (mol/mol) ratio of about 2:1 for this process (see also Table I). The amount of gaseous CO2 in the headspace of the culture increased gradually from atmospheric values (0.03%) to about 1% during the course of the H2 production period. This corresponds to a rate of CO2 accumulation less than 0.5% of the rate of H2 accumulation (v/v), and is negligible compared with the amount of CO2 that accumulated in the liquid phase. Furthermore, the accumulation of fermentation by-products such as formate and ethanol was not detected.
Table I.
Substrate levels during H2 production in C. reinhardtii
| Substrate | Amount upon S Deprivation (0 h) | Amount upon Culture Sealing | Amount after 80 h of H2 Production | Changea during H2 Production |
|---|---|---|---|---|
| H2, mL | 0 | 0 | 140 | +140 |
| H2, mmol | 0 | 0 | 4.67 | +4.67 |
| CO2, mmol | 1.77 | 1.25 | 3.5 | +2.25 |
| Acetate, mmol | 15 | 7.6 | 8.2 | +0.6 (+8%) |
| Protein, mmol amino acids | 1.36 | 2.00 | 0.97 | −1.03 (−52%) |
| Starch, mmol Glc | 16 × 10−3 | 52 × 10−3 | 39 × 10−3 | −13 × 10−3 |
| (−25%) |
Values correspond to 1-L cultures with densities of 6 × 106 cells/mL at the time of sulfur deprivation (t = 0 h). H2 volume (mL) conversion to molarity (mmol) at 25°C assumed 29.97 L/mol at NREL (atmospheric pressure of 620 mm Hg at 1,600-m altitude) and 24.45 L/mol at Berkeley (atmospheric pressure of 760 mm Hg at sea level). Protein weight conversion to moles assumed an average amino acid molecular mass of 110 g/mol.
Change is defined as the absolute (or % in parentheses) difference between the entries of columns 4 and 3.
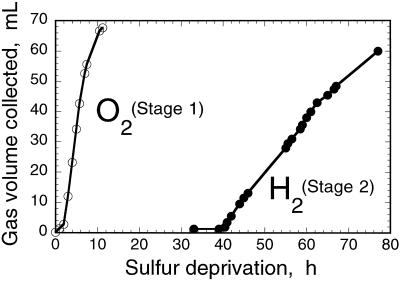
Figure 3 shows the result of experiments conducted at the University of California (Berkeley), in which S-deprived cultures were supplemented with 25 mm NaHCO3, pH 7.6, to serve as the substrate of oxygenic photosynthesis. C. reinhardtii cultures grown in a Roux bottle (850-mL capacity), and having a density of about 3 × 106 cells/mL, were incubated in the S-deprived medium in the light. Cultures were sealed at 0 h and O2 gas collection was measured with the inverted graduated cylinder setup (stage 1). In stage 1, the rate of O2 gas accumulation (estimated from the slope of the line in Fig. 3, O2) was about 12 mL O2 h−1 (equivalent to 25 mmol O2 mol−1 Chl s−1). This rate, not corrected for cellular respiration, is comparable to the average of the rates measured with a Clark-type O2 electrode between 0 and 10 h of S deprivation (Fig. 1, P). H2 gas accumulation was measured with the same setup at later times, following the onset of anaerobiosis in the sealed cultures (stage 2). The rate of H2 gas accumulation (Fig. 3, H2) was estimated to be about 2 mL H2 h−1 (equivalent to 4.1 mmol H2 mol−1 Chl s−1), which is less than 20% of the rate of O2 gas collected in the inverted graduated cylinder (Fig. 3, O2). The above results show a H2/O2 (mol/mol) ratio of 0.17:1. If the entire electron-transport capacity of the photosynthetic apparatus were directed toward H2 production in stage 2, then one would expect a theoretically maximum H2/O2 (mol/mol) ratio of 2:1 (Benemann et al., 1973; Bishop et al., 1977; McBride et al., 1977; Greenbaum, 1982, 1988; Miura, 1995). The results in Figures 2 and 3 suggest that this maximal yield of H2 production was not attained. Furthermore, the rate-limiting step in the above-described stage 1 → stage 2 H2-production process is not presently known.
Figure 3.
Stage 1 → stage 2 temporal separation of photosynthetic O2 and H2 gas production by C. reinhardtii cells suspended in a S-free medium. Gases were collected in inverted graduated cylinders by the displacement of water. ○, O2 (stage 1); ●, H2 (stage 2).
