Key Points
Question
What is the association between compensated hypercapnia and hypercapnic acidosis on hospital mortality in mechanically ventilated patients with acute cerebral injury?
Findings
In this cross-sectional study including 30 742 patients with cerebral injury admitted to intensive care units in Australia and New Zealand, hospital mortality was higher in patients with hypercapnic acidosis compared with patients with compensated hypercapnia or normocapnia. In patients with hypercapnic acidosis, the adjusted odds ratio for hospital mortality increased with increasing partial pressure of carbon dioxide, while in patients with compensated hypercapnia, the adjusted odds did not change with increasing partial pressure of carbon dioxide.
Meaning
In mechanically ventilated patients with cerebral injury, hypercapnic acidosis is associated with increased mortality, and compensated hypercapnia appears to have no such association.
Abstract
Importance
Clinical studies investigating the effects of hypercapnia and hypercapnic acidosis in acute cerebral injury are limited. The studies performed so far have mainly focused on the outcomes in relation to the changes in partial pressure of carbon dioxide and pH in isolation and have not evaluated the effects of partial pressure of carbon dioxide and pH in conjunction.
Objective
To review the association of compensated hypercapnia and hypercapnic acidosis during the first 24 hours of intensive care unit admission on hospital mortality in adult mechanically ventilated patients with cerebral injury.
Design, Setting, and Participants
Multicenter, binational retrospective review of patients with cerebral injury (traumatic brain injury, cardiac arrest, and stroke) admitted to 167 intensive care units in Australia and New Zealand between January 2000 and December 2015. Patients were classified into 3 groups based on combination of arterial pH and arterial carbon dioxide (normocapnia and normal pH, compensated hypercapnia, and hypercapnic acidosis) during the first 24 hours of intensive care unit stay.
Main Outcomes and Measures
Hospital mortality.
Results
A total of 30 742 patients (mean age, 55 years; 21 827 men [71%]) were included. Unadjusted hospital mortality rates were highest in patients with hypercapnic acidosis. Multivariable logistic regression analysis and Cox proportional hazards analysis in 3 diagnostic categories showed increased odds of hospital mortality (cardiac arrest odds ratio [OR], 1.51; 95% CI, 1.34-1.71; stroke OR, 1.43; 95% CI, 1.27-1.6; and traumatic brain injury OR, 1.22; 95% CI, 1.06-1.42; P <.001) and hazard ratios (HR) (cardiac arrest HR, 1.23; 95% CI, 1.14-1.34; stroke HR, 1.3; 95% CI, 1.21-1.4; traumatic brain injury HR, 1.13; 95% CI, 1-1.27), in patients with hypercapnic acidosis compared with normocapnia and normal pH. There was no difference in mortality between patients who had compensated hypercapnia compared with patients who had normocapnia and normal pH. In patients with hypercapnic acidosis, the adjusted OR of hospital mortality increased with increasing partial pressure of carbon dioxide, while no such increase was noted in patients with compensated hypercapnia.
Conclusions and Relevance
Hypercapnic acidosis was associated with increased risk of hospital mortality in patients with cerebral injury. Hypercapnia, when compensated to normal pH during the first 24 hours of intensive care unit admission, may not be harmful in mechanically ventilated patients with cerebral injury.
This cross-sectional study reviews the association of compensated hypercapnia and hypercapnic acidosis during the first 24 hours of intensive care unit admission on hospital mortality in adult mechanically ventilated patients with cerebral injury.
Introduction
Cerebral injury may be caused by traumatic brain injury, stroke, and cardiac arrest. Hypercapnia and hypocapnia are avoided in such patients to prevent secondary brain injury.1 Clinical studies investigating the effects of hypocapnia and hypercapnia on traumatic brain injury and cardiac arrest revealed that hypocapnia and hypercapnia were associated with increased in-hospital mortality.2,3,4,5,6 Clinical studies evaluating the effects of arterial partial pressure of carbon dioxide (Pco2) derangements in stroke are very few.7,8,9 Two studies investigated the effect of hypocapnia, with differing results.7,8 A retrospective observational study in patients with subarachnoid hemorrhage showed higher mortality with hypocapnia,8 and a randomized clinical trial with a sample size of 50 patients with pituitary apoplexy reported no difference in mortality when hypocapnia was compared with normocapnia.7 The study by Westermaier et al9 in patients with aneurysmal subarachnoid hemorrhage found benefit in outcome with hypercapnia.
In addition to Pco2, pH level appears to have a significant effect on clinical outcomes in patients with acute cerebral injury. Acidic pH has been shown to have an increased risk of mortality and unfavorable outcomes in patients with severe traumatic brain injury,10 out-of-hospital cardiac arrest,11 and ischemic stroke.12,13
All these studies have mainly focused on the changes in Pco2 and pH in isolation and have not evaluated the effects of Pco2 and pH in conjunction. To our knowledge, no study has investigated for differences in outcomes between compensated hypercapnia and hypercapnic acidosis. Given this limited data and lack of evaluation of the outcomes in combination of Pco2 and pH, we aimed to review the effects of compensated hypercapnia and hypercapnic acidosis during the first 24 hours of intensive care unit (ICU) admission on clinical outcomes in adult mechanically ventilated patients with cerebral injury caused by cardiac arrest, stroke, and traumatic brain injury.
