Abstract
Hepatic ischemia/reperfusion (I/R) injury is a major complication of liver surgery, including liver resection, liver transplantation, and trauma surgery. Much has been learned about the inflammatory injury response induced by I/R, including the cascade of proinflammatory mediators and recruitment of activated leukocytes. In this review, we discuss the complex network of events that culminate in liver injury after I/R, including cellular, protein, and molecular mechanisms. In addition, we address the known endogenous regulatory mediators that function to maintain homeostasis and resolve injury. Finally, we cover more recent insights into how the liver repairs and regenerates after I/R injury, a setting in which physical mass remains unchanged, but functional liver mass is greatly reduced. In this regard, we focus on recent work highlighting a novel role of CXC chemokines as important regulators of hepatocyte proliferation and liver regeneration after I/R injury.
Key words: Hepatic injury, Necrosis, Hepatic inflammation, Liver regeneration
INTRODUCTION
Ischemia is defined as a deficient supply of blood to an organ and causes shortage of oxygen and may disrupt cellular metabolism. Reperfusion is the restoration of blood flow and, in many organs, is followed by tissue injury that results from the previous lack of oxygen and nutrient delivery and buildup of metabolic by-products. Ischemia/reperfusion (I/R) injury of the liver is a major complication in many clinical scenarios, such as liver resection, liver transplantation, and trauma1–3. Vascular occlusion techniques such as the Pringle maneuver and total hepatic vascular exclusion are used for avoiding excessive blood loss during liver surgery, whereas liver transplantation involves perfusion of liver grafts with preservation fluid prior to cold storage. A better understanding of hepatic I/R injury may lead to improvements to the clinical care of many patients, particularly those undergoing surgery with extended ischemia times or marginal liver grafts for transplantation. Experimental models of I/R injury have provided a solid foundation of the cellular and molecular mechanisms of the hepatic injury response. However, less is known about the precise manner of liver repair and regeneration after I/R injury. In this review, we discuss the underlying mechanisms of liver I/R injury and present the current understanding of the process of liver recovery and regeneration after I/R injury.
INITIAL PHASE OF I/R INJURY RESPONSE IN THE LIVER
Jaeschke and colleagues proposed that there are two distinct phases of liver injury after warm I/R. The early phase of liver injury is characterized by rapid Kupffer cell activation after reperfusion4,5. Activated Kupffer cells release reactive oxygen species (ROS), including superoxide anion and hydrogen peroxide, which induce oxidative stress and parenchymal and vascular injuries6. Indeed, suppression or depletion of Kupffer cells reduces the severity of injury7. Although the degree of this initial phase of liver injury is relatively modest, it triggers a subsequent cascade of events including a sequence of proinflammatory mediators leading to the recruitment of activated leukocytes and significant liver injury (Fig. 1). The factors contributing to the initiation of I/R injury are discussed below.
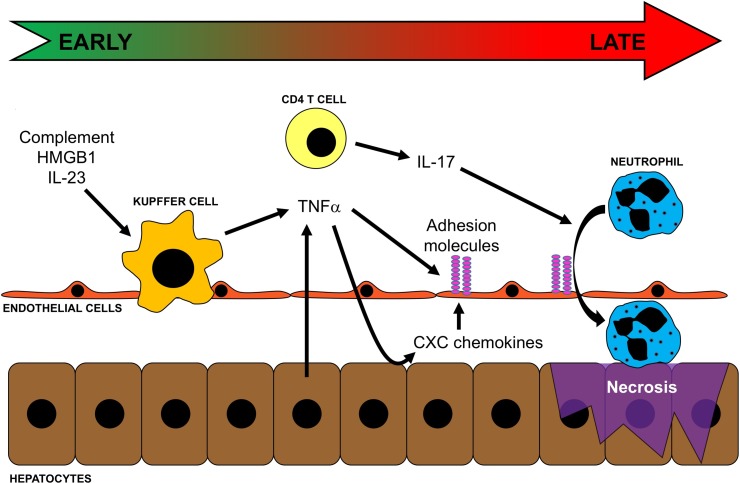
Figure 1.
Events contributing to inflammatory liver injury after ischemia/reperfusion (I/R). Oxidant stress induced by I/R results in the release of complement, high-mobility group box 1 (HMGB1), and IL-23, which activate Kupffer cells and induce their production of TNF-α. TNF-α propagates the inflammatory response by upregulating adhesion molecules on endothelial cells and inducing expression of CXC chemokines by hepatocytes. At the same time, CD4 T cells are transiently recruited to the liver and, along with the increased expression of CXC chemokines and adhesion molecules, facilitates neutrophil recruitment into the liver parenchyma. Neutrophils then directly injure hepatocytes via oxidants and proteases, leading to necrotic cell death.
Complement
Complement consists of a number of circulating small proteins and plays a central role for immune defense and inflammation against pathogens. The complement cascade is stimulated by the cellular proteins that are released after reperfusion. In fact, deposition of activated complement components is detected in human liver as well as in rodent models after hepatic I/R and contributes to initiating the inflammatory response of I/R injury8,9. Deletion of serum complement before ischemia attenuates Kupffer cell-induced oxidative stress after reperfusion, and complement-depleted animals had decreased neutrophil accumulation and hepatic injury after I/R10. Other studies showed that C3 deficiency and C5aR antagonist also decrease I/R injury associated with lower neutrophil recruitment11,12. Complement is activated through three distinct pathways: the classical, alternative, and mannose-binding lectin pathways. Inhibition of the classical and alternative pathways using soluble complement receptor type 1 (sCR1) improves microvascular perfusion, decreases leukocyte recruitment, and attenuates hepatic injury8,10,13. Taken together, complement is one of the most upstream mediators of I/R injury and functions to activate Kupffer cells.
