Abstract
Objectives:
To identify the prevalence of carbapenem-resistant Klebsiella pneumoniae (CRKP) and the most common types of cabapenemases among CRKP in the Southern (Asir) province hospitals, Saudi Arabia.
Methods:
The cross-sectional study was conducted between late April and September in 2015. A total of 54 Klebsiella pneumoniae (K. pneumoniae) isolates with reduced sensitivity to carbapenems were obtained from various clinical specimens of the 2 largest hospitals in the Southern province. Minimum inhibitory concentrations (MICs) of carbapenems were confirmed using E-test. Molecular detection of the most common carbapenemase genes (blaIMP, bla-carbapenem-hydrolyzing oxacillinase [OXA-48], blaVIM, bla-New Delhi metallo-ß-lactamas [NDM], and blaKPC) was performed using multiplex-polymerase chain reaction.
Results:
The current study found that increasing age and intensive care unit admission were associated with CRKP isolation. The major type of carbapenemases was OXA-48 with 81.5% (n=44) and it seems to reach an endemic level. New Delhi metallo-ß-lactamas (NDM) was the second most frequent carbapenemase by 7.4% (n=4) of isolates while Verona integron-encoded metallo-ß-lactamase (VIM) was reported only in one isolate.
Conclusion:
Saudi Arabia receives large numbers of visitors and migrant workers from OXA-48 and NDM endemic countries such as Turkey, India, and Pakistan every year.
Although Enterobacteriaceae are found normally in intestines, extended-spectrum b-lactamases (ESBLs) producing gram negative isolates including Enterobacteriaceae represent a great challenge to the international medical community. These bacteria can hydrolyze many antimicrobial agents including penicillins as well as the third generation of cephalosporins and monobactams. These species include Enterobacter cloacae (E. cloacae), Klebsiella pneumoniae (K. pneumoniae) and Escherichia coli (E. coli).1 On the other hand, Carbapenems have the ability to resist many b-lactamase enzymes and are therefore considered the last treatment option for serious infections caused by ESBLs-producing Enterobacteriaceae.2 The overuse of these antibiotics has contributed to the emergence of carbapenem-resistant Enterobacteriaceae as well as the first carbapenemase producer in Enterobacteriaceae (NmcA) that was reported in 1993 among E. cloacae isolates.3,4 Carbapenem-resistant Enterobacteriaceae (CRE) are capable of inactivating carbapenem via the production of carbapenemase enzymes. Many carbapenemases have been identified and categorized into many classes. The Ambler classes A (KPC), B (VIM, IMP, NDM) and D (OXA-48) categories are considered the most clinically important carbapenmases. These classes are commonly found in K. pneumoniae isolates that have been associated with serious nosocomial infections.2,4 The dissemination of KPC, VIM, IMP, NDM and OXA-48 among K. pneumoniae has also been reported in different countries. For example, NDM-1 (New Delhi metallo-b-lactamase) was first identified in India and was recently reported in Asia, North America, Europe, and Australia.5 Similarly, OXA-48 had first been identified in K. pneumoniae in Turkey and it has recently been reported in Europe and the Middle East.6 According to the Center for Disease Control and Prevention (CDC) in the USA, approximately 8.7% of Klebsiella nosocomial isolates in 2006-2007 were carbapenem-resistant compared to less than 1% in 2000.7 Consequently, carbapenem-resistant K. pneumoniae (CRKP) isolates are becoming an increasingly global concern. Although few studies have been conducted on CRKP isolates in the Arabian Peninsula, almost all countries in the Gulf Cooperation Council (GCC) share the same ESBL and cabapenemase-producing Enterobacteriaceae, most of which were recovered from nosocomial infections. Moreover, a recent review of b-lactamase producing gram-negative bacilli from GCC states showed that b-lactamases genes such as OXA-48, CTX-M-15, and NDM-1 are the most common and widespread b-lactamases.8 In addition, several studies have shown the emergence of OXA-48, NDM-1, and VIM in many K. pneumoniae isolates in Saudi Arabian Riyadh hospitals. Most of those isolates were recovered from critically ill patients in intensive care units (ICUs) and were associated with high mortality rates.2,9 Recently, Zowawi et al8 highlighted that only limited studies have been conducted in Saudi Arabia to identify and characterize ESBL genotypes. Zowawi et al8 also suggest that the developing regional surveillance of antibiotic resistance is urgent to detect multi-drug resistant (MDR) isolates (namely CRKP).