Methods
We conducted a retrospective review of all patients who had cerebral injury and were mechanically ventilated during a 16-year period (January 2000 to December 2015) admitted to 167 ICUs in Australia and New Zealand. Data were collected from the Australian and New Zealand Intensive Care Society Adult Patient Database. The Australian and New Zealand Intensive Care Society Adult Patient Database is a high-quality database run by the Australian and New Zealand Intensive Care Society Center for Outcome and Resource Evaluation. It collates complete patient information that is required to calculate patient severity during the first 24 hours of ICU admission from more than 80% of ICUs across Australia and New Zealand as part of quality assurance and benchmarking process among participating ICUs. Ethics approval was obtained from Monash University research ethics committee. The ethics committee waived informed consent from the patients because the data were gathered as part of routine quality assurance benchmarking process for the participating ICUs.
Adult patients with cerebral injury receiving mechanical ventilation during the first 24 hours of their admission to the ICU were included in the study. Cerebral injury was diagnosed if the patients had admission diagnosis of 1 of 3 diagnostic categories: traumatic brain injury, cardiac arrest, or stroke (including intracerebral hemorrhage, subdural, subarachnoid hemorrhage, and ischemic stroke). For this analysis, we used the arterial blood gas that provided the highest scoring Acute Physiology, Age, Chronic Health Evaluation III subscore and as such is likely to have represented the worst pH/Pco2 combination in the first 24 hours of ICU admission. Patients were classified into 3 groups based on a combination of arterial pH and arterial carbon dioxide levels: normocapnia (Pco2, 35-45 mm Hg) and normal pH (7.35-7.45) (group 1), compensated hypercapnia (normal pH [7.35-7.45] with elevated carbon dioxide [Pco2 >45 mm Hg]) (group 2), and hypercapnic acidosis (Pco2 >45 mm Hg and pH <7.35) (group 3) during the first 24 hours of ICU stay. Patients with metabolic acidosis, metabolic alkalosis, and respiratory alkalosis were excluded because the focus of the study was to investigate the effects of compensated hypercapnia and hypercapnic acidosis on the outcomes after cerebral injury. Patients in group 1 were considered a reference group to which patients in group 2 and 3 were compared. The primary outcome measure included hospital mortality. Secondary outcome measures included ICU mortality, duration of ICU and hospital stay, and survival to discharge home.
All analysis was performed using SAS, version 9.4 (SAS Institute Inc). Data were initially assessed for normality. Group comparisons were made using χ2 tests for equal proportion, analysis of variance for normally distributed variables, and Kruskal-Wallis tests otherwise, with results reported as number values (percentages), means (SDs), and medians (interquartile range), respectively. Given the retrospective nature of this study, to account for differing patient characteristics, logistic regression models were constructed using all available baseline information that related to the patient (age, sex, and chronic comorbidities) or hospital (location, level, admission source, and time of admission) to identify each patient’s probability (propensity) of presenting to the ICU with either hypercapnic acidosis or compensated hypercapnia (eTables 1 and 2 in the Supplement). These models were constructed using both stepwise selection and backward elimination techniques, with only variables that were significant (P < .01) from both methods included. To investigate the independent effect of hypercapnia and hypercapnic acidosis on hospital mortality, hierarchical multivariable regression models were used using logistic regression for hospital death and Cox proportional hazards regression for time to death. These models adjusted for patient severity, propensity to be hypercapnic, propensity to be hypercapnic acidotic, baseline Glasgow Coma Scale (GCS) Score stratified into 3 groups (GCS score 3-7, 8-12, and 13-15), and year of admission, with patients nested in sites and sites treated as a random effect. Subgroup analysis relating to diagnosis, neurological severity (baseline GCS score), and operative status were performed using hierarchical multivariable logistic regression models. To determine whether the association between hypercapnic status and mortality differed according to the 3 diagnostic categories (diagnostic subgroup, neurological severity [baseline GCS score], or operative status), interaction terms with hypercapnic status were fitted. Duration of survivals have been presented as Kaplan-Meier curves with log-rank tests comparing equality of strata. Duration of stay variables (hospital and ICU length of stay) were log-transformed and analyzed using hierarchical mixed linear modeling, again adjusting for the covariates outlined here, with results presented as geometric means (95% CI). To account for survival bias, duration variables were further stratified by survival status. To facilitate a measure of patient severity independent of hypercapnia and neurological severity, each patient’s predicted risk of death was calculated in accordance with the Australia and New Zealand Risk of Death methods,14 with the components of pH, oxygen, and GCS score removed. Australia and New Zealand Risk of Death is an updated mortality prediction model specifically calibrated for use in Australia and New Zealand ICUs that has been derived from components of the Acute Physiology, Age, Chronic Health Evaluation II and III scoring systems, with additional diagnostic variables, and has been shown to have significantly better calibration and discrimination than Acute Physiology, Age, Chronic Health Evaluation III. To further ensure that our observed results were not driven by imbalances in patient severity, an additional matched sensitivity analysis was performed with patients from each of the 3 hypercapnic diagnostic categories matched for patient severity. Given the magnitude of the data set, to more closely align statistical and clinical significance, a 2-sided P value of .01 was used to indicate statistically significant results.