Toll-Like Receptor
Toll-like receptors (TLRs) have been shown as a key mediator for initiating the innate immune response after I/R. TLRs are present on a variety of liver cell types including Kupffer cells, dendritic cells, and hepatocytes. TLRs recognize danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) to propagate the inflammatory response. Following I/R, which is a noninfectious setting, hepatocellular reactive oxygen stress mediates the release of high-mobility group box 1 (HMGB1), which is the most widely studied DAMP14. HMGB1 levels increase as early as 1 h after reperfusion and work as a key endogenous TLR4 ligand. Neutralization of HMGB1 or TLR4 deficiency reduces I/R injury15,16. TLR signaling in nonparenchymal cells, Kupffer cells, and dendritic cells is required for upregulating proinflammatory mediators, including TNF-α, IL-6, and ICAM-1, whereas hepatocyte TLR4 facilitates the release of HMGB1 from hypoxic hepatocyte17,18. Downstream components of the TLR4 signaling pathway include the adaptor molecules, MyD88 and TIR domain-containing adaptor inducing IFN-β (TRIF), which bind to intracellular receptor domains. MyD88-deficient mice are not protected from I/R liver injury16. However, the TRIF-dependent pathway activates its downstream signaling interferon regulator factor 3 (IRF3), leading to the production of type 1 IFN and CXCL10, which modulates I/R injury by upregulating proinflammatory cytokines16,19,20.
CD4 T Lymphocytes
Very rapidly after reperfusion, CD4 T lymphocytes are recruited to the postischemic liver21. The role of these cells in I/R injury has been studied using T-cell-deficient mice and antibody depletion of CD4 T lymphocytes, both models demonstrating that a loss of CD4 T lymphocytes significantly dampens the injury after I/R21. Furthermore, adoptive transfer of CD4 T lymphocytes into T-cell-deficient mice restores the injury response21. Depletion or deficiency of CD4 T lymphocytes appears to regulate liver I/R injury by decreasing recruitment of neutrophils22. After I/R injury, recruited CD4 T lymphocytes release IL-17, which facilitates the recruitment of neutrophils22. IL-17 has been recognized to induce chemokine secretion from other cell types, including epithelial cells, fibroblasts, and endothelial cells23,24. Upregulation of IL-17 after I/R increases chemokine levels, which contributes to the recruitment of neutrophils25. CD4 T lymphocytes also appear to contribute directly to neutrophil recruitment. One study has shown that approximately 30% of recruited CD4 T cells were colocalized with platelets in hepatic sinusoids and that CD4 T lymphocytes activate endothelial cells and increase platelet adherence and neutrophil migration26. Platelet adherence on ICAM-1-expressing endothelial cells induced microvascular injury and hepatocyte cell death after I/R27.
T cells may be activated by antigen-dependent and antigen-independent pathways. Although hepatic I/R is thought to be a sterile injury, there is evidence of antigen-dependent CD4 T-lymphocyte activation. In OT-II mice, in which T cells only express a TCR that recognizes ovalbumin, hepatic I/R injury was significantly reduced28. However, the magnitude of this change was modest compared to studies of antigen-independent pathways of CD4 T-lymphocyte activation. After hepatic I/R injury, liver-recruited CD4 T lymphocytes are activated by ROS, IL-6, and TNF-α derived from Kupffer cells29. In addition, the CD154–CD40 costimulation pathway plays an important role in the involvement of T cells after I/R30. Disruption of CD154 ameliorated fulminant liver injury that is correlated with depressed T-cell sequestration, decreased VEGF expression, inhibition of TNF-α and T-helper type 1 cytokine production, and induction of antiapoptotic but depression of proapoptotic proteins31,32.
Inflammatory Cytokines
After I/R, proinflammatory cytokines play a central role in propagating the inflammatory response throughout the liver. The complicated cytokine cascade after I/R begins with the upregulation of IL-12 and IL-23. IL-12 expression was observed 1 h after reperfusion and disappeared within 4 h33. The source of IL-12 and IL-23 has not been identified yet, but Kupffer cells and hepatic stellate cells likely produce these cytokines34. Both the neutralization of IL-12 and IL-23 and in mice lacking the p40 subunit, which is common to both IL-12 and IL-23, the robust increase in TNF-α and IFN-γ after I/R was diminished, resulting in less neutrophil accumulation and liver injury33,35. Therefore, IL-12 and IL-23 appear to be early response cytokines that amplify the inflammatory response by stimulating the expression of TNF-α and IFN-γ.
IL-1β is another early response cytokine that has been shown to propagate the inflammatory response after I/R in the liver. Antagonism of IL-1β signaling with the IL-1 receptor antagonist significantly reduces TNF-α expression, liver inflammation, and injury after I/R36. A subsequent study showed that gene deletion of the type I IL-1 receptor had no effect on liver injury37.
TNF-α has been long recognized as perhaps the most important mediator in the hepatic inflammatory response to I/R38. TNF-α is released by a variety of cells in the liver, but its release by Kupffer cells is most prominent, and it is detected rapidly after reperfusion38,39. TNF-α stimulates hepatocytes and Kupffer cells to produce neutrophil chemoattractants, particularly CXC chemokines40. In addition, TNF-α upregulates the adhesion molecules ICAM-1, VCAM-1, and P-selectin on vascular endothelial cells41. Neutralization of TNF-α nearly abrogates I/R injury by suppressing the inflammatory response and resultant liver injury38,41.
Nuclear Factor κB (NF-κB)
NF-κB is a major transcription factor involved in mediating inflammatory gene expression in a wide range of cell types. NF-κB is composed of proteins of the Rel family, which share a homologous amino acid sequence in their amino termini called the Rel homology domain that is necessary for dimerization, binding to DNA, and binding to IκB (inhibitor of NF-κB) proteins42,43. NF-κB complexes are made up of homo- or heterodimers, but the majority of NF-κB in the liver consists of the p50/p65 heterodimer. In unstimulated cells, NF-κB is sequestered in the cytoplasm by binding to IκB proteins. IκB proteins mask the nuclear localization sequence of NF-κB, thereby preventing its translocation into the nucleus. There are two pathways for NF-κB activation after I/R injury. In the classical pathway, cell stimulation results in the serine phosphorylation of IκB by the IκB kinase complex (IKK complex). Phosphorylated IκB then becomes the target of ubiquitin ligase, which polyubiquitinates the protein for subsequent proteasomal degradation44,45. In addition to this well-characterized pathway, there appears to be an alternative pathway for activating NF-κB that does not involve IκB degradation. This alternative pathway was originally described in hypoxic T cells and involves the phosphorylation of IκBα on tyrosine residue 42 that leads to its dissociation from NF-κB46. Experimental data suggest that the activation of NF-κB via this mechanism occurs predominantly after hypoxia, whereas the classical pathway occurs primarily after cytokine stimulation47–51. For both pathways, once NF-κB is free from IκB, it translocates to the nucleus where it binds DNA and initiates the transcription of target genes.