The aim of this study was initially to identify the prevalence of CRKP in the Southern (Asir) province of Saudi Arabia. Ultimately, we aim to identify the most common types of cabapenemases among CRKP in this geographical region.
Methods
This cross-sectional study was conducted between late April and September in 2015 in the 2 largest hospitals in Abha city in the Southern province of Saudi Arabia. These hospitals were the Asir Central Hospital (ACH) and the Armed Force Hospital Southern Region (AFHSR). Ethical approval was obtained from the Ethics Committee of both hospitals before commencement of the study. Clinical information of the isolates was collected and recorded in a Microsoft Excel database. The isolates were coded to facilitate cross-referencing between samples. Though, no patient names were supplied.
Inclusion and exclusion criteria
Isolates of K. pneumoniae that showed reduced sensitivity to carbapenems from male or female patients (all ages) from all hospital units were included. Klebsiella pneumoniae isolates that were sensitive to carbapenems were excluded. Duplicate isolates from the same patient were also excluded, unless they were isolated from different specimens with a distinguishable susceptibility pattern.
Collection and identification of isolates
Fifty-four K. pneumoniae isolates that showed reduced sensitivity to carbapenems were collected from different clinical specimens from the 2 hospitals. All isolates were identified in a microbiology laboratory using the Vitek-2 identification system (BioMerieux, France) and the GN card according to the standard identification methods used by both hospitals. From pure culture on MacConkey agar plates, all identified K. pneumoniae isolates were transferred to 1.5 ml Eppendorf tubes contain LB Broth with 20% (v/v) glycerol and were maintained at -80ºC for long-term storage.
Control strains
The following strains were used as positive controls: K. pneumoniae NCTC 13438 as a positive control for KPC, NCTC 13443 for NDM-1, NCTC 13440 for VIM-1, NCTC 13442 for OXA-48 and E. coli NCTC 13476 for IMP. Also, E. coli NCTC 10418 was used as a negative control.
Antibiotic susceptibility testing (AST)
Antimicrobial susceptibility testing was carried out using the Vitek-2 system (BioMerieux, France), according to the manufacturer’s instructions. The antimicrobial agents included: Ampicillin, Piperacillin-Tazobactam, Amoxicillin-Clavulanate, Ceftazidime, Imipenem, Cefepime, Meropenem, Amikacin, Gentamicin, Ciprofloxacin, Tigecycline, Trimethoprim-Sulfamethoxazole.
Minimum inhibitory concentration value of imipenem and meropenem was tested against all isolates using an E-test (BioMérieux, France). E-tests were performed and interpreted according to the manufacturer’s instructions. The E-test MIC concentration gradient of both antibiotics was between 0.0025 µg/ml to ≥32 µg/ml.
Phenotypic detection of KPC/MBLs enzymes and AmpC activity
A carbapenemase detection set (D70C disc, Mast Group LTD, Merseyside, UK) was used to identify carbapenemase (KPC/MBLs enzymes and AmpC activity) for all isolates. This method was performed and interpreted according to manufacturer’s instructions.
Molecular detection of carbapenemase genes using multiplex-PCR
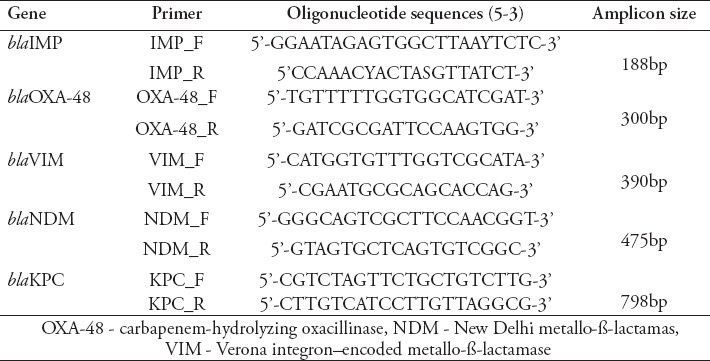
All K. pneumoniae isolates were tested for common carbapenemase genes (blaIMP, blaOXA-48, blaVIM, blaNDM, and blaKPC). Multiplex-PCR was performed according to the protocol of Zarakolu et al10 using a series of primers (Table 1) that were obtained from Macrogen (Seoul, South Korea).