Results
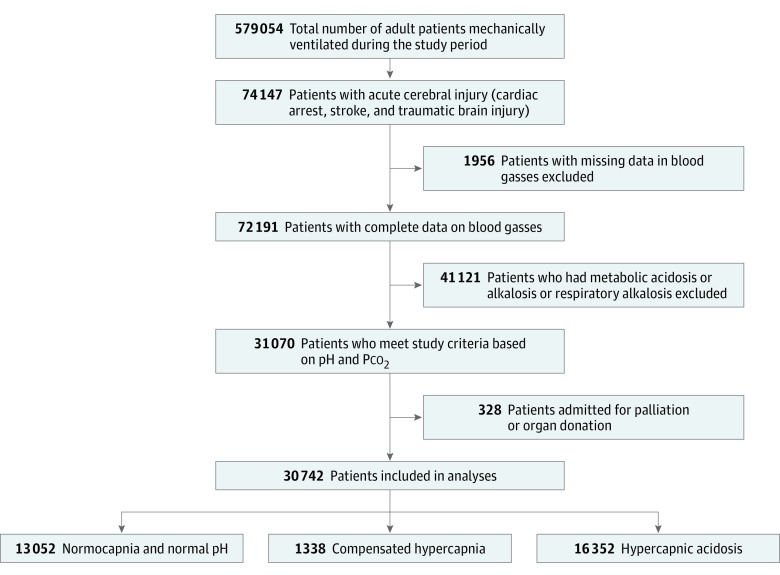
A total of 30 742 patients were included in the study (Figure 1). A comparison of demographics, comorbidities, physiological and laboratory variables, and ICU admission diagnostic category data are presented in Table 1. Patients with normocapnia and normal pH were younger and had lower comorbidities compared with other groups. Patients with compensated hypercapnia and hypercapnic acidosis differed from patients with normocapnia and normal pH levels in heart rate, blood pressure, and temperature, as well as renal and liver function test results during the first 24 hours of their ICU admission. Plasma glucose levels were higher in the hypercapnic acidosis group. Lower Pao2 and higher Pco2 levels were noted in patients with compensated hypercapnia and hypercapnic acidosis (Table 1). Acute Physiology, Age, Chronic Health Evaluation III scores and customized Australia and New Zealand Risk of Death were lower in patients with normocapnia and normal pH levels (Table 1). Unadjusted hospital mortality was higher, and discharge home was lower in patients with hypercapnic acidosis (Table 2). Patients with compensated hypercapnia had a higher discharge home rate compared with patients with hypercapnia acidosis or normocapnia and normal pH (Table 2). Unadjusted hospital mortality rates were highest in patients with hypercapnic acidosis in all 3 diagnostic categories (eFigures 1-4 in the Supplement and Table 2). Hospital mortality increased in patients with hypercapnic acidosis with increasing Pco2 (Figure 2). No such increased mortality was noted in patients with compensated hypercapnia. In patients with traumatic brain injury, lower hospital mortality was noted in patients with compensated hypercapnia (eFigure 4 in the Supplement and Table 2).
Figure 1. Study Profile.
Table 1. Comparisons of Demographics, Comorbidities, and Physiological and Laboratory Data.
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| Normocapnia and Normal pH (n = 13 052) |
Compensated Hypercapnia (n = 1338) |
Hypercapnic Acidosis (n = 16 352) |
||
| Age, y, mean (SD) | 53.1 (20.2) | 54.9 (19.9) | 56.2 (18.9) | <.001 |
| Men | 8949 (68.6) | 996 (74.4) | 11929 (73) | <.001 |
| Worst GCS score, mean (SD) | 8.15 (4.44) | 8.47 (4.66) | 6.27 (4.55) | <.001 |
| GCS score 3-7 | 6462 (50) | 630 (47) | 11125 (68) | <.001 |
| GCS score 8-12 | 3237 (25) | 398 (30) | 2925 (18) | <.001 |
| GCS score 13-15 | 3170 (24) | 290 (22) | 1935 (12) | <.001 |
| Missing data on GCS score | 183 (1.4) | 20 (1.5) | 367 (2.2) | <.001 |
| Comorbidities | ||||
| Chronic respiratory disease | 245 (1.9) | 72 (5.4) | 1119 (6.8) | <.001 |
| Chronic cardiovascular disease | 773 (5.9) | 123 (9.2) | 1620 (9.9) | <.001 |
| Chronic liver disease | 117 (0.9) | 22 (1.6) | 171 (1) | .03 |
| Chronic renal failure | 169 (1.3) | 25 (1.9) | 472 (2.9) | <.001 |
| Immunosuppression | 149 (1.1) | 18 (1.3) | 312 (1.9) | <.001 |
| Physiological data | ||||
| Highest body temperature, °C | 37.5 (0.9) | 37.6 (0.9) | 37 (1.4) | <.001 |
| Highest heart rate, bpm | 95.7 (22.8) | 98.5 (23.9) | 107 (25.7) | <.001 |
| Highest respiratory rate, PM | 19.1 (6.3) | 20.4 (7.1) | 21.4 (7.3) | <.001 |
| Highest mean arterial pressure, mm Hg | 105 (18) | 106 (19) | 101 (21) | <.001 |
| Laboratory data | ||||
| Lowest sodium, mEq/L | 138 (4.05) | 139 (4.59) | 138 (4.75) | <.001 |
| Highest, mEq/L | 4.19 (0.526) | 4.23 (0.582) | 4.69 (0.86) | <.001 |
| Lowest potassium plasma, mEq/L | 22.4 (2.9) | 25.3 (3.9) | 19.8 (4.8) | <.001 |
| Highest creatinine, mg/dL, median (IQR) | 0.85 (0.70-1.04) | 0.80 (0.74-1.17) | 1.18 (0.88-1.75) | <.001 |