The role of NF-κB in hepatic I/R injury is complex and is dependent on the cell type (Fig. 2). During the initial phase of injury, NF-κB is activated by oxidant stress and proinflammatory stimuli to increase the expression of proinflammatory cytokines, chemokines, and adhesion molecules. In Kupffer cells, NF-κB activation promotes the expression of TNF-α and IL-6 after I/R52. In hepatocytes, NF-κB activation drives their production of TNF-α, contributing to the inflammatory injury53. NF-κB activation in endothelial cells leads to the expression of chemokines of the IL-8 family and the adhesion molecules E-selectin, ICAM-1, and VCAM-154,55.
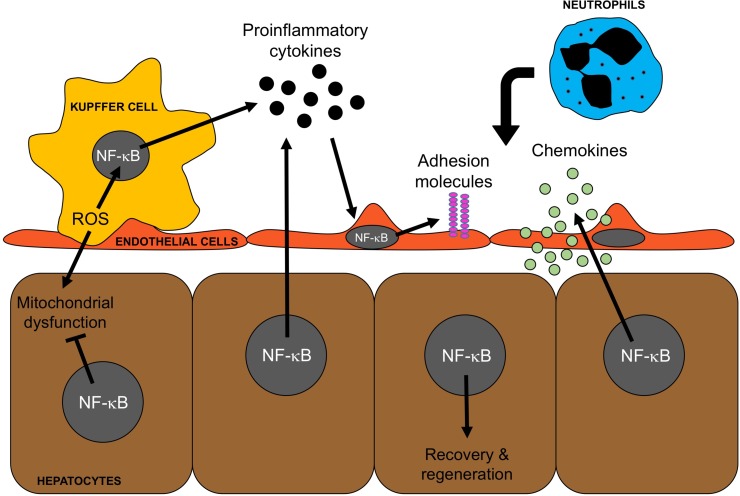
Figure 2.
Cell-specific roles of nuclear factor κB (NF-κB) in liver injury and recovery and regeneration. NF-κB is a driver of the inflammatory response: I/R induces the generation of reactive oxygen species (ROS) in Kupffer cells, which activates NF-κB resulting in the production and release of proinflammatory cytokines, such as TNF-α, by Kupffer cells. These proinflammatory cytokines activate other cell types to produce inflammatory mediators, such as adhesion molecules and chemokines, which facilitate the recruitment of neutrophils. NF-κB is a key modulator of hepatocyte survival: ROS generated by Kupffer cells also impacts hepatocytes and contributes to mitochondrial dysfunction. NF-κB activation in hepatocytes leads to the production of several cytoprotective proteins that mitigate mitochondrial dysfunction and contribute to hepatocyte survival and proliferation.
LATE PHASE OF I/R INJURY RESPONSE IN THE LIVER
The late phase of I/R injury is characterized by the process of neutrophil recruitment to the postischemic liver and their damage to hepatocytes via oxidants and proteases. The mechanisms of neutrophil recruitment are dependent on the ability of circulating neutrophils to adhere to the vascular endothelium and transmigrate from the blood vessel lumen into the tissue parenchyma. These processes require the coordinated efforts of both chemotactic agents and vascular cell adhesion molecules.
Chemokines and Chemoattractants
Chemokines are a group of small (8–10 kDa), basic, heparin-binding proteins that are secreted by a variety of cell types56,57. Chemokines are identified based on the structural location of the cysteine residues in the amino terminus such that there are four families of chemokines: CXC, CC, CX3C, and C (where X is any amino acid). CXC chemokines can be further identified based on the presence of a Glu-Leu-Arg (ELR) amino acid motif at the amino terminus of the peptide, which determines receptor specificity. ELR+ CXC chemokines bind to the receptors CXCR1 and CXCR2, while ELR− CXC chemokines bind to the receptors CXCR3 and CXCR4. After hepatic I/R, ELR+ CXC chemokines are highly expressed and contribute to the recruitment of neutrophils40,58. ELR+ CXC chemokines not only are chemotactic for neutrophils but also activate neutrophils59,60, which may contribute to hepatocellular damage. Neutralization of ELR+ CXC chemokines greatly attenuates neutrophil accumulation and subsequent liver injury40,58. Gene deletion of the receptor CXCR2 completely abolishes acute neutrophil accumulation after I/R61. However, these studies showed that neutrophil accumulation was just delayed, suggesting that other, nonchemokine chemoattractants are involved in the recruitment of neutrophils. One such agent is leukotriene B4 (LTB4), which has been shown to be expressed after liver I/R injury62.
Adhesion Molecules
Several adhesion molecules expressed on both vascular endothelial cells and neutrophils are responsible for the physical capture, arrest, and transmigration of neutrophils from the vascular space into the liver parenchyma. Initial capture of neutrophils within the hepatic microvasculature is mediated by P- and L-selectin. P-selectin is expressed on the vascular endothelium and binds to its ligand expressed on the neutrophil surface, while L-selectin is expressed on the neutrophil surface and binds to its ligand expressed on endothelial cells63–66. These selectins function to capture the neutrophils and reduce their velocity such that more firm adhesive interactions can take place. The latter are facilitated by integrins expressed on the neutrophil surface and ICAM-1 and VCAM-1 expressed on the endothelial cells, which mediate adhesive arrest of neutrophils on endothelial cells, and their extravasation into the liver parenchyma67,68.
