Abstract
Thyroid hormones (THs), namely, 3,5,3′-triiodo-l-thyronine (T3) and 3,5,3′,5′-tetraiodo-l-thyronine (thyroxine or T4), influence a variety of physiological processes that have important implications in fetal development, metabolism, cell growth, and proliferation. While THs elicit several beneficial effects on lipid metabolism and improve myocardial contractility, these therapeutically desirable effects are associated to a thyrotoxic state that severely limits the possible use of THs as therapeutic agents. Therefore, several efforts have been made to develop T3 analogs that could retain the beneficial actions (triglyceride, cholesterol, obesity, and body mass lowering) without the adverse TH-dependent side effects. This goal was achieved by the synthesis of TRβ-selective agonists. In this review, we summarize the current knowledge on the effects of one of the best characterized TH analogs, the TRβ1-selective thyromimetic, GC-1. In particular, we review some of the effects of GC-1 on different liver disorders, with reference to its possible clinical application. A brief comment on the possible therapeutic use of GC-1 in extrahepatic disorders is also included.
Key words: Thyromimetics, Hepatocellular carcinoma, Regenerative medicine, Liver cell proliferation, Thyroid hormone receptor
FROM THYROID HORMONES TO SELECTIVE THYROMIMETICS
Thyroid hormones (THs), 3,5,3′-triiodo-l-thyronine (T3) and 3,5,3′,5′-tetraiodo-l-thyronine (thyroxine or T4), influence a variety of effects, such as fetal development, cell growth, and homeostasis. They also affect processes and pathways mediating carbohydrate, lipid, protein, and mineral metabolism in almost all tissues1. Particular interest has been directed toward the beneficial effects of the administration of exogenous THs on lipid metabolism, such as increased metabolic rate, weight loss, lipolysis, and lowering serum cholesterol levels, as well as on the improvement in myocardial contractility2,3. Unfortunately, these therapeutically desirable effects are associated to a thyrotoxic state, which includes induction of tachycardia, arrhythmia, muscle catabolism, reduced bone mineralization, alteration of central nervous system (CNS) development, and mood disorders1,4–6. Such adverse effects strongly limit the use of THs. For this reason, since the 1950s, the possibility of identifying TH derivatives that could uncouple the therapeutic actions of THs from their deleterious effects has been actively pursued.
Although the existence of nongenomic mechanisms initiated at the cell membrane that could be involved in few cardiovascular and metabolic actions has been postulated7, most of the effects of THs are mediated by nuclear receptors (TRs): thyroid hormone receptor-α (TRα) and thyroid hormone receptor-β (TRβ). TRs encode for several mRNA isoforms, generated by the use of different promoters and alternative splicing8,9. TR isoforms are widely present in most organs/tissues; however, their distribution is quite heterogeneous among the different tissues and/or during developmental stages. Studies in mice with inactivation or mutation of different TR isoforms demonstrated that TRα is the dominant receptor in the brain and skeletal system and mediates most of the synergism between T3 and the sympathetic signaling pathway in the heart. On the other hand, TRβ is the most abundant TH isoform in the liver, where it mediates most of the T3 effects on lipid metabolism and regulation of metabolic rate10–12.
Based on these observations, many works have been undertaken to develop compounds that selectively act on the different isoforms of TRs, thus leading to the activation of different T3-mediated pathways1,12.
The liver is one of the major target organs of THs, and research over the past few decades reported that disruption of cellular TH signaling triggers a variety of liver-associated diseases with a spectrum ranging from hepatic steatosis to hepatocellular carcinoma (HCC)13. A promising therapeutic strategy for liver diseases might rely on TRβ1-selective thyromimetics, such as GC-1, CGS23425, KB-141, KB2115, DITPA, and MB07344, the active form of the prodrug MB07811. Indeed, all these TH analogs possess TH-related biological effects without overt cardiotoxic effects14–17.
THE DAWN OF GC-1
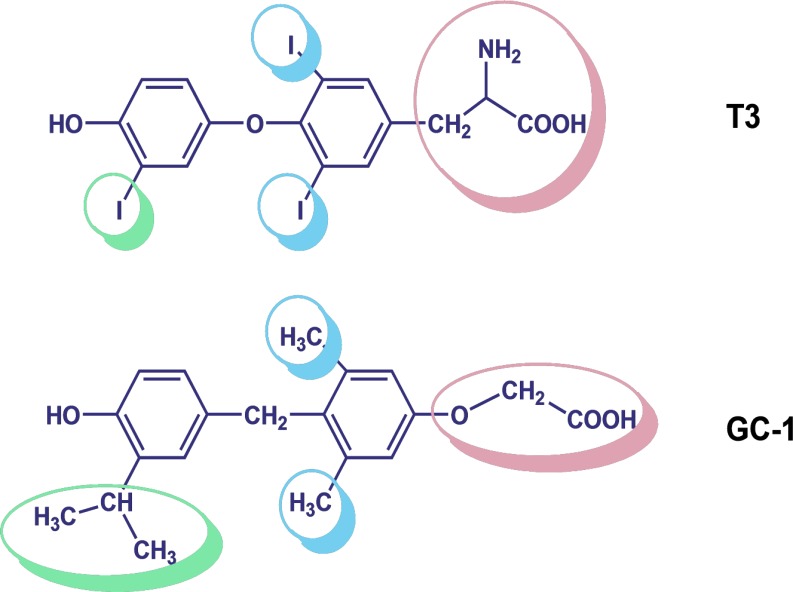
In 1998, the synthesis of 3,5-dimethyl-4(4′-hydroxy-3′-isopropylbenzyl)-phenoxy) acetic acid, the TRβ1-selective agonist, was announced18. Importantly, this novel thyromimetic compound, named GC-1 (commercially known as Sobetirome and QRX-431), represented a scaffold compound for the development, design, and synthesis of structurally diverse thyromimetic ligands. The principal structural changes presented by GC-1, with respect to the natural hormone T3 (Fig. 1), were (1) the three iodine residues of T3 were replaced by the methyl and isopropyl groups; (2) a methylene linkage replaced the biaryl-ether linkage between the two phenol groups; and (3) the amino acid side chain at the 1 position was changed with an oxyacetic group. Using a radioligand displacement assay, it has been demonstrated that because of the abovementioned modifications, GC-1 showed affinity for TRβ1 comparable to that of T3, whereas this novel thyromimetic compound demonstrated a 10-fold lower affinity to TRα1 compared with T3. The TRβ selectivity was observed, both in binding experiments and in dose–response cellular transactivation experiments18. Successively, a new synthetic route was described that allowed to prepare multigram quantities of GC-1 for use in animal models19. A few studies were also performed in order to shed light into the β-selectivity mechanism of GC-1. Initially, X-ray crystallographic studies revealed that TRβ-selective binding of GC-1 is dependent on a single TR subtype-specific residue in the TR ligand-binding cavity (LBC). This critical residue is Asn331 in TRβ that is replaced by Ser277 in TRα20,21. Subsequently, the X-ray structures of both TR isoforms bound to T3 and GC-1, supported by molecular dynamics simulations of the complex, allowed to formulate a more accurate mechanism for the β-selectivity of GC-1. This structural investigation, performed by Bleicher et al., proposed that the key factors for isoform-selective binding of GC-1 are the presence of the oxyacetic acid ester oxygen, the absence of the amino group relative to T3, the Ser/Asn active site (Ser277 in human TRα is substituted by Asn331 in TRβ), and the alternate conformations of a conserved Arg residue in the binding pocket (Arg228α/Arg282β)22. While in TRβ a single, stable “productive” conformation is observed, in which GC-1 interacts effectively with Arg282, in TRα, both “productive” and “nonproductive” conformations are present. In the “productive” conformation, identical to that of TRβ, the ligand interacts strongly with the Arg228 residue and with Ser277. However, TRα Arg228 also possesses the ability to flip away from the ligand in “nonproductive” conformations, resulting in the loss of a crucial interaction for the stabilization of the ligand22.