| Worst inspired oxygen concentration, %/100 | 0.53 (0.24) | 0.548 (0.25) | 0.729 (0.26) | <.001 |
| Worst arterial oxygen partial pressure, mm Hg, median, (IQR) | 127 (92-205) | 107 (78-164) | 104 (76-174) | <.001 |
| Worst arterial CO2 partial pressure, mm Hg | 39 (2.8) | 52.2 (18.3) | 56.7 (14.8) | <.001 |
| Worst arterial pH | 7.4 (0.03) | 7.39 (0.03) | 7.19 (0.13) | <.001 |
| Highest hemoglobin, g/dL | 12.5 (2.0) | 12.6 (2.2) | 13.1 (2.5) | <.001 |
| Highest white blood cell count, /μL | 14 100 (7800) | 13 900 (11 500) | 17 800 (11 600) | <.001 |
| Lowest platelets, × 103/μL | 203 (79) | 201 (90) | 197 (88) | <.001 |
| Plasma albumin, mmol/L | 31.3 (5.9) | 30.9 (6.0) | 29.8 (6.9) | <.001 |
| Worst plasma bilirubin, mmol/L, median (IQR) | 13 (9-18) | 13 (9-18) | 12 (8-18) | <.001 |
| Plasma glucose, mg/dL | 161.08 (63.06) | 159.28 (64.86) | 209.01 (106.31) | <.001 |
| Severity of illness at ICU admission | ||||
| APACHE III score | 57.9 (28) | 61.8 (30.5) | 91.4 (37.3) | <.001 |
| ANZROD, % | 29.3 | 33.8 | 51.3 | <.001 |
| Diagnostic category | ||||
| Traumatic brain injury (n = 9507) | 4902 (37.6) | 482 (36) | 4123 (25.2) | <.001 |
| Cerebrovascular accidents (n = 9477) | 6219 (47.6) | 548 (41) | 2710 (16.6) | <.001 |
| Cardiac arrest (n = 11 785) | 1931 (14.8) | 308 (23) | 9519 (58.2) | <.001 |
Abbreviations: ANZROD, The Australian and New Zealand Risk of Death; APACHE, Acute physiology and chronic health evaluation; bpm, beats per minute; GCS, Glasgow Coma Scale; ICU, intensive care unit; IQR, interquartile range.
SI conversion factor: To convert creatine to millimoles per liter, multiply by 88.4; glucose to millimoles per liter, multiply by 0.0555; hemoglobin to grams per liter, multiply by 10; sodium to micromoles per liter, multiply by 1; potassium to millimoles per liter, multiply by 1; platelets to × 109 per liter, multiply by 1; white blood cell count to × 109 per liter, multiply by 0.001.
Table 2. Comparison of Outcomes in Patients With Cerebral Injury Based on Their pH and Carbon Dioxide (Unadjusted) and of Hospital Mortality (Unadjusted) Based on Diagnostic Categories.
| Variable | Normocapnia and Normal pH (n = 13052) |
Compensated Hypercapnia (n = 1338) |
Hypercapnic Acidosis (n = 16352) |
P Value |
|---|---|---|---|---|
| Status at discharge, No. (%) | ||||
| Died in hospital | 3614 (27.7) | 400 (29.9) | 8223 (50.3) | NA |
| Discharged home | 4757 (36.4) | 505 (37.7) | 5064 (31) | NA |
| Discharged to rehabilitation | 2224 (17) | 205 (15.3) | 1300 (8) | NA |
| Discharged to other hospital | 2368 (18.1) | 221 (16.5) | 1697 (10.4) | NA |
| Duration of stay | ||||
| Hospital LOS, d, median (IQR) | 8.9 (8.2-9.6) | 8 (7.2-8.8) | 7 (6.4-7.5) | <.001 |
| Hospital LOS, survivors, d, geometric mean (95% CI) | 10.9 (9.9-12) | 10 (8.9-11.2) | 10.6 (9.6-11.6) | .016 |
| Hospital LOS, deaths, d, geometric mean (95% CI) | 4.9 (4.5-5.4) | 4.3 (3.7-5) | 3.5 (3.2-3.9) | <.001 |
| ICU LOS, d, geometric mean (95% CI) | 3.1 (2.9-3.3) | 2.8 (2.5-3) | 2.8 (2.6-2.9) | <.001 |
| ICU LOS, survivors, d, geometric mean (95% CI) | 3.5 (3.3-3.8) | 3.2 (2.9-3.5) | 4.2 (3.9-4.5) | <.001 |
| ICU LOS, deaths, d, geometric mean (95% CI) | 2.5 (2.2-2.7) | 2.2 (1.9-2.6) | 1.7 (1.5-1.8) | <.001 |
| Diagnostic category, No./total No. (%) | ||||
| Traumatic brain injury (n = 9507) | 535/4908 (10.9) | 36/480 (7.5) | 852/4116 (20.7) | <.001 |
| Stroke (n = 9477) | 2321/6223 (37.3) | 234 /548(42.7) | 1589/2712 (58.6) | <.001 |
| Cardiac arrest (n = 11758) | 758/1929 (39.3) | 130/308 (42.2) | 5782/9526 (60.7) | <.001 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; LOS, length of stay, NA, not applicable.