Table 1.
Primers used in this study.
DNA extraction
From overnight cultures, 2 colonies were transferred into microcentrifuge tubes, each containing 300 µl of sterile distilled water with 5-10% Chelex 100 (HiMedia, India). The cell suspension was mixed thoroughly and heated for 10 minutes at 96°C. The cell suspension was then transferred into an ice tray for 2 minutes. Finally, the suspension was centrifuged for 5 minutes at 13,000g to create supernatant containing bacterial DNA. Two microliters of the supernatant was used for PCR reaction.
Multiplex PCR conditions
Multiplex PCR was carried out for all isolates according to previously described methods in Zarakolu et al.10 Briefly, a 25 µl PCR reaction containing 12.5µl of Taq PCR master mix (Qiagen, Germany), 0.5 µl sterile RNase free water and 2 µM of each primer (1 µl of 50 µM) and 2 µl of DNA template underwent PCR amplification. Amplification steps include a 5 min denaturation at 95ºC, followed by 36 cycles of 94°C for 45 sec, 53°C for 45 sec, and 72°C for 1 minute. Final extension was at 72°C for 6 min.
Agarose gel electrophoresis
The resulting amplicons were separated using a 1.5% agarose (Promega, USA) gel containing 0.5 µg/µl Ethidium Bromide. The gel was electrophoresed in 1x TBE buffer at 100 V for 55 min in an electrophoreses system (Bio-Rad, USA). A 50 bp ladder was used as a molecular size marker. DNA bands were visualized with a gel documentation system from Syngene (UK).
Statistical analysis
All data were stored in Microsoft Excel, Version 2016. Data management and statistical analyses were also performed in Excel. The descriptive statistics of the data and variables are shown in the form of frequencies and percentages.
Results
During the period of examination, 54 K. pneumoniae isolates that showed reduced sensitivity to carbapenems were recovered from the 2 hospitals (ACH and AFHSR) in the Southern province of Saudi Arabia. Thirty-four (63%) patients were from ACH and 20 patients (37%) were from AFHSR. Seventy-four percent of the patients (n=40) were male and 26% (n=14) were female (Table 2). Ages ranged from one month to 90 years (mean age = 49.39 year). Isolates were collected from blood (n=13), sputum (n=10), urine (n=8), wound swab (n=6), tracheal aspirate (n=5) and body fluid (n=5). Most K. pneumoniae isolates (n=23) were isolated from patients in ICUs followed by the male medical ward (MMW) (n=7) and the male surgical ward (MSW) (n=7). Two isolate samples each were collected from the male neuro ward (MNW), emergency room (ER), outpatient department (OPD), and female surgical ward (FSW) (Table 2).
Table 2.
Demographic information and clinical characteristics of CRKP patients in the Southern province of Saudi Arabia.
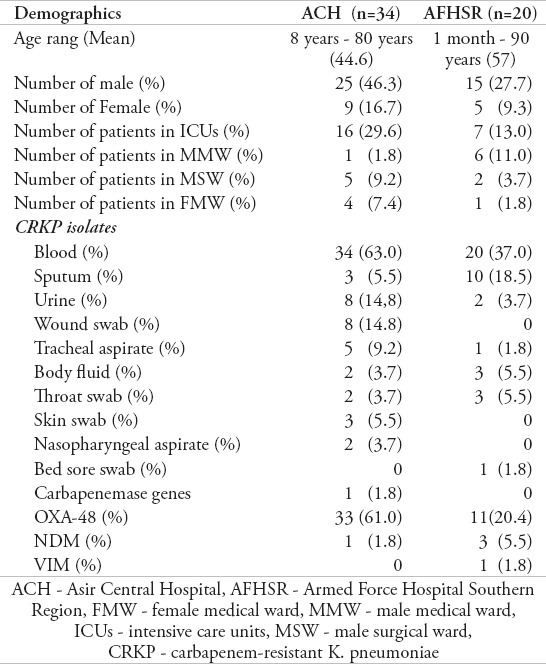
ACH - Asir Central Hospital, AFHSR - Armed Force Hospital Southern Region, FMW - female medical ward, MMW - male medical ward, ICUs - intensive care units, MSW - male surgical ward, CRKP - carbapenem-resistant K. pneumoniae
Antibiotic susceptibility test (AST)
All 54 K. pneumoniae isolates were resistant to amoxicillin-clavulanate and ampicillin. Also, all isolates showed a high level of resistance against ciprofloxacin, piperacillin-tazobactam, ceftazidime, cefepime, amikacin, and gentamicin. Approximately, 63% and 57.4% of isolates were resistant to the carbapenems, imipenem, and meropenem, respectively. Conversely, tigecycline was relatively effective against 31 (57.4%) of the tested isolates.
