Key Points
Question
Is daily antituberculosis therapy superior to thrice-weekly dosing in reducing failures and acquired rifampicin resistance among HIV-positive patients with culture-confirmed pulmonary tuberculosis in the era of antiretroviral therapy?
Findings
In this randomized clinical trial including 331 patients, favorable responses were 91% vs 77%, while hepatotoxic effects occurred in 9% vs 2% with daily and intermittent antituberculosis therapy regimens, respectively. Acquired rifampicin resistance emerged only in the group receiving intermittent antituberculosis therapy, despite antiretroviral therapy.
Meaning
Daily administration of antituberculosis therapy resulted in higher cure rates and prevented acquired rifampicin resistance compared with a thrice-weekly regimen, but with slightly higher hepatotoxicity, which was manageable under trial conditions.
This randomized clinical trial tests the safety and efficacy of daily vs intermittent antituberculosis therapy in HIV-positive patients.
Abstract
Importance
The benefit of daily over thrice-weekly antituberculosis therapy among HIV-positive patients with pulmonary tuberculosis (TB) who are receiving antiretroviral therapy remains unproven.
Objective
To compare the efficacy and safety of daily, part-daily, and intermittent antituberculosis therapy regimens in the treatment of HIV-associated pulmonary TB.
Design, Setting, and Participants
This open-label, randomized clinical trial was conducted by the National Institute for Research in Tuberculosis, south India. Adults infected with HIV with newly diagnosed, culture-positive, pulmonary TB were enrolled between September 14, 2009, and January 18, 2016.
Interventions
Patients were randomized to daily, part-daily, and intermittent antituberculosis therapy regimens, stratified by baseline CD4 lymphocyte count and sputum smear grade. Antiretroviral therapy was initiated as per national guidelines. Clinical and sputum microbiological examinations of patients were performed monthly until 18 months after randomization. Adverse events were recorded using standard criteria.
Main Outcomes and Measures
The primary outcome was favorable response, defined as treatment completion with all available sputum cultures negative for Mycobacterium tuberculosis during the last 2 months of treatment. Unfavorable responses included treatment failures, dropouts, deaths, and toxic effects among regimens.
Results
Of 331 patients (251 [76%] male; mean [SD] age, 39 [9] years; mean [SD] HIV viral load, 4.9 [1.2] log10 copies/mL; and median [interquartile range] CD4 lymphocyte count, 138 [69-248] cells/μL), favorable responses were experienced by 91% (89 of 98), 80% (77 of 96), and 77% (75 of 98) in the daily, part-daily, and intermittent regimens, respectively. With the difference in outcome between daily and intermittent regimens crossing the O’Brien-Fleming group sequential boundaries and acquired rifampicin resistance emergence (n = 4) confined to the intermittent group, the data safety monitoring committee halted the study. A total of 18 patients died and 18 patients dropped out during the treatment period in the 3 regimens. Six, 4, and 6 patients in the daily, part-daily, and intermittent regimens, respectively, had TB recurrence.
Conclusions and Relevance
Among HIV-positive patients with pulmonary TB receiving antiretroviral therapy, a daily anti-TB regimen proved superior to a thrice-weekly regimen in terms of efficacy and emergence of rifampicin resistance.
Trial Registration
clinicaltrials.gov Identifier: NCT00933790
Introduction
Globally, tuberculosis (TB) remains the leading cause of morbidity and mortality among individuals infected with HIV. When this trial commenced in 2009, World Health Organization (WHO) guidelines recommended daily or part-daily (daily in the intensive phase alone) antituberculosis therapy (ATT) for TB, while the Revised National TB Control Program in India used the thrice-weekly regimen until 2016. With few trial data from head-to-head comparison of these regimens among HIV-positive patients with TB, there was no evidence to guide treatment. Trials using intermittent (thrice-weekly) ATT, conducted at the National Institute for Research in Tuberculosis (NIRT) in India, reported cure rates of 83% and 90% among HIV-positive patients with TB, before and after the era of antiretroviral therapy (ART), compared with 96% among HIV-negative patients with TB in similar settings.