Neutrophil-Derived Reactive Oxygen Species and Proteases
Whereas ROS are produced within hepatocytes during the initial phase of injury, it is the production and release of ROS by neutrophils that cause the most direct injury to hepatocytes. Once recruited into the liver, neutrophils directly bind to hepatocytes by engagement of integrins on neutrophils and ICAM-1 on hepatocytes69,70. This direct contact triggers the activation of NADPH oxidase in neutrophils. Oxidation of NADPH liberates an electron that reduces molecular oxygen to form superoxide anion. Superoxide dismutase catalyzes superoxide anion to hydrogen peroxide (H2O2), which is a highly diffusible oxidant and can also be further reduced to hydroxyl radical (HO•). In addition, myeloperoxidase released from neutrophil granules generates hypochlorous acid (HOCl) from H2O2, another diffusible oxidant. ROS produced by neutrophils diffuse into hepatocytes and cause mitochondrial dysfunction with calcium accumulation and mitochondrial permeability transition leading to cell death71. Inhibition of NADPH oxidase significantly attenuates liver injury after I/R72.
In addition to the generation of oxidants, activated neutrophils release a number of mediators by granule exocytosis. In addition to myeloperoxidase, mentioned above, neutrophil degranulation releases large amounts of proteases (i.e., elastase, cathepsin G, heparanase, collagenase) and hydrolytic enzymes that may be directly cytotoxic to hepatocytes. Serine proteases, such as elastase and cathepsin G, may directly damage membrane components of hepatocytes, where metalloproteinases primarily degrade basement membrane and matrix components. The role of proteases in I/R injury has been demonstrated by treatment with protease inhibitors that limit liver injury73,74.
Modes of Hepatocyte Cell Death
There has been considerable controversy over the mode of hepatocyte cell death after I/R injury. Two modes of cell death have been described following hepatic I/R injury: apoptosis and necrosis. These modes of cell death are very different in mechanism and morphology. Hallmarks of apoptosis include cell shrinkage, chromatin condensation, nuclear fragmentation, and formation of apoptotic bodies. Necrosis can be characterized by mitochondrial and cell swelling and resultant loss of plasma membrane integrity and vacuolization. A critical aspect of both forms of cell death is mitochondrial dysfunction. Interestingly, a given stimulus can induce apoptosis or necrosis based on the intensity of the stimulus and/or the effect of the stimulus on intracellular ATP levels75–77. For example, peroxynitrite, a ROS, can induce apoptosis at a low concentration and necrosis at higher concentrations75. Alternatively, induction of apoptotic pathways can lead to necrosis if intracellular ATP is depleted76,77. There have been a number of studies that have reported widespread and substantial hepatocyte apoptosis after I/R injury. At issue with many of these studies are the methods in which apoptosis is differentiated from necrosis, as it has been established that cells undergoing necrosis will often stain positively for apoptotic markers, such as TUNEL78,79. These studies have shown that although apoptotic pathways are activated in hepatocytes after I/R, the final mode of cell death in the overwhelming majority of hepatocytes is necrosis.
RESOLUTION OF INFLAMMATORY INJURY AFTER I/R
Like most other biological processes, inflammation is homeostatic, and there exist a number of regulatory mechanisms that help prevent uncontrolled inflammation. Several anti-inflammatory mediators have been shown to be expressed after I/R and to play key roles in the resolution of the injury response.
IL-6 is a multifunctional cytokine produced by Kupffer cells and macrophages and is released during inflammation. Gene deletion of IL-6 resulted in increased hepatic I/R injury, and therapeutic treatment with IL-6 reduced I/R injury80. These effects were associated with reduced expression of TNF-α and P-selectin80. In addition, IL-6 enhanced the activation of signal transducer and activator of transcription 3 (STAT3) and led to hepatocyte proliferation after I/R injury81. Thus, IL-6 appears to function as a factor that resolves inflammatory injury and promotes repair and regeneration.
IL-13 is an anti-inflammatory cytokine that limits inflammation by inhibitory effects on the transcription factor NF-κB82. However, in the context of hepatic I/R injury, gene deletion of IL-13 was shown to result in significantly greater hepatocellular injury without any change in NF-κB activation83. These studies also demonstrated that IL-13 knockout animals had much greater endothelial cell injury and that, in vitro, IL-13 protected hepatocytes from H2O2-induced cytotoxicity. IL-13 therefore appears to have prominent protective effects on hepatocytes and liver endothelial cells.
Secretory leukocyte protease inhibitor (SLPI) is a small protein produced by a variety of cells that potently inhibits enzymes with serine protease activity84. Expression of SLPI increases in the liver after I/R, and neutralization of SLPI dramatically increases I/R liver injury85. These effects were due to the inhibitory effects on NF-κB and the reduced expression of proinflammatory mediators and neutrophil recruitment. Treatment with exogenous SLPI had profound effects in limiting liver inflammation and injury after I/R85. SLPI appears to be an important endogenous mediator that resolves inflammation and injury after liver I/R.
LIVER REPAIR AND REGENERATION AFTER I/R INJURY
General Mechanism of Liver Regeneration
The liver is a unique organ in terms of its regenerative capacity. Liver parenchymal cells rarely proliferate in their quiescent phase; however, once functional liver mass is reduced due to physical or functional loss, hepatocytes gain the potential of proliferation for maintaining organ function until the original size is restored. The regulation of liver regeneration is mediated by the interaction between cytokines, growth factors, and metabolic pathways86. Cytokines play an important role for priming the quiescent hepatocyte, which is the G0 phase to the G1 phase for entering into cell cycle. TNF-α and IL-6 are released from Kupffer cells and activate NF-κB and STAT3 to lead transcription of target genes for liver regeneration. Once primed, hepatocytes respond to several growth factors, especially hepatocyte growth factor (HGF) and epidermal growth factor receptor (EGFR) ligands, resulting in the transition from the G1 to the S phase of DNA replication. HGF is released from the extracellular matrix as well as produced by hepatic stellate cells and endothelial cells after hepatectomy. HGF and its receptor MET signaling activate ERK1/2 and are essential for cell cycle progression87. In addition to HGF, the EGFR ligands EGF, transforming growth factor-α (TGF-α), and heparin-binding EGF-like growth factor (HB-EGF) are also mitogenic for hepatocytes88.
