Abstract
Alcoholic liver disease encompasses the progressive stages of liver dysfunction that culminates in alcoholic cirrhosis (AC) and in severe cases alcoholic hepatitis (AH). Currently, prognostic scores have limited specificity and sensitivity. Plasma keratin-18 (K18) levels are elevated during liver disease and may be biomarkers of outcome. The objective of this study was to determine if total K18 (M65) or caspase-cleaved K18 (M30) levels were different between AC and AH patients. M65 and M30 levels were measured in the plasma of consented healthy controls and patients with AC and AH. Cell death was assessed by TUNEL staining and caspase activity. M65 and M30 values were significantly higher in AC patients compared to healthy controls and further increased in AH patients. The M65 values and the M30/M65 ratios of nonsurviving AH patients were significantly elevated above their surviving counterparts and healthy controls. Statistical analysis indicated that M30/M65 ratios outperformed current indices for accurately distinguishing the prognosis of AH patients. These scores occurred with minimal increase in plasma cell death markers such as ALT and AST. Serum caspase activity, TUNEL staining, and M30 immunohistochemistry in biopsies indicated that serum and tissue values may not correlate well with overall cell death. In conclusion, both M65 and M30 differentiate AH from AC patients, and M65 values and the M30/M65 ratio are capable of predicting early stage mortality; however, they may not accurately reflect pure hepatocyte cell death in these populations, as they do not strongly correlate with traditional cell death markers.
Key words: Alcoholic hepatitis (AH), Liver injury, Necrosis, Apoptosis, Cirrhosis
INTRODUCTION
Alcoholic hepatitis (AH) is the most severe form of liver dysfunction associated with chronic alcoholism1. Patients with AH experience far higher mortality rates and undergo a series of symptoms consistent with acute-on-chronic liver failure1. There are a number of different metrics for prognostication of outcome in AH patients2–6; however, none of these values have high specificity and sensitivity, likely due to the complex nature of AH. Combinations of tests have been proven to be more effective at stratifying patients based on potential mortality6, but may be cumbersome to use clinically, especially in acute situations. As such, new biomarkers, especially those that could be combined with current metrics, are in need for both early diagnosis and prognosis of AH patients.
Keratin-18 (K18) is a multifunctional intermediate filament protein present in simple epithelial tissues7. During hepatocellular necrosis, K18 is released passively from necrotic cells into the blood8. During apoptosis, K18 is cleaved by caspases9, and this cleaved form of K18 is also released into the blood8. ELISA tests have been developed for both K18 and cleaved K18 and are referred to as the M65 and M30 ELISA, respectively. Plasma or serum values of both M65 and M30 have been used extensively to better define cell death in many forms of liver disease10–15, as the liver expresses K18 in hepatocytes7. The ratio between M30 and M65 has been used as a metric of cell death in both humans10,12,15 and animal models16,17. Both M65 and M30 fragments have fairly long half lives in vivo that give a wider analytical window for detecting rapid processes, particularly apoptosis. Moreover, plasma M30 is predictive of nonalcoholic steatohepatitis (NASH) in patients suspected of having fatty liver, indicating significant diagnostic utility18. Modification of other metrics with M65 or M30 values can enhance predictive capacity of other prognostic metrics in liver diseases19. Despite its established use in other diseases and despite the fact that K18 is a known component of Mallory bodies present in patients with alcoholic cirrhosis (AC), there are no current studies that have investigated plasma M65 or M30 levels in the context of a clinical diagnosis, despite the fact that apoptotic cells are typically found in AH livers20–22.
Given this information, this study was initiated to better understand the role of cell death in patients with AH. We hypothesized that plasma M65 and M30 values would be elevated enough to potentially distinguish AC from AH and potentially predict outcome in these patients. Surprisingly, M65 and M30 levels were dramatically and substantially elevated in both AC and AH patients and had significant clinical value in predicting short-term mortality in AH patients. Even still, these values correlated poorly with traditional plasma parameters of liver cell death and may be related to other, as of yet unknown, pathologies that occur during AH.
MATERIALS AND METHODS
Patient Recruitment
Patients were recruited to the study in association with the Kansas University Medical Center Liver Center after informed consent. All studies were approved by The University of Kansas Medical Center Institutional Review Board before the onset of patient recruitment, and all studies are in accordance with the Helsinki Accords.
AH patient enrollment was based on patient history, plasma biochemistry, and clinical diagnosis. Transjugular biopsy was not used as criteria for the diagnosis of AH, as it is not part of The University of Kansas Medical Center standard operating procedure for the diagnosis of AH.
The following were the primary inclusion criteria for AH: AST above the upper limit of normal but AST < 400, AST > ALT, bilirubin > 3.0 mg/dl, a recent history of alcohol abuse (<2 months) as evaluated by clinical research staff, and a clinical diagnosis of AH by the attending physician.
All patients were recruited from an in-patient setting under the care of a hepatologist. Although this study was initiated before the current standards recommended by the National Institute on Alcohol Abuse and Alcoholism23, 22 of 24 patients in the AH group had severe AH at the point of admission according to the MELD score (MELD > 20), and 23 of 24 patients fit the guidelines for AH, with 1 patient having an AST-to-ALT ratio slightly less than 1.5. Pentoxifylline and/or corticosteroid treatment patients were grouped based on whether they had received treatment at any point in the preceding 7 days before admission to the study. Entrance criteria for AC patients included a history of chronic alcohol abuse and past or current diagnosis of AC based on liver biopsy or clinical features. All AC patients were evaluated by clinical research staff as abstinent for a minimum of 2 months, as a way to further distinguish them from the AH population. Patient data are provided in Table 1. Clinical data were obtained by The University of Kansas Medical Center hospital staff. AH samples were generally obtained between 2 and 5 days after admission to the hospital. An additional cohort of healthy volunteers was procured at The University of Kansas Medical Center from local volunteers. As patient-matched biopsies were generally not attainable for a majority of patients, and liver biopsy is not part of routine care for the diagnosis and management of AH at the Kansas University Hospital, banked pathology specimens were obtained and biochemically matched as close as possible for both AC and AH populations (Table 2). AC patient samples were taken from explanted livers directly at the time of explantation. Patient livers were stored in formalin for fixation before processing. AH patient biopsies were acquired by transjugular biopsy performed by interventional radiography and obtained by us from the hospital’s pathology department.
Table 1.