Acquired rifampicin resistance (ARR), a phenomenon observed in HIV-positive patients with TB treated with once-weekly, twice-weekly, and thrice-weekly rifampicin-based intermittent regimens, was rarely encountered in HIV-negative patients with TB. A cross-protocol analysis studying the impact of HIV and ART on ARR among patients with pulmonary TB receiving intermittent ATT revealed that HIV infection per se (odds ratio [OR], 2.02) and pretreatment isoniazid resistance (OR, 9.97) significantly contributed to ARR, with ART reducing but not eliminating the trend. Li et al demonstrated that intermittent ATT dosing after the intensive phase in HIV-TB coinfection did not contribute to ARR, making a part-daily regimen a feasible alternative to a fully daily regimen. The meta-analysis by Khan et al concluded that a daily intensive phase of ATT could reduce TB failures and prevent ARR in HIV-positive patients with TB. However, they noted the absence of any randomized clinical trial (RCT) addressing the question of dosing schedules on TB treatment outcomes in HIV. Hence, an RCT was conducted comparing the efficacy of daily, part-daily, and intermittent (thrice-weekly) TB regimens in reducing failures and emergence of ARR among HIV-positive patients from south India newly diagnosed with sputum culture–confirmed, rifampicin-sensitive TB.
Methods
Study Design and Overview
This open-label, parallel-group, active comparator RCT was conducted at centers of NIRT in Chennai, Vellore, and Madurai, south India, between September 14, 2009, and January 18, 2016, after obtaining Institutional Ethics Committee approval and written informed consent from all study participants (full protocol in Supplement 1). Interim analysis was planned when 33%, 66%, and 100% of enrolled patients had their outcomes available, applying the O’Brien-Fleming stopping rules.
Participants
Adults 18 years or older who were HIV positive and newly diagnosed with pulmonary TB by either a smear test or Xpert MTB/RIF test (Cepheid) were enrolled. Patients taking protease inhibitor–based ART, with known hypersensitivity or resistance to rifampicin, pregnant or lactating women, those with major psychiatric illness, and those who were moribund or had significant liver or renal function abnormalities at baseline were excluded (eAppendix in Supplement 2).
Investigations
Testing for HIV (2 separate third-generation rapid tests) was performed at baseline, adhering to national guidelines. Four sputum samples collected before treatment, 3 during treatment, and 2 during follow-up were examined for acid-fast bacilli by fluorescence microscopy, processed by the modified Petroff method, and cultured on Lowenstein-Jensen medium for Mycobacterium tuberculosis detection. Drug susceptibility testing was performed using the minimal inhibitory concentration method for isoniazid , rifampicin, and ethambutol and the resistance ratio method for streptomycin. High-performance liquid chromatography was used for mycobacterial species identification. Baseline cultures were stored for subsequent DNA fingerprinting, for comparison of strains at the time of failure (eAppendix in Supplement 2). Chest radiographs were interpreted by a panel of at least 2 chest physicians and a consensus opinion taken.
Complete blood cell count (automated hematology analyzer; ABX; HORIBA), liver and renal function tests, random plasma glucose (automated analyzer; Olympus Corp), CD4 lymphocyte count (FACScount flow cytometer; Becton Dickinson), and plasma HIV RNA load (Amplicor automated viral load monitor; Roche) measurements were done before treatment, at the end of the intensive phase, at treatment completion, and whenever clinically indicated.
Randomization
Patients fulfilling the clinical and laboratory criteria were randomly allocated 1:1:1 to the 3 ATT regimens, using a permuted block randomization scheme, with the treatment assignment list contained in concealed envelopes (eAppendix in Supplement 2). Stratification was based on a baseline CD4 lymphocyte count of less than 150 cells/μL vs greater than or equal to 150 cells/μL and sputum smear grading of 0 and 1+ vs 2+ and 3+.
Intervention
The 3 ATT regimens were (1) daily (daily in both intensive and continuation phases)—2EHRZ7/4HR7; (2) part-daily (daily intensive phase and intermittent continuation phase)—2EHRZ7/4HR3; and (3) intermittent (thrice weekly throughout)—2EHRZ3/4HR3 (eAppendix in Supplement 2).
The regimens were given under direct observation during weekdays and self-administered on weekends and holidays, supplemented with pyridoxine, 100 mg, and a tablet of co-trimoxazole double strength daily unless clinically contraindicated. Patients who had never received ART were initiated on lamivudine and efavirenz along with 1 of 3 drugs (zidovudine, stavudine, or tenofovir) between 2 and 8 weeks after ATT (eAppendix in Supplement 2), as per prevailing guidelines.