Liver Repair and Regeneration After I/R
For decades, the gold standard experimental model for the study of liver regeneration has been partial hepatectomy. In this model, a large segment of the liver (typically ∼60%) is resected, and the remnant liver is undamaged. However, postischemic liver is highly damaged and stressed and, therefore, represents a much different biological milieu. While many of the same mechanisms for liver regeneration that occur after hepatectomy are operant after I/R injury, unlike hepatectomy, the remaining hepatocytes after I/R are highly stressed by the insult and inflammation response, which can impact hepatocyte proliferation61,89. The trigger of liver regeneration after I/R injury remains unknown, but hepatocyte proliferation begins after liver injury. Proliferation of hepatocytes after I/R begins in the perivascular regions and is correlated with the expression of stathmin, which controls cell proliferation and progression through mitosis, and is observed when I/R-induced increases in the expression of SSAT and p21 subside89. Furthermore, the postischemic liver has a large amount of dead tissue that must be cleared and remodeled; physical liver mass remains unchanged, but functional liver mass is greatly reduced. As such, necrotic cells are cleared and replaced with regenerating hepatocytes, which occurs along a frontal boundary (Fig. 3)61,89. During liver repair after injury, nonparenchymal cells, such as macrophages and hepatic stellate cells, are involved in liver tissue remodeling, and their interactions are highly coordinated90,91. In other models of severe liver injury, activation of hepatic progenitor cells occurs concomitant with a ductular reaction when loss of hepatocytes is massive and parenchymal proliferative capacity is impaired, and the expansion of hepatic progenitor cells is correlated with the severity of hepatocyte loss after liver injury92. Activation and differentiation of progenitor cells are governed by a complex microenvironment regulated by macrophages and hepatic stellate cells93,94. The nature of these interactions after I/R injury has not yet been elucidated.
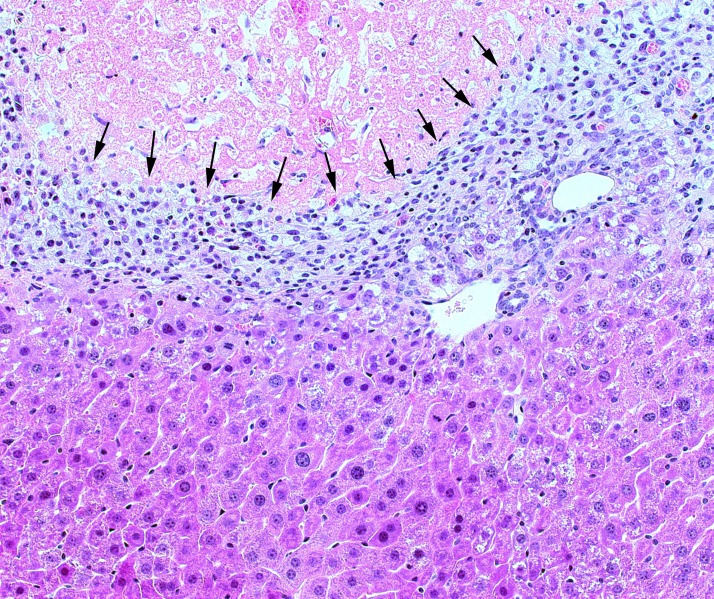
Figure 3.
Remodeling of liver tissue after I/R injury. During liver repair and regeneration after I/R injury, a frontal boundary (arrows) of phagocytes, stellate cells, and others clears and remodels necrotic tissue (above boundary) such that regenerating hepatocytes can restore functional mass and normal architecture (below boundary).
NF-κB in Regeneration After I/R Injury
Whereas activation of NF-κB occurs during the acute injury phase and is linked to the production of proinflammatory mediators, NF-κB activation in hepatocytes also occurs after injury and is hepatoprotective and proregenerative (Fig. 2)95–99. There is abundant evidence that the primary function of NF-κB in hepatocytes is aligned with cell survival and proliferation. The original report of NF-κB p65 knockout mice documented that these mice died in utero due to massive hepatocyte apoptosis100. Suppression of NF-κB in the model of partial hepatectomy results in increased apoptosis and decreased proliferation98. After I/R injury, controlled hypothermia during the ischemia period was hepatoprotective and associated with increased NF-κB activation selectively in hepatocytes101. In a model of orthotopic liver transplantation, specific inhibition of NF-κB resulted in increased injury and significant hepatocyte apoptosis96.
CXC Chemokines in Regeneration After I/R
As mentioned above, ELR+ CXC chemokines play an important role in the recruitment of neutrophils during the injury response40,58. These same mediators directly impact hepatocytes and their path to proliferation or cell death. The role of CXC chemokines in hepatocyte proliferation and liver regeneration was first described by Colletti and colleagues102,103. Their findings demonstrated that ELR+ CXC chemokines stimulated hepatocyte proliferation and contributed to liver regeneration after partial hepatectomy. More specifically, these chemokines were signaling via the receptor CXCR2 such that blockade or knockout of CXCR2 reduced proliferation and decreased liver regeneration102,103. However, after I/R injury, inhibition or knockout of CXCR2 resulted in increased activation of NF-κB and STAT3 and increased hepatocyte proliferation and liver regeneration61. These findings were exactly the opposite of those observed in hepatectomy and were later found to be related to the concentration of ELR+ CXC chemokines. After partial hepatectomy, chemokine levels increased 3- to 5-fold, whereas after I/R, liver chemokine levels increased 30- to 100-fold61,104. In vitro studies showed that low concentrations of ELR+ chemokines induced hepatocyte proliferation but that high concentrations induced significant cytotoxicity61. Other reports demonstrated that overexpression of the ELR+ CXC chemokine, keratinocyte-derived chemokine (KC) in the liver (>100-fold) results in massive hepatocellular necrosis within 48 h105. The divergent effects of ELR+ CXC chemokines for inducing hepatocyte proliferation versus cell death depending on ligand concentration were also confirmed by exogenous chemokine treatments. Animals receiving high doses of chemokines had reduced hepatocyte proliferation and liver regeneration after hepatectomy, whereas low dose of chemokines promoted hepatocyte proliferation and regeneration104. CXCR2 appears to be the primary receptor in hepatocytes that mediates effects of ELR+ CXC chemokines. CXCR1 also is functional and appears to function in opposition to CXCR2. CXCR1 is not constitutively expressed in quiescent hepatocytes, but I/R injury induces its expression, particularly in hepatocytes106. Blockade or knockout of CXCR1 was found to result in a slight delay in liver repair, although without any effect on hepatocyte proliferation106. Although the effects of CXCR1 blockade or knockout on liver repair were not striking, it appears as though it functions in a negative feedback manner to regulate CXCR2, whereas dual blocking experiments have established CXCR2 as the dominant receptor functionally with regard to effects on hepatocytes.