Patient Values for AC and AH Patients at Sample Acquisition
| Alcoholic Cirrhosis (AC) | Total Alcoholic Hepatitis (AH) | Surviving Alcoholic Hepatitis (SV) | Nonsurviving Alcoholic Hepatitis (NS) | |
|---|---|---|---|---|
| n | 27 | 24 | 14 | 10 |
| Age | 51 ± 2.2 | 44 ± 3* | 39 ± 3 | 51 ± 4 |
| Sex (% male) | 66% | 56% | 57% | 60% |
| AST (U/L) | 77 ± 12 | 115 ± 16 | 126 ± 24 | 99 ± 22 |
| ALT (U/L) | 38 ± 8 | 47 ± 8 | 50 ± 10 | 43 ± 15 |
| GDH (U/L) | 33 ± 11 | 40 ± 9 | 28 ± 11 | 55 ± 15 |
| Bilirubin (mg/dl) | 3.3 ± 0.65 | 19.0 ± 2.2* | 15.7 ± 2.3* | 23.5 ± 4.0* |
| Creatinine (mg/dl) | 1.2 ± 0.1 | 1.5 ± 0.2* | 1.3 ± 0.3 | 1.8 ± 0.3* |
| INR | 1.5 ± 0.13 | 2.3 ± 0.2* | 2.3 ± 0.3* | 2.3 ± 0.2* |
| WBC | 6.6 ± 0.5 | 10.6 ± 1.0* | 9.6 ± 1.4* | 11.9 ± 1.7* |
| LDH (U/L) | 303 ± 34 | 509 ± 80 | 513 ± 85* | 503 ± 163 |
| CK (U/L) | 153 ± 80 | 247 ± 102 | 244 ± 110 | 252 ± 204 |
| ALP (U/L) | 53 ± 8 | 73 ± 16 | 67 ± 10 | 82 ± 36 |
| ABIC score | 6.9 ± 0.3 | 8.2 ± 0.4* | 7.4 ± 0.5 | 9.3 ± 0.5*† |
| MELD score | 15.3 ± 1.2 | 31.1 ± 1.6* | 29.1 ± 2.7* | 34.0 ± 2.1* |
| 30-Day mortality | 0% | 41.6% | 0% | 100% |
The ABIC score was calculated as in Dominguez and coworkers5, and the MELD score was calculated as in Sheth et al.42. ANOVA was used to compare all three groups (AC, SV, and NS), and a t-test (nonparametric or parametric as appropriate) was used to compare two groups (AC, AH or SV, NS).
*p < 0.05, AH versus AC; †p < 0.05, NS versus SV.
Table 2.
Patient Biopsy Values
| Alcoholic Cirrhosis (AC) | Alcoholic Hepatitis (AH) | |
|---|---|---|
| n | 6 | 3 |
| Age | 54 ± 2 | 38 ± 5* |
| Sex (% male) | 66% | 66% |
| AST (U/L) | 49.1 ± 17.4 | 132 ± 20* |
| ALT (U/L) | 45.6 ± 9.9 | 51 ± 12 |
| Bilirubin (mg/dl) | 12.4 ± 5.4 | 10.2 ± 1.9 |
| Creatinine (mg/dl) | 1.8 ± 0.8 | 0.6 ± 0.07 |
| INR | 1.7 ± 0.1 | 1.5 ± 0.1 |
| WBC | 4.6 ± 0.6 | 11.3 ± 3.0* |
Biopsies were identified by the KUMC Liver Center staff from patients previously diagnosed with AC or AH. Data are mean ± SE.
p < 0.05.
Murine Studies
All studies were approved by the local Institutional Animal Care and Use Committee before onset. C57BL/6J mice (8–10 weeks of age) were treated with 700 mg/kg galactosamine and 100 μg/kg Salmonella enteritidis lipopolysaccharide (Gal/LPS) and then sacrificed 6 h later24. Blood was acquired via the vena cava, and tissue was stored in formaldehyde for fixation and subsequent processing.
Immunohistochemistry
Tissue samples for AH or nonliver immunohistochemistry were acquired from the University of Kansas Medical Center Department of Pathology as an additional cohort. Diagnosis of AH in biopsy samples was based on the pathologist’s report and histological features. The M30 antibody (Sigma-Aldrich, St. Louis, MO, USA) and the TUNEL assay kit (Roche, Basel, Switzerland) were used as previously described25,26.
Plasma Cell Death Values
M65 Epideath and M30 Apoptosense ELISAs were used according to the manufacturer’s instructions (VLVBio, Stockholm, Sweden). Internal quality controls present in the kit were run with all the samples to confirm assay performance. Plasma caspase 3 activity values were measured as described previously using a fluorogenic assay16.
Plasma Clinical Chemistry
Creatine kinase (CK) was assessed according to the manufacturer’s suggested protocol (Biovision, San Francisco, CA, USA) with the exception that samples had previously been frozen and thawed before the performance of the assay. Lactate dehydrogenase (LDH) was measured using a previously established protocol14.
Statistical Analysis
Data were generally nonnormal as determined by the Shapiro–Wilk test. Mann–Whitney U-test was used to directly compare the two groups. One-way ANOVA on ranks with Dunn’s post hoc was used to compare multiple groups against control. Receiver operating characteristic curves were used to assess correlations. All statistical tests were run using SigmaPlot (Systat Software, San Jose, CA, USA). For all values, p < 0.05 was considered significant.
RESULTS
Patient Recruitment
To better understand the role of cell death in AH patients, we recruited three distinct subpopulations of patients to this study: healthy controls, AC patients, and AH patients. Patient values (Table 1) mimic plasma biochemistry panels found in studies with large patient numbers27,28. Diagnosis of AC or AH was based partially on clinical history in addition to clinical chemistry. Healthy controls confirmed an absence of any acute or chronic drinking before admission to the study. Both MELD and ABIC scores were significantly higher in the AH population versus the AC controls, largely due to worsened liver function (elevated INR and bilirubin) as expected with the acute liver dysfunction. Generally these values were similar between groups, with the nonsurvivor group of AH patients having a higher rate of kidney dysfunction, in agreement with a previous study29.
