Abstract
Acetaminophen (APAP) overdose is the most common cause of acute liver failure in the US, and decades of intense study of its pathogenesis resulted in the development of the antidote N-acetylcysteine, which facilitates scavenging of the reactive metabolite and is the only treatment in clinical use. However, the narrow therapeutic window of this intervention necessitates a better understanding of the intricacies of APAP-induced liver injury for the development of additional therapeutic approaches that can benefit late-presenting patients. More recent investigations into APAP hepatotoxicity have established the critical role of mitochondrial dysfunction in mediating liver injury as well as clarified mechanisms of APAP-induced hepatocyte cell death. Thus, it is now established that mitochondrial oxidative and nitrosative stress is a key mechanistic feature involved in downstream signaling after APAP overdose. The identification of specific mediators of necrotic cell death further establishes the regulated nature of APAP-induced hepatocyte cell death. In addition, the discovery of the role of mitochondrial dynamics and autophagy in APAP-induced liver injury provides additional insight into the elaborate cell signaling mechanisms involved in the pathogenesis of this important clinical problem. In spite of these new insights into the mechanisms of liver injury, significant controversy still exists on the role of innate immunity in APAP-induced hepatotoxicity.
Key words: Acetaminophen (APAP), Programmed necrosis, Mitochondria, Sterile inflammation, Neutrophils
INTRODUCTION
Acetaminophen (APAP) is one of the most common analgesic and antipyretic drugs in use globally1. Though the drug is safe and effective at therapeutic doses, the therapeutic window is narrow, and an overdose is highly hepatotoxic. Because of the ubiquitous nature and broad availability of the drug, this has resulted in APAP hepatotoxicity being the most frequent cause of acute liver failure (ALF) in the US2 and other Western countries3. While a number of APAP overdose cases are due to suicide attempts, the availability of combination products, where the presence of APAP may not be easily recognized, has led to an increase in unintentional and chronic APAP overdose, accounting for over 50% of cases of APAP-related ALF4. Thus, APAP hepatotoxicity contributes to around 70,000 hospitalizations each year in the US5. Overall, APAP overdose is responsible for 46% of all cases of ALF in the US and has now grown to be a significant public health problem6. Decades of investigations into the mechanisms of APAP-induced liver injury have provided significant insight into the role of APAP metabolism and formation of a reactive metabolite in initiating the cascade of events ultimately leading to liver injury.
METABOLISM OF ACETAMINOPHEN
When consumed at therapeutic doses, the majority (80%–90%) of APAP is conjugated with glucuronic acid or sulfate and excreted through the kidneys7. A minor component is acted upon by cytochrome P450 enzymes such as Cyp2E1 and Cyp1A2 to form a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI)8. Though highly reactive, NAPQI is rarely harmful after consumption of therapeutic doses because it is rapidly conjugated with abundant glutathione stores in the liver and excreted through the bile. However, this contrasts to the scenario after consumption of an overdose of APAP where the sulfation pathway is saturated7, and NAPQI generation is significantly elevated in spite of the high capacity of the glucuronidation pathway9. Excessive generation of NAPQI results in its robust reaction with hepatic glutathione stores and the subsequent rapid depletion of glutathione within the liver. This leaves free reactive NAPQI available for reaction with protein sulfhydryl groups to form APAP protein adducts7. Metabolism of APAP can be influenced by genotype differences, and variations in glucuronidation are seen in different populations due to polymorphisms in the UDP-glucuronosyltransferase (UGT) enzymes. It was recently shown that UGT2B15 *2/*2 genotype subjects showed higher APAP protein adduct concentrations than *1/*2 and *1/*1 individuals10. Formation of APAP protein adducts and their release into the circulation are now areas of intense study due to the clinical implications in the management of patients with APAP overdose, mainly since APAP protein adducts have been suggested to be biomarkers useful for diagnosing an APAP overdose11. However, protein adducts are also detectable in the vast majority of subjects taking therapeutic doses of APAP12, and protein-derived APAP–cysteine can be detected after repeated supratherapeutic ingestion of APAP in the absence of hepatotoxicity13. While the clinical utility of APAP protein adduct measurements in this context has been questioned14, a recently developed competitive immunoassay (AcetaSTAT) has been suggested to identify patients with APAP-induced acute liver injury or failure15. Because of these clinical implications, a better understanding of protein adduct formation and its relationship to hepatocyte necrosis is warranted. Mechanistically, it was considered earlier that glutathione levels need to be significantly depleted before NAPQI would react with proteins16. However, recent evidence suggests that this may not be the case, since APAP protein adducts were found to be generated even at therapeutic doses of APAP7,12,17,18; in fact, protein adducts were formed before significant GSH depletion7,18,19. Rather than assessing the overall protein adduct formation in the cell, which was initially considered to be critical for cell death20, it now appears that protein adduct formation on mitochondrial proteins is most relevant for toxicity21–23.
MITOCHONDRIA, PROTEIN ADDUCTS, AND APAP HEPATOTOXICITY
Mitochondria are essential organelles with the primary responsibility of cellular energy generation. However, mitochondria can also play significant roles in cellular signaling, for example, through generation of reactive oxygen species (ROS) (Fig. 1). This is facilitated, in part, by the translocation of cytosolic proteins to the mitochondria, a recurring paradigm in a number of cellular signaling contexts. Mitochondrial proteins are significant targets of NAPQI, and mitochondrial protein adducts were unique to APAP treatment in contrast to the nontoxic regioisomer 3′-hydroxyacetanilide (AMAP) in mice22,24,25. However, AMAP can be hepatotoxic in human hepatocytes23,26, and this correlated with the formation of mitochondrial protein adducts and compromised mitochondrial function23. Mitochondrial protein adducts also seem responsible for the APAP-induced mitochondrial dysfunction23, though critical target proteins responsible for these effects are not well characterized yet. However, general proteomic techniques have identified a number of mitochondrial proteins with adducts of APAP, including ATP synthase and glutathione peroxidase27, and a mitochondrial proteomic approach using blue native PAGE showed changes in proteins such as HMG CoA synthase, accompanied by inhibition of enzyme activity after APAP treatment28. Additional mitochondrial proteins such as glycine amidinotransferase, the redox-sensitive chaperone PARK7, peroxiredoxin 6, and the voltage-gated ion channel VDAC2 were found to be modified by NAPQI in 3D cultures of human hepatocytes and nonparenchymal cells29. In spite of the identification of these mitochondrial protein targets, a direct effect of their modification on compromising mitochondrial function is not evident, and some of these changes could be consequences rather than causes of mitochondrial dysfunction. Nonetheless, NAPQI binding to mitochondrial proteins correlates with APAP toxicity30, and hence the effect could be a cumulative one, with mitochondrial function being affected once a threshold of mitochondrial protein modification is attained.