Outcome Measures
The primary outcome measure was proportion of patients with a favorable response at the end of ATT, defined as treatment completion accompanied by negative sputum cultures (n = 6) collected during the last 2 months of treatment. Unfavorable responses included (1) bacteriological failures (with or without emergence of ARR) and clinical failures (mandating change or restart of regimen, (2) deaths (all-cause mortality), except unnatural death, (3) toxic effects necessitating permanent substitution of allocated regimen, and (4) patients who dropped out (irretrievable >6 weeks). Surveillance for the primary outcome was done through a detailed clinical and sputum examination, performed monthly or when clinically indicated, for either persistence or recrudescence of TB symptoms or signs. The team performed appropriate evaluation and management, and outcomes were classified accordingly (eAppendix in Supplement 2). Treatment-emergent adverse drug reactions were reported as per modified Division of AIDS criteria. Similarly, recurrences were classified as bacteriological or clinical (eAppendix in Supplement 2). Retrieval mechanisms were inbuilt for effective case holding (eAppendix in Supplement 2). An independent end point review committee, blind to the assigned regimen, adjudicated the outcomes after scrutinizing patient details.
Statistical Analysis
Sample Size
We hypothesized that a daily and a part-daily regimen would have a 95% rifampicin resistance–free survival compared with 80% with the intermittent regimen (superiority design). With a power of 80%, a type I error of 5%, and increasing the sample size by 20% to compensate for loss due to death, default, and exclusions after randomization, we arrived at a sample size of 420 (140 patients in each group).
Comparisons and Analyses
The primary comparisons for efficacy were between daily vs intermittent regimens and part-daily vs intermittent regimens; the contrast was fixed prior to trial initiation. The modified intent-to-treat analysis was used, including all randomized patients except those with pretreatment cultures that were negative for M tuberculosis, were rifampicin resistant, or grew nontuberculous mycobacteria. To adjust for multiple comparisons, the Bonferroni correction was applied, taking a two-sided P value of .025 as the significance level. The per-protocol analysis was simultaneously performed, including patients in modified intent-to-treat analysis, but with an adherence of greater than 80% of scheduled drug intake. The χ2 test was used for proportions, and the t test and Wilcoxon test were used for analyzing continuous variables. Kaplan-Meier mean survival estimates for time to an unfavorable event were calculated, and the log-rank test was used for significance testing between regimens. A Cox hazards model was built, taking into account all potential risk factors for an unfavorable response, that could simultaneously analyze categorical and continuous variables, accounting for individual period of observation, and provide the hazard rates and ratios for each of the risk factors. Furthermore, the γ frailty model was used to measure heterogeneity of baseline parameters among patients allocated to the 3 regimens. We used SPSS statistical software, version 20.0 (IBM) for all analyses.
Results
The data safety monitoring committee, after careful deliberation of the findings of the second interim analysis, when 331 patients had been recruited, halted the trial and permitted publication of the findings; preliminary results were presented at the AIDS 2016 conference.
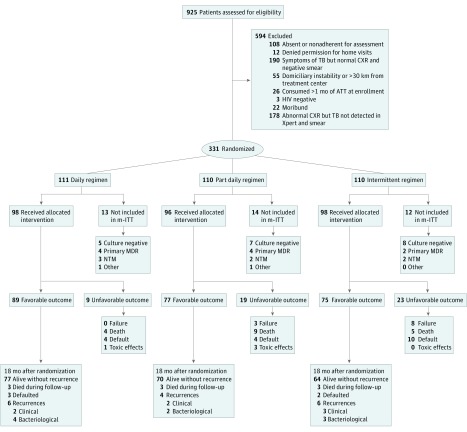
Between September 14, 2009, and January 18, 2016, 925 patients were screened and 331 were randomly allocated to daily (n = 111), part-daily (n = 110), and intermittent (n = 110) ATT regimens (Figure 1). Of the 331 patients allocated, 251 (76%) were male; mean (SD) age was 39 (9) years; mean (SD) HIV viral load was 4.9 (1.2) log10 copies/mL, and median (interquartile range [IQR]) CD4 lymphocyte count was 138 (69-248) cells/μL. Baseline characteristics were comparable across the 3 groups of patients (Table 1).
Figure 1. Flow of Patients From Screening Until 18 Months After Randomization.
Adults aged 18 years or older who were HIV positive and newly diagnosed with pulmonary tuberculosis (TB) were enrolled. Patients fulfilling the clinical and laboratory criteria were randomly allocated to 1 of 3 regimens. ATT indicates antituberculosis therapy; CXR, radiograph of the chest; MDR, multidrug-resistant; mITT, modified intent-to-treat analysis; NTM, nontuberculous mycobacteria; Xpert, Xpert MTB/RIF test (Cepheid).