High Levels of Circulating M65 and M30 Levels in AC and AH Patients
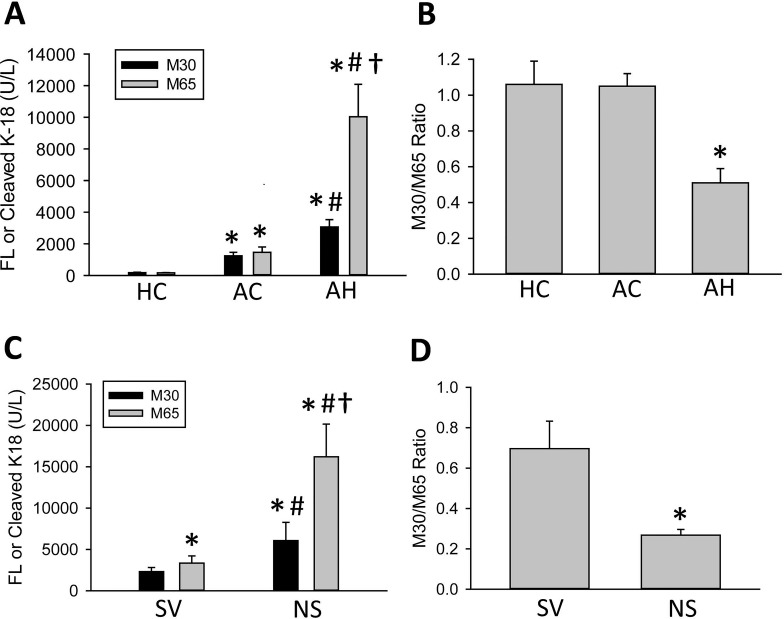
K18 release into the blood is prominently involved in a number of diseases featuring high degrees of cell death including acetaminophen toxicity10,12, NASH18, and cholestatic liver injury14,16. Plasma M65 and M30 levels were measured in healthy volunteers and in AC and AH patients using M65 EpiDeath and M30 Apoptosense assays (Fig. 1A). AC patients had significant elevations in both M30 and M65. These values were highly similar, which would typically be considered indicative of a mainly apoptotic injury index. Surprisingly, though, AH patients had significantly elevated values of both M30 and M65, even above the AC population (Fig. 1A). Moreover, M65 was significantly elevated above M30 in the AH population (Fig. 1A). These data would indicate that AC patients have considerable levels of apoptotic cell death, whereas AH patients have an additional degree of oncotic necrosis in addition to apoptosis (Fig. 1B).
Figure 1.
M30 and M65 measurements in representative patient populations. (A) M30 and M65 were measured in healthy controls (HC) and in patients with alcoholic cirrhosis (AC) and alcoholic hepatitis (AH). (B) The M30/M65 ratios were calculated for these patients. (C, D) AH patients were split by 30-day survival, and the M30 and M65 values and the M30/M65 ratios were recalculated for surviving (SV) and nonsurviving (NS) patients. All data represent mean ± SE. (A, B) *p < 0.05 versus HC, #p < 0.05 versus matched AC, †p < 0.05 versus all other values. (C) *p < 0.05 versus SV M30, #p < 0.05 versus matched SV M65, †p < 0.05 versus NS M30. (D) *p < 0.05 versus SV M30/M65 ratio.
Elevated Levels of M65 in Nonsurviving AH Patients
Mortality is high in AH patients with reported values between 10% and 50% over the first 90 days27,28. In order to assess whether M65 or M30 might have prognostic value in the treatment of AH patients, the AH group was subdivided into surviving and nonsurviving patients (Fig. 1C). M65 and M30 values were significantly elevated in the nonsurviving AH population (Fig. 1C). The M30/M65 ratio was significantly lower in the nonsurviving AH patients, indicating that these AH patients may undergo increased necrosis and release of M65 into plasma (Fig. 1D). Given this information, we hypothesized that either M65 or the M30/M65 ratio might be a clinically useful measurement in AH patients.
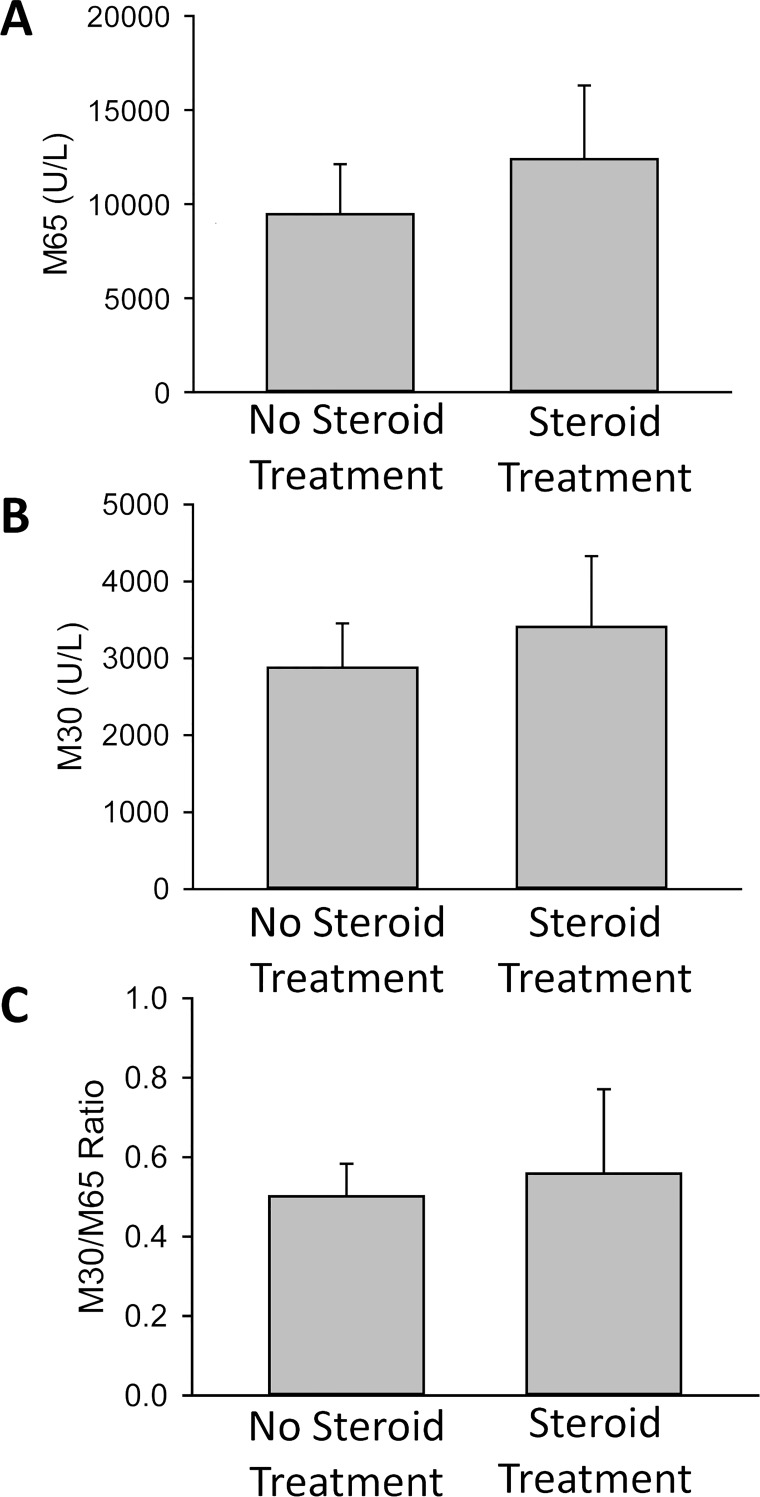
No Overt Effect of Corticosteroid and Pentoxifylline Treatment for AH on M30/M65 Levels
To determine whether the treatment had an effect on the M30 and M65 values, patients were divided based on whether they had received corticosteroids or pentoxifylline for treatment at any point in the preceding 7 days. However, no significant differences were observed in the M65 (Fig. 2A) and M30 levels (Fig. 2B), or the M30/M65 ratio (Fig. 2C) between patients with steroid or pentoxifylline treatment and no treatment. As there was some heterogeneity in the onset of treatment due to the complex nature of AH diagnosis and treatment, these data would need to be confirmed in a controlled study powered to examine differences; however, our initial data do not suggest an overt effect of treatment on the M30 or M65 levels in AH.