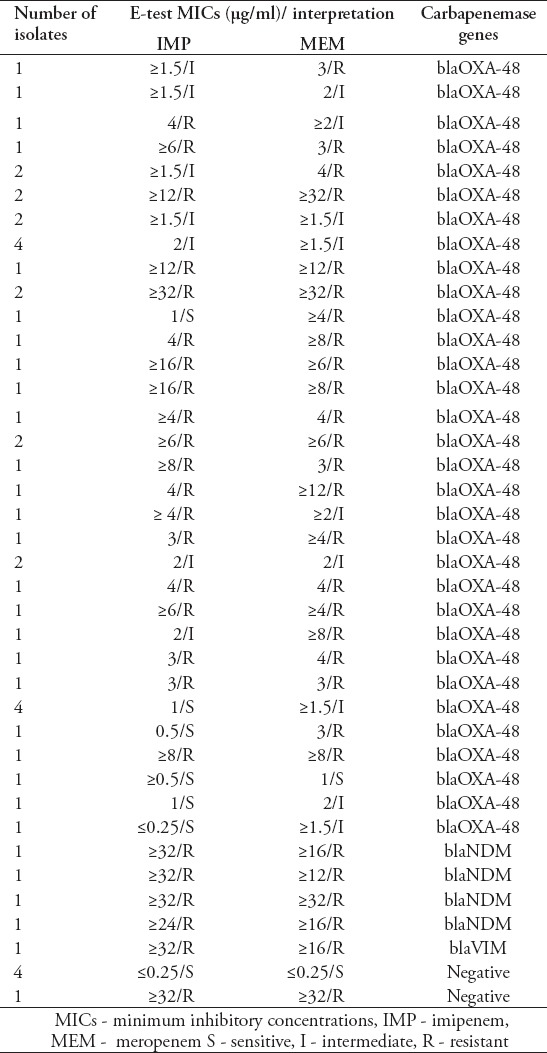
The MICs of imipenem and meropenem ranged from ≤0.25 to ≥32 µg/ml. Twenty-eight (52%) isolates were resistant to imipenem with MICs values between 3 and ≥32 µg/ml. Fifty-nine percent of isolates (n=32) were resistant to meropenem with MICs values between 3 and ≥32 µg/ml. Seventeen isolates showed intermediate susceptibility to meropenem with MICs values between 1.5 and 2 µg/ml and only 5 of these isolates were susceptible to MICs ≥1 µg /ml (Table 3).
Table 3.
Carbapenem resistance pattern and carbapenemase genes of CRKP isolates in the Southern province of Saudi Arabia.
Phenotypic detection of KPC, MBLs enzymes, and AmpC activity
Carbapenemase detection set (mast discs) from the Mast Group LTD was used to identify carbapenemase (KPC, MBLs enzymes) and AmpC activity among the 54 K. pneumoniae isolates. This method can help to identify these enzymes and AmpC activity based on a simple calculation of zone size and comparison of combined disks (Figure 1). All K. pneumoniae isolates were tested using this method and revealed that 5 isolates were MBLs-positive, while 10 isolates were equivocal. All the remainig isolates (n=39) were negative for KPC, MBLs, and AmpC.
Figure 1.