Structural and Functional Properties of the H2-Producing Photosynthetic Apparatus
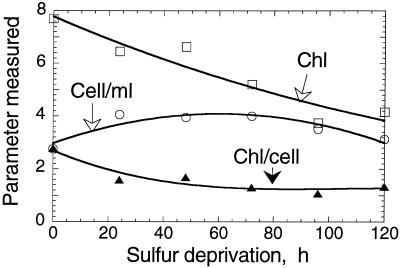
The Chl content of the cells and the composition of the thylakoid membrane in C. reinhardtii changed upon S deprivation. Figure 4 shows that the cell density of the culture increased transiently from about 3 × 106 cells/mL at 0 h to about 4 × 106 cells/mL at 60 h, and subsequently declined to 3 × 106 cells/mL at 120 h of S deprivation. Concomitantly, the Chl content of the culture declined steadily from about 8 μm to about 4 μm over the duration of this experiment. The Chl content per cell declined from about 2.8 × 10−15 mol Chl/cell to about 1 × 10−15 mol Chl/cell after 120 h of S deprivation. These results show that some cell division does occur during the first 60 h of S deprivation, but that a gradual loss of Chl also occurs throughout the deprivation period. Interestingly, the Chl a/b ratio of the cells increased only slightly (by about 10%–20%) in the 0- to 120-h S deprivation period.
Figure 4.
Chl concentration, cell density, and Chl content per cell in a S-deprived C. reinhardtii culture. Initial values at t = 0 h were: Chl = 7.7 μm, Cell/mL = 2.8 × 106, Chl/cell = 2.8 × 10−15 mol/cell.
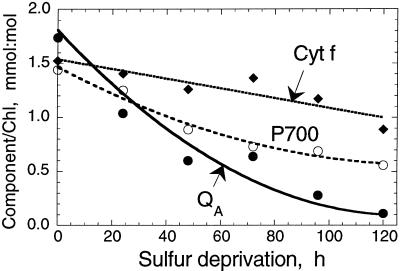
The concentration of integral thylakoid membrane complexes (PSII, Cyt b6-f, and PSI) in the thylakoid membrane of S-deprived C. reinhardtii was investigated spectrophotometrically as follows: (a) from the amplitude of the light-minus-dark absorbance change at 320 nm (measuring the photochemical reduction of the primary quinone acceptor QA of PSII); (b) from the amplitude of the light-minus-dark absorbance change at 700 nm (measuring the photochemical oxidation of the reaction center P700 of PSI); and (c) from the hydroquinone-reduced minus ferricyanide-oxidized difference spectra of cytochrome f in isolated thylakoid membranes (Melis et al., 1996). Figure 5 shows that the amount of all three functional components declined with time under S deprivation, with PSII (QA) declining faster than P700 and Cyt f.
Figure 5.
Concentration of functional PSII (QA), cytochrome b6-f complex (Cyt f), and PSI (P700) as a function of time in S-deprived C. reinhardtii.
The loss of PSII centers functional in charge separation (Fig. 5, QA, half-time of 40 h) was considerably slower than the loss of O2 evolution activity in the cells (Fig. 1, P, half-time of 20 h). These results are consistent with the notion that S deprivation first causes a conversion of PSII centers from the QB-reducing to the QB-nonreducing form (Wykoff et al., 1998), followed by a slower loss of PSII centers from the chloroplast thylakoids. This notion was supported by results of western-blot analyses with antibodies specific for the various reaction center proteins of PSII and PSI (not shown). Thus, the response of the cells to S deprivation suggests a strategy designed first to prevent the generation of O2, thus avoiding severe oxidative damage under conditions of limited protein biosynthesis, and, second, to recycle existing proteins, releasing S internally to be used in the biosynthesis of proteins indispensable for the survival of the organism.