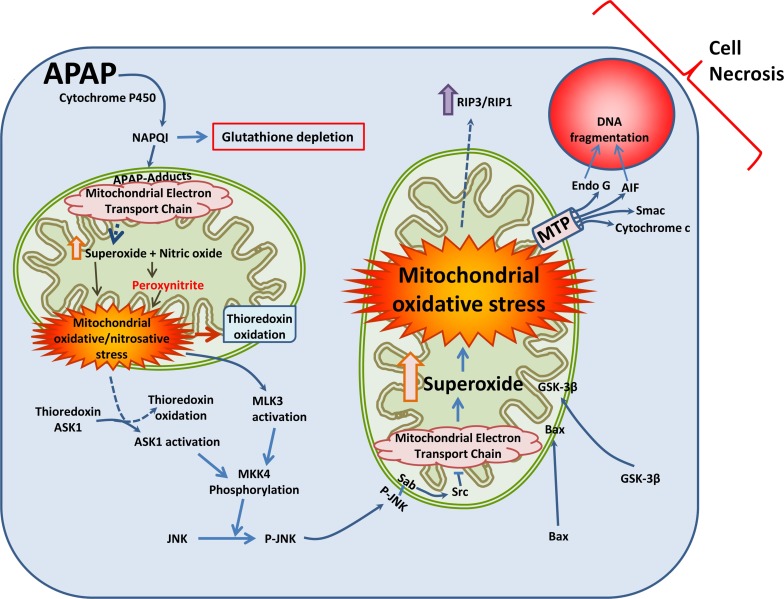
Figure 1.
Mechanism of acetaminophen (APAP)-induced hepatocyte cell death. At high concentrations, APAP in hepatocytes is metabolized by components of the cytochrome P450 system to a reactive intermediate, N-acetyl-p-benzoquinone imine (NAPQI). High concentrations of NAPQI deplete cellular glutathione stores and subsequently form APAP protein adducts, especially on mitochondrial proteins. Components of the electron transport chain such as ATP synthase are affected, which compromises respiratory chain function and enhances generation of free radicals such as superoxide. This reacts with nitric oxide (NO) within the mitochondria to produce highly reactive peroxynitrite, which nitrates mitochondrial proteins such as manganese superoxide dismutase (MnSOD). This compromises mitochondrial antioxidant defenses, causing mitochondrial oxidant stress and oxidation of proteins such as mitochondrial thioredoxin. In the cytosol, oxidation of thioredoxin results in its detachment from its binding partner apoptosis signal-regulating kinase 1 (ASK1), which is then activated. ASK1, along with activated mixed-lineage kinase 3 (MLK3) then activate c-jun N-terminal kinase (JNK) to its phosphorylated form through MKK4 phosphorylation. Phosphorylated JNK translocates to the mitochondria and binds to Sab on the outer mitochondrial membrane, which, through a Src-mediated pathway, further inhibits mitochondrial electron transport. This amplifies mitochondrial oxidant stress, which is further exacerbated by translocation of Bax and glycogen synthase kinase-3β (GSK-3β) from the cytosol to the mitochondria. These events activate the mitochondrial permeability transition, which releases mitochondrial intermembrane proteins such as endonuclease G and apoptosis-inducing factor (AIF), along with cytochrome c and Smac. Translocation of AIF and endonuclease G to the nucleus then induces nuclear DNA fragmentation, which along with activation of receptor-interacting protein kinases 3/1 (RIP3/RIP1) finally induce programmed necrosis.
MITOCHONDRIAL REACTIVE OXYGEN AND REACTIVE NITROGEN SPECIES
The main characteristic of mitochondrial dysfunction induced by APAP adducts is increased generation of ROS such as superoxide31, as well as peroxynitrite32, which can modify proteins by nitration of their tyrosine residues33 (Fig. 1). The importance of mitochondrial superoxide in mediating APAP hepatotoxicity is further illustrated by the significant exacerbation of liver injury in mice with a partial deficiency of manganese superoxide dismutase (SOD2)34,35, which would usually scavenge superoxide within the mitochondria. Similarly, the mitochondria-targeted SOD mimetic mito-TEMPO effectively protected against APAP hepatotoxicity36. The source of the mitochondrial superoxide production is likely the respiratory chain, since APAP has been shown to inhibit respiration through complex II by 47% in isolated mouse hepatocytes, while complex I activity was affected to a lesser extent37,38. A more recent study also demonstrated that NAPQI directly inhibited complex II activity in a concentration-dependent manner, attaining >90% inhibition with concentrations in the μM range39. In spite of these data, however, the quest for the exact source of superoxide within the electron transport chain is still ongoing. Nevertheless, as mentioned earlier, the reaction of superoxide with nitric oxide (NO) and the subsequent generation of peroxynitrite within the mitochondria are critical mediators of APAP-induced mitochondrial dysfunction32,40. While all three nitric oxide synthases (NOSs) have been suggested to be potential sources of NO for this reaction41–43, current data point toward neuronal NOS (nNOS) as a source. Pharmacological inhibition of nNOS protected hepatocytes in culture against APAP-induced cell death44, and mice deficient in nNOS showed less liver injury after an APAP overdose41. A mitochondrial source for NO has been postulated for a number of years45, though the exact identity of this NOS has remained unresolved. While earlier studies suggested that nNOS is unlikely to be the mitochondrial NOS (mtNOS)46, subsequent studies suggest that mtNOS is nNOS or a spliced variant of it (likely nNOSα or nNOSμ), at least in the heart47. Hence, it is possible that the source of mitochondrial NO to form peroxynitrite with superoxide from the respiratory chain could be nNOS. Whatever the source of NO, generation of peroxynitrite within the mitochondria results in modification of a number of proteins by nitration of tyrosine residues. When these targets are mitochondrial DNA32 or critical antioxidant enzymes such as superoxide dismutase, whose activity is compromised by nitration after APAP overdose48, it can have further cascading consequences. The importance of peroxynitrite in mediating APAP-induced liver injury is also illustrated by the fact that direct scavenging of peroxynitrite by mitochondrial GSH49,50 or resveratrol51 also prevented protein nitration and protected against APAP-induced liver injury.