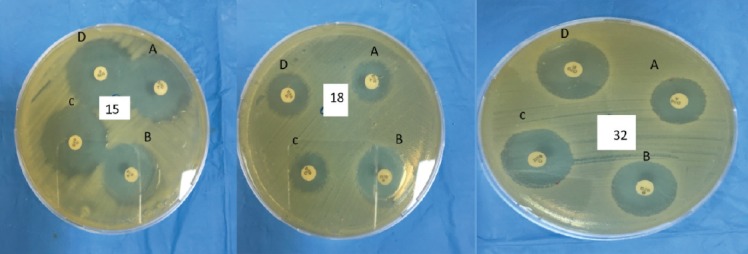
Phenotypic detection of carbapenemase (KPC and MBLs) using carbapenemase detection set (Mast discs). A: meropenem 10µg disc; B: meropenem 10µg + MbL inhibitor disc; C: meropenem 10µg + KBC inhibitor disc; D: meropenem 10µg + AmpC inhibitor disc. Comparing the inhibition zone of the meropenem disc (A) to the inhibition zones of each meropenem disc plus inhibitors for the following isolates: Klebsiella pneumoniae isolate 15 has zone diameter (B-A <5mm, C-A and D-A <4mm) MBLs, KPC and AmpC are negative, isolate 18 zone dimeter (B-A >5mm, C-A and D-A <4mm) MBLs is positive while KPC and AmpC are negative, isolate 32 with zone dimeter ( C-A >4mm B-A and D-A >3mm) is equivocal.
Molecular detection of carbapenemase genes
All K. pneumoniae isolates were tested in multiplex PCR for the presence of the most common carbapenemase genes (blaIMP, blaOXA-48, blaVIM, blaNDM, and blaKPC). Forty-four (81.5%) K. pneumoniae isolates harbored blaOXA-48 and 4 isolates were positive for blaNDM. Only one isolate harbored blaVIM. No producers of blaIMP and blaKPC were detected among all tested isolates (Tables 2 & 3).
Discussion
Rapid dissemination of CRKP is the main cause of treatment failure and increases morbidity and mortality rates in hospital patients.11 In Saudi Arabia, a recent study reported that multidrug resistant K. pneumoniae isolates that produce OXA-48 and NDM carbapenemases were isolated from patients in Riyadh hospitals.2 Carbapenem-resistant K. pneumoniae has also been reported in most countries in the Gulf Cooperation Council (GCC).8,12,13 The Southern province of Saudi Arabia has many hospitals that serve large populations, though this geographical region has not undergone screening programs that include CRKP. Therefore, the central aim of this study is to identify the most common carbapenemases in K. pneumoniae isolated from the hospitals of the Southern province. We have determined the prevalence of CRKP in the 2 largest hospitals in the Southern province between April and September of 2015 and found that the isolation of CRKP is associated with old age (54% of CRKP patients were older than 50 years). This finding is consistent with those of Kofteridis et al,14 which showed that increasing age is a significant risk factor associated with CRKP isolation. It is noteworthy that our findings indicate that ICU patients are more vulnerable population to CRKP infections. Previous studies have also reported that ICU admission and pre-ICU admission are associated with CRKP colonization and infection.14,15 In the current study, greater than one-third of K. pneumoniae isolates (42.6%, n=23) were recovered from ICUs.
Antibiotic susceptibility testing (AST), using automated methods, is still used routinely in many clinical laboratories to determine susceptibility profiles of several bacterial pathogens. E-test is often used as a confirmatory testing method to confirm results produced by automated methods such as the Vitek 2 test.16 Here, E-test was used to confirm carbapenems resistance among K. pneumoniae isolates. In some isolates, there were discrepancies in the susceptibility results for imipenem and meropenem between the Vitek 2 system and E-test methods. These differences, particularly with Vitek 2, has been previously observed and is mainly attributed to false-susceptible and false-resistant errors caused by the Vitek 2 AST card and out of date software.16-18 Although E-test appeared to be more reliable than the Vitek-2 system in the detection of carbapenemase-producers; E-test failed to detect some OXA-48 producers based on MIC values (namely, OXA-48-producer with imipenem and meropenem MICs ≥0.5 µg/ml and 1 µg/ml) (Table 3). This issue has been reported in other studies which stated that OXA-48 producers sometimes show susceptibility or low resistance to carbapenems, which makes the detection of OXA-48 producers based only on MIC values more challenging.19,20