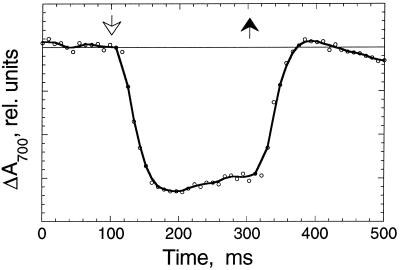
In the absence of functional PSII, the photobiological production of H2 requires the presence and operation of PSI. PSI is capable of generating reduced intermediates (e.g. reduced ferredoxin) with a sufficiently negative midpoint redox potential for the generation of molecular H2 (Redding et al., 1999). Figure 5 (Cyt f and P700) shows that significant amounts of Cyt f and P700 are retained in the thylakoid membrane throughout the 120-h S-deprivation period. Cytochrome b6-f and PSI are needed for the transport of electrons from organic substrate in a chlororespiration-type process (Moller and Lin, 1986; see also below) to ferredoxin and the reversible hydrogenase. PSI activity during this H2-production process, supported by electrons from endogenous substrate, was shown by in vivo measurements of the photooxidation and recovery kinetics of P700 in S-deprived cells that were suspended in the presence of the PSII electron transport inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU).
Figure 6 shows such a kinetic trace in which actinic excitation (administered at 100 ms) caused a negative absorbance change at 700 nm (oxidation of P700 in the sample). When actinic excitation was turned off at 300 ms, P700 was reduced promptly in the dark, with kinetics in the millisecond time range. The fast recovery of P700 in the dark suggests an abundance of electrons in the intersystem electron transport chain (plastoquinone, cytochrome b6-f, and plastocyanine). The presence or absence of DCMU had no effect on the observed light-induced oxidation or dark recovery kinetics (results not shown), which is consistent with the absence of electron donation by PSII. This repetitive light-induced oxidation and dark-recovery pattern was kinetically identical in all samples examined throughout the 120-h S-deprivation period, demonstrating the active operation of an electron-transport pathway that involves electron donation from organic substrate to the thylakoid membrane of C. reinhardtii, probably at the level of the plastoquinone pool.
Figure 6.
In vivo light-induced absorbance change measurements of P700 (ΔA700) in C. reinhardtii S-deprived for 48 h. Cells were suspended in the presence of 20 μm DCMU. The time response of the apparatus was limited through the use of electronic filters to 15 ms. Saturating blue actinic excitation (250 μmol photons m−2 s−1) came on at 100 ms (white arrow) and went off at 300 ms (black arrow).
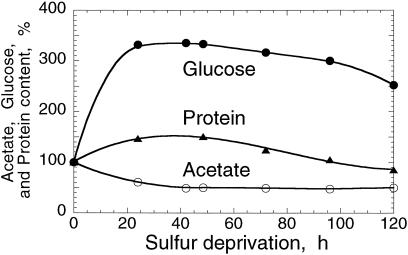
The role of various metabolites and the identity of the organic substrate that serves as the source of electrons for this photobiological H2 production were investigated. Acetate and starch are likely candidates for a chlororespiratory substrate in C. reinhardtii (Gibbs et al., 1986). Figure 7 (acetate) shows that the amount of acetate in the culture medium declined by about 50% during the 0- to 30-h period after S deprivation. However, it remained stable at this level during the 30- to 120-h period and even started to increase slightly thereafter (data points beyond 120 h not shown). These results suggest that acetate is consumed by respiration for as long as there is O2 in the culture medium (0–30 h), but it does not contribute significantly to the source of electrons in the H2-production process (30–120 h).
Figure 7.
Acetate (○), protein (▴), and starch (●, measured as total Glc) contents in C. reinhardtii as a function of time in the absence of S. The absolute values at zero time, corresponding to culture densities of 6 × 106 cells/mL, were: acetate = 15 μmol/mL, starch = 16 nmol Glc/mL, and protein = 150 μg/mL.
Consistent with this interpretation are measurements of the pH in the culture medium. The pH increased (from 7.5–8.2) during the 0- to 30-h period of aerobic incubation in the absence of S, consistent with the uptake and utilization of acetate, and the concomitant release of hydroxide anion as a by-product of this reaction. Once anaerobiosis was established (t > 30 h), however, this pH increase was gradually reversed (from 8.2–8.0), which is consistent with the notion of a light-dependent catabolic pathway that resulted in the formation of H2 gas and CO2. The majority of the released CO2 was trapped in the culture medium (Fig. 2), presumably as bicarbonate anion (CO2 + H2O → HCO3− + H+) due to the high pH value of the solution in the culture medium.
The amount of starch in the cells (equivalent to 16 nmol Glc mL−1 culture), increased transiently by about 330% in the first 25 h of S deprivation (Ball et al., 1990), and subsequently declined slightly during the S deprivation period (Fig. 7, Glc). Starch catabolism cannot be the source of the organic substrate that feeds electrons into the reversible hydrogenase pathway, because the absolute starch content of the culture (micromole quantities of Glc per liter) is not sufficient to account for the millimole quantities of H2 produced (see below). Quantitation of cellular protein in the S-deprived cultures showed that the amount of protein (150 μg per mL culture) also increased transiently to about 150% of the initial in the 0- to 30-h period. Thereafter, and concomitant with the H2 production activity, the level of protein in the culture declined to about 80% of the initial value at 120 h of S deprivation (Fig. 7, protein).