MITOCHONDRIA AS A SIGNAL INTEGRATION PLATFORM IN APAP HEPATOTOXICITY
In addition to being a source of free radicals which initiate signaling events, the mitochondria also act as signal-integrating platforms, where cytosolic proteins are translocated to amplify damage induced by the initial oxidative stress. This is probably enabling a threshold effect, such that hepatocytes exposed to varying levels of free radicals can respond differently to modulate the liver’s functional response to the insult. This paradigm would enable necrosis to be limited to cells exposed to highest levels of APAP around the centrilobular area, sparing cells further away, which could stimulate recovery and regeneration and allow subsequent repopulation and recovery of liver function. Thus, the initial oxidative stress in mitochondria induced by APAP adducts subsequently results in oxidation of thioredoxin (Trx) within the mitochondria52, and NAPQI was also shown to modify and inhibit the activity of Trx 1 and 253 (Fig. 1). In addition, treatment with a recombinant human serum albumin–Trx 1 fusion protein (HSA-Trx) was also shown to protect against APAP-induced liver injury when administered up to 4 h after APAP54. The oxidation of Trx 1 causes its detachment from its binding partner apoptosis signal-regulating kinase 1 (ASK1), resulting in ASK1 activation by phosphorylation55. Mixed-lineage kinase 3 (MLK3) is another upstream MAPK3 that is activated by oxidant stress during APAP hepatotoxicity56. Activated ASK1 and MLK3 phosphorylate MKK457, a MAPK2 kinase, which then phosphorylates c-jun N-terminal kinase (JNK) in the cytosol58,59. The importance of ASK1 in this process is illustrated by the fact that ASK1 knockout mice are protected against APAP-induced activation of JNK55. In addition, treatment of mice with a pharmacological inhibitor of ASK1 also prevented APAP-induced liver injury60.
Activation of JNK then initiates a cascading effect, with translocation of phosphorylated JNK to the mitochondrial outer membrane58, where it binds with the Sab protein and initiates an Src-mediated inhibition of the electron transport and increases ROS production to amplify mitochondrial dysfunction61 and peroxynitrite formation59. Disruption of the interaction between P-JNK and mitochondria has been shown to be protective against APAP-induced liver injury62, illustrating the importance of these steps in APAP-induced programmed necrosis of hepatocytes. In parallel with translocation of activated JNK to the mitochondria, cytosolic Bax also moves to the mitochondria63,64, and this contributes to subsequent release of mitochondrial proteins to the cytosol detailed below, though this has no effect on peroxynitrite formation63. However, Bax-deficient animals, though protected initially from hepatocyte necrosis, succumbed to liver injury at later time points due to the sustained mitochondrial oxidant stress63, suggesting that in the absence of Bax, alternate mechanisms of mitochondrial protein release are activated in the face of sustained oxidant insult. A likely mechanism is that matrix swelling causes rupture of the outer membrane and release of the intermembrane proteins63. Another cytosolic protein translocating to the mitochondria after APAP is the glycogen synthase kinase-3β (GSK-3β), which is a major regulator of glycogen synthase but has been shown to regulate other processes, including cell death65. GSK-3β also plays a role in APAP-induced liver injury, since silencing GSK-3β was shown to attenuate JNK activation and inhibit APAP hepatotoxicity65. Ultimately, both JNK and Bax translocation contributes to release of mitochondrial proteins into the cytosol and then their translocation to the nucleus as detailed below, to ultimately result in hepatocyte necrotic cell death (Fig. 1).
RELEASE OF MITOCHONDRIAL PROTEINS AND SUBSEQUENT DNA FRAGMENTATION
Translocation of phosphorylated JNK and Bax to the mitochondria amplifies mitochondrial oxidant stress and peroxynitrite formation and induces opening of the mitochondrial permeability transition pore (MPTP) (Fig. 1). Induction of the MPTP compromises mitochondrial ATP production, depolarizes the mitochondrial membrane, and consequently shuts down mitochondrial function66–68. The MPTP has been suggested to consist of Bax, along with the protein Bak on the mitochondrial outer membrane69,70, with the c-subunit ring of the F1FO ATP synthase71 being one of the regulatory components within the inner membrane. However, the role of the c-subunit ring has been recently called into question72, so further studies may be needed to confirm the MPTP structure. Another well-characterized regulatory component of the MTP is cyclophilin D73, though its relevance with respect to pore opening induced by APAP seems to depend on the APAP dose. Pharmacological inhibition of cyclophilin D using cyclosporine A provided only temporary protection in vitro66, but cyclophilin D-deficient mice were protected against liver injury when treated with a moderate APAP overdose of 200 mg/kg68. However, no protection was evident when higher doses (600 mg/kg) were used74, again suggesting that in the face of sustained mitochondrial insult due to either higher doses or long-term exposure, alternate regulatory molecules are probably recruited to induce downstream features of the cell signaling cascade to ultimately result in hepatocyte necrosis. Another trigger for the induction of the MPTP in the context of APAP-induced liver injury is lysosomal iron, whose translocation to the mitochondria has been shown to occur in mouse hepatocytes treated with APAP, where it then induced opening of the MPTP75. This iron release could be due to lysosomal instability, which has been documented after APAP treatment76, and lysosomal iron translocation seems to occur through the calcium uniporter, since treatment with either an iron chelator or an inhibitor of the uniporter prevented mitochondrial free radical generation and membrane depolarization77. While the induction of the MPTP has been considered to be a catastrophic feature of APAP-induced cell signaling, recent research suggests that this too could be adjusted depending on the dose of APAP, where treatment of animals with a low 150-mg/kg overdose of APAP resulted in transient JNK activation and reversible induction of the MPTP20, from which cells may be able to recover. This suggests that the cellular response to APAP, be it JNK activation, Bax translocation, or induction of the MPTP, can be calibrated to the dose of APAP the cells are exposed to.