CONCLUSIONS
Hepatic I/R injury may occur in a wide variety of clinical scenarios and is a major cause of morbidity and mortality. The injury is caused by a complex cascade of inflammatory mediators that recruits activated leukocytes into the liver. The mechanisms of liver repair and regeneration after I/R involve cell-mediated clearance of dead cells and tissue remodeling, and these processes are modulated by a number of different mediators, with some of these, such as ELR+ CXC chemokines, functioning in novel new ways to regulate hepatocyte proliferation versus cell death. Our understanding of these reparative and regenerative mechanisms after liver injury remains incomplete and warrants further investigation. Such studies may identify new therapeutic modalities that could improve patient care.
REFERENCES
- 1. Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178(5):454–8. [PubMed] [Google Scholar]
- 2. Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–38. [DOI] [PubMed] [Google Scholar]
- 3. Kim YI. Ischemia-reperfusion injury of the human liver during hepatic resection. J Hepatobiliary Pancreat Surg. 2003;10(3):195–9. [DOI] [PubMed] [Google Scholar]
- 4. Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15(5):277–84. [DOI] [PubMed] [Google Scholar]
- 5. Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260(3 Pt 1):G355–62. [DOI] [PubMed] [Google Scholar]
- 6. Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79(2):115–36. [DOI] [PubMed] [Google Scholar]
- 7. Shiratori Y, Kiriyama H, Fukushi Y, Nagura T, Takada H, Hai K, Kamii K. Modulation of ischemia-reperfusion-induced hepatic injury by Kupffer cells. Dig Dis Sci. 1994;39(6):1265–72. [DOI] [PubMed] [Google Scholar]
- 8. Chavez-Cartaya RE, DeSola GP, Wright L, Jamieson NV, White DJ. Regulation of the complement cascade by soluble complement receptor type 1. Protective effect in experimental liver ischemia and reperfusion. Transplantation 1995;59(7):1047–52. [DOI] [PubMed] [Google Scholar]
- 9. Straatsburg IH, Boermeester MA, Wolbink GJ, van Gulik TM, Gouma DJ, Frederiks WM, Hack CE. Complement activation induced by ischemia-reperfusion in humans: A study in patients undergoing partial hepatectomy. J Hepatol. 2000;32(5):783–91. [DOI] [PubMed] [Google Scholar]
- 10. Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264(4 Pt 1):G801–9. [DOI] [PubMed] [Google Scholar]
- 11. He S, Atkinson C, Qiao F, Cianflone K, Chen X, Tomlinson S. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Invest. 2009;119(8):2304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arumugam TV, Woodruff TM, Stocks SZ, Proctor LM, Pollitt S, Shiels IA, Reid RC, Fairlie DP, Taylor SM. Protective effect of a human C5a receptor antagonist against hepatic ischaemia-reperfusion injury in rats. J Hepatol. 2004;40(6):934–41. [DOI] [PubMed] [Google Scholar]
- 13. Lehmann TG, Koeppel TA, Munch S, Heger M, Kirschfink M, Klar E, Post S. Impact of inhibition of complement by sCR1 on hepatic microcirculation after warm ischemia. Microvasc Res. 2001;62(3):284–92. [DOI] [PubMed] [Google Scholar]
- 14. Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204(12):2913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173(12):7115–9. [DOI] [PubMed] [Google Scholar]
- 17. Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175(11):7661–8. [DOI] [PubMed] [Google Scholar]
- 18. Nace GW, Huang H, Klune JR, Eid RE, Rosborough BR, Korff S, Li S, Shapiro RA, Stolz DB, Sodhi CP, Hackam DJ, Geller DA, Billiar TR, Tsung A. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology 2013;58(1):374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, Kupiec-Weglinski JW. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology 2008;47(1):199–206. [DOI] [PubMed] [Google Scholar]
- 20. Zhai Y, Shen XD, Gao F, Zhao A, Freitas MC, Lassman C, Luster AD, Busuttil RW, Kupiec-Weglinski JW. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology 2008;47(1):207–14. [DOI] [PubMed] [Google Scholar]
- 21. Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100(2):279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2005;289(5):G969–76. [DOI] [PubMed] [Google Scholar]
- 23. Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162(4):2347–52. [PubMed] [Google Scholar]
- 24. Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108(3):430–8. [DOI] [PubMed] [Google Scholar]
- 25. Caldwell CC, Tschoep J, Lentsch AB. Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol. 2007;82(3):457–64. [DOI] [PubMed] [Google Scholar]
- 26. Khandoga A, Hanschen M, Kessler JS, Krombach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology 2006;43(2):306–15. [DOI] [PubMed] [Google Scholar]
- 27. Khandoga A, Biberthaler P, Enders G, Axmann S, Hutter J, Messmer K, Krombach F. Platelet adhesion mediated by fibrinogen-intercelllular adhesion molecule-1 binding induces tissue injury in the postischemic liver in vivo. Transplantation 2002;74(5):681–8. [DOI] [PubMed] [Google Scholar]
- 28. Kuboki S, Sakai N, Tschop J, Edwards MJ, Lentsch AB, Caldwell CC. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation 2008;86(5):710–8. [DOI] [PubMed] [Google Scholar]
- 30. Shen X, Wang Y, Gao F, Ren F, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology 2009;50(5):1537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ke B, Shen XD, Gao F, Tsuchihashi S, Farmer DG, Briscoe D, Busuttil RW, Kupiec-Weglinski JW. The CD154-CD40 T-cell co-stimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation 2005;79(9):1078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, Busuttil RW, Kupiec-Weglinski JW. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation 2002;74(3):315–9. [DOI] [PubMed] [Google Scholar]
- 33. Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, Edwards MJ. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology 1999;30(6):1448–53. [DOI] [PubMed] [Google Scholar]
- 34. Leifeld L, Cheng S, Ramakers J, Dumoulin FL, Trautwein C, Sauerbruch T, Spengler U. Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B. Hepatology 2002;36(4 Pt 1):1001–8. [DOI] [PubMed] [Google Scholar]