A quantitative summary of H2 production and substrate utilization data is given in Table I. Concomitant with the production of 4.67 mmol of H2, cells released 2.25 mmol of CO2 and a small amount of acetate into the medium. In addition, they consumed (presumably through catabolism) over 50% of the cellular protein, equivalent to about 1 mmol of amino acid. Starch content declined by about 25%, equivalent to 13 μmol of Glc, which is negligibly small to account for the production of 4.67 mmol of H2. A quantitative treatment of the results (i.e. the amount of H2 actually produced versus the protein consumed) suggests a H2/amino acid ratio of 4.5:1. On average, there are 10 gram atoms of H per amino acid for the 20-amino acid constituent of proteins, suggesting that protein consumption alone could suffice to provide the reductant needed for the light-dependent H2 production process.
These results do not preclude the possibility that consumption of other cellular constituents and metabolites may also, directly or indirectly, contribute reductant to the reversible hydrogenase pathway, leading to H2 production under these conditions. However, such a rigorous and detailed analysis is beyond the scope of the present work.
DISCUSSION
The ability of green algae to produce H2 directly from water has been recognized for over 55 years (Gaffron and Rubin, 1942). This activity is catalyzed by the reversible hydrogenase, an enzyme that is induced in the cells after exposure to a short period of anaerobiosis. However, the activity is rapidly lost as soon as the light is turned on, because of immediate deactivation of the reversible hydrogenase by photosynthetically generated O2. Although continuous purging of H2-producing cultures with inert gases has allowed for the sustained production of H2 for up to 160 h (Reeves and Greenbaum, 1985), such purging is expensive and impractical for large-scale mass cultures of algae. The use of exogenous reductants such as sodium dithionite, as well as the addition of herbicides to inhibit photosynthetic O2 evolution, create irreversible conditions that may lead to cell death. Consequently, the absence of a physiological way of surmounting the O2 sensitivity of hydrogenases has discouraged research on applied algal H2-production systems.
However, the results presented in this paper show a novel two-stage method to temporally separate O2 evolution and H2 production activities, thus allowing H2 production for extended periods of time without resorting to the use of the above-mentioned mechanical or chemical manipulations. The new method demonstrates, for the first time to our knowledge, the successful operation of a single-organism, two-stage photobiological H2 evolution process in a green alga. It is based on the concept of substrate S as a reversible switch to metabolically regulate the activity of the O2-evolving PSII complex (Wykoff et al., 1998). The reversibility of the method was tested successfully by cycling a single algal culture between the two stages (oxygenic photosynthesis and H2 production) for up to three full cycles (results not shown).
Why do C. reinhardtii cells produce molecular H2 under these conditions? The most likely explanation is that H2 evolution is the only mechanism available to the algae for generating sufficient amounts of ATP required for the survival of the organism under S-depleted anaerobic conditions. The main processes for ATP formation, mitochondrial respiration and oxygenic photosynthesis, are not available to sealed and S-deprived C. reinhardtii cells due to the lack of O2 and inactivation of PSII function, respectively. Electron transport from organic substrate through the plastoquinone pool and the Cyt b6-f complex can generate the required pH gradient across the thylakoid membrane for the generation of ATP. Light-dependent electron transport by PSI through ferredoxin and the reversible hydrogenase produces molecular H2 and sustains the electron transport process and thus the pH gradient. This overall process occurs at the expense of reductant that is eventually released into the environment in the form of gaseous H2. Cyclic electron transport around the Cyt b6-f complex and PSI, primed by electron donation from organic substrate, may also contribute to the generation of ATP. The consumption of protein under these conditions is important not only because it generates organic substrate to sustain the H2 production and ATP formation processes but also to release bio-organic S. The latter would thus become available for the de novo biosynthesis of proteins essential for the survival of the cells.