Induction of the MPTP by the various stimuli detailed above ultimately results in release of a number of critical mitochondrial proteins into the cytosol, some of which, such as apoptosis-inducing factor (AIF) and endonuclease G, have nuclear localization signals78, which result in their translocation to the nucleus79. Others, such as cytochrome c, are essential for mitochondrial electron transport, and their loss from the mitochondria further contributes to disruption of ATP production and mitochondrial dysfunction. Endonuclease G cleavage of DNA in the nucleus results in DNA fragmentation indistinguishable from that seen during apoptosis32, and translocation of AIF to the nucleus results in chromatin condensation and DNA fragmentation80. Its importance in APAP-induced DNA fragmentation is illustrated by the reduced liver injury seen in partial AIF-deficient mice81. Recent data also indicate that the extent of mitochondrial protein release and DNA fragmentation could also dictate severity of liver injury in response to APAP between substrains of the common C57BL/6 mouse species used as an animal model of APAP-induced liver injury82.
Thus, mitochondrial protein adduct formation, oxidative stress, and, ultimately, induction of the MPTP are central to APAP-induced liver injury, and it is now evident that the organelle also plays a significant role in the recovery and regeneration process after injury. Recent evidence indicates that activation of mitochondrial biogenesis subsequent to liver injury plays a critical role in recovery after injury, and induction of mitochondrial biogenesis protects against APAP hepatotoxicity83. Another feature of the recovery process is the removal of damaged mitochondria by a form of autophagy termed mitophagy84,85, and inhibition of mitophagy by lysosomal cholesterol accumulation was shown to sensitize mice to APAP hepatotoxicity86. From the spatial perspective, timely removal of damaged mitochondria by mitophagy may prevent induction of the cell death cascade, especially in hepatocytes at the border of the necrotic area85. Activation of autophagy was shown to protect against APAP-induced hepatotoxicity, and inhibition of autophagy aggravated liver injury84. Autophagy was also shown to help in the removal of APAP protein adducts, which may be most critical in protecting cells during long-term APAP treatment87. Thus, while mitochondria are critical elements involved in the signaling cascade toward APAP-induced cell death, the organelle also plays an important role in the recovery and regeneration of liver function, especially in hepatocytes away from the central vein at the borders of the area of necrosis83.
HEPATOCYTE CELL DEATH MECHANISMS AFTER APAP TOXICITY
The mode of cell death after APAP-induced liver injury was controversial initially, since a number of mechanistic steps in the process, such as mitochondrial Bax translocation, release of cytochrome c64,79,88, and nuclear DNA fragmentation32, were similar to that seen during apoptosis (Fig. 1). However, a fundamental feature of apoptosis, namely, caspase activation, was absent64,89,90. In addition, APAP-induced cell death has all the characteristics of necrosis both in vitro and in vivo66,91–93. The lack of protection against liver injury by caspase inhibitors in APAP hepatotoxicity89–91,94 provides further evidence of the lack of apoptosis in this context. Furthermore, on closer examination, characteristics of DNA fragmentation are also distinct between apoptosis and APAP-induced necrosis95–97. Part of the reason for the early disregard for necrotic cell death was probably due to the fact that in contrast to apoptosis, necrosis was considered to be an unregulated form of cell death, which occurred once cellular integrity was compromised due to a multitude of insults. This viewpoint has shifted due to emerging evidence that necrotic cell death can also be regulated, based on the discovery of a number of molecular mediators involved in the process, termed necroptosis98. A central feature here is the formation of a multiprotein complex termed the “necrosome,” which includes, among others, the receptor-interacting protein kinases 1 and 3 (RIP1 and RIP3)98 and activation of the pseudokinase mixed-lineage kinase domain-like protein (MLKL)99. RIP1 and RIP3 form heterodimeric scaffolds in the complex, and while RIP1–RIP1 interactions are dispensable for necroptosis, RIP1–RIP3 or RIP3–RIP3 interactions are required for induction of necroptosis100. Activated RIP3 phosphorylates MLKL101, and the phosphorylated MLKL translocates to the cell membrane where MLKL interferes with membrane integrity, causing necrotic cell death99. RIP3 levels were found to be elevated after APAP overdose in mice102,103, and RIP3 deficiency provided early protection against liver injury, though this was not sustained102. While RIP1 has also been implicated in APAP-induced liver injury56,102,104, it has been suggested to act independently of the necrosome complex105. In addition, the fact that MLKL105 and tumor necrosis factor-α (TNF-α)106 do not seem to be involved in APAP-induced cell death suggests that APAP-induced cell death should be termed “programmed necrosis” rather than necroptosis107.
STERILE INFLAMMATION AND APAP HEPATOTOXICITY
The extensive cell necrosis after an APAP overdose leads to release of damage-associated molecular patterns (DAMPs) including mitochondrial DNA, nuclear DNA fragments, high-mobility group box 1 (HMGB1) protein, and many others108–110 (Fig. 2). DAMPs bind to pattern recognition receptors such as toll-like receptors (TLRs) on inflammatory cells and transcriptionally activate cytokine formation in inflammatory cells111,112. Some of these inflammatory mediators are constitutively active, for example, TNF-α and interleukin-1α (IL-1α), whereas others are generated as a pro-form that requires proteolytic cleavage by caspase 1, for example, IL-1β and IL-18111. DAMPs like ATP can stimulate the purinergic receptor P2X7 on macrophages and activate the Nalp3 inflammasome, which triggers the activation of caspase 1112. The proinflammatory cytokines and chemokines formed can activate neutrophils and monocytes and recruit these cells into the liver where they then may aggravate the existing cell necrosis112. While there is general agreement in the literature that APAP-induced cell necrosis causes DAMP release, proinflammatory mediator formation, and recruitment of inflammatory cells into the liver, it is highly controversial whether this sterile inflammatory response actually aggravates the injury or is beneficial by removing cell debris and promoting regeneration111–113 (Fig. 2).