Figure 1.
Chemical structures of 3,5,3′-triiodo-l-thyronine (T3) and GC-1.
GC-1 AND METABOLIC DISORDERS
Lipid disorders, which contribute to hypertension, diabetes, and atherosclerosis, are considered to be among the most relevant health problems in developed countries nowadays23. Although treatment with 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase) inhibitors (known as statins) is considered a cornerstone for lowering cholesterol, new strategies to optimize plasma cholesterol levels are still needed. Developing new types of drugs would be especially helpful in those patients that do not tolerate or do not respond to the treatment with statins. Therefore, considering the selective action of GC-1, it is not surprising that this compound has been widely studied as a promising prototype of new drugs for the treatment of a variety of metabolic disturbances, including high lipid levels.
In one of the first investigations aimed at evaluating the effects of GC-1 in vivo, this TRβ-selective agonist was administered to hypothyroid mice and hypercholesteremic rats, and its effect was compared with that of equimolar doses of T324. In this proof-of-concept study, GC-1 showed extremely encouraging results, as its administration elicited cholesterol- and triglyceride-lowering effects. Importantly, no harmful effects on heart weight, heart rate, and mRNAs coding for proteins related to cardiac contraction, such as myosin heavy chain α (MHCα), MHCβ, and sarcoplasmic reticulum calcium adenosine triphosphatase (Serca2), were observed24. This study also demonstrated, for the first time, that GC-1 has the ability to combine both organ and TRβ1 selectivity, which may then enhance hepatic targeting.
Further experiments were performed in cynomolgus monkeys, which were chosen because their lipid metabolic profile more closely resembles that of humans25. Data reported in this article showed that, in addition to cholesterol-lowering activity, GC-1 reduced levels of lipoprotein (a), an important risk factor, which is often elevated in patients with premature atherosclerosis26.
Moreover, a comparison between GC-1 and atorvastatin, based on literature data, revealed that GC-1 has a much greater potency in both monkeys and rats23. Although it became soon obvious that GC-1 decreases serum cholesterol levels, the mechanisms behind this reduction remain elusive.
Successive studies elucidated the pathways involved in the cholesterol-lowering effect of this selective thyromimetic. Indeed, experiments by Johansson and coworkers27 performed in euthyroid chow-fed mice demonstrated that GC-1 not only reduced serum cholesterol and triglyceride levels but also attenuated diet-induced hypercholesterolemia. By increasing expression of the hepatic high-density lipoprotein (HDL) receptor (SR-B1), as well as stimulating the activity of the rate-limiting enzyme of bile acid synthesis, cholesterol 7α-hydroxylase (CYP7A1), and fecal bile acid excretion, GC-1 was shown to induce several steps in the reverse cholesterol transport (RCT). Moreover, in this study, GC-1 was found to be even more efficient than T3 when administered at equimolar doses27. Although the reduction of cholesterol levels by THs and TH analogs has been also attributed to their ability to increase the expression of hepatic low-density lipoprotein receptor (LDLR)28, successive studies by Lin et al.29 pointed out that the lipid-lowering effects of GC-1 are independent of the mechanism of LDLRs. This conclusion, stemming from studies performed in LDLR−/− mice, suggests that GC-1 may represent a promising cholesterol-lowering therapeutic approach for the treatment of homozygous familial hypercholesterolemia (hFH)29. This rare genetic disorder, caused by a complete lack of functional LDLRs, is characterized by severe hypercholesterolemia, atherosclerosis, and, unfortunately, unresponsiveness to current therapies30.
Finally, in a recent study, long-term GC-1 treatment of apolipoprotein E (ApoE)-deficient mice, fed an atherogenic diet, significantly reduced the levels of cholesteryl esters in the aortas, highlighting the preventive effect of GC-1 on the development of atherosclerosis31.
The rising prevalence of obesity represents another important medical problem with major impact on morbidity and mortality. Also in this case, current treatments have shown limited efficacy and safety. Therefore, improved therapeutic strategies are urgently needed32. Pharmacological modification of adaptive thermogenesis in the treatment of obesity has been well described33,34. In an early study, the level of uncoupling protein 1 (UCP1), the key thermogenic protein in brown adipose tissue (BAT), the primary site of facultative thermogenesis35, was reported to be induced by GC-1 treatment36. Furthermore, Grover et al.25 demonstrated that GC-1 decreased body weight in primates, without significant change in heart rate or arterial blood pressure. Successively, treatment with this TRβ1-specific agonist was reported to increase the metabolic rate and to reduce fat mass in rats, without any apparent loss of skeletal muscles37.
Finally, in a very recent study, constant and controlled administration of GC-1 from an innovative nanochannel membrane device (NMD) for drug delivery was shown to reverse very high-fat diet (VHFD)-induced fat accumulation in the liver, induce weight loss, reduce fat mass, and normalize serum cholesterol and glycemia as well38.
Taken together, these data obtained in several mammalian species (mice, rats, and monkeys) strongly indicate that GC-1 has promising properties as an agent lowering cholesterol and triglyceride levels, as well as inducing fat loss.
GC-1 USE IN NONALCOHOLIC FATTY LIVER DISEASE
Although TRβ1-selective thyromimetics have been primarily designed with the aim of developing new therapies against hypercholesterolemia, their possible use for the treatment of other metabolic disorders has also been investigated, including nonalcoholic fatty liver disease (NAFLD)39. The embracing term NAFLD was adopted to cover the full spectrum of metabolic fatty disorders, ranging from simple steatosis to steatohepatitis, advanced fibrosis, and cirrhosis. The acronym NASH (nonalcoholic steatohepatitis), as originally coined by Ludwig et al.40 in 1980, instead describes a liver disease that histologically mimics alcoholic hepatitis but occurs in the absence of significant alcohol consumption and refers to a well-defined stage within the spectrum of NAFLD. NAFLD, the most common liver disorder in Western countries, has been reported to affect 17%–46% of adults. NAFLD incidence has been estimated to affect 20–86/1,000 person-year based on elevated liver enzymes and/or on ultrasound, and 34/1,000 per year by proton magnetic resonance spectroscopy (1H-MRS). Type 2 diabetes mellitus (T2DM) closely associates with the severity of NAFLD, progression to NASH, advanced fibrosis, and the development of HCC. In fact, large-scale epidemiological studies have repeatedly associated obesity and T2DM with the risk of HCC. The occurrence of HCC has also been reported in NAFLD/cryptogenic cirrhosis. Importantly, NAFLD is the second leading indication for HCC-related transplantation in the US. Moreover, NAFLD-associated cirrhosis is among the top three indications for liver transplantation41. In the past, histological characteristics of NASH used to be controversial and varied in the literature. As proposed by Neuschwander-Tetri and Caldwell42, the histopathological features of NASH encompass zone 3 of the liver lobule. In this area, macrovesicular steatosis in combination with hepatocyte ballooning, and a mixed inflammatory infiltrate often associated with characteristic perisinusoidal and pericellular fibrosis, predominate. NAFLD has been recognized as the most common cause of chronic liver disease in many countries and for this reason poses an important health problem42,43. Recently, it became evident that hepatic steatosis should not be regarded as an innocuous, benign condition, but rather as an important cause of advanced liver disease44.