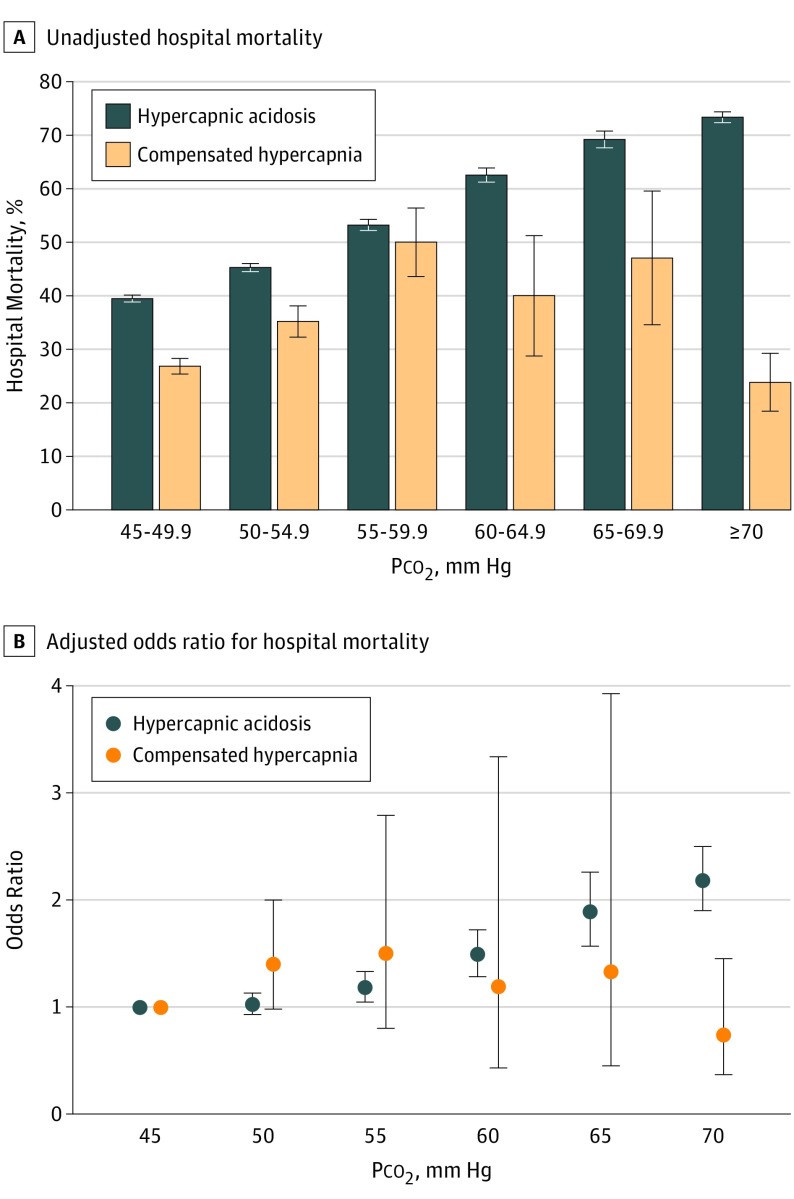
Figure 2. Hospital Mortality With Increasing Partial Pressure of Carbon Dioxide in Patients With Hypercapnic Acidosis and Compensated Hypercapnia.
Hospital mortality with increasing partial pressure of carbon dioxide (Pco2) in patients with hypercapnic acidosis and compensated hypercapnia unadjusted (A) and adjusted odds ratio for hospital mortality (B). Error bars represent 95% CI. P = .008 for interaction between hypercapnic status and Pco2.
Multivariable analysis using both logistic regression (hospital mortality) and Cox proportional hazards regression (time to death) confirmed patients with hypercapnic acidosis had an increased risk of death compared with patients with normocapnia and normal pH levels (Table 3). While this increased risk of death was evident across the 3 diagnostic categories, there was a statistically significant interaction between hypercapnic status and diagnosis indicating that the increase in risk of hospital mortality for patients with hypercapnic acidosis did differ according to diagnostic category, with the greatest risk for patients with cardiac arrest (odds ratio [OR], 1.51; 95% CI, 1.34-1.71); stroke (OR, 1.43; 95% CI, 1.27-1.6); and traumatic brain injury (OR, 1.22; 95% CI, 1.06-1.42; P <.001) (Table 3). Patients with compensated hypercapnia did not have an increased risk of hospital mortality compared with patients with normocapnia and normal pH levels (Table 3). These findings were subsequently confirmed from sensitivity analysis matching patients for baseline severity eTable 3 in the Supplement.
Table 3. Adjusted Hospital Mortality (Logistic Regression and Cox-Proportional Hazards) in Patients With Cerebral Injury.
| Diagnostic Category | Logistic Regression Analysisa | Cox Proportional Hazardsb | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | HR (95% CI) | P Value | |
| Cardiac arrest | ||||
| Hypercapnic group | ||||
| Normocapnia and normal pH | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Compensated hypercapnia vs normocapnia and normal pH | 1.04 (0.78-1.38) | 0.98 (0.81-1.19) | ||
| Hypercapnic acidosis vs normocapnia and normal pH | 1.51 (1.34-1.71) | 1.23 (1.14-1.34) | ||
| Stroke | ||||
| Hypercapnic group | ||||
| Normocapnia and normal pH | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Compensated hypercapnia vs normocapnia and normal pH | 0.98 (0.8-1.21) | 1.04 (0.9-1.2) | ||
| Hypercapnic acidosis vs normocapnia and normal pH | 1.43 (1.27-1.6) | 1.3 (1.21-1.4) | ||
| Traumatic brain injury | ||||
| Hypercapnic group | ||||
| Normocapnia and normal pH | 1 [Reference] | .004 | 1 [Reference] | .07 |
| Compensated hypercapnia vs normocapnia and normal pH | 0.74 (0.5-1.11) | 0.85 (0.6-1.21) | ||
| Hypercapnic acidosis vs normocapnia and normal pH | 1.22 (1.06-1.42) | 1.13 (1-1.27) | ||
Abbreviations: HR, hazard ratio; OR, odds ratio.
P value for interaction between diagnostic group and hypercapnic group: P < .001.
P value for interaction between diagnostic group and hypercapnic group: P < .001.
In subgroup analysis based on baseline GCS score, patients with hypercapnic acidosis had consistent increased risk of hospital mortality for patients with cardiac arrest and stroke, with no significant evidence that the nature of the association between hypercapnic status and mortality differed according to baseline GCS score (eTable 4 in the Supplement). Patients with traumatic brain injury with hypercapnic acidosis had increased risk of hospital mortality (compared with normocapnia and normal pH levels) in patients with GCS score between 3-7; this was not the case for those with GCS score 8-12 and 13-15, with the interaction between hypercapnic status and GCS score not statistically significant (eTable 4 in the Supplement).