Figure 2.
AH patients were divided into groups that were treated with corticosteroids and/or pentoxifylline within the previous 7 days (n = 15) and those who had not been treated (n = 9). (A) M65 and (B) M30 levels were measured, and (C) M30/M65 ratios were calculated. Data represent means ± SE.
M65 and M30/M65 Ratio Predict Outcome in AH Patients
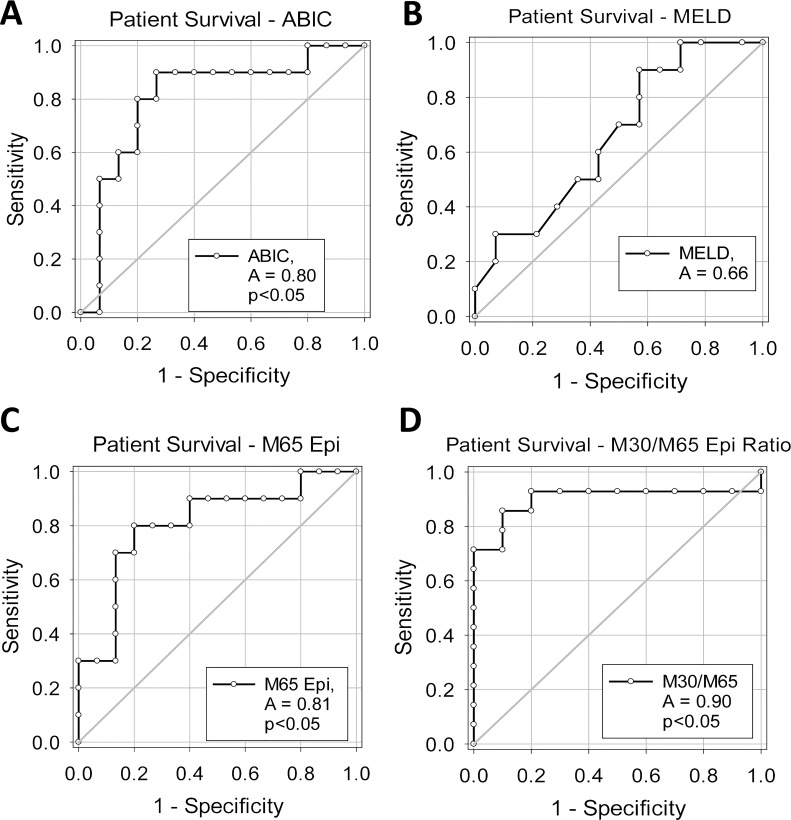
Short-term mortality in the AC population in this study was limited, and thus no assessment was made in this population. On the contrary, AH patients had a mortality rate of ∼40%, consistent with other studies1. Patient plasma biochemistry values for surviving and nonsurviving AH patients were similar, with the nonsurviving AH patients trending higher in bilirubin and creatinine and being significantly higher in age (Table 1); however, there was no significant difference in the ALT, AST, or INR values. The ABIC score was elevated in the nonsurviving population, whereas the MELD score was similar between groups. The ABIC score (Fig. 3A) and the MELD score, (Fig. 3B) along with M65 (Fig. 3C) and the M30/M65 (Fig. 3D) ratio were assessed for their capacity to predict short-term mortality (30-day mortality) in AH patients. The ABIC score was significantly associated with short-term mortality (c-statistic, 0.80; p < 0.05). Although the MELD score was not statistically significant in this study, it did produce a positive AUROC (c-statistic, 0.66). M65 produced a similar value as the ABIC (Fig. 3C) (c-statistic, 0.81; p < 0.05), although the highest score was produced by the M30/M65 ratio (Fig. 3D) (c-statistic, 0.90; p < 0.05). The cutoff values indicate that the M30/M65 ratio had 90% sensitivity and 86% specificity when a cutoff value of 0.3884 was applied (Table 3). As such, the M30/M65 ratio may be a highly specific and sensitive prognostic marker and should be evaluated in a larger population.
Figure 3.
Receiver operator characteristic (ROC) curve analysis for patient survival. ROC curve analyses for 30-day survival for (A) ABIC score, (B) MELD score, (C) M65 values, and (D) M30/M65 ratio.
Table 3.
Cutoff Values for Surviving and Nonsurviving AH Patients
| Cutoff | Specificity | Sensitivity | |
|---|---|---|---|
| M65 | 8,403 U/L | 80% | 79% |
| M30 | 2,634 U/L | 60% | 60% |
| M30/M65 ratio | 0.3884 | 90% | 86% |
| ABIC | 8.555 | 80% | 79% |
| MELD | 32.5 | 60% | 58% |
Representative patient profiles for the M65 and M30/M65 values in relation to admission to hospital, admission to study, and known date of death are listed in Table 4. Samples were typically acquired 2–5 days after admission, consistent with other studies. Mortality was generally due to end-stage liver disease (ESLD) or cardiac death secondary to ESLD in these populations (Table 4). Given that M65 and M30/M65 could accurately predict future mortality in patients at early time points consistent with the initial clinical diagnosis, we concluded that M65 and the M30/M65 ratio were both candidates for clinical biomarkers of AH severity.