Accurate and prompt identification of CRKP is crucial for controlling its spread. There are many phenotypic detection methods that have been developed for the identification and differentiation of different carbapenemase types. Mast carbapenemase detection set (Mast Group LTD), for instance, is a new method that was developed to identify and differentiate carbapenemases based on a simple calculation using zone size comparison of combined disks, incorporating specific enzyme inhibitors. Here, we use this testing method and showed acceptable discriminatory power between carbapenemase enzymes (particulrly KPC and MBLs), although 10 OXA-48-positive K. pneumoniae isolates had equivocal results while the rest of OXA-48 isolates were negative. The main limitations of this testing method are its inability to distinguish the type of enzyme acquisition (namely VIM, IMP, NDM) and its inability to definitively identify OXA-48.21
These limitations may be explained by the fact that no inhibitor is yet available for class D (OXA-48) carbapenemase that can be used in any phenotypic detection method.20 This method could be used as a screening method for the detection of CRKP when more advanced techniques are not available. However, negative and equivocal results should be confirmed using molecular methods. Consequently, molecular detection and characterization of carbapenemase-producers have become the methods of choice because they can be performed rapidly with an extremely high level of accuracy. A multiplex-PCR assay that was recently introduced by Zarakolu et al10 was used to identified the most common carbapenemases (namely KPC, IMP, VIM, NDM and OXA-48). Klebsiella pneumoniae isolates producing OXA-48 carbapenemase was first identified in the Middle-East (in Turkey) and has rapidly spread globally.22 To date, OXA-48 is considered the most common carbapenemase in the Middle-Eastern countries (Figure 2). Moreover, OXA-48-positive and NDM-positive K. pneumoniae have also been isolated from Saudi hospitals as well as many countries in the Arabian Peninsula, and both enzymes have been previously described as major carbapenemases of Enterobacteriaceae in countries in the Arabian Peninsula.2,8,23,24 In this study, we identified the OXA-48 in 81.5% (n=44) of K. pneumoniae isolates, which is reflective of the high prevalence of OXA-48-positive K. pneumoniae in Saudi Arabia including the Southern province. Although NDM was first reported in India and its dissemination varies geographically (Figure 2), Middle Eastern countries have been described as the second reservoir of NDM producing isolates.2,25 In this study, 7.4% (n=4) of K. pneumoniae isolates were NDM-positive while VIM was reported in only one isolate and no IMP or KPC isolates were detected. Interestingly, these results are consistent with those of Shibl et al,2 who detected OXA-48 in 78% of K. pneumoniae isolates, NDM in 20%, and found only a single isolate that was VIM-positive in Riyadh hospitals.2 This result may be explained by the fact that Saudi Arabia receives large number of visitors and migrant workers from OXA-48 and NDM endemic countries such as Turkey, India, and Pakistan. Moreover, this study also revealed that 3 types of enzymes (VIM, IPM and KPC) were not the major types of carbapenemases in Saudi Arabian hospitals (Figure 2).
Figure 2.
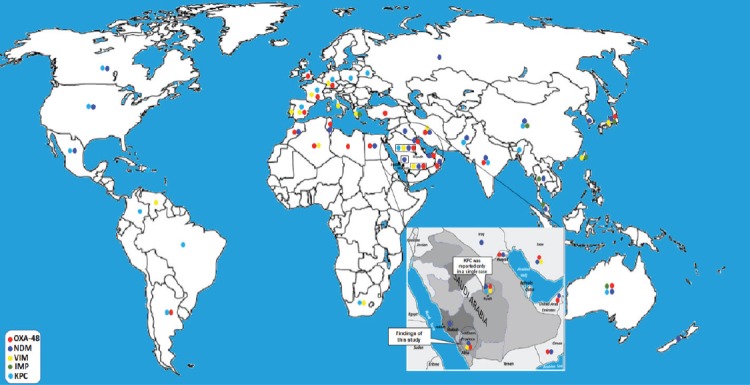
Worldwide distribution of carbapenemase-producing Klebsiella pneumoniae.
Although the first identification of KPC enzyme in K. pneumoniae was in North Carolina, USA in 1996,26,27 these isolates have spread across 38 states and recently have been reported in Canada, Europe, Australia, China, India; and many south America countries such as Brazil, Mexico, Colombia, and Argentina (Figure 2).24-26,28 Even though only one KPC-positive K. pneumoniae isolate was incidentally reported in Riyadh, Saudi Arabia (Figure 2),29 we should have concern regarding the wide-spread of KPC- producers in Saudi Arabian hospitals. This concern is justified by the fact that the annual movement of more than 100,000 Saudi students30 to and from the USA may contribute to the transmission and spread of KPC-producing isolates in Saudi Arabia. Consequently, effective surveillance of KPC-producers will help to identify them quickly and allow appropriate infection control to take place. This will help to prevent the emergence and spread of these epidemic isolates.