- 35. Husted TL, Blanchard J, Schuster R, Shen H, Lentsch AB. Potential role for IL-23 in hepatic ischemia/reperfusion injury. Inflamm Res. 2006;55(5):177–8. [DOI] [PubMed] [Google Scholar]
- 36. Shito M, Wakabayashi G, Ueda M, Shimazu M, Shirasugi N, Endo M, Mukai M, Kitajima M. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation 1997;63(1):143–8. [DOI] [PubMed] [Google Scholar]
- 37. Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161(5):1797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85(6):1936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wanner GA, Ertel W, Muller P, Hofer Y, Leiderer R, Menger MD, Messmer K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock 1996;5(1):34–40. [DOI] [PubMed] [Google Scholar]
- 40. Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest. 1995;95(1):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock 1998;10(3):182–91. [DOI] [PubMed] [Google Scholar]
- 42. Baldwin AS Jr. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–83. [DOI] [PubMed] [Google Scholar]
- 43. Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–86. [DOI] [PubMed] [Google Scholar]
- 44. Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9(13):1586–97. [DOI] [PubMed] [Google Scholar]
- 45. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. [DOI] [PubMed] [Google Scholar]
- 46. Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell 1996;86(5):787–98. [DOI] [PubMed] [Google Scholar]
- 47. Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54(6):1425–30. [PubMed] [Google Scholar]
- 48. Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IkappaB degradation. Hepatology 1998;28(4):1022–30. [DOI] [PubMed] [Google Scholar]
- 49. Mukhopadhyay A, Manna SK, Aggarwal BB. Pervanadate-induced nuclear factor-kappaB activation requires tyrosine phosphorylation and degradation of IkappaBalpha. Comparison with tumor necrosis factor-alpha. J Biol Chem. 2000;275(12):8549–55. [DOI] [PubMed] [Google Scholar]
- 50. Fan C, Li Q, Ross D, Engelhardt JF. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem. 2003;278(3):2072–80. [DOI] [PubMed] [Google Scholar]
- 51. Okaya T, Lentsch AB. Hepatic expression of S32A/S36A IkappaBalpha does not reduce postischemic liver injury. J Surg Res. 2005;124(2):244–9. [DOI] [PubMed] [Google Scholar]
- 52. Li JD, Peng Y, Peng XY, Li QL, Li Q. Suppression of nuclear factor-kappaB activity in Kupffer cells protects rat liver graft from ischemia-reperfusion injury. Transplant Proc. 2010;42(5):1582–6. [DOI] [PubMed] [Google Scholar]
- 53. Luedde T, Assmus U, Wustefeld T, Meyer zu Vilsendorf A, Roskams T, Schmidt-Supprian M, Rajewsky K, Brenner DA, Manns MP, Pasparakis M, Trautwein C. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest. 2005;115(4):849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lakshminarayanan V, Drab-Weiss EA, Roebuck KA. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-kappaB to the interleukin-8 promoter in endothelial and epithelial cells. J Biol Chem. 1998;273(49):32670–8. [DOI] [PubMed] [Google Scholar]
- 55. Read MA, Neish AS, Luscinskas FW, Palombella VJ, Maniatis T, Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity 1995;2(5):493–506. [DOI] [PubMed] [Google Scholar]
- 56. Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–48. [DOI] [PubMed] [Google Scholar]
- 57. Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250(2):91–104. [DOI] [PubMed] [Google Scholar]
- 58. Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: Roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology 1998;27(2):507–12. [DOI] [PubMed] [Google Scholar]
- 59. Djeu JY, Matsushima K, Oppenheim JJ, Shiotsuki K, Blanchard DK. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8. J Immunol. 1990;144(6):2205–10. [PubMed] [Google Scholar]
- 60. Schroder JM. The monocyte-derived neutrophil activating peptide (NAP/interleukin 8) stimulates human neutrophil arachidonate-5-lipoxygenase, but not the release of cellular arachidonate. J Exp Med. 1989;170(3):847–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Edwards MJ, Lentsch AB. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology 2008;48(4):1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hughes H, Farhood A, Jaeschke H. Role of leukotriene B4 in the pathogenesis of hepatic ischemia-reperfusion injury in the rat. Prostaglandins Leukot Essent Fatty Acids 1992;45(2):113–9. [DOI] [PubMed] [Google Scholar]
- 63. Sawaya DE Jr, Zibari GB, Minardi A, Bilton B, Burney D, Granger DN, McDonald JC, Brown M. P-selectin contributes to the initial recruitment of rolling and adherent leukocytes in hepatic venules after ischemia/reperfusion. Shock 1999;12(3):227–32. [DOI] [PubMed] [Google Scholar]
- 64. Singh I, Zibari GB, Brown MF, Granger DN, Eppihimer M, Zizzi H, Cruz L, Meyer K, Gonzales E, McDonald JC. Role of P-selectin expression in hepatic ischemia and reperfusion injury. Clin Transplant. 1999;13(1 Pt 2):76–82. [DOI] [PubMed] [Google Scholar]
- 65. Yadav SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology 1999;29(5):1494–502. [DOI] [PubMed] [Google Scholar]
- 66. Yadav SS, Howell DN, Gao W, Steeber DA, Harland RC, Clavien PA. L-selectin and ICAM-1 mediate reperfusion injury and neutrophil adhesion in the warm ischemic mouse liver. Am J Physiol. 1998;275(6 Pt 1):G1341–52. [DOI] [PubMed] [Google Scholar]
- 67. Granger DN, Kubes P. The microcirculation and inflammation: Modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55(5):662–75. [PubMed] [Google Scholar]
- 68. Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57(3):368–74. [PubMed] [Google Scholar]
- 69. Boury NM, Czuprynski CJ. Listeria monocytogenes infection increases neutrophil adhesion and damage to a murine hepatocyte cell line in vitro. Immunol Lett. 1995;46(1–2):111–6. [DOI] [PubMed] [Google Scholar]
- 70. Nagendra AR, Mickelson JK, Smith CW. CD18 integrin and CD54-dependent neutrophil adhesion to cytokine-stimulated human hepatocytes. Am J Physiol. 1997;272(3 Pt 1):G408–16. [DOI] [PubMed] [Google Scholar]
- 71. Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev. (Orlando) 2012;26(2):103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: Attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G760–7. [DOI] [PubMed] [Google Scholar]