Table 4.
Patient Time Frames and Relative M65 and M30/M65 Values
| Postadmission Acquisition Time (Days) | Days to Mortality From Hospital Admission | M65 at Acquisition (U/L) | M30/M65 at Acquisition (Ratio) | Cause of Death | |
|---|---|---|---|---|---|
| Patient 1 | 4 | 25 | 15,125 | 0.29 | Cardiac/ALF |
| Patient 45 | 10 | 17 | 31,000 | 0.27 | ESLD |
| Patient 47 | 2 | 10 | 11,580 | 0.18 | ESLD |
| Patient 55 | 4 | 13 | 22,050 | 0.24 | Cardiac/ALF |
| Patient 56 | 3 | 17 | 29,019 | 0.23 | Unknown |
Representative patient time frames demonstrating sample acquisition time and mortality dates from hospital admission in addition to M65 and the M30/M65 ratio. ALF, acute liver failure; ESLD, end-stage liver failure.
Cell Death Markers in ALD Patients
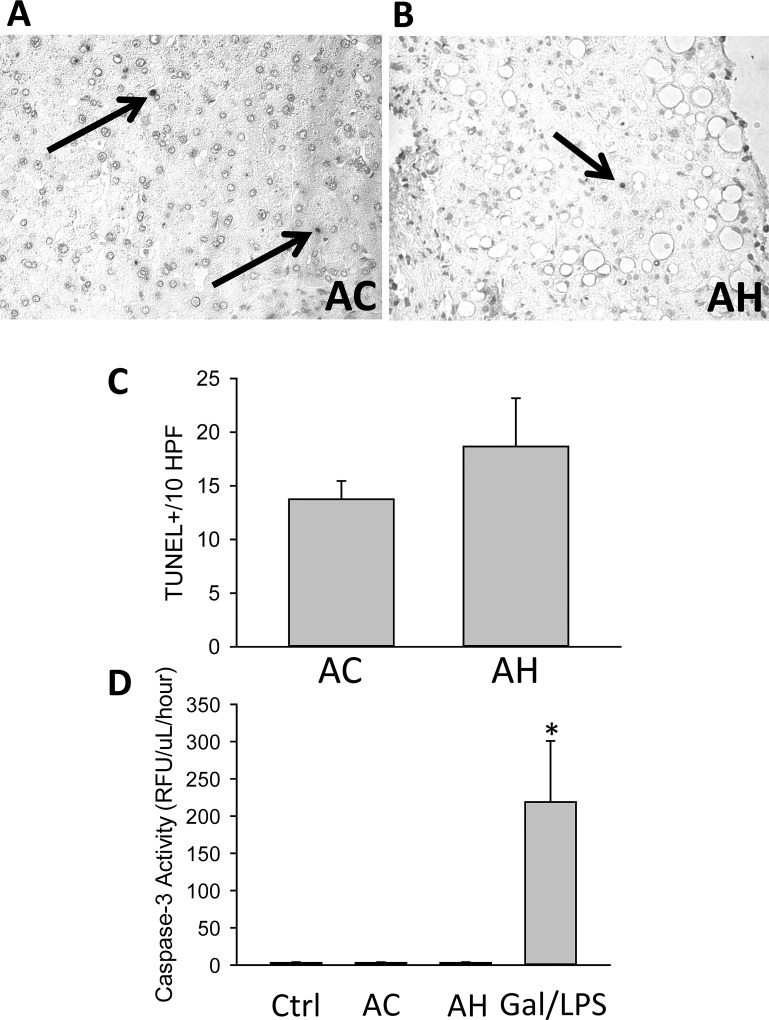
As the values of M30 and M65 were far higher than we hypothesized at the start of this investigation, we wanted to verify the presence of cell death and M30 in liver biopsies. The TUNEL assay was performed on AC and AH patient liver biopsies, and TUNEL+ cells were counted in 10 high-power fields (HPF). While commonly perceived to be a method of detection of apoptotic cells, the TUNEL assay is known to stain both apoptotic and necrotic cells in the liver30,31. Despite the dramatically elevated levels of M65 and M30, only minimal levels of TUNEL+ cells were found in the livers of AH (Fig. 4A) and AC (Fig. 4B) biopsies, and TUNEL staining appeared purely nuclear, with minimal cytoplasmic leakage. This profile is considered to be apoptotic30,31. Quantification did not yield a significant difference between the groups, although AH patients had slightly higher values (Fig. 4C), consistent with other studies21. Assessment of plasma caspase 3 activity, a gold standard marker of apoptosis, yielded no significant difference between control samples and AC or AH patients and was essentially absent when compared to an established model of murine liver apoptosis (Fig. 4D). To further determine if M65 or M30 leakage was directly related to cell death, we attempted to correlate M65 or M30 with cell death as measured by a number of other plasma biomarkers of liver cell death and cell function, with a focus on the AH population. M65 and M30 values positively correlated with one another in AH patients (Table 5). Of note, neither M65 nor M30 values correlated well with the established markers of liver injury ALT or AST, or bilirubin, the traditional marker of liver function, nor did they correlate with WBCs; however, M65 showed a very minor but statistically significant correlation with glutamate dehydrogenase (GDH) levels (Table 5). As GDH is more specific for mitochondrial injury, this may indicate that the release of M65 or M30 is related to mitochondrial dysfunction present in some AH patients32,33. We also assessed CK and LDH as measurements of non-liver tissue necrosis (Table 1). While both CK and LDH were mildly elevated in AH patients, this was not a significant difference, and there was no difference between surviving and nonsurviving AH patients.
Figure 4.
Cell death markers in patients with AH and AC. TUNEL staining was performed in liver sections from (A) AC and (B) AH patients. Arrows indicate TUNEL+ cells. (C) The numbers of TUNEL+ cells in 10 high-power fields were quantified. (D) In addition, serum caspase 3 activities were measured in HC and in AC and AH patients. Serum from galactosamine/LPS-treated mice was used as a positive control.
Table 5.