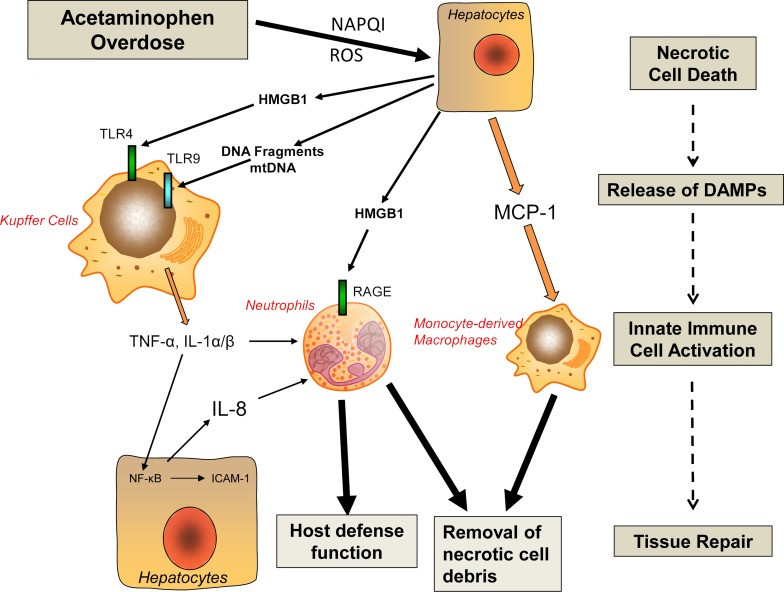
Figure 2.
Sterile inflammation and liver regeneration. A sterile inflammatory response is initiated by release of damage-associated molecular patterns (DAMPs) from necrotic cells. DAMPs activate pattern recognition receptors such as toll-like receptors (TLRs), which induces the formation of cytokines and chemokines and the recruitment of inflammatory cells (see text for details). In APAP-induced liver injury, the preponderance of experimental and clinical evidence suggests that this sterile inflammatory response does not aggravate the original injury but causes the removal of necrotic cells and promotes regeneration (see text for details). HMGB1, high-mobility group box 1 protein; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; mtDNA, mitochondrial DNA; RAGE, receptor for advanced glycation end products; ROS, reactive oxygen species.
There are two main areas of controversy. First, it is controversial whether neutrophils, the first responder to the initial cell necrosis, contribute to the injury. The principal support for this hypothesis comes from studies that showed neutropenia being protective against APAP hepatotoxicity114. However, this approach was criticized as prolonged neutropenia causes a preconditioning effect that is protective independent of the actual neutrophils115. In addition, interventions that inactivated neutrophils independent of neutropenia such as CD18 antibodies116, inhibitors of NADPH oxidase117, and use of mice deficient in CD1894, intercellular adhesion molecule-1117, or NADPH oxidase118 all were not protective against APAP-induced liver injury. Furthermore, there was neither neutrophil activation nor a neutrophil-mediated oxidant stress observed in the liver during the injury phase94,117. Most importantly, similar results regarding neutrophil activation were obtained in human patients119. Together, the findings strongly argue against a direct involvement of neutrophils in the injury phase.
A second area of controversy is the role of certain proinflammatory mediators, in particular IL-1β. It was reported that DNA fragments promote pro-IL-1β formation during APAP hepatotoxicity120. In addition, the Nalp3 inflammasome was activated leading to caspase 1 activation and processing of pro-IL-1β to the active cytokine120. The pathophysiological relevance of these mechanisms and IL-1β was established by the reduced APAP-induced liver injury in TLR9-, Nalp3-, and caspase 1-deficient mice120. However, while the effect of TLR9 was confirmed by others121, the protection in mice deficient in Nalp3 or caspase 1 was not reproducible122. In addition, caspase inhibitors prevented IL-1β formation but did not protect, and adding high doses of exogenous IL-1β enhanced neutrophil recruitment but did not affect the APAP-induced liver injury123. More recently, another study showed that neither mice deficient in IL-1β nor treatment with an anti-IL-lβ antibody protected against APAP hepatotoxicity124. Interestingly, these authors did not also find a protection in caspase 1- or Nalp3-deficient mice124. The very limited IL-1β formation as reported in mice123,124 was also confirmed in APAP overdose patients111, suggesting that inflammasome activation and IL-1β formation are of limited importance for APAP-induced liver injury in mice and humans. However, Zhang and coworkers124 suggested a role for IL-1α in the pathophysiology. IL-1α is generated by Kupffer cells through TLR4 stimulation but not by TLR9- or TLR3-dependent mechanisms124. In their hands, an anti-IL-1α antibody protected and mice deficient in IL-1α or the IL-1 receptor experienced less injury after an APAP overdose124. By again performing long-term neutropenia experiments, the authors concluded that IL-1α-activated neutrophils contribute to the injury process124. Although these conclusions appeared to be justified based on the reported experiments, it again raised the issue of questionable neutropenia experiments111,115, and the findings contradicted previous data showing that IL-1 receptor-deficient mice were not protected123, that total elimination of Kupffer cells actually enhanced APAP-induced liver injury125,126, and that TLR9120,121 and TLR3127 are important for the pathophysiology. Thus, there is not only a controversy between investigators who conclude that the sterile inflammatory response after APAP overdose is mainly involved in regeneration and those who believe that it aggravates the initial injury, but there are also extensive contradictions between reported results and suggested mechanisms among investigators who believe in an inflammatory injury component in APAP-induced hepatotoxicity. Although it is not always obvious why so many contradictory results are being reported, it might be useful to focus on the clinically relevant aspects. In APAP overdose patients, proinflammatory cytokine formation is limited128 and neutrophil activation does not occur during the injury phase but more during regeneration119. Consistent with these neutrophil findings, monocyte-derived macrophages that are recruited during APAP hepatotoxicity display a proregenerative phenotype in mice and in humans, suggesting that the inflammatory response is mainly geared toward recovery from the tissue injury129–131.
FUTURE PERSPECTIVES
Over the last several decades, significant progress has been made in the understanding of the intracellular signaling mechanisms leading to APAP-induced cell death in hepatocytes in experimental animals and humans. Although more can be learned about various aspects of these mechanisms, it is important to keep in mind the potential effects of intervention strategies on drug metabolism, which can lead to misinterpretations. It is also critical to connect any newly discovered mediators and pathways to the established mechanisms. Furthermore, instead of assuming that a sterile inflammatory has to cause an innate immune cell-mediated injury, intracellular signaling mechanisms need to be more considered as targets for inflammatory mediators. Not only is APAP overdose a clinically relevant model to study hepatocyte cell death and liver injury, but it is also increasingly used to test potential therapeutic intervention strategies. The relevance of the studies will depend on the solid understanding of the toxicity mechanisms.