Despite the high prevalence of this liver disorder and its potential for serious sequelae, currently no effective and approved therapeutic strategy exists. The understanding of the pathogenesis of NAFLD has unquestionably improved with the help of several animal models; indeed, these models have provided important information with respect to the complex metabolic and genetic factors that lead to this liver disease45.
Feeding a choline-devoid methionine-deficient (CMD) diet is a particularly useful and frequently employed nutritional model, resulting in liver injury that shows close pathological and biochemical similarities to human NASH46–48. Employing this experimental model, Perra et al.49 demonstrated that, similar to T3, GC-1 was able to prevent the development of hepatic steatosis and promote a rapid regression of preexisting fat accumulation, suggesting a potential therapeutic application of GC-1. The disappearance of hepatic triglycerides was accompanied by a concomitant decrease in lipid peroxidation49. Moreover, GC-1 was highly effective in reducing the burden of hepatic steatosis also in other animal experimental models, such as ob/ob mice and Western diet-fed LDLR−/− mice50. With regard to human data, a recent report on a patient with NASH complicated by Graves’ disease indicated that hyperthyroidism may improve the pathological condition of NASH51. On the other hand, although the amelioration of fatty liver by GC-1 was evident in ob/ob mice, its effects on glycemia and insulin sensitivity were highly variable and time and dosage dependent50. Moreover, although GC-1 treatment prevented the development of hepatic steatosis in high-fat diet-fed rats, decreasing hepatic triglyceride content by up to 75%, it also caused hyperglycemia and insulin resistance52. Thus, although many evidences indicate that GC-1 could be used as a useful antisteatogenic agent, the latter studies suggest caution prior to implicating its beneficial effect on NASH.
GC-1 AND HEPATOCYTE PROLIFERATION
Hepatic regenerative medicine suffers from limited therapeutic options for the treatment of acute or chronic hepatic insufficiency, which will ultimately progress to end-stage liver disease. As a consequence, treatment of end-stage limited disease is often liver transplantation. However, because of the shortage of organs, alternatives, such as generation of de novo organs, cell therapy, stem cell differentiation, and bioartificial livers53, are needed. Another strategy is to stimulate liver regeneration to promote an increase in functional hepatocyte mass within a diseased liver54. Such regenerative therapy could have potential applicability in treatment after acute and chronic liver injuries as well as for use in transplantation settings. In this context, the use of such regeneration-promoting agents in living-related donors and in recipients after transplantation is promising.
Several studies have shown that T3 is a strong inducer of liver cell proliferation in rats and mice55–57. Unlike that observed in liver regeneration following partial hepatectomy (PH)58 or cell loss due to necrosis, the hepatomitogenic effect of T3 occurs in the absence of activation of transcription factors, such as AP-1, NF-κB, or STAT3, or increased expression of immediate early genes, such c-fos, c-jun, or c-myc, while causing a very rapid increase in the mRNA and protein levels of cyclin D157. These studies suggest that T3-induced hepatocyte proliferation occurs through pathways different from those underlying liver regeneration. Whether the mitogenic signals are mediated by extragenomic mechanisms or take place via TRβ transcriptional activation remains unclear.
Using both wild-type and hepatocyte-specific β-catenin knockout mice, the involvement of β-catenin, the key effector of the Wnt signaling pathway59,60, in T3-induced hepatocyte proliferation has also been assessed61,62. Notably, T3 showed a potent hepatomitogenic effect not only in intact liver but also during the regenerative response in rodents after 70% partial or 90% subtotal hepatectomy63–66.
Recently, TRβ agonists like GC-1 have been shown to be agents, which may be useful as regenerative therapies61,62,66. Indeed, GC-1 mimics the effect of T3 as a powerful inducer of hepatocyte proliferation in rats. In particular, GC-1-induced hepatocyte proliferation was associated with a rapid increase in cyclin D1 mRNA levels, a strong enhancement of the nuclear levels of E2F1 and p107, an increase in proliferating cell nuclear antigen (PCNA), and no change in the expression of c-jun and c-fos 67. Moreover, no tissue injury was reported. Shortly afterward, a strong mitogenic response induced by GC-1, identical to that observed for T3, was also described in mouse hepatocytes68. On the other hand, a significant decrease in hepatocyte proliferation after GC-1 treatment occurs in both liver-specific β-catenin knockout and liver-specific double knockouts of Wnt co-receptors LRP5 and LRP6 (LRP5-6-LKO), when compared to wild-type littermates. The latter results led to the conclusion that, similar to what was observed with T3, β-catenin activation is, at least in part, responsible for GC-1-induced hepatocyte proliferation62.
Not only does GC-1 promote hepatocyte proliferation, but pretreatment with this thyromimetic accelerated cyclin D1 expression and earlier transition of hepatocytes into the S phase, following PH62. These studies pave the way for the use of thyromimetics, and especially TRβ agonists, like GC-1, that are more selective in their action on hepatocytes, in hepatic regenerative medicine, particularly acute liver injuries, or in transplantation settings. Although these studies provide evidence of a potential usefulness of GC-1 in regenerative medicine, the safety of these agents, and especially their effect on hepatic tumor cell proliferation, needs to be further investigated before their use in chronic hepatic injuries or preneoplastic conditions could be proposed.
ANTIPRENEOPLASTIC/NEOPLASTIC EFFECTS OF GC-1
HCC is the second cause of global cancer-related deaths69,70. Despite the significant advances in pharmacological therapies, the prognosis for this tumor type has not improved in recent years. This is due to the fact that most HCC cases are diagnosed at advanced stages, when no effective systemic therapy exists for these patients. Although the approval of the multikinase inhibitor sorafenib has provided some hope, the benefits obtained from the treatment with this drug remained disappointing, as demonstrated by the modest improvement in median overall survival71. For this reason, new therapeutic options are needed for HCC.