- 73. Kushimoto S, Okajima K, Uchiba M, Murakami K, Harada N, Okabe H, Takatsuki K. Role of granulocyte elastase in ischemia/reperfusion injury of rat liver. Crit Care Med. 1996;24(11):1908–12. [DOI] [PubMed] [Google Scholar]
- 74. Li XK, Matin AF, Suzuki H, Uno T, Yamaguchi T, Harada Y. Effect of protease inhibitor on ischemia/reperfusion injury of the rat liver. Transplantation 1993;56(6):1331–6. [DOI] [PubMed] [Google Scholar]
- 75. Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: Two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA 1995;92(16):7162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57(10):1835–40. [PubMed] [Google Scholar]
- 77. Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185(8):1481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: Apoptosis or necrosis? Hepatology 2001;33(2):397–405. [DOI] [PubMed] [Google Scholar]
- 79. Yang M, Antoine DJ, Weemhoff JL, Jenkins RE, Farhood A, Park BK, Jaeschke H. Biomarkers distinguish apoptotic and necrotic cell death during hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2014;20(11):1372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Camargo CA Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology 1997;26(6):1513–20. [DOI] [PubMed] [Google Scholar]
- 81. Tiberio L, Tiberio GA, Bardella L, Cervi E, Cerea K, Dreano M, Garotta G, Fra A, Montani N, Ferrari-Bravo A, Callea F, Grigolato P, Giulini SM, Schiaffonati L. Mechanisms of interleukin-6 protection against ischemia-reperfusion injury in rat liver. Cytokine 2006;34(3–4):131–42. [DOI] [PubMed] [Google Scholar]
- 82. Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest. 1997;100(10):2443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kato A, Okaya T, Lentsch AB. Endogenous IL-13 protects hepatocytes and vascular endothelial cells during ischemia/reperfusion injury. Hepatology 2003;37(2):304–12. [DOI] [PubMed] [Google Scholar]
- 84. Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci USA 1986;83(18):6692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lentsch AB, Yoshidome H, Warner RL, Ward PA, Edwards MJ. Secretory leukocyte protease inhibitor in mice regulates local and remote organ inflammatory injury induced by hepatic ischemia/reperfusion. Gastroenterology 1999;117(4):953–61. [DOI] [PubMed] [Google Scholar]
- 86. Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 2006;43(2 Suppl 1):S45–53. [DOI] [PubMed] [Google Scholar]
- 87. Borowiak M, Garratt AN, Wustefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA 2004;101(29):10608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kang LI, Mars WM, Michalopoulos GK. Signals and cells involved in regulating liver regeneration. Cells 2012;1(4):1261–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;289(4):C826–35. [DOI] [PubMed] [Google Scholar]
- 90. Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mochizuki A, Pace A, Rockwell CE, Roth KJ, Chow A, O’Brien KM, Albee R, Kelly K, Towery K, Luyendyk JP, Copple BL. Hepatic stellate cells orchestrate clearance of necrotic cells in a hypoxia-inducible factor-1alpha-dependent manner by modulating macrophage phenotype in mice. J Immunol. 2014;192(8):3847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T. Liver regeneration in acute severe liver impairment: A clinicopathological correlation study. Liver Int. 2006;26(10):1225–33. [DOI] [PubMed] [Google Scholar]
- 93. Boulter L, Lu WY, Forbes SJ. Differentiation of progenitors in the liver: A matter of local choice. J Clin Invest. 2013;123(5):1867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roskams T. Relationships among stellate cell activation, progenitor cells, and hepatic regeneration. Clin Liver Dis. 2008;12(4):853–60, ix. [DOI] [PubMed] [Google Scholar]
- 95. Bellas RE, FitzGerald MJ, Fausto N, Sonenshein GE. Inhibition of NF-kappa B activity induces apoptosis in murine hepatocytes. Am J Pathol. 1997;151(4):891–6. [PMC free article] [PubMed] [Google Scholar]
- 96. Bradham CA, Schemmer P, Stachlewitz RF, Thurman RG, Brenner DA. Activation of nuclear factor-kappaB during orthotopic liver transplantation in rats is protective and does not require Kupffer cells. Liver Transpl Surg. 1999;5(4):282–93. [DOI] [PubMed] [Google Scholar]
- 97. Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002;110(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101(4):802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xu Y, Bialik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, Brenner DA, Czaja MJ. NF-kappaB inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Am J Physiol. 1998;275(4 Pt 1):C1058–66. [DOI] [PubMed] [Google Scholar]
- 100. Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 1995;376(6536):167–70. [DOI] [PubMed] [Google Scholar]
- 101. Kuboki S, Okaya T, Schuster R, Blanchard J, Denenberg A, Wong HR, Lentsch AB. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G201–7. [DOI] [PubMed] [Google Scholar]
- 102. Colletti LM, Green M, Burdick MD, Kunkel SL, Strieter RM. Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock 1998;10(4):248–57. [DOI] [PubMed] [Google Scholar]
- 103. Ren X, Carpenter A, Hogaboam C, Colletti L. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Pathol. 2003;163(2):563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wilson GC, Kuboki S, Freeman CM, Nojima H, Schuster RM, Edwards MJ, Lentsch AB. CXC chemokines function as a rheostat for hepatocyte proliferation and liver regeneration. PLoS One 2015;10(3):e0120092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Stefanovic L, Stefanovic B. Mechanism of direct hepatotoxic effect of KC chemokine: Sequential activation of gene expression and progression from inflammation to necrosis. J Interferon Cytokine Res. 2006;26(10):760–70. [DOI] [PubMed] [Google Scholar]
- 106. Clarke C, Kuboki S, Sakai N, Kasten KR, Tevar AD, Schuster R, Blanchard J, Caldwell CC, Edwards MJ, Lentsch AB. CXC chemokine receptor-1 is expressed by hepatocytes and regulates liver recovery after hepatic ischemia/reperfusion injury. Hepatology 2011;53(1):261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]