ACKNOWLEDGMENTS
Work in the authors’ laboratory was supported by the National Institutes of Health grants R01 DK070195 and R01 AA12916, and by grants from the National Institute of General Medical Sciences (P20 GM103549 and P30 GM118247) of the National Institutes of Health. The authors declare no conflicts of interest.
REFERENCES
- 1. Lee WM. Acetaminophen-related acute liver failure in the United States. Hepatol Res. 2008;38(Suppl 1):S3–8. [DOI] [PubMed] [Google Scholar]
- 2. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005;42(6):1364–72. [DOI] [PubMed] [Google Scholar]
- 3. Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369(26):2525–34. [DOI] [PubMed] [Google Scholar]
- 4. Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: An updated review. Arch Toxicol. 2015;89(2):193–9. [DOI] [PubMed] [Google Scholar]
- 5. Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40(6):585–92. [DOI] [PubMed] [Google Scholar]
- 6. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761–94, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30(9):2174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10(4):267–78. [DOI] [PubMed] [Google Scholar]
- 9. Xie Y, McGill MR, Cook SF, Sharpe MR, Winefield RD, Wilkins DG, Rollins DE, Jaeschke H. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in HepaRG cells. Xenobiotica 2015;45(10):921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Court MH, Zhu Z, Masse G, Duan SX, James LP, Harmatz JS, Greenblatt DJ. Race, gender, and genetic polymorphism contribute to variability in acetaminophen pharmacokinetics, metabolism, and protein-adduct concentrations in healthy African-American and European-American volunteers. J Pharmacol Exp Ther. 2017;362(3):431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davern TJ 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM, Acute Liver Failure Study G. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology 2006;130(3):687–94. [DOI] [PubMed] [Google Scholar]
- 12. Heard K, Green JL, Anderson V, Bucher-Bartelson B, Dart RC. Paracetamol (acetaminophen) protein adduct concentrations during therapeutic dosing. Br J Clin Pharmacol. 2016;81(3):562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Malley GF, Mizrahi F, Giraldo P, O’Malley RN, Rollins D, Wilkins D. Protein-derived acetaminophen-cysteine can be detected after repeated supratherapeutic ingestion of acetaminophen in the absence of hepatotoxicity. J Med Toxicol. 2015;11(3):317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bond GR. Acetaminophen protein adducts: A review. Clin Toxicol. (Phila) 2009;47(1):2–7. [DOI] [PubMed] [Google Scholar]
- 15. Roberts DW, Lee WM, Hinson JA, Bai S, Swearingen CJ, Stravitz RT, Reuben A, Letzig L, Simpson PM, Rule J, Fontana RJ, Ganger D, Reddy KR, Liou I, Fix O, James LP. An immunoassay to rapidly measure acetaminophen protein adducts accurately identifies patients with acute liver injury or failure. Clin Gastroenterol Hepatol. 2017;15(4):555–62.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187(1):211–7. [PubMed] [Google Scholar]
- 17. Heard KJ, Green JL, James LP, Judge BS, Zolot L, Rhyee S, Dart RC. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: Dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269(3):240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: A human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 2011;53(3):974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187(1):195–202. [PubMed] [Google Scholar]
- 21. Hu J, Ramshesh VK, McGill MR, Jaeschke H, Lemasters JJ. Low dose acetaminophen induces reversible mitochondrial dysfunction associated with transient c-Jun N-terminal kinase activation in mouse liver. Toxicol Sci. 2016;150(1):204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264(17):9814–9. [PubMed] [Google Scholar]
- 23. Xie Y, McGill MR, Du K, Dorko K, Kumer SC, Schmitt TM, Ding WX, Jaeschke H. Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol Appl Pharmacol. 2015;289(2):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Myers TG, Dietz EC, Anderson NL, Khairallah EA, Cohen SD, Nelson SD. A comparative study of mouse liver proteins arylated by reactive metabolites of acetaminophen and its nonhepatotoxic regioisomer, 3’-hydroxyacetanilide. Chem Res Toxicol. 1995;8(3):403–13. [DOI] [PubMed] [Google Scholar]
- 25. Matthews AM, Hinson JA, Roberts DW, Pumford NR. Comparison of covalent binding of acetaminophen and the regioisomer 3’-hydroxyacetanilide to mouse liver protein. Toxicol Lett. 1997;90(1):77–82. [DOI] [PubMed] [Google Scholar]
- 26. Hadi M, Dragovic S, van Swelm R, Herpers B, van de Water B, Russel FG, Commandeur JN, Groothuis GM. AMAP, the alleged non-toxic isomer of acetaminophen, is toxic in rat and human liver. Arch Toxicol. 2013;87(1):155–65. [DOI] [PubMed] [Google Scholar]
- 27. Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273(28):17940–53. [DOI] [PubMed] [Google Scholar]
- 28. Andringa KK, Bajt ML, Jaeschke H, Bailey SM. Mitochondrial protein thiol modifications in acetaminophen hepatotoxicity: Effect on HMG-CoA synthase. Toxicol Lett. 2008;177(3):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruderer R, Bernhardt OM, Gandhi T, Miladinovic SM, Cheng LY, Messner S, Ehrenberger T, Zanotelli V, Butscheid Y, Escher C, Vitek O, Rinner O, Reiter L. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol Cell Proteomics 2015;14(5):1400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144(3):279–88. [DOI] [PubMed] [Google Scholar]
- 31. Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, Jaeschke H. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci. 2010;117(2):515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315(2):879–87. [DOI] [PubMed] [Google Scholar]
- 33. Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30(5):463–88. [DOI] [PubMed] [Google Scholar]
- 34. Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37(2):193–200. [DOI] [PubMed] [Google Scholar]
- 35. Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011;251(3):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Du K, Farhood A, Jaeschke H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol. 2017;91(2):761–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burcham PC, Harman AW. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem. 1991;266(8):5049–54. [PubMed] [Google Scholar]
- 38. Ramsay RR, Rashed MS, Nelson SD. In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch Biochem Biophys. 1989;273(2):449–57. [DOI] [PubMed] [Google Scholar]
- 39. Lee KK, Imaizumi N, Chamberland SR, Alder NN, Boelsterli UA. Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology 2015;61(1):326–36. [DOI] [PubMed] [Google Scholar]
- 40. Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: Role of mitochondrial oxidant stress. Toxicol Sci. 2001;62(2):212–20. [DOI] [PubMed] [Google Scholar]