In recent years, growing evidence has demonstrated that THs and TRs are implicated in tumor development. Indeed, two case-control studies suggested that hypothyroidism represents a risk factor in HCC development. In the first study, Hassan et al.72 observed a significant elevated risk association between hypothyroidism and HCC in women that was independent of established HCC risk factors, whereas in the second one, hypothyroidism was significantly more prevalent in HCC patients with an unknown etiology73. In other studies, aberrant expression and/or TR mutations have been observed in various human cancers, including not only HCC74 but also breast cancer, colon cancer, lung cancer, pituitary tumor, renal clear cell carcinomas, and thyroid cancer75,76. Furthermore, support for the role of TRs in HCC development stems from the observation that TRs, in particular the TRβ isoform, are downregulated in both human and rat HCC77–79. Notably, in experimental rat models, TRβ downregulation was observed very early in the hepatocarcinogenic process and discriminated the most aggressive preneoplastic lesions from those endowed with a low proliferative capacity78.
In vivo studies showed that a 14-day treatment with GC-1 accelerated the regression of chemically induced hepatic preneoplastic lesions80 in rats subjected to the resistant hepatocyte (R-H) model of hepatocarcinogenesis81. Similar to what was observed with T3, a progressive loss of fetal markers such as the placental form of glutathione S-transferase (GST-P) and γ glutamyl transpeptidase (GGT) and reacquisition of glucose 6-phosphatase (G6Pase) and adenosine triphosphatase (ATPase), two proteins expressed in normal differentiated liver, occurred in preneoplastic lesions following GC-1 treatment. This shift toward a fully differentiated phenotype preceded the loss of the preneoplastic lesions. In this context, it should be noted that the analysis of TRβ1 expression in hepatic preneoplastic lesions generated by the R-H model and characterized by TRβ1 downregulation in a short-term treatment with T3 induced an increased expression of TRβ1 and of its target genes78. Moreover, an increased expression of TRβ1 after treatment with T3 was also observed in two HCC cell lines (HuH7 and Mahlavu). Importantly, no effect of T3 was observed upon TRβ1 silencing, indicating the TRβ1 dependency of this effect78. Although no data regarding the effect of GC-1 administration on TRβ expression are available, it is likely that reactivation of TRβ1 and of its target genes might occur after treatment with this and other thyromimetics. Of note, an antipreneoplastic effect of GC-1 was also observed in the CMD model of hepatocarcinogenesis80. Finally, very recent results showed that a short treatment of mice with GC-1 exerted a clear antitumoral effect on HCC generated by hydrodynamic tail vein injection of hMet-S45Y-β-catenin, using the sleeping beauty transposon–transposase (Monga SP, paper submitted). Under these experimental conditions, the antitumorigenic effect of GC-1 was attributed to its ability to inactivate Met signaling.
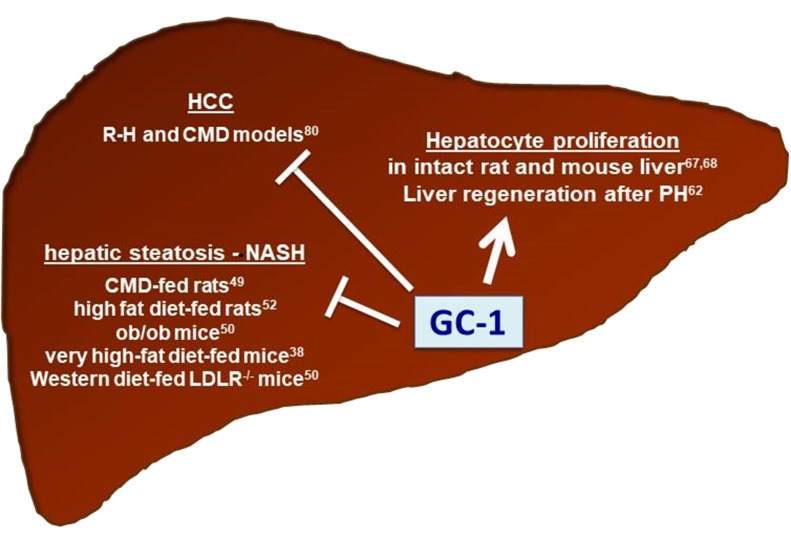
Regardless of the precise mechanisms implicated in GC-1 antineoplastic activity, overall, these results indicate that thyromimetics not only appear to be safe and, therefore, useful in regenerative medicine, but they can also be proposed as antitumorigenic agents for HCC. A schematic representation of the effects of GC-1 on NASH, HCC, and hepatocyte proliferation is provided in Figure 2.
Figure 2.
Effect of GC-1 on hepatic steatosis/nonalcoholic steatohepatitis (NASH), hepatocellular carcinoma (HCC), and hepatocyte proliferation in distinct experimental models.
GC-1 AND HUMAN CLINICAL STUDIES
So far, the most appealing effects of TH to the pharmaceutical industry are associated with their ability to lower lipids and to induce weight loss through increased energy expenditure82–84. These effects were observed both in patients with hypo- and hyperthyroidism85–87 and in TR knockout models88. Unfortunately, significant adverse effects elicited by THs1,4–6 strongly limited their clinical use. As already mentioned, a novel approach for the treatment of dyslipidemia appeared with selective thyromimetics, which possess TH-related biological effects without deleterious cardiotoxic effects14–16. Importantly, administration of GC-1 to rats had less chronotropic and inotropic effects on the heart when compared with T324 and did not result in bone loss typical of T3-induced thyrotoxicosis89. Since GC-1 was found to lower plasma cholesterol levels in primates, having lipoprotein profiles similar to humans25, it was suggested that it may have efficacy in humans. The first clinical results with GC-1 were presented at a scientific meeting90 and in public announcements (QuatRx Web site). The results of the phase 1 clinical trials showed that not only was GC-1 well tolerated by patients, but also its cholesterol-lowering effect was confirmed. Importantly, LDL cholesterol was reduced by up to 41%91. Weight loss was not reported in clinical trials of GC-116,90. The efficacy of GC-1 in ameliorating the lipid profile in patients was not associated with any obvious deleterious effect. Unfortunately, no attempts to use GC-1 as a hepatomitogen or an antineoplastic agent in HCC therapy have been performed in humans yet.
ONLY LIVER? THE POSSIBLE EFFECT OF GC-1 ON OTHER ORGANS
TH has important actions related to health and disease in all tissues of the body including the CNS, where TH plays a key role in regulating brain development and maintaining specialized cell populations and structures such as myelin92.
Compared to TH’s actions in the periphery, much less is known at the molecular and mechanistic levels about its actions in the brain. Acting through nuclear hormone receptors, TH has been shown to promote the expression of a number of oligodendrocyte-specific genes including myelin basic protein (MBP), myelin-associated glycoprotein (MAG), proteolipid protein (PLP), and cyclic nucleotide 30-phosphodiesterase (CNP)93–95.
Even though TRα is the most abundant TR isoform in the brain, several studies suggest an important role for TRβ1 in oligodendrocyte maturation. Indeed, recent studies in rodent models of demyelination suggest that TH has the capacity to promote remyelination. However, the endogenous TH is not a viable candidate for myelin repair as it lacks a therapeutic index (TI) separating desirable therapeutic effects from deleterious systemic thyrotoxic effects, particularly on heart, bone, and skeletal muscle16. Selective modulation of the TRβ has been shown to circumvent many of these undesirable effects, thus widening the safety margin between therapeutic and harmful effects25,89,91,96.