The establishment of anaerobiosis by S deprivation is an energy-dependent process that requires a carbon substrate for respiration. The main substrate for respiration in the initial 30 h of the S deprivation treatment is clearly acetate, as seen in Figure 7. As the culture becomes anaerobic, acetate consumption stops and does not appear to play a role in the H2 production process. Thus, the primary role of acetate is to help enhance cellular respiration and to establish anaerobiosis. This contention was supported by preliminary stage 1 → stage 2 H2 production measurements conducted with C. reinhardtii cultures grown and suspended in the absence of acetate. In the latter, a delay in the onset of anaerobiosis in the culture was observed, attributable in part to a slower inactivation of photosynthetic O2 evolution (half-time of about 60 h) and in part to lower rates of respiration in the absence of exogenous acetate (results not shown).
The H2 production process is light dependent and utilizes the chlororespiratory and reversible hydrogenase pathways under anaerobic conditions. The fermentative metabolism of C. reinhardtii in the light was studied extensively by Gibbs and co-workers (Gfeller and Gibbs, 1984; Gibbs et al., 1986; Maione and Gibbs, 1986). The main products of starch photofermentation in the presence of DCMU (an inhibitor of PSII electron transport and O2 evolution, whose addition brings about results similar to those described here) were found to be H2 and CO2 in a ratio of 2.8:1 (mol/mol) (Gfeller and Gibbs, 1984). Formate and ethanol were present in much smaller amounts, and no acetate accumulation was detected. In contrast to Gibbs' results, we did not observe a stoichiometric photoconversion of starch into H2 and CO2 under our experimental conditions, although we did observe a H2:CO2 production ratio of about 2:1 (mol/mol). As seen in Figure 7 and Table I, little starch appeared to have been mobilized during the H2-producing stage of the culture. However, significant consumption of protein took place concomitantly with H2 production, suggesting that protein is a primary substrate and a source of electrons for the chlororespiratory-type process that eventually feeds electrons into the reversible hydrogenase pathway. Clearly, more work is needed to accurately define the metabolic pathways involved and the stoichiometries of the substrate catabolized and H2 and CO2 generated in this photobiological H2 production process.
In summary, the ability of green algae to photoproduce H2 gas has been a biological curiosity for many years. Until now, only traces of H2 could be detected for very short periods of time using a Clark-type H2 electrode or a mass spectrometer. The present work shows, for the first time to our knowledge, that it is possible to produce and accumulate significant volumes of H2 gas using C. reinhardtii in a sustainable photobiological process that can be employed continuously for several days. The process depends on physiological treatment of the algal culture, not on mechanical or chemical manipulation of the cells. This single-organism, two-stage biophotolysis and H2 production process may serve as the basis for further research and development efforts that could generate renewable H2 for the fuel and chemical industries.
ACKNOWLEDGMENTS
We thank Dr. John R. Benemann for his critical reading of the manuscript and Dr. Elias Greenbaum for sharing his unpublished data. M.F. gratefully acknowledges support from the Swiss National Science Foundation in the form of a grant for prospective researchers.
Footnotes
The work was supported by the U.S. Department of Energy Hydrogen Research and Development Program under Department of Energy-University of California, Berkeley, Cooperative Agreement (no. DE–FC36–98GO10278 to A.M. and contract no. DE–AC36–98–GO10337 to M.L.G. and M.S.).