- 41. Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S, James LP, MacMillan-Crow LA, Hinson JA. Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Ther. 2012;340(1):134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, Cohen SD, Gordon MK, Gerecke DR, Zhou P, Laskin DL. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: Potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol Appl Pharmacol. 2002;184(1):27–36. [PubMed] [Google Scholar]
- 43. Salhanick SD, Orlow D, Holt DE, Pavlides S, Reenstra W, Buras JA. Endothelially derived nitric oxide affects the severity of early acetaminophen-induced hepatic injury in mice. Acad Emerg Med. 2006;13(5):479–85. [DOI] [PubMed] [Google Scholar]
- 44. Banerjee S, Melnyk SB, Krager KJ, Aykin-Burns N, Letzig LG, James LP, Hinson JA. The neuronal nitric oxide synthase inhibitor NANT blocks acetaminophen toxicity and protein nitration in freshly isolated hepatocytes. Free Radic Biol Med. 2015;89:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lacza Z, Pankotai E, Busija DW. Mitochondrial nitric oxide synthase: Current concepts and controversies. Front Biosci. (Landmark Ed) 2009;14:4436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lacza Z, Snipes JA, Zhang J, Horvath EM, Figueroa JP, Szabo C, Busija DW. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic Biol Med. 2003;35(10):1217–28. [DOI] [PubMed] [Google Scholar]
- 47. Gonzalez DR, Treuer AV, Dulce RA. Neuronal nitric oxide synthase in heart mitochondria: A matter of life or death. J Physiol. 2009;587(Pt 12):2719–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther. 2011;337(1):110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: Protection by glutathione. J Pharmacol Exp Ther. 2002;303(2):468–75. [DOI] [PubMed] [Google Scholar]
- 50. Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 2010;51(1):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Du K, McGill MR, Xie Y, Bajt ML, Jaeschke H. Resveratrol prevents protein nitration and release of endonucleases from mitochondria during acetaminophen hepatotoxicity. Food Chem Toxicol. 2015;81:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ramachandran A, Lebofsky M, Yan HM, Weinman SA, Jaeschke H. Hepatitis C virus structural proteins can exacerbate or ameliorate acetaminophen-induced liver injury in mice. Arch Toxicol. 2015;89(5):773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jan YH, Heck DE, Dragomir AC, Gardner CR, Laskin DL, Laskin JD. Acetaminophen reactive intermediates target hepatic thioredoxin reductase. Chem Res Toxicol. 2014;27(5):882–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanaka R, Ishima Y, Maeda H, Kodama A, Nagao S, Watanabe H, Chuang VT, Otagiri M, Maruyama T. Albumin fusion prolongs the antioxidant and anti-inflammatory activities of thioredoxin in mice with acetaminophen-induced hepatitis. Mol Pharm. 2014;11(4):1228–38. [DOI] [PubMed] [Google Scholar]
- 55. Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 2008;135(4):1311–21. [DOI] [PubMed] [Google Scholar]
- 56. Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82(5):1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang J, Min RWM, Le K, Zhou S, Aghajan M, Than TA, Win S, Kaplowitz N. The role of MAP2 kinases and p38 kinase in acute murine liver injury models. Cell Death Dis. 2017;8(6):e2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283(20):13565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246(1–2):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, Jaeschke H. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2015;286(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286(40):35071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huo Y, Win S, Than TA, Yin S, Ye M, Hu H, Kaplowitz N. Antcin H protects against acute liver injury through disruption of the interaction of c-Jun-N-terminal kinase with mitochondria. Antioxid Redox Signal. 2017;26(5):207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324(1):8–14. [DOI] [PubMed] [Google Scholar]
- 64. El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: Roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191(2):118–29. [DOI] [PubMed] [Google Scholar]
- 65. Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285(11):8244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 2004;40(5):1170–9. [DOI] [PubMed] [Google Scholar]
- 67. Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42(1):110–6. [DOI] [PubMed] [Google Scholar]
- 68. Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45(2):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Karch J, Molkentin JD. Identifying the components of the elusive mitochondrial permeability transition pore. Proc Natl Acad Sci USA 2014;111(29):10396–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J, Kinnally KW, Molkentin JD. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2013;2:e00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA 2014;111(29):10580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chinopoulos C. Mitochondrial permeability transition pore: Back to the drawing board. Neurochem Int. 2017. [DOI] [PubMed] [Google Scholar]
- 73. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005;434(7033):658–62. [DOI] [PubMed] [Google Scholar]
- 74. LoGuidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology 2011;54(3):969–78. [DOI] [PubMed] [Google Scholar]
- 75. Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci. 2010;117(1):101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Woolbright BL, Ramachandran A, McGill MR, Yan HM, Bajt ML, Sharpe MR, Lemasters JJ, Jaeschke H. Lysosomal instability and cathepsin B release during acetaminophen hepatotoxicity. Basic Clin Pharmacol Toxicol. 2012;111(6):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hu J, Kholmukhamedov A, Lindsey CC, Beeson CC, Jaeschke H, Lemasters JJ. Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: Protection by starch-desferal and minocycline. Free Radic Biol Med. 2016;97:418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Norberg E, Orrenius S, Zhivotovsky B. Mitochondrial regulation of cell death: Processing of apoptosis-inducing factor (AIF). Biochem Biophys Res Commun. 2010;396(1):95–100. [DOI] [PubMed] [Google Scholar]
- 79. Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94(1):217–25. [DOI] [PubMed] [Google Scholar]
- 80. Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. AIF-mediated programmed necrosis: A highly regulated way to die. Cell Cycle 2007;6(21):2612–9. [DOI] [PubMed] [Google Scholar]