Whereas GC-1 was designed as a cardiac-sparing drug for treating hypercholesterolemia by activating TRβ in the liver91, it is unique within the thyromimetic field in possessing significant distribution in the brain24,97.
To investigate its therapeutic potential against CNS disorders, GC-1 has been tested in preclinical models of X-linked adrenoleukodystrophy (X-ALD), a lipid storage disease of the CNS and the adrenal gland caused by mutations in a membrane lipid transporter (ABCD1) that lead to the toxic accumulation of C24 and C26 very long chain fatty acids (VLCFAs) in all cells98. One proposed therapeutic strategy for genetic complementation of ABCD1 is pharmacological upregulation of ABCD2, a gene encoding a homologous peroxisomal transporter. Indeed, a very recent report99 showed that TH and GC-1 treatment rapidly induces transcription of ABCD2, accompanied by an expected reduction of both periphery and CNS levels of VLCFAs, thus supporting a new therapeutic strategy for X-ALD.
Furthermore, GC-1 has been shown to promote oligodendrogenesis from human and rodent oligodendrocyte progenitor cells (OPCs) in vitro and enhance oligodendrogenesis during development with attending increased production of myelin proteins in vivo. These results support the idea that thyromimetic agents that distribute to the CNS may be useful candidates for treating demyelinating disorders, such as multiple sclerosis (MS)100.
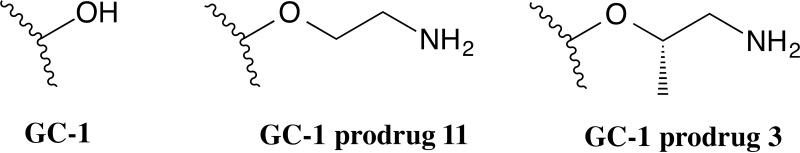
Lipid solubility is a key factor in passive diffusion through the blood–brain barrier (BBB). From a structural point of view, GC-1 contains a carboxylate group, negatively charged at physiological pH, which is crucial for the high-affinity binding to the TRβ, but could be unfavorable for CNS drug distribution due to either its lipophobic character or the electrostatic repulsion at negatively charged tight junctions of BBB endothelial cells. Therefore, to further enhance the BBB penetration and CNS distribution of GC-1, a prodrug strategy was recently employed to mask the carboxylate group101. A series of ester prodrugs for GC-1 were thus synthesized and tested in vivo to evaluate their CNS penetration and ability to deliver GC-1 to the brain102. Among them, the ethanolamino ester of GC-1, namely, prodrug 11 (Fig. 3), was found to have the greatest CNS penetration with minimized peripheral (serum and liver) exposure of the parent drug, suggesting that prodrugs able to deliver more GC-1 to the brain may provide a therapeutic option for treating demyelinating disorders such as MS.
Figure 3.
Chemical structures of GC-1 prodrugs 11 and 3.
The same authors very recently reported the development of an additional series of prodrugs that feature improved CNS distribution compared to the originally reported GC-1 ethanolamino ester 11, and, in the process, they discovered that these ester promoieties undergo an intramolecular rearrangement to form the corresponding amides, which were found to be the pharmacologically active forms of the prodrugs102. In particular, prodrug 3 (Fig. 3) revealed superior properties compared to previously reported GC-1 ethanolamine-derived prodrug 11. In addition, systemic dosing of prodrug 3 was found to stimulate transcription of the TR target gene Hairless (Hr) in the brain with greater potency than unmodified GC-1, further confirming an increased potency of target engagement in the brain. Together, these results indicated that prodrug 3, and more generally amide-based prodrugs of sobetirome, may offer advantages for thyromimetic targeting of the CNS.
CONCLUSIONS
In the last two decades, an improved understanding of TR structure and function and TH analog chemistry has led to the creation of potent thyromimetics with receptor subtype-selective activities. Among them, GC-1 quickly became one of the standard TRβ-selective thyromimetics in the field. Despite the fact that GC-1 was found to be an effective therapeutic for reducing LDL cholesterol with only minor side effects, and with the potential for managing weight loss, its clinical trials in humans as a cholesterol-lowering drug were halted and are unlikely to move forward in the near future. In addition, new insights into a potential application of GC-1 for treating demyelinating disorders, such as X-ALD and MS, are rapidly emerging. Distribution to the CNS is an essential property for a therapeutic agent that stimulates myelin repair, and GC-1 is the only clinical stage thyromimetic that is known to cross the BBB. To this aim, ester and amide prodrug strategies applied to GC-1 proved to be able to deliver increased concentrations of the active drug to the CNS. Hence, GC-1 prodrugs warrant further studies as therapeutic agents for demyelinating disorders. This analog might also prove beneficial in the area of hepatic regenerative medicine. Indeed, because shortage of organs for transplantation represents a major problem, a strategy to be pursued is to stimulate liver regeneration to promote functional hepatocyte mass within a diseased liver. Such regenerative therapy could have potential applicability in the treatment of acute and chronic liver injuries, as well as for use in transplantation settings. Because there is no evidence of harmful side effects produced by short uses of GC-1, its hepatomitogenic activity, clearly demonstrated in rodent liver, can prove particularly beneficial in living-related donors and in recipients posttransplantation. Finally, in view of the proposed local hypothyroidism that characterizes HCCs, it is conceivable that treatment with TH analogs may prove to be beneficial also in HCC therapy. Indeed, the possibility that the reactivation of the TH/TR axis could lead to the induction of a differentiation program of neoplastic cells represents a fascinating challenge that deserves great attention.
ACKNOWLEDGMENTS
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC; Grant IG-15279 to A.C.) and Fondazione Banco di Sardegna (to A.C). M.A.K. is a recipient of a fellowship from Fondazione Umberto Veronesi.