Table 1. Baseline Characteristics of Patients Randomized to 3 Antituberculosis Therapy Regimens.
| Characteristic | Regimen | ||||
|---|---|---|---|---|---|
| Daily (n = 111) | Part-Daily (n = 110) | Intermittent (n = 110) | |||
| Demographic characteristics | |||||
| Age, mean (SD), y | 38 (9) | 39 (9) | 40 (9) | ||
| Male, No. (%) | 86 (77) | 81 (74) | 84 (76) | ||
| Weight, mean (SD), kg | 42.78 (8.28) | 42.60 (7.54) | 44.50 (7.47) | ||
| BMI, mean (SD) | 16.79 (2.71) | 16.87 (2.66) | 17.36 (2.74) | ||
| Site of lesion, No. | |||||
| Pulmonary | 70 | 77 | 68 | ||
| Pulmonary with extrapulmonary | 41 | 33 | 42 | ||
| Opportunistic infections at baseline, No. | 37 | 36 | 32 | ||
| Pneumocystitis carinii pneumonia | 8 | 2 | 4 | ||
| Oral candidiasis | 15 | 23 | 19 | ||
| Herpes zoster or herpes simplex | 6 | 8 | 7 | ||
| Hepatitis B and C | 8 | 2 | 3 | ||
| Mycobacterium avium intracellulare | 2 | 1 | 1 | ||
| History of alcohol intake, No. | 54 | 46 | 52 | ||
| Smoker, No. | 40 | 37 | 47 | ||
| Transmission, No. | |||||
| Heterosexual | 90 | 87 | 88 | ||
| Homosexual | 1 | 2 | 3 | ||
| Intravenous drug user | 7 | 2 | 3 | ||
| Others | 7 | 8 | 8 | ||
| Biochemical and hematological parameters | |||||
| RBC count, median (range), ×106 cells/μL | 3.55 (3.01-4.12) | 3.54 (2.98-4.13) | 3.62 (3.14-4.15) | ||
| WBC count, median (range), ×103 cells/μL | 6.4 (4.4-8.9) | 6.7 (4.7-9.5) | 6.8 (4.9-8.3) | ||
| Platelets, median (IQR), ×103 cells/μL | 3.09 (2.36-4.07) | 3.12 (1.94-4.24) | 3.15 (2.44-4.23) | ||
| Hemoglobin, mean (SD), g/dL | 9.81 (2.14) | 9.43 (2.08) | 9.88 (2.05) | ||
| Hematocrit, mean (SD), % | 30.07 (9.97) | 28.96 (8.8) | 29.66 (6.66) | ||
| Plasma glucose, median (IQR), mg/dL | 102 (92-120) | 105 (96-124) | 102 (90-125) | ||
| Serum creatinine, mean (SD), mg/dL | 0.77 (0.19) | 0.73 (0.21) | 0.73 (0.18) | ||
| Blood urea, mean (SD), mg/dL | 21.40 (8.90) | 20.91 (9.18) | 21.35 (7.67) | ||
| Serum bilirubin, median (IQR), mg/dL | 0.5 (0.4-0.7) | 0.4 (0.4-0.7) | 0.5 (0.4-0.7) | ||
| Serum AST, median (IQR), IU/L | 37 (24-66) | 35 (23-61) | 34 (25-52) | ||
| Serum ALT, median (IQR), IU/L | 24 (16-43) | 22 (13-33) | 20 (14-35) | ||
| Serum AP, median (IQR), IU/L | 123 (86-218) | 108 (74-189) | 125 (82-240) | ||
| Immunological parameters | |||||
| Receiving ART at ATT initiation, No. | 23 | 16 | 22 | ||
| Not receiving ART during ATT, No. | 11 | 22 | 13 | ||
| Stavudine, lamivudine, efavirenz or zidovudine, lamivudine, efavirenz regimen, No. | 60 | 51 | 54 | ||
| Tenofovir, lamivudine, efavirenz regimen, No. | 40 | 37 | 43 | ||
| CD4 lymphocyte count, median (IQR), cells/μL | 130 (65-226) | 144 (71-261) | 137 (68-258) | ||
| CD8 lymphocyte count, median (IQR), cells/μL | 600 (334-957) | 573 (337-1048) | 707 (356-1018) | ||