In further subgroups, analyses based on diagnostic subgroups (ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, subdural or extradural hemorrhage, isolated traumatic brain injury, and traumatic brain injury associated with other injuries), patients with hypercapnic acidosis consistently had an increase in risk (compared with normocapnia and normal pH levels) and there were no significant interactions among patients with stroke or patients with traumatic brain injury to indicate that the nature of the association between hypercapnic status and mortality differed according to diagnostic subgroup (eTable 5 in the Supplement). Patients with compensated hypercapnia had no increased risk of hospital mortality compared with patients who had normocapnia and normal pH levels. Finally, in the subgroup analyses, based on the requirement of surgical intervention, patients with hypercapnic acidosis retained the highest risk of hospital mortality with no significant interactions between the requirement or nonrequirement of surgical intervention in patients with stroke or traumatic brain injury (eTable 6 in the Supplement).
The adjusted odds ratio of hospital mortality significantly differed between patients who had hypercapnic acidosis and compensated hypercapnia with increasing Pco2 (Figure 2). In patients with hypercapnic acidosis, the adjusted odds ratio of hospital mortality increased with increasing Pco2. In patients with compensated hypercapnia, the adjusted odds of hospital mortality did not change with increasing Pco2 (Figure 2).
Discussion
The main results of this study show that hospital mortality in patients with cerebral injury is higher in patients with hypercapnic acidosis compared with patients who had normocapnia or compensated hypercapnia. This increased mortality was consistent across all the 3 diagnostic categories and persisted after adjusting for the variables that principally contribute to hospital mortality. While mortality increased with increasing Pco2 in patients with hypercapnic acidosis, it did not increase in patients with compensated hypercapnia.
The effects of hypercapnia in acute cerebral injury have been described in several studies.15,16,17,18,19,20 Most suggest normocapnia after cerebral injury is associated with better clinical outcomes, and hypocapnia and hypercapnia are associated with poor clinical outcomes.2,6,15,16,19 However, in patients with cerebral injury secondary to cardiac arrest, there is some evidence to suggest that mild hypercapnia may be beneficial. Experimental studies have demonstrated mild to moderate hypercapnia to be neuroprotective after transient global cerebral ischemia reperfusion injury, while severe hypercapnia resulted in worsening of cerebral edema.20 The study by Schneider et al5 showed that in patients admitted to ICUs after cardiac arrest, presence of hypercapnia during the first 24 hours of ICU stay was associated with a greater likelihood of discharge home among survivors.5 A pilot randomized clinical trial showed induction of mild hypercapnia after cardiac arrest reduced neuron-specific enolase, a biomarker of cerebral injury, compared with normocapnia.17 Hypercapnia has been shown to increase cerebral blood flow in patients who were successfully resuscitated after cardiac arrest and in patients with subarachnoid hemorrhage.9,18,21 Hypercapnia was also shown to have anticonvulsive22 and antiinflammatory properties23 that may be helpful to improve neurological recovery after cerebral injury. The results of our study show that compensated hypercapnia was not associated with an increase in adverse outcomes and that patient outcomes are comparable with those in patients with normocapnia and normal pH levels. However, in our study, we did not find improved outcomes in the compensated hypercapnia group compared with the normocapnic group. Further, prospective studies will be required to evaluate whether compensated hypercapnia may be beneficial in a subcategory of patients with cerebral injury.
The effect of pH on clinical outcomes was predominantly studied in patients with out-of-hospital cardiac arrest.11,24 A pH of at least 7.05 was found to be an independent predictor for a favorable outcome.11 However, this study did not differentiate acidosis caused by carbon dioxide (hypercapnic acidosis) and metabolic acidosis. The study by Takaki et al24 investigating the predictors of neurologic recovery in patients resuscitated after cardiac arrest found that blood pH had a stronger predictive power than CO2 in patients after out-of-hospital cardiac arrest.24
In patients with ischemic stroke, acidosis is common and has been demonstrated to be associated with toxic calcium influx into the cell and programmed cell death25 and poorer outcomes.12,13 Although our data do not provide information about the level of acidosis at the time of onset of the acute cerebral injury, prolonged acidosis into the first 24 hours of intensive care admission is likely to represent significant exposure to this mechanism for neuronal influx of calcium ions and higher levels of consequent injury caused by the excitotoxic action of glutamate release triggered by high intracellular levels of calcium 2.26 Our data are also limited with respect to time at onset or pattern of brain injury for the patients with cardiac arrest and brain hemorrhage. However, although not reported in the literature, it is possible that similar mechanisms of secondary injury might be active in the cardiac arrest group experiencing prolonged periods of critically low brain blood flow and acidosis secondary to anaerobic metabolism and in the hemorrhage group in the context of mass effect or vasospasm causing focal ischemia.
Strengths
This study has several strengths. First, it involved more than 30 000 mechanically ventilated patients with cerebral injury from 167 ICUs (constituting about 80% of ICUs) in 2 countries, making its findings highly generalizable for all ICUs in Australia and New Zealand. This study specifically aimed to delineate the effects of hypercapnia with and without the effects of concurrent acidosis, which, to our knowledge, has not been studied in any earlier studies. It is likely that our findings have external validity in other developed countries with intensive care practices similar to Australia and New Zealand. To our knowledge, this is the largest study relating hypercapnic status to mortality published thus far, and the large sample size of our study enables identification of small but significant differences in outcomes. The Australian and New Zealand Intensive Care Society Adult Patient Database is recognized as a high-quality clinical registry with excellent data quality. Analyses arising from this data have been published in multiple high-impact journals.27,28 Data collection from the participating ICUs is robust and quality-controlled, with an established data dictionary to ensure uniformity and accuracy of the data collected.