Linear Correlation in AH Patients
| Correlation | R 2 Value |
|---|---|
| M65/M30 | 0.78* |
| M65/MELD | 0.09 |
| M65/ABIC | 0.13 |
| M65/AST | 0.01 |
| M65/ALT | 0.01 |
| M65/GDH | 0.16* |
| M65/age | 0.08 |
| M65/creatinine | 0.13 |
| M65/WBC | 0.01 |
| M65/bilirubin | 0.04 |
Correlation was assessed by linear regression between M65 assay and other measurements.
p < 0.05.
Limited M30 Staining in Human AH or AC Livers
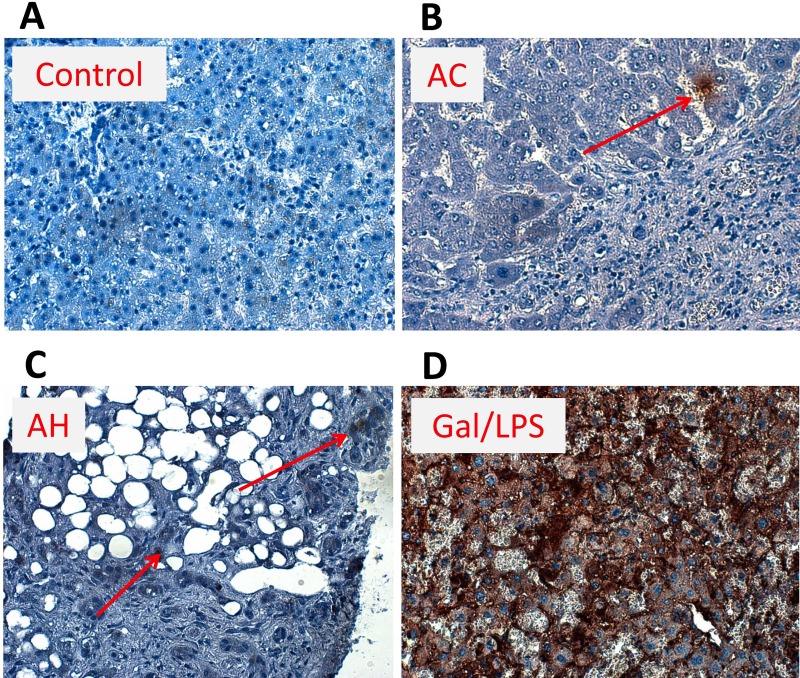
Given the low TUNEL values associated with both AC and AH patients, we wanted to understand if M30 was elevated in the liver tissue in AH or AC patients8. We performed immunohistochemistry for M30 (Fig. 5) in livers of a healthy control, AH and AC patients, as well as galactosamine/LPS-treated mice as a positive control25. While there was limited M30 staining in both AH and AC livers in all examined biopsies (representative biopsies shown), some cells did stain positive for M30. This relatively limited staining profile was especially apparent when contrasted to livers of galactosamine/LPS-treated mice, where a majority of cells showed some positivity for M30 (Fig. 5). As such, mechanisms other than pure cell death should be considered for the release of M65/M30 in these populations, as there appears to be a stark disparity between traditional understanding of cell death and the massive release of M65 and M30 present in these patients.
Figure 5.
M30 staining in ALD and control patients. M30 (caspase-cleaved K18 fragment) immunohistochemistry was performed in liver sections from HC and patients with AC and AH, and in galactosamine/LPS (Gal/LPS)-treated mice. Representative sections are shown (magnification: 200×). Arrows indicate positive cells. Arrows were not used in the Gal/LPS sample due to the ubiquitous staining.
DISCUSSION
This study was initiated to better understand whether cell death markers could be potentially useful in stratifying patients and predicting outcomes in a population with ALD. In this study, we found that M65 and M30 levels were significantly elevated above control values in both AC and AH patients (Fig. 1). Furthermore, M65 values were significantly elevated above M30 values in all AH patients, as well as in nonsurviving versus surviving AH patients (Fig. 1). Treatment with corticosteroids and pentoxifylline did not have a significant effect on M65, M30, or the M30/M65 ratio (Fig. 2). The M30/M65 ratio was thus significantly decreased and may have considerable value as a prognostic score (Figs. 1 and 3). Despite this utility, the reasoning for the high degree of M65/M30 release remains under investigation, as M65 and M30 serum values did not correlate well with other serum cell death markers, and histological examination of AC and AH livers did not reveal the massive cell death that is typically associated with the M65 and M30 values listed in this study (Figs. 4 and 5).
Transjugular biopsy is not a common practice for the diagnosis of AH at the University of Kansas Medical Center or at many major US medical centers, as the American Association for the Study of Liver Disease has not fully endorsed biopsy in this patient population yet and thus was not used as part of the diagnosis of patients1,34. While this raises the possibility that some patients may not have true AH, it is more in line with common clinical practice in the US1,34,35. Using an ELISA assay to detect K18 levels in the blood, we found surprisingly high levels, consistent with major apoptotic and necrotic cell death (Fig. 1). These measurements of plasma M65 and M30, assays thought to specifically indicate cell death, may be highly useful clinically, despite the fact that their dramatic elevation appears out of synch with traditional markers of cell death.
Keratin 18 Levels in Alcoholic Hepatitis
Keratin levels have been measured in a number of human liver diseases as a marker of cell death10,12,14,15,18. Similar measurements have been taken in alcoholics, although there have never been measurements in the context of a clinical diagnosis nor attempts to predict survival using these specific tests36. One of the most interesting aspects of this study is the dramatic elevation seen in plasma M65 and M30 values in AH patients (Fig. 1). Previous measurements in cholestatic patients14, acetaminophen-induced liver injury12, and in NASH18 indicate that M65 and M30 values are only elevated above control values in the event of major necrosis or elevated apoptosis. Not only were M65 values elevated to as much as 31,000 U/L (range: 700–31,000 U/L in AH group) in this study in AH patients, but this also occurred in a patient with an ALT activity of 13 U/L (normal range: 7–56 U/L) and an AST activity of 67 U/L (normal range: 10–40 U/L). Of note, this patient died within 28 days of entering the study. Other patients presented in this study followed similar time courses with M65 values in excess of 10,000 U/L, AST and ALT values below 200, and mortality within 2–3 weeks (Table 4). As such, there appears to be a disparity between commonly understood markers of cell death in this population as neither serum markers of liver such as ALT or AST seem to concur with the M65/M30 values. Biopsies of a separate population seemed to confirm these data, as staining for M30 in AH patients or AC patients produced only minimal TUNEL+ cells (Fig. 4) or M30+ cells in their livers (Fig. 5). The only positive correlation with other cell death markers was with GDH, although the absolute value was minimal (R 2 = 0.16)32. The mechanism behind M65 and M30 release in these patients is undetermined, as is the reason why GDH correlates with M65 and M30 and other markers do not. Potentially, this could be the result of M30 release from a number of different organ systems simultaneously. Evaluating CK levels in serum did not show a significant increase in AH compared to AC patients, suggesting that neither skeletal nor heart muscle injury appears to be a major contributing factor, although there was a mild increase in CK and LDH consistent with the increases found in AST (Table 1). M65 and M30 could also be acting as indirect markers of the accumulation of Mallory hyaline internally in hepatocytes, as the inclusion of Mallory bodies is considered a marker of advanced disease states and known to be composed largely of K8 and K1820. Even still, Mallory bodies are also present in other chronic liver diseases, including NASH, which rarely presents with elevation of M65 to this degree37. Both hypotheses should be tested in the future given the potential diagnostic and prognostic capacity of M65 and the M30/M65 ratio (Figs. 1 and 3), and the potential advances in the understanding of the basic science of AH.