Study limitation
Limited funds have prevented the collection of more CRKP isolates from all hospitals in the Southern province. It was also not possible to use more advanced molecular typing technologies such as multi-locus sequence typing (MLST) in order to investigate the molecular epidemiology of CRKP in this geographical region.
In conclusion, the major type of carbapenemase was OXA-48 and it seems to have reached an endemic level. Further studies are needed to identify the most common clones of CRKP, in particular OXA-48-producers in Saudi Arabia.
Acknowledgment
The authors would like to thank the King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia for technical and financial support. Many thanks to those at the clinical microbiology laboratories of Asir Central Hospital and Armed Force Hospital Southern Region for helping in sample collection. Also, many thanks to the staff at the Special Infectious Agents Unit (SIAU), King Fahad Medical Research Center (KFMRC) and King Abdulaziz University for their support during the practical work. We would also like to thank Professor John Perry, the Freeman hospital (Newcastle upon Tyne, United Kingdom) for providing control strains as a generous gift. Finally, we would like to thank Mr. Ayman Al-Zahrani for his help in drawing the figure 2 illustrating the worldwide distribution of carbapenem-resistant Klebsiella pneumoniae.
Footnotes
Disclosure.
References
- 1.Rawat D, Nair D. Extended-spectrum beta-lactamases in Gram Negative Bacteria. J Glob Infect Dis. 2010;2:263–274. doi: 10.4103/0974-777X.68531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibl A, Al-Agamy M, Memish Z, Senok A, Khader SA, Assiri A. The emergence of OXA-48- and NDM-1-positive Klebsiella pneumoniae in Riyadh, Saudi Arabia. Int J Infect Dis. 2013;17:e1130–e1133. doi: 10.1016/j.ijid.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Naas T, Nordmann P. Analysis of a carbapenem-hydrolyzing class A beta-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc Natl Acad Sci U S A. 1994;91:7693–7697. doi: 10.1073/pnas.91.16.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djahmi N, Dunyach-Remy C, Pantel A, Dekhil M, Sotto A, Lavigne JP. Epidemiology of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Mediterranean countries. Biomed Res Int. 2014;2014:305784. doi: 10.1155/2014/305784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: Epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7:895. doi: 10.3389/fmicb.2016.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potron A, Poirel L, Rondinaud E, Nordmann P. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Euro Surveill Euro Surveill. 2013;18:20549. doi: 10.2807/1560-7917.es2013.18.31.20549. [DOI] [PubMed] [Google Scholar]
- 7.Balkhy HH, El-Saed A, Al Johani SM, Francis C, Al-Qahtani AA, Al-Ahdal MN, et al. The epidemiology of the first described carbapenem-resistant Klebsiella pneumoniae outbreak in a tertiary care hospital in Saudi Arabia: how far do we go? Eur J Clin Microbiol Infect Dis. 2012;31:1901–1909. doi: 10.1007/s10096-011-1519-0. [DOI] [PubMed] [Google Scholar]
- 8.Zowawi HM, Balkhy HH, Walsh TR, Paterson DL. Beta-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin Microbiol Rev. 2013;26:361–380. doi: 10.1128/CMR.00096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uz Zaman T, Aldrees M, Al Johani SM, Alrodayyan M, Aldughashem FA, Balkhy HH. Multi-drug carbapenem-resistant Klebsiella pneumoniae infection carrying the OXA-48 gene and showing variations in outer membrane protein 36 causing an outbreak in a tertiary care hospital in Riyadh, Saudi Arabia. Int J Infect Dis. 2014;28:186–192. doi: 10.1016/j.ijid.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Zarakolu P, Eser OK, Aladag E, Al-Zahrani IA, Day KM, Atmaca O, et al. Epidemiology of carbapenem-resistant Klebsiella pneumoniae colonization: a surveillance study at a Turkish university hospital from 2009 to 2013. Diagn Microbiol Infect Dis. 2016;85:466–470. doi: 10.1016/j.diagmicrobio.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Pavelkovich A, Balode A, Edquist P, Egorova S, Ivanova M, Kaftyreva L, et al. Detection of carbapenemase-producing enterobacteriaceae in the baltic countries and st. Petersburg area. Biomed Res Int. 2014;2014:548960. doi: 10.1155/2014/548960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. NDM-1 OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin Microbiol Infect. 2012;18:E144–E148. doi: 10.1111/j.1469-0691.2012.03796.x. [DOI] [PubMed] [Google Scholar]