REFERENCES
- 1. Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–142. [DOI] [PubMed] [Google Scholar]
- 2. Greenspan FS. The thyroid gland. In: Greenspan FS, Baxter JD editors. Basic and clinical endocrinology. Norwalk (CT): Appleton & Lange; 1994. p. 160–223. [Google Scholar]
- 3. Motomura K, Brent GA. Mechanisms of thyroid hormone action. Implications for the clinical manifestation of thyrotoxicosis. Endocrinol Metab Clin North Am. 1998;27:1–23. [DOI] [PubMed] [Google Scholar]
- 4. Galioni EF, Gorman JW, Guzvich P, Pouteau J, Rubinger JH, Strisower B. Long-term effect of dried thyroid on serum-lipoprotein and serum-cholesterol levels. Lancet 1957;272:120–3. [DOI] [PubMed] [Google Scholar]
- 5. Dillmann WH. Biochemical basis of thyroid hormone action in the heart. Am J Med. 1990;88:626–30. [DOI] [PubMed] [Google Scholar]
- 6. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–9. [DOI] [PubMed] [Google Scholar]
- 7. Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12:111–21. [DOI] [PubMed] [Google Scholar]
- 8. Lazar MA. Thyroid hormone receptors: Multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–9. [DOI] [PubMed] [Google Scholar]
- 9. Brent GA. The molecular basis of thyroid hormone action. N Engl J Med. 1994;331:847–53. [DOI] [PubMed] [Google Scholar]
- 10. Forrest D, Vennström B. Functions of thyroid hormone receptors in mice. Thyroid 2000;10:41–52. [DOI] [PubMed] [Google Scholar]
- 11. Gullberg H, Rudling M, Saltó C, Forrest D, Angelin B, Vennström B. Requirement for thyroid hormone receptor beta in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol. 2002;16:1767–77. [DOI] [PubMed] [Google Scholar]
- 12. Ribeiro MO. Effects of thyroid hormone analogs on lipid metabolism and thermogenesis. Thyroid 2008;18:197–203. [DOI] [PubMed] [Google Scholar]
- 13. Chi HC, Chen CY, Tsai MM, Tsai CY, Lin KH. Molecular functions of thyroid hormones and their clinical significance in liver-related diseases. Biomed Res Int. 2013;2013:601361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Webb P. Selective activators of thyroid hormone receptors. Expert Opin Investig Drugs 2004;13:489–500. [DOI] [PubMed] [Google Scholar]
- 15. Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid 2008;18:239–53. [DOI] [PubMed] [Google Scholar]
- 16. Baxter JD, Webb P. Thyroid hormone mimetics: Potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8:308–20. [DOI] [PubMed] [Google Scholar]
- 17. Tancevski I, Eller P, Patsch JR, Ritsch A. The resurgence of thyromimetics as lipid-modifying agents. Curr Opin Investig Drugs 2009;10:912–8. [PMC free article] [PubMed] [Google Scholar]
- 18. Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol. 1998;5:299–306. [DOI] [PubMed] [Google Scholar]
- 19. Chiellini G, Nguyen NH, Yoshihara HA, Scanlan TS. Improved synthesis of the iodine-free thyromimetic GC-1. Bioorg Med Chem Lett. 2000;10:2607–11. [DOI] [PubMed] [Google Scholar]
- 20. Wagner RL, Huber BR, Shiau AK, Kelly A, Cunha Lima ST, Scanlan TS, Apriletti JW, Baxter JD, West BL, Fletterick RJ. Hormone selectivity in thyroid hormone receptors. Mol Endocrinol. 2001;15:398–410. [DOI] [PubMed] [Google Scholar]
- 21. Ye L, Li YL, Mellström K, Mellin C, Bladh LG, Koehler K, Garg N, Garcia Collazo AM, Litten C, Husman B, Persson K, Ljunggren J, Grover G, Sleph PG, George R, Malm J. Thyroid receptor ligands. 1. Agonist ligands selective for the thyroid receptor beta1. J Med Chem. 2003;46:1580–8. [DOI] [PubMed] [Google Scholar]
- 22. Bleicher L, Aparicio R, Nunes FM, Martinez L, Gomes Dias SM, Figueira AC, Santos MA, Venturelli WH, da Silva R, Donate PM, Neves FA, Simeoni LA, Baxter JD, Webb P, Skaf MS, Polikarpov I. Structural basis of GC-1 selectivity for thyroid hormone receptor isoforms. BMC Struct Biol. 2008;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baxter JD, Webb P, Grover G, Scanlan TS. Selective activation of thyroid hormone signaling pathways by GC-1: A new approach to controlling cholesterol and body weight. Trends Endocrinol Metab. 2004;15:154–7. [DOI] [PubMed] [Google Scholar]
- 24. Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH. The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 2000;141:3057–64. [DOI] [PubMed] [Google Scholar]
- 25. Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: Selective actions relative to 3,5,3′-triiodo-L-thyronine. Endocrinology 2004;145:1656–61. [DOI] [PubMed] [Google Scholar]
- 26. Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 2000;102:1082–5. [DOI] [PubMed] [Google Scholar]
- 27. Johansson L, Rudling M, Scanlan TS, Lundåsen T, Webb P, Baxter J, Angelin B, Parini P. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA 2005;102:10297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ness GC, Zhao Z. Thyroid hormone rapidly induces hepatic LDL receptor mRNA levels in hypophysectomized rats. Arch Biochem Biophys. 1994;315:199–202. [DOI] [PubMed] [Google Scholar]
- 29. Lin JZ, Martagón AJ, Hsueh WA, Baxter JD, Gustafsson JÅ, Webb P, Phillips KJ. Thyroid hormone receptor agonists reduce serum cholesterol independent of the LDL receptor. Endocrinology 2012;153:6136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, Ose L, Averna M, Boileau C, Borén J, Bruckert E, Catapano AL, Defesche JC, Descamps OS, Hegele RA, Hovingh GK, Humphries SE, Kovanen PT, Kuivenhoven JA, Masana L, Nordestgaard BG, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AF, Steinhagen-Thiessen E, Stroes ES, Taskinen MR, Tybjærg-Hansen A, Wiklund O; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia in children and adolescents: Gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36:2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kannisto K, Rehnmark S, Slätis K, Webb P, Larsson L, Gåfvels M, Eggertsen G, Parini P. The thyroid receptor β modulator GC-1 reduces atherosclerosis in ApoE deficient mice. Atherosclerosis 2014;237:544–54. [DOI] [PubMed] [Google Scholar]
- 32. Grundy SM. Metabolic complications of obesity. Endocrine 2000;13:155–65. [DOI] [PubMed] [Google Scholar]
- 33. Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 2000;404:652–60. [DOI] [PubMed] [Google Scholar]
- 34. Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. Int J Obes. (Lond) 2007;31:204–12. [DOI] [PubMed] [Google Scholar]
- 35. Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64:1–64. [DOI] [PubMed] [Google Scholar]
- 36. Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA. Thyroid hormone–sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform-specific. J Clin Invest. 2001;108:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villicev CM, Freitas FR, Aoki MS, Taffarel C, Scanlan TS, Moriscot AS, Ribeiro MO, Bianco AC, Gouveia CH. Thyroid hormone receptor beta-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J Endocrinol. 2007;193:21–9. [DOI] [PubMed] [Google Scholar]