Differentiation of AH from AC patients as shown in this study does not present with many challenges due to the fact that AC patients had significantly less liver dysfunction and were categorically nondrinking for an extended period of time. We chose to include this group though both as a baseline for M65 and M30 levels in advanced ALD and to determine if levels in AH patients increased significantly above patients with AC without active AH. Our data largely confirm a study published during the preparation of this article indicating that the dramatic elevations in plasma M65 and M30 levels in AH patients might be a useful noninvasive diagnostic marker of AH38. In a small subset of the data from this study with a limited population, we found even in decompensated nondrinking cirrhotics without AH, but with MELD > 20, M65 values were substantially higher in the AH population than high MELD cirrhotics (data not shown). These data should be confirmed in larger patient settings.
Hepatic Cell Death in Alcoholic Hepatitis
Many studies have used the M30/M65 ratio as an index of apoptosis to necrosis during liver disease10,15–17. Human plasma M65 and M30 values typically correlate very well with other cell death markers, although this was not the case in this study12,15. Given this information, the use of M65 and M30 as a biomarker of acute hepatic cell death, not as a biomarker of AH severity, in the context of AH, is questionable according to data in this study (Figs. 1, 4, and 5), as their values are at odds with a majority of the other measures of cell death. While a number of different acute liver failure diseases feature prominent hepatic parenchymal necrosis, apoptosis, or both, AH appears to occur through a phenotype shift in hepatocytes toward a nonfunctional parenchyma. Simultaneous elevations in bilirubin and INR (Table 1) indicate that while the liver is not technically dying of massive cell death, it does experience limited hepatocyte functionality, which leads to the acute failure. This may be related to recent data illustrating how the deposition of laminin across the AH liver results in inefficient production of mature hepatocytes, especially in therapy-resistant patients33. Regardless, the reason for the massive M30 and M65 release compared to relatively low values of traditional cell death measurements was not conclusively determined in this study. Recent studies have indicated that AH results in systemic inflammation that affects multiple organ systems39–41. Tissue damage from systemic inflammation associated with AH is another possible and likely source of K18 release. While measurements of LDH and AST, which are widely expressed, did not correlate with M30 or M65, it remains a possibility that cell death in nonhepatic tissues is a potential additional source of M30 and M65 release. Future studies focused on understanding the role of both apoptotic and necrotic cell death, as well as studies focused on the mechanisms of K18 release in AH patients, may yield valuable clinical information given the highly predictive nature of K18 fragments.
This study had two potential drawbacks in the sample collection process. Samples were largely acquired within 2–5 days of the initial presentation, which is consistent with other studies, and only a single sample was acquired from each patient. Future studies should attempt to collect patient blood closest to presentation and monitor patient outcomes over the first 7 days to compare M30/M65 levels with more dynamic scores6. While we have reported here that standard corticosteroid or pentoxifylline treatment did not have a significant effect on M65 and M30 levels or the M30/M65 ratio, the study was not sufficiently powered or designed to fully assess this. Longitudinal samples within a randomized clinical trial should be used to fully evaluate this matter in the near future for the M30/M65 ratio or M65 values to be considered as a potential prognostic indicator for AH.
In summary, our data indicate that the M65 and M30 levels in patients might be useful as diagnostic criteria of AH in larger AC populations. Future studies aimed at understanding additional cellular sources or additional mechanisms of M65 and M30 release in AH patients may increase our understanding on how this relates to the progressive failure of AH livers. Larger studies validating the data here on the predictive capacity of M30/M65 in AH patients may have a lasting and beneficial effect on patient outcomes and our understanding of basic liver pathophysiology during AH.
ACKNOWLEDGMENTS
This work was supported by a CTSA grant from the NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research #UL1TR000001 (formerly #UL1RR033179) and the Robert Hanlon Trust. In addition, this work was supported in part by the National Institutes of Health grant R01 AA12916 (to H.J.) and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 (to B.L.W.) from the National Institute of Environmental Health Sciences. The authors declare no conflicts of interest.