LITERATURE CITED
- Adams MWW. The structure and mechanism of iron-hydrogenases. Biochim Biophys Acta. 1990;1020:115–145. doi: 10.1016/0005-2728(90)90044-5. [DOI] [PubMed] [Google Scholar]
- Albracht SPJ. Nickel hydrogenases: in search of the active site. Biochim Biophys Acta. 1994;1188:167–204. doi: 10.1016/0005-2728(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Uemura I, Miyake J, Asada Y. Fermentative metabolism to produce hydrogen gas and organic compounds in a cyanobacterium, Spirulina platensis. J Ferment Bioenerg. 1997;83:17–20. [Google Scholar]
- Appel J, Schulz R. Hydrogen metabolism in organisms with oxygenic photosynthesis: hydrogenases as important regulatory devices for a proper redox poising? J Photochem Photobiol. 1998;47:1–11. [Google Scholar]
- Arnon D. Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–5. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI, Mitsui A, Paneque A. Photoproduction of hydrogen gas coupled with photosynthetic phosphorylation. Science. 1961;134:1425–1425. [Google Scholar]
- Ball SG, Dirick L, Decq A, Martiat J-C, Matagne RF. Physiology of starch storage in the monocellular alga Chlamydomonas reinhardtii. Plant Science. 1990;66:1–9. [Google Scholar]
- Benemann JR. Hydrogen biotechnology: progress and prospects. Nature Biotechnol. 1996;14:1101–1103. doi: 10.1038/nbt0996-1101. [DOI] [PubMed] [Google Scholar]
- Benemann JR, Berenson JA, Kaplan NO, Kamen MD. Hydrogen evolution by a chloroplast-ferredoxin-hydrogenase sustem. Proc Natl Acad Sci USA. 1973;70:2317–2320. doi: 10.1073/pnas.70.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benemann JR, Weare NM. Hydrogen evolution by nitrogen-fixing Anabaena cylindrica cultures. Science. 1974;184:174–175. doi: 10.1126/science.184.4133.174. [DOI] [PubMed] [Google Scholar]
- Bishop NI, Frick M, Jones LW. Photohydrogen production in green algae: water serves as the primary substrate for hydrogen and oxygen production. In: Mitsui A, Miyachi S, San Pietro A, Tamura S, editors. Biological Solar Energy Conversion. New York: Academic Press; 1977. pp. 3–22. [Google Scholar]
- Boichenko VA, Greenbaum E, Seibert M. Hydrogen production by photosynthetic microorganisms. In: Archer MD, Barber J, editors. Photoconversion of Solar Energy: Molecular to Global Photosynthesis. Vol. 2. London: Imperial College Press; 1999. (in press) [Google Scholar]
- Davies JP, Yildiz F, Grossman AR. Mutants of Chlamydomonas with aberrant responses to sulfur deprivation. Plant Cell. 1994;6:53–63. doi: 10.1105/tpc.6.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffron H, Rubin J. Fermentative and photochemical production of hydrogen in algae. J Gen Physiol. 1942;26:219–240. doi: 10.1085/jgp.26.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller RP, Gibbs M. Fermentative metabolism of Chlamydomonas reinhardtii: I. Analysis of fermentative products from starch in dark and light. Plant Physiol. 1984;75:212–218. doi: 10.1104/pp.75.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi ML, Togasaki RK, Seibert M. Oxygen sensitivity of algal H2-production. Appl Biochem Biotech. 1997;63:141–151. doi: 10.1007/BF02920420. [DOI] [PubMed] [Google Scholar]
- Gibbs M, Gfeller RP, Chen C. Fermentative metabolism of Chlamydomonas reinhardtii: II. Photoassimilation of acetate. Plant Physiol. 1986;82:160–166. doi: 10.1104/pp.82.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum E. Photosynthetic hydrogen and oxygen production: kinetic studies. Science. 1982;196:879–880. doi: 10.1126/science.215.4530.291. [DOI] [PubMed] [Google Scholar]
- Greenbaum E. Energetic efficiency of hydrogen photoevolution by algal water-splitting. Biophys J. 1988;54:365–368. doi: 10.1016/S0006-3495(88)82968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DO, Markov SA, Watanabe Y, Rao KK. The potential applications of cyanobacterial photosynthesis for clean technologies. Photosynth Res. 1995;46:159–167. doi: 10.1007/BF00020426. [DOI] [PubMed] [Google Scholar]
- Hallenbeck PC, Benemann JR. Hydrogen from algae. In: Barber J, editor. Photosynthesis in Relation to Model Systems. New York: Elsevier/North-Holland Biomedical Press; 1979. pp. 331–364. [Google Scholar]
- Happe T, Mosler B, Naber JD. Induction, localization and metal content of hydrogenase in the green alga Chlamydomonas reinhardtii. Eur J Biochem. 1994;222:769–774. doi: 10.1111/j.1432-1033.1994.tb18923.x. [DOI] [PubMed] [Google Scholar]
- Kessler E. Effect of anaerobiosis on photosynthetic reactions and nitrogen metabolism of algae with and without hydrogenase. Arch Microbiol. 1973;93:91–100. doi: 10.1007/BF00424940. [DOI] [PubMed] [Google Scholar]