- 38. Filgueira CS, Nicolov E, Hood RL, Ballerini A, Garcia-Huidobro J, Lin JZ, Fraga D, Webb P, Sabek OM, Gaber AO, Phillips KJ, Grattoni A. Sustained zero-order delivery of GC-1 from a nanochannel membrane device alleviates metabolic syndrome. Int J Obes. (Lond) 2016;40:1776–1783. [DOI] [PubMed] [Google Scholar]
- 39. Huang YY, Gusdon AM, Qu S. Cross-talk between the thyroid and liver: A new target for nonalcoholic fatty liver disease treatment. World J Gastroenterol. 2013;19:8238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 41. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 42. Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003;37:1202–19. [DOI] [PubMed] [Google Scholar]
- 43. Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology 2002;122:1649–57. [DOI] [PubMed] [Google Scholar]
- 44. den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: A mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol. 2004;24:644–9. [DOI] [PubMed] [Google Scholar]
- 45. Koteish A, Mae Diehl A. Animal models of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:679–90. [DOI] [PubMed] [Google Scholar]
- 46. Lombardi B, Pani P, Schlunk FF. Choline-deficiency fatty liver: Impaired release of hepatic triglycerides. J Lipid Res. 1968;9:437–46. [PubMed] [Google Scholar]
- 47. Veteläinen R, van Vliet A, van Gulik TM. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J Gastroenterol Hepatol. 2007;22:1526–33. [DOI] [PubMed] [Google Scholar]
- 48. Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perra A, Simbula G, Simbula M, Pibiri M, Kowalik MA, Sulas P, Cocco MT, Ledda-Columbano GM, Columbano A. Thyroid hormone (T3) and TRbeta agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 2008;22:2981–9. [DOI] [PubMed] [Google Scholar]
- 50. Martagón AJ, Lin JZ, Cimini SL, Webb P, Phillips KJ. The amelioration of hepatic steatosis by thyroid hormone receptor agonists is insufficient to restore insulin sensitivity in ob/ob mice. PLoS One 2015;10:e0122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miyake T, Matsuura B, Furukawa S, Todo Y, Yamamoto S, Yoshida O, Imai Y, Watanabe T, Yamamoto Y, Hirooka M, Tokumoto Y, Kumagi T, Abe M, Seike H, Miyauchi S, Hiasa Y. Hyperthyroidism improves the pathological condition of nonalcoholic steatohepatitis: A case of nonalcoholic steatohepatitis with Graves’ disease. Intern Med. 2016;55:2019–23. [DOI] [PubMed] [Google Scholar]
- 52. Vatner DF, Weismann D, Beddow SA, Kumashiro N, Erion DM, Liao XH, Grover GJ, Webb P, Phillips KJ, Weiss RE, Bogan JS, Baxter J, Shulman GI, Samuel VT. Thyroid hormone receptor-β agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am J Physiol Endocrinol Metab. 2013;305:E89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Monga SP. Hepatic regenerative medicine: Exploiting the liver’s will to live. Am J Pathol. 2014,184:306–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karp SJ. Clinical implications of advances in the basic science of liver repair and regeneration. Am J Transpl. 2009;9:1973–80. [DOI] [PubMed] [Google Scholar]
- 55. Short J, Brown RF, Husakova A, Gilbertson JR, Zemel R, Lieberman I. Induction of deoxyribonucleic acid synthesis in the liver of the intact animal. J Biol Chem. 1972;247:1757–66. [PubMed] [Google Scholar]
- 56. Francavilla A, Carr BI, Azzarone A, Polimeno L, Wang Z, Van Thiel DH, Subbotin V, Prelich JG, Starzl TE. Hepatocyte proliferation and gene expression induced by triiodothyronine in vivo and in vitro. Hepatology 1994;20:1237–41. [PubMed] [Google Scholar]
- 57. Pibiri M, Ledda-Columbano GM, Cossu C, Simbula G, Menegazzi M, Shinozuka H, Columbano A. Cyclin D1 is an early target in hepatocyte proliferation induced by thyroid hormone (T3). FASEB J. 2001;15:1006–13. [DOI] [PubMed] [Google Scholar]
- 58. Higgins GM, Anderson RM. Experimental pathology of the liver. Arch Pathol 1931;12:186–202. [Google Scholar]
- 59. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell 2009;17:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fanti M, Singh S, Ledda-Columbano GM, Columbano A, Monga SP. Tri-iodothyronine induces hepatocyte proliferation by protein kinase A-dependent β-catenin activation in rodents. Hepatology 2014;59:2309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alvarado TF, Puliga E, Preziosi M, Poddar M, Singh S, Columbano A, Nejak-Bowen K, Monga SP. Thyroid hormone receptor β agonist induces β-catenin-dependent hepatocyte proliferation in mice: Implications in hepatic regeneration. Gene Expr. 2016;17:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bockhorn M, Frilling A, Benko T, Best J, Sheu SY, Trippler M, Schlaak JF, Broelsch CE. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur Surg Res. 2007;39:58–63. [DOI] [PubMed] [Google Scholar]
- 64. Columbano A, Simbula M, Pibiri M, Perra A, Deidda M, Locker J, Pisanu A, Uccheddu A, Ledda-Columbano GM. Triiodothyronine stimulates hepatocyte proliferation in two models of impaired liver regeneration. Cell Prolif. 2008;41:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Malik R, Habib M, Tootle R, Hodgson H. Exogenous thyroid hormone induces liver enlargement, whilst maintaining regenerative potential—A study relevant to donor preconditioning. Am J Transplant. 2005;5:1801–7. [DOI] [PubMed] [Google Scholar]
- 66. Taki-Eldin A, Zhou L, Xie HY, Chen KJ, Zhou WH, Zhang W, Xing CY, Yang Z, Zhang K, Zheng SS. Tri-iodothyronine enhances liver regeneration after living donor liver transplantation in rats. J Hepatobiliary Pancreat Sci. 2011;18:806–14. [DOI] [PubMed] [Google Scholar]
- 67. Columbano A, Pibiri M, Deidda M, Cossu C, Scanlan TS, Chiellini G, Muntoni S, Ledda-Columbano GM. The thyroid hormone receptor-beta agonist GC-1 induces cell proliferation in rat liver and pancreas. Endocrinology 2006;147:3211–8. [DOI] [PubMed] [Google Scholar]
- 68. Kowalik MA, Perra A, Pibiri M, Cocco MT, Samarut J, Plateroti M, Ledda-Columbano GM, Columbano A. TRbeta is the critical thyroid hormone receptor isoform in T3-induced proliferation of hepatocytes and pancreatic acinar cells. J Hepatol 2010;53:686–92. [DOI] [PubMed] [Google Scholar]
- 69. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 70. Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- 72. Hassan MM, Kaseb A, Li D, Patt YZ, Vauthey JN, Thomas MB, Curley SA, Spitz MR, Sherman SI, Abdalla EK, Davila M, Lozano RD, Hassan DM, Chan W, Brown TD, Abbruzzese JL. Association between hypothyroidism and hepatocellular carcinoma: A case-control study in the United States. Hepatology 2009;49:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reddy A, Dash C, Leerapun A, Mettler TA, Stadheim LM, Lazaridis KN, Roberts RO, Roberts LR. Hypothyroidism: A possible risk factor for liver cancer in patients with no known underlying cause of liver disease. Clin Gastroenterol Hepatol. 2007;5:118–23. [DOI] [PubMed] [Google Scholar]