Limitations
Our study is a retrospective study with inherent limitations. The worst value of Adult Patient Database Pco2 and pH used in classifying patients was limited to the 24 hours after ICU admission. Thus, patients may have had more deranged blood gasses (abnormal Pco2 and pH) prior to ICU admission or after 24 hours after ICU admission, and the absence of this data precluded evaluation of association of hypercapnic status before or after 24 hours of ICU admission on hospital mortality. However, most studies published thus far have also evaluated the association of hypercapnia on clinical outcomes using data during the first 24 hours of patient’s hospital presentation. Our results are therefore comparable with existing literature.2,5,11,24,29 Some patients presenting with compensated hypercapnia and hypercapnic acidosis may have had renal compensation prior to the ICU admission. From the data available, it was not possible to evaluate the proportion of patients with prior renal compensation before admission to ICU with cerebral injury or the etiology of hypercapnic acidosis that could have contributed to the increased mortality. We also did not have data on physiological indices, such as cerebral blood flow or intracranial pressure measurement, and neuroimaging data that could have helped in understanding the possible mechanisms in the increased mortality noted in patients with hypercapnic acidosis patients. The outcomes of patients with cerebral injury is known to be dependent on several factors such as duration and type of cardiac arrest,30 pattern or severity of brain injury, volume of intracerebral blood, GCS score, and computed tomography characteristics.31,32 Given the retrospective nature of our study, we did not have data on some of these variables that could have further aided in understanding the association of compensated hypercapnia and hypercapnic acidosis on hospital mortality. Furthermore, retrospective studies such as this could have unknown confounders that may have accounted for the observed differences in the outcomes. Nevertheless, the current ICU severity scoring system used in Australia and New Zealand14 is known to have excellent calibration and discrimination in intensive care patients, particularly in patients with cerebral injuries. This may have compensated for the lack of availability of some known severity markers.
Conclusions
Hypercapnic acidosis was associated with increased risk of hospital mortality in patients with cerebral injury. Hypercapnia when compensated to normal pH during the first 24 hours of ICU admission may not be harmful in mechanically ventilated patients with cerebral injury.
eFigure 1. Kaplan-Meier Survival Curves All Cerebral Injury Patients
eFigure 2. Kaplan-Meier Survival Curves for Cardiac Arrest Patients
eFigure 3. Kaplan-Meier Survival Curves for Stroke Patients
eFigure 4. Kaplan-Meier Survival Curves for Traumatic Brain Injury Patients
eTable 1. Prediction Model to Determine Each Patient’s Probability to Present to Intensive Care Units With Hypercapnic Acidosis
eTable 2. Prediction Model to Determine Each Patient’s Probability to Present to Intensive Care Units With Compensated Hypercapnia
eTable 3. Adjusted Hospital Mortality for Cohort of Cerebral Injury Patients Matched for Admission Severity Scores
eTable 4. Adjusted Hospital Mortality (logistic regression) in Patients with Cerebral Injury Stratified Based on Glasgow Coma Scale (GCS)
eTable 5. Adjusted Hospital Mortality (logistic regression) in Subgroups of Cerebral Injury Based on Diagnostic Subgroups
eTable 6. Adjusted Hospital Mortality (logistic regression) in Subgroups of Cerebral Injury Patients Who Required Neurosurgical Intervention and Who Did Not Require Neurosurgical Intervention
References
- 1.Maas AI, Dearden M, Teasdale GM, et al. ; European Brain Injury Consortium . EBIC-guidelines for management of severe head injury in adults. Acta Neurochir (Wien). 1997;139(4):286-294. [DOI] [PubMed] [Google Scholar]
- 2.Davis DP, Idris AH, Sise MJ, et al. Early ventilation and outcome in patients with moderate to severe traumatic brain injury. Crit Care Med. 2006;34(4):1202-1208. [DOI] [PubMed] [Google Scholar]
- 3.Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127(21):2107-2113. [DOI] [PubMed] [Google Scholar]
- 4.Roberts BW, Kilgannon JH, Chansky ME, Trzeciak S. Association between initial prescribed minute ventilation and post-resuscitation partial pressure of arterial carbon dioxide in patients with post-cardiac arrest syndrome. Ann Intensive Care. 2014;4(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider AG, Eastwood GM, Bellomo R, et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation. 2013;84(7):927-934. [DOI] [PubMed] [Google Scholar]
- 6.Warner KJ, Cuschieri J, Copass MK, Jurkovich GJ, Bulger EM. The impact of prehospital ventilation on outcome after severe traumatic brain injury. J Trauma. 2007;62(6):1330-1336. [DOI] [PubMed] [Google Scholar]
- 7.Christensen MS, Paulson OB. Prolonged artificial hyperventilation in severe cerebral apoplexy. Clinical results and cerebrospinal fluid findings in a controlled study. Eur Neurol. 1972;8(1):137-141. [DOI] [PubMed] [Google Scholar]
- 8.Solaiman O, Singh JM. Hypocapnia in aneurysmal subarachnoid hemorrhage: incidence and association with poor clinical outcomes. J Neurosurg Anesthesiol. 2013;25(3):254-261. [DOI] [PubMed] [Google Scholar]