- Kessler E. Algal Physiology and Biochemistry. Oxford: Blackwell; 1974. Hydrogenase, photoreduction and anaerobic growth of algae; pp. 454–473. [Google Scholar]
- Kessler E. Hydrogen metabolism of eukaryotic organisms. In: Schlegel HG, Gottschalk G, Pfennig N, editors. Microbial Production and Utilization of Gases. Germany: Göttingen; 1976. pp. 247–254. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maione TE, Gibbs M. Hydrogenase-mediated activities in isolated chloroplasts of Chlamydomonas reinhardii. Plant Physiol. 1986;80:360–368. doi: 10.1104/pp.80.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AC, Lien S, Togasaki RK, San Pietro A. Mutational analysis of Chlamydomonas reinhardi: application to biological solar energy conversion. In: Mitsui A, Miyachi S, San Pietro A, Tamura S, editors. Biological Solar Energy Conversion. New York: Academic Press; 1977. pp. 77–86. [Google Scholar]
- Melis A. Spectroscopic methods in photosynthesis: photosystem stoichiometry and chlorophyll antenna size. Phil Trans R Soc Lond B. 1989;323:397–409. [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. [Google Scholar]
- Melis A, Murakami A, Nemson JA, Aizawa K, Ohki K, Fujita Y. Chromatic regulation in Chlamydomonas reinhardtii alters photosystem stoichiometry and improves the quantum efficiency of photosynthesis. Photosynth Res. 1996;47:253–265. doi: 10.1007/BF02184286. [DOI] [PubMed] [Google Scholar]
- Melis A, Neidhardt J, Benemann JR. Dunaliella salina (Chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J Appl Phycol. 1999;10:515–525. [Google Scholar]
- Meyer J, Gagnon J. Primary structure of hydrogenase I from Clostridium pasterianum. Biochemistry. 1991;30:9697–9704. doi: 10.1021/bi00104a018. [DOI] [PubMed] [Google Scholar]
- Miura Y. Hydrogen production by biophotolysis based on microalgal photosynthesis. Proc Biochem. 1995;30:1–7. [Google Scholar]
- Moller IM, Lin W. Membrane-bound NAD(P) H dehydrogenases in higher plant cells. Annu Rev Plant Physiol. 1986;37:309–334. [Google Scholar]
- Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC. X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science. 1998;282:1853–1858. doi: 10.1126/science.282.5395.1853. [DOI] [PubMed] [Google Scholar]
- Redding K, Cournac L, Vassiliev IR, Golbeck JH, Peltier G, Rochaix J-D. Photosystem I is indispensable for photoautotrophic growth, CO2 fixation, and H2 photoproduction in Chlamydomonas reinhardtii. J Biol Chem. 1999;274:10466–10473. doi: 10.1074/jbc.274.15.10466. [DOI] [PubMed] [Google Scholar]
- Reeves ME, Greenbaum E. Long-term endurance and selection studies in hydrogen and oxygen photoproduction by Chlamydomonas reinhardtii. Enzyme Microb Technol. 1985;10:169–174. [Google Scholar]
- Roessler PG, Lien S. Activation and de novo synthesis of hydrogenase in Chlamydomonas. Plant Physiol. 1984;76:1086–1089. doi: 10.1104/pp.76.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel HG, Schneider K. In: Hydrogenases: Their Catalytic Activity, Structure and Function. Schlegel HG, Schneider K, editors. Germany: Göttingen; 1978. pp. 15–44. [Google Scholar]
- Seibert M, Flynn T, Benson D, Tracy E, Ghirardi ML. Development of selection and screening procedures for rapid identification of H2-producing algal mutants with increased O2 tolerance. In: Zaborsky O, editor. Biohydrogen. New York: Plenum Press; 1998. pp. 227–234. [Google Scholar]
- Voordouw G, Brenner S. Nucleotide sequence of the gene encoding the hydrogenase from Desulfovibrio vulgaris. Eur J Biochem. 1985;148:515–520. doi: 10.1111/j.1432-1033.1985.tb08869.x. [DOI] [PubMed] [Google Scholar]
- Voordouw G, Strang JD, Wilson FR. Organization of the genes encoding [Fe] hydrogenase in Desulfovibrio vulgaris. J Bacteriol. 1989;171:3881–3889. doi: 10.1128/jb.171.7.3881-3889.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver PF, Lien S, Seibert M. Photobiological production of hydrogen. Sol Energy. 1980;24:3–45. [Google Scholar]
- Wykoff DD, Davies JP, Melis A, Grossman AR. The regulation of photosynthetic electron-transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol. 1998;117:129–139. doi: 10.1104/pp.117.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Davies JP, Grossman AR. Characterization of sulfate transport in Chlamydomonas reinhardtii during sulfur-limited and sulfur-sufficient growth. Plant Physiol. 1994;104:981–987. doi: 10.1104/pp.104.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]