- 81. Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122(2):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Duan L, Davis JS, Woolbright BL, Du K, Cahkraborty M, Weemhoff J, Jaeschke H, Bourdi M. Differential susceptibility to acetaminophen-induced liver injury in sub-strains of C57BL/6 mice: 6N versus 6J. Food Chem Toxicol. 2016;98(Pt B):107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Du K, Ramachandran A, McGill MR, Mansouri A, Asselah T, Farhood A, Woolbright BL, Ding WX, Jaeschke H. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol. 2017;108(Pt A):339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 2012;55(1):222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ni HM, Williams JA, Jaeschke H, Ding WX. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol. 2013;1:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Baulies A, Ribas V, Nunez S, Torres S, Alarcon-Vila C, Martinez L, Suda J, Ybanez MD, Kaplowitz N, Garcia-Ruiz C, Fernandez-Checa JC. Lysosomal cholesterol accumulation sensitizes to acetaminophen hepatotoxicity by impairing mitophagy. Sci Rep. 2015;5:18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, Jaeschke H, Ding WX. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J Hepatol. 2016;65(2):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: Mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol. 2002;181(2):133–41. [DOI] [PubMed] [Google Scholar]
- 89. Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78(15):1670–6. [DOI] [PubMed] [Google Scholar]
- 90. Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156(3):179–86. [DOI] [PubMed] [Google Scholar]
- 91. Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: Apoptosis or oncotic necrosis? Toxicol Sci. 2002;67(2):322–8. [DOI] [PubMed] [Google Scholar]
- 92. Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24(2):85–9. [DOI] [PubMed] [Google Scholar]
- 93. Jacob M, Mannherz HG, Napirei M. Chromatin breakdown by deoxyribonuclease1 promotes acetaminophen-induced liver necrosis: An ultrastructural and histochemical study on male CD-1 mice. Histochem Cell Biol. 2007;128(1):19–33. [DOI] [PubMed] [Google Scholar]
- 94. Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30(9):1280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 2003;125(4):1246–57. [DOI] [PubMed] [Google Scholar]
- 96. Jaeschke H, Williams CD, Farhood A. No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology 2011;53(2):718–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61(4):1659–65. [PubMed] [Google Scholar]
- 98. Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3(115):re4. [DOI] [PubMed] [Google Scholar]
- 99. Zhang J, Yang Y, He W, Sun L. Necrosome core machinery: MLKL. Cell Mol Life Sci. 2016;73(11–12):2153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21(11):1709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012;148(1–2):213–27. [DOI] [PubMed] [Google Scholar]
- 102. Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology 2013;58(6):2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Deutsch M, Graffeo CS, Rokosh R, Pansari M, Ochi A, Levie EM, Van Heerden E, Tippens DM, Greco S, Barilla R, Tomkotter L, Zambirinis CP, Avanzi N, Gulati R, Pachter HL, Torres-Hernandez A, Eisenthal A, Daley D, Miller G. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis. 2015;6:e1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhang YF, He W, Zhang C, Liu XJ, Lu Y, Wang H, Zhang ZH, Chen X, Xu DX. Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett. 2014;225(3):445–53. [DOI] [PubMed] [Google Scholar]
- 105. Dara L, Johnson H, Suda J, Win S, Gaarde W, Han D, Kaplowitz N. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology 2015;62(6):1847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology 1998;27(4):1021–9. [DOI] [PubMed] [Google Scholar]
- 107. Dara L, Liu ZX, Kaplowitz N. Questions and controversies: The role of necroptosis in liver disease. Cell Death Discov. 2016;2:16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122(4):1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56(5):1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110. Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett. 2010;192(3):387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Woolbright BL, Jaeschke H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J Hepatol. 2017;66(4):836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology 2012;143(5):1158–72. [DOI] [PubMed] [Google Scholar]
- 113. Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int. 2012;32(1):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 2006;43(6):1220–30. [DOI] [PubMed] [Google Scholar]
- 115. Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: Another perspective. Hepatology 2007;45(6):1588–9; author reply 9. [DOI] [PubMed] [Google Scholar]
- 116. Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: Role of neutrophils. Toxicol Sci. 2000;54(2):509–16. [DOI] [PubMed] [Google Scholar]
- 117. Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2006;216(1):98–107. [DOI] [PubMed] [Google Scholar]
- 118. James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: Role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 2003;37(12):1289–97. [DOI] [PubMed] [Google Scholar]
- 119. Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275(2):122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119(2):305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Marques PE, Oliveira AG, Pereira RV, David BA, Gomides LF, Saraiva AM, Pires DA, Novaes JT, Patricio DO, Cisalpino D, Menezes-Garcia Z, Leevy WM, Chapman SE, Mahecha G, Marques RE, Guabiraba R, Martins VP, Souza DG, Mansur DS, Teixeira MM, Leite MF, Menezes GB. Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice. Hepatology 2015;61(1):348–60. [DOI] [PubMed] [Google Scholar]
- 122. Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol. 2011;252(3):289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol. 2010;247(3):169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang C, Feng J, Du J, Zhuo Z, Yang S, Zhang W, Wang W, Zhang S, Iwakura Y, Meng G, Fu YX, Hou B, Tang H. Macrophage-derived IL-1alpha promotes sterile inflammation in a mouse model of acetaminophen hepatotoxicity. Cell Mol Immunol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Campion SN, Johnson R, Aleksunes LM, Goedken MJ, van Rooijen N, Scheffer GL, Cherrington NJ, Manautou JE. Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am J Physiol Gastrointest Liver Physiol. 2008;295(2):G294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15(12):1504–13. [DOI] [PubMed] [Google Scholar]
- 127. Cavassani KA, Moreira AP, Habiel D, Ito T, Coelho AL, Allen RM, Hu B, Raphelson J, Carson WFt, Schaller MA, Lukacs NW, Omary MB, Hogaboam CM, Kunkel SL. Toll like receptor 3 plays a critical role in the progression and severity of acetaminophen-induced hepatotoxicity. PLoS One 2013;8(6):e65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Woolbright BL, McGill MR, Sharpe MR, Jaeschke H. Persistent generation of inflammatory mediators after acetaminophen overdose in surviving and non-surviving patients. Hepatology 2015;62(Suppl):500A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, Wagner B, Barnardo A, Pomplun S, Auzinger G, Bernal W, Heaton N, Vergani D, Thursz MR, Wendon J. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 2012;56(2):735–46. [DOI] [PubMed] [Google Scholar]
- 130. Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology 2002;35(5):1093–103. [DOI] [PubMed] [Google Scholar]
- 131. Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84(6):1410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]