REFERENCES
- 1. Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62:S38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193–9. [PubMed] [Google Scholar]
- 3. Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005;41:353–8. [DOI] [PubMed] [Google Scholar]
- 4. Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L, Boleslawski E, Deltenre P, Canva V, Pruvot FR, Mathurin P. The Lille model: A new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348–54. [DOI] [PubMed] [Google Scholar]
- 5. Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R, Arroyo V, Caballería J, Ginès P, Bataller R. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–56. [DOI] [PubMed] [Google Scholar]
- 6. Louvet A, Labreuche J, Artru F, Boursier J, Kim DJ, O’Grady J, Trépo E, Nahon P, Ganne-Carrié N, Naveau S, Diaz E, Gustot T, Lassailly G, Cannesson-Leroy A, Canva-Delcambre V, Dharancy S, Park SH, Moreno C, Morgan TR, Duhamel A, Mathurin P. Combining data from liver disease scoring systems better predicts outcomes of patients with alcoholic hepatitis. Gastroenterology 2015;149:398–406. [DOI] [PubMed] [Google Scholar]
- 7. Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, Marberger M, Bivén K, Shoshan MC, Linder S. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64:1751–6. [DOI] [PubMed] [Google Scholar]
- 9. Caulín C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bechmann LP, Marquitan G, Jochum C, Saner F, Gerken G, Canbay A. Apoptosis versus necrosis rate as a predictor in acute liver failure following acetaminophen intoxication compared with acute-on-chronic liver failure. Liver Int. 2008;28:713–6. [DOI] [PubMed] [Google Scholar]
- 11. Craig DG, Lee P, Pryde EA, Masterton GS, Hayes PC, Simpson KJ. Circulating apoptotic and necrotic cell death markers in patients with acute liver injury. Liver Int. 2011;31:1127–36. [DOI] [PubMed] [Google Scholar]
- 12. Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Adebayo D, Morabito V, Andreola F, Pieri G, Luong TV, Dhillon A, Mookerjee R, Jalan R. Mechanism of cell death in acute-on-chronic liver failure: A clinico-pathologic-biomarker study. Liver Int. 2015;35:2564–74. [DOI] [PubMed] [Google Scholar]
- 14. Woolbright BL, Dorko K, Antoine DJ, Clarke JI, Gholami P, Li F, Kumer SC, Schmitt TM, Forster J, Fan F, Jenkins RE, Park BK, Hagenbuch B, Olyaee M, Jaeschke H. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 2015;283:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weemhoff JL, Woolbright BL, Jenkins RE, McGill MR, Sharpe MR, Olson JC, Antoine DJ, Curry SC, Jaeschke H. Plasma biomarkers to study mechanisms of liver injury in patients with hypoxic hepatitis. Liver Int. 2017;37:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang M, Antoine DJ, Weemhoff JL, Jenkins RE, Farhood A, Park BK, Jaeschke H. Biomarkers distinguish apoptotic and necrotic cell death during hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2014;20:1372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006;44:27–33. [DOI] [PubMed] [Google Scholar]
- 19. Bechmann LP, Jochum C, Kocabayoglu P, Sowa JP, Kassalik M, Gieseler RK, Saner F, Paul A, Trautwein C, Gerken G, Canbay A. Cytokeratin 18-based modification of the MELD score improves prediction of spontaneous survival after acute liver injury. J Hepatol. 2010;53:639–47. [DOI] [PubMed] [Google Scholar]
- 20. Savolainen VT, Lalu K, Penttilä A, Virtanen I, Karhunen PJ. Cytokeratin inclusions in alcoholic liver disease and their relation to the amount of alcohol intake. Liver 1994;14:281–7. [DOI] [PubMed] [Google Scholar]
- 21. Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–53. [DOI] [PubMed] [Google Scholar]
- 22. Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, Trinchet JC, Beaugrand M, Guillet JG, Guettier C. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol. 2001;34:254–60. [DOI] [PubMed] [Google Scholar]
- 23. Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, McClain C, McCullough A, Mitchell MC, Morgan TR, Nagy L, Radaeva S, Sanyal A, Shah V, Szabo G; NIAAA Alcoholic Hepatitis Consortia. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: Recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–6. [PubMed] [Google Scholar]
- 25. Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology 2004;40:998–1007. [DOI] [PubMed] [Google Scholar]
- 26. Woolbright BL, Li F, Xie Y, Farhood A, Fickert P, Trauner M, Jaeschke H. Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol Lett. 2014;228:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O’Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH, STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–28. [DOI] [PubMed] [Google Scholar]
- 28. Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, Augustin S, Mookerjee RP, Michelena J, Smyrk TC, Buob D, Leteurtre E, Rincón D, Ruiz P, García-Pagán JC, Guerrero-Marquez C, Jones PD, Barritt AS 4th, Arroyo V, Bruguera M, Bañares R, Ginès P, Caballería J, Roskams T, Nevens F, Jalan R, Mathurin P, Shah VH, Bataller R. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014;146:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Altamirano J, Fagundes C, Dominguez M, García E, Michelena J, Cárdenas A, Guevara M, Pereira G, Torres-Vigil K, Arroyo V, Caballería J, Ginès P, Bataller R. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65–71. [DOI] [PubMed] [Google Scholar]
- 30. Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: A cautionary note. Hepatology 1995;21:1465–8. [DOI] [PubMed] [Google Scholar]
- 31. Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 2003;125:1246–57. [DOI] [PubMed] [Google Scholar]
- 32. McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dubuquoy L, Louvet A, Lassailly G, Truant S, Boleslawski E, Artru F, Maggiotto F, Gantier E, Buob D, Leteurtre E, Cannesson A, Dharancy S, Moreno C, Pruvot FR, Bataller R, Mathurin P. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut 2015;64:1949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14–32. [DOI] [PubMed] [Google Scholar]
- 35. Verbeke L, Laleman W, Nevens F. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;373:281. [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez-Quintela A, García J, Campos J, Perez LF, Alende MR, Otero E, Abdulkader I, Tomé S. Serum cytokeratins in alcoholic liver disease: Contrasting levels of cytokeratin-18 and cytokeratin-19. Alcohol 2006;38:45–9. [DOI] [PubMed] [Google Scholar]
- 37. Younossi ZM, Page S, Rafiq N, Birerdinc A, Stepanova M, Hossain N, Afendy A, Younoszai Z, Goodman Z, Baranova A. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg. 2011;21:431–9. [DOI] [PubMed] [Google Scholar]
- 38. Bissonnette J, Altamirano J, Devue C, Roux O, Payancé A, Lebrec D, Bedossa P, Valla D, Durand F, Ait Oufella H, Sancho-Bru P, Caballeria J, Ginès P, Boulanger CM, Bataller R, Rautou PE. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology 2017;66:555–63. [DOI] [PubMed] [Google Scholar]
- 39. Hmoud BS, Patel K, Bataller R, Singal AK. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: A meta-analysis of randomized trials. Liver Int. 2016;36:721–8. [DOI] [PubMed] [Google Scholar]
- 40. Michelena J, Altamirano J, Abraldes JG, Affò S, Morales-Ibanez O, Sancho-Bru P, Dominguez M, García-Pagán JC, Fernández J, Arroyo V, Ginès P, Louvet A, Mathurin P, Mehal WZ, Caballería J, Bataller R. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015;62:762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vergis N, Atkinson SR, Knapp S, Maurice J, Allison M, Austin A, Forrest EH, Masson S, McCune A, Patch D, Richardson P, Gleeson D, Ryder SD, Wright M, Thursz MR. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology 2017;152:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]