- 13.Sonnevend A, Ghazawi AA, Hashmey R, Jamal W, Rotimi VO, Shibl AM, et al. Characterization of Carbapenem-Resistant Enterobacteriaceae with High Rate of Autochthonous Transmission in the Arabian Peninsula. PLoS One. 2015;10:e0131372. doi: 10.1371/journal.pone.0131372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kofteridis DP, Valachis A, Dimopoulou D, Maraki S, Christidou A, Mantadakis E, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization: a case-case-control study. J Infect Chemother. 2014;20:293–297. doi: 10.1016/j.jiac.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Jiao Y, Qin Y, Liu J, Li Q, Dong Y, Shang Y, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Glob Health. 2015;109:68–74. doi: 10.1179/2047773215Y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenover FC, Kalsi RK, Williams PP, Carey RB, Stocker S, Lonsway D, et al. Carbapenem resistance in Klebsiella pneumoniae not detected by automated susceptibility testing. Emerg Infect Dis. 2006;12:1209–1213. doi: 10.3201/eid1208.060291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bratu S, Landman D, Haag R, Recco R, Eramo A, Alam M, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165:1430–1435. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 18.Bobenchik AM, Deak E, Hindler JA, Charlton CL, Humphries RM. Performance of Vitek 2 for antimicrobial susceptibility testing of Enterobacteriaceae with Vitek 2 (2009 FDA) and 2014 CLSI breakpoints. J Clin Microbiol. 2015;53:816–823. doi: 10.1128/JCM.02697-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodford N, Eastaway AT, Ford M, Leanord A, Keane C, Quayle RM, et al. Comparison of BD Phoenix, Vitek 2, and MicroScan automated systems for detection and inference of mechanisms responsible for carbapenem resistance in Enterobacteriaceae. J Clin Microbiol. 2010;48:2999–3002. doi: 10.1128/JCM.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakthavatchalam YD, Anandan S, Veeraraghavan B. Laboratory detection and clinical implication of oxacillinase-48 like Carbapenemase: The Hidden Threat. J Glob Infect Dis. 2016;8:41–50. doi: 10.4103/0974-777X.176149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsakris A, Poulou A, Bogaerts P, Dimitroulia E, Pournaras S, Glupczynski Y. Evaluation of a new phenotypic OXA-48 disk test for differentiation of OXA-48 carbapenemase-producing Enterobacteriaceae clinical isolates. J Clin Microbiol. 2015;53:1245–1251. doi: 10.1128/JCM.03318-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poirel L, Heritier C, Tolun V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamal WY, Albert MJ, Rotimi VO. High Prevalence of New Delhi Metallo-beta-Lactamase-1 (NDM-1) Producers among Carbapenem-Resistant Enterobacteriaceae in Kuwait. PLoS One. 2016;11:e0152638. doi: 10.1371/journal.pone.0152638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer FP, Castelberg C, Quiblier C, Bloemberg GV, Hombach M. Evaluation of carbapenemase screening and confirmation tests with Enterobacteriaceae and development of a practical diagnostic algorithm. J Clin Microbiol. 2015;53:95–104. doi: 10.1128/JCM.01692-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014;20:821–830. doi: 10.1111/1469-0691.12719. [DOI] [PubMed] [Google Scholar]
- 26.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen LF, Anderson DJ, Paterson DL. Overview of the epidemiology and the threat of Klebsiella pneumoniae carbapenemases (KPC) resistance. Infect Drug Resist. 2012;5:133–141. doi: 10.2147/IDR.S26613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Qadheeb NS, Althawadi S, Alkhalaf A, Hosaini S, Alrajhi AA. Evolution of tigecycline resistance in Klebsiella pneumoniae in a single patient. Ann Saudi Med. 2010;30:404–407. doi: 10.4103/0256-4947.67087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor C, Albasri W. The Impact of Saudi Arabia King Abdullah's Scholarship Program in the U.S. Open Journal of Social Sciences. 2014;2:109–118. [Google Scholar]