- 9.Westermaier T, Stetter C, Kunze E, et al. Controlled transient hypercapnia: a novel approach for the treatment of delayed cerebral ischemia after subarachnoid hemorrhage? J Neurosurg. 2014;121(5):1056-1062. [DOI] [PubMed] [Google Scholar]
- 10.Gupta AK, Zygun DA, Johnston AJ, et al. Extracellular brain pH and outcome following severe traumatic brain injury. J Neurotrauma. 2004;21(6):678-684. [DOI] [PubMed] [Google Scholar]
- 11.Momiyama Y, Yamada W, Miyata K, et al. Prognostic values of blood pH and lactate levels in patients resuscitated from out-of-hospital cardiac arrest. Acute Med Surg. 2017;4(1):25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsura K, Ekholm A, Asplund B, Siesjö BK. Extracellular pH in the brain during ischemia: relationship to the severity of lactic acidosis. J Cereb Blood Flow Metab. 1991;11(4):597-599. [DOI] [PubMed] [Google Scholar]
- 13.Nedergaard M, Goldman SA, Desai S, Pulsinelli WA. Acid-induced death in neurons and glia. J Neurosci. 1991;11(8):2489-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul E, Bailey M, Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand Risk of Death model. J Crit Care. 2013;28(6):935-941. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie N, Williams TA, Tohira H, Ho KM, Finn J. A systematic review and meta-analysis of the association between arterial carbon dioxide tension and outcomes after cardiac arrest. Resuscitation. 2017;111:116-126. [DOI] [PubMed] [Google Scholar]
- 16.Roberts BW, Karagiannis P, Coletta M, Kilgannon JH, Chansky ME, Trzeciak S. Effects of PaCO2 derangements on clinical outcomes after cerebral injury: A systematic review. Resuscitation. 2015;91:32-41. [DOI] [PubMed] [Google Scholar]
- 17.Eastwood GM, Schneider AG, Suzuki S, et al. Targeted therapeutic mild hypercapnia after cardiac arrest: a phase II multi-centre randomised controlled trial (the CCC trial). Resuscitation. 2016;104:83-90. [DOI] [PubMed] [Google Scholar]
- 18.Vaahersalo J, Bendel S, Reinikainen M, et al. ; FINNRESUSCI Study Group . Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurologic outcome. Crit Care Med. 2014;42(6):1463-1470. [DOI] [PubMed] [Google Scholar]
- 19.Warner KJ, Cuschieri J, Copass MK, Jurkovich GJ, Bulger EM. Emergency department ventilation effects outcome in severe traumatic brain injury. J Trauma. 2008;64(2):341-347. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, Cao B, Niu L, et al. Effects of permissive hypercapnia on transient global cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2010;112(2):288-297. [DOI] [PubMed] [Google Scholar]
- 21.Eastwood GM, Tanaka A, Bellomo R. Cerebral oxygenation in mechanically ventilated early cardiac arrest survivors: the impact of hypercapnia. Resuscitation. 2016;102:11-16. [DOI] [PubMed] [Google Scholar]
- 22.Tolner EA, Hochman DW, Hassinen P, et al. Five percent CO2 is a potent, fast-acting inhalation anticonvulsant. Epilepsia. 2011;52(1):104-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Croinin D, Ni Chonghaile M, Higgins B, Laffey JG. Bench-to-bedside review: permissive hypercapnia. Crit Care. 2005;9(1):51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takaki S, Kamiya Y, Tahara Y, Tou M, Shimoyama A, Iwashita M. Blood pH is a useful indicator for initiation of therapeutic hypothermia in the early phase of resuscitation after comatose cardiac arrest: a retrospective study. J Emerg Med. 2013;45(1):57-64. [DOI] [PubMed] [Google Scholar]
- 25.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Duan B, Wang DG, et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48(4):635-646. [DOI] [PubMed] [Google Scholar]
- 27.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629-1638. [DOI] [PubMed] [Google Scholar]
- 28.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308-1316. [DOI] [PubMed] [Google Scholar]
- 29.Bennett KS, Clark AE, Meert KL, et al. ; Pediatric Emergency Care Medicine Applied Research Network . Early oxygenation and ventilation measurements after pediatric cardiac arrest: lack of association with outcome. Crit Care Med. 2013;41(6):1534-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sathianathan K, Tiruvoipati R, Vij S. Prognostic factors associated with hospital survival in comatose survivors of cardiac arrest. World J Crit Care Med. 2016;5(1):103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987-993. [DOI] [PubMed] [Google Scholar]
- 32.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329-337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Kaplan-Meier Survival Curves All Cerebral Injury Patients
eFigure 2. Kaplan-Meier Survival Curves for Cardiac Arrest Patients
eFigure 3. Kaplan-Meier Survival Curves for Stroke Patients
eFigure 4. Kaplan-Meier Survival Curves for Traumatic Brain Injury Patients
eTable 1. Prediction Model to Determine Each Patient’s Probability to Present to Intensive Care Units With Hypercapnic Acidosis
eTable 2. Prediction Model to Determine Each Patient’s Probability to Present to Intensive Care Units With Compensated Hypercapnia
eTable 3. Adjusted Hospital Mortality for Cohort of Cerebral Injury Patients Matched for Admission Severity Scores
eTable 4. Adjusted Hospital Mortality (logistic regression) in Patients with Cerebral Injury Stratified Based on Glasgow Coma Scale (GCS)
eTable 5. Adjusted Hospital Mortality (logistic regression) in Subgroups of Cerebral Injury Based on Diagnostic Subgroups
eTable 6. Adjusted Hospital Mortality (logistic regression) in Subgroups of Cerebral Injury Patients Who Required Neurosurgical Intervention and Who Did Not Require Neurosurgical Intervention