- 74. Lin KH, Shieh HY, Chen SL, Hsu HC. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol Carcinog. 1999;26:53–61. [DOI] [PubMed] [Google Scholar]
- 75. González-Sancho JM, García V, Bonilla F, Muñoz A. Thyroid hormone receptors/THR genes in human cancer. Cancer Lett. 2003;192:121–32. [DOI] [PubMed] [Google Scholar]
- 76. Aranda A, Martínez-Iglesias O, Ruiz-Llorente L, García-Carpizo V, Zambrano A. Thyroid receptor: Roles in cancer. Trends Endocrinol Metab. 2009;20:318–24. [DOI] [PubMed] [Google Scholar]
- 77. Liao CH, Yeh CT, Huang YH, Wu SM, Chi HC, Tsai MM, Tsai CY, Liao CJ, Tseng YH, Lin YH, Chen CY, Chung IH, Cheng WL, Chen WJ, Lin KH. Dickkopf 4 positively regulated by the thyroid hormone receptor suppresses cell invasion in human hepatoma cells. Hepatology 2012;55:910–20. [DOI] [PubMed] [Google Scholar]
- 78. Frau C, Loi R, Petrelli A, Perra A, Menegon S, Kowalik MA, Pinna S, Leoni VP, Fornari F, Gramantieri L, Ledda-Columbano GM, Giordano S, Columbano A. Local hypothyroidism favors the progression of preneoplastic lesions to hepatocellular carcinoma in rats. Hepatology 2015;61:249–59. [DOI] [PubMed] [Google Scholar]
- 79. Martínez-Iglesias OA, Alonso-Merino E, Gómez-Rey S, Velasco-Martín JP, Martín Orozco R, Luengo E, García Martín R, Ibáñez de Cáceres I, Fernández AF, Fraga MF, González-Peramato P, Varona C, Palacios J, Regadera J, Aranda A. Autoregulatory loop of nuclear corepressor 1 expression controls invasion, tumor growth, and metastasis. Proc Natl Acad Sci USA 2016;113:E328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Perra A, Kowalik MA, Pibiri M, Ledda-Columbano GM, Columbano A. Thyroid hormone receptor ligands induce regression of rat preneoplastic liver lesions causing their reversion to a differentiated phenotype. Hepatology 2009;49:1287–96. [DOI] [PubMed] [Google Scholar]
- 81. Solt DB, Medline A, Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977;88:595–618. [PMC free article] [PubMed] [Google Scholar]
- 82. Hansson P, Valdemarsson S, Nilsson-Ehle P. Experimental hyperthyroidism in man: Effects on plasma lipoproteins, lipoprotein lipase and hepatic lipase. Horm Metab Res. 1983;15:449–52. [DOI] [PubMed] [Google Scholar]
- 83. Moreno M, Lanni A, Lombardi A, Goglia F. How the thyroid controls metabolism in the rat: Different roles for triiodothyronine and diiodothyronines. J Physiol. 1997;505:529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, Linemeyer DL. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA 2007;104:15490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mason RL, Hunt HM, Hurxthal L. Blood cholesterol values in hyperthyroidism and hypothyroidism. N Engl J Med. 1930;203:1273–8. [Google Scholar]
- 86. Abrams JJ, Grundy SM. Cholesterol metabolism in hypothyroidism and hyperthyroidism in man. J Lipid Res. 1981;22:323–38. [PubMed] [Google Scholar]
- 87. Silva JE. The thermogenic effect of thyroid hormone and its clinical implications. Ann Intern Med. 2003;139:205–13. [PubMed] [Google Scholar]
- 88. Brent GA. Tissue-specific actions of thyroid hormone: Insights from animal models. Rev Endocr Metab Disord. 2000;1:27–33. [DOI] [PubMed] [Google Scholar]
- 89. Freitas FR, Moriscot AS, Jorgetti V, Soares AG, Passarelli M, Scanlan TS, Brent GA, Bianco AC, Gouveia CH. Spared bone mass in rats treated with thyroid hormone receptor TR beta-selective compound GC-1. Am J Physiol Endocrinol Metab. 2003;285:E1135–41. [DOI] [PubMed] [Google Scholar]
- 90. Lin VH, Klepp HM, Hanley RM. Sobetirome is a thyroid hormone receptorβ- and liver-selective thyromimetic that can effect substantial LDL-C lowering without significant changes in heart rate or the thyroid axis in euthyroid men. 90th Annual Meeting of the Endocrine Society, 2008;OR36–OR33. [Google Scholar]
- 91. Scanlan TS. Sobetirome: A case history of bench-to-clinic drug discovery and development. Heart Fail Rev. 2010;15:177–82. [DOI] [PubMed] [Google Scholar]
- 92. Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–59. [DOI] [PubMed] [Google Scholar]
- 93. Farsetti A, Mitsuhashi T, Desvergne B, Robbins J, Nikodem VM. Molecular basis of thyroid hormone regulation of myelin basic protein gene expression in rodent brain. J Biol Chem. 1991;266:23226–32. [PubMed] [Google Scholar]
- 94. Rodriguez-Peña A, Ibarrola N, Iñiguez MA, Muñoz A, Bernal J. Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J Clin Invest. 1993;91:812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tosic M, Torch S, Comte V, Dolivo M, Honegger P, Matthieu JM. Triiodothyronine has diverse and multiple stimulating effects on expression of the major myelin protein genes. J Neurochem. 1992;59:1770–7. [DOI] [PubMed] [Google Scholar]
- 96. Borngraeber S, Budny MJ, Chiellini G, Cunha-Lima ST, Togashi M, Webb P, Baxter JD, Scanlan TS, Fletterick RJ. Ligand selectivity by seeking hydrophobicity in thyroid hormone receptor. Proc Natl Acad Sci USA 2003;100:15358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Takahashi N, Asano Y, Maeda K, Watanabe N. In vivo evaluation of 1-benzyl-4-aminoindole-based thyroid hormone receptor β agonists: Importance of liver selectivity in drug discovery. Biol Pharm Bull. 2014;37:1103–8. [DOI] [PubMed] [Google Scholar]
- 98. Genin EC, Gondcaille C, Trompier D, Savary S. Induction of the adrenoleukodystrophy-related gene (ABCD2) by thyromimetics. J Steroid Biochem Mol Biol. 2009;116:37–43. [DOI] [PubMed] [Google Scholar]
- 99. Hartley MD, Kirkemo LL, Banerji T, Scanlan TS. A thyroid hormone based strategy for correcting the biochemical abnormality in X-linked adrenoleukodystrophy (X-ALD). Endocrinology 2017;158(5):1328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Baxi EG, Schott JT, Fairchild AN, Kirby LA, Karani R, Uapinyoying P, Pardo-Villamizar C, Rothstein JR, Bergles DE, Calabresi PA. A selective thyroid hormone β receptor agonist enhances human and rodent oligodendrocyte differentiation. Glia 2014;62:1513–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Placzek AT, Ferrara SJ, Hartley MD, Sanford-Crane HS, Meinig JM, Scanlan TS. Sobetirome prodrug esters with enhanced blood-brain barrier permeability. Bioorg Med Chem. 2016;24:5842–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ferrara SJ, Meinig JM, Placzek AT, Banerji T, McTigue P, Hartley MD, Sanford-Crane HS, Banerji T, Bourdette D, Scanlan TS. Ester-to-amide rearrangement of ethanolamine-derived prodrugs of sobetirome with increased blood-brain barrier penetration. Bioorg Med Chem. 2017;25:2743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]