| CD4/CD8 ratio, median (range) | 0.23 (0.11-0.4) | 0.23 (0.16-0.40) | 0.20 (0.11-0.38) | ||
| Viral load, mean (SD), log10 copies/mL | 4.91 (1.29) | 5.00 (1.14) | 4.93 (1.26) | ||
| ATT-ART interval, median (IQR), d | 16 (2-36) | 17 (5-47) | 15 (1-34) | ||
| CD4 lymphocyte count <100 cells/μL, No. (%) | 45 (41) | 38 (34) | 43 (39) | ||
| Chest radiograph characteristics | |||||
| >3 Zones, No./total No. (%) | 71/111 (64) | 82/110 (75) | 67/110 (61) | ||
| Bilateral lesions, No. | 71 | 71 | 65 | ||
| Lower lung TB, No. | 69 | 66 | 57 | ||
| Cavitation, No. | 23 | 18 | 16 | ||
| Pleural, No. | 24 | 18 | 17 | ||
| Military, No. | 13 | 9 | 10 | ||
| Mediastinal adenitis, No. | 31 | 30 | 25 | ||
| Others, No. | 2 | 3 | 2 | ||
| Microbiological characteristics | |||||
| Sputum smear grade 2+, No./total No. | 52/111 | 52/110 | 52/110 | ||
| Culture grade ≥2+, No./total No. | 79/111 | 68/110 | 66/110 | ||
| Sensitive to all first-line drugs, No./total No. (%) | 92/99 (93) | 82/97 (85) | 85/97 (88) | ||
| Isoniazid resistance, No. | 5 | 11 | 11 | ||
| MDR TB at baseline (isoniazid and rifampicin resistance), No. | 4 | 4 | 2 | ||
Abbreviations: ALT, alanine transaminase; AP, alkaline phosphatase; ART, antiretroviral therapy; AST, aspartate transaminase; ATT, antituberculosis therapy; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared; IQR, interquartile range; MDR, multidrug-resistant; RBC, red blood cell; TB, tuberculosis; WBC, white blood cell.
SI conversion factors: To convert RBC count to ×1012/L, multiply by 1.0. To convert WBC count to ×109/L, multiply by 0.001. To convert platelet count to ×109/L, multiply by 1.0. To convert hemoglobin to g/L, multiply by 10.0. To convert hematocrit to proportion of 1, multiply by 0.01. To convert plasma glucose to mmol/L, multiply by 0.0555. To convert serum creatinine to μmol/L, multiply by 76.25. To convert blood urea to mmol/L, multiply by 0.357. To convert serum bilirubin to μmol/L, multiply by 17.104. To convert serum AST to μkat/L, multiply by 0.0167. To convert serum ALT to μkat/L, multiply by 0.0167. To convert serum AP to μkat/L, multiply by 0.0167.
Outcome Analysis
The CONSORT diagram summarizes the flow of patients from screening until 18 months after randomization (Figure 1). At the end of the intensive phase, sputum smear results were negative for 117 of 181 patients (65%) receiving daily ATT vs 52 of 90 patients (58%) receiving intermittent ATT (difference, 6.9%; 95% CI, −5.2% to −19.1%; P = .33), while culture results were negative for 177 of 183 patients (97%) receiving daily ATT vs 77 of 89 patients (87%) receiving intermittent ATT (difference, 10.2%; 95% CI, 3.5%-19.0%; P = .002) (eFigure 1 in Supplement 2).
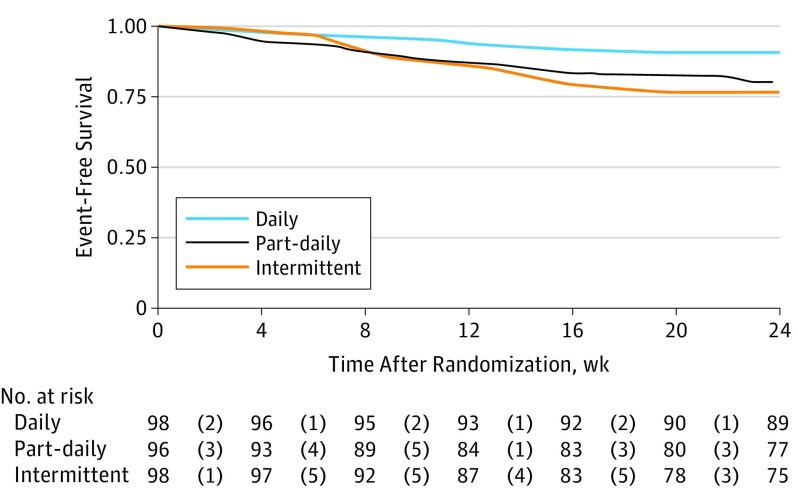
In the modified intent-to-treat analysis, favorable responses at the end of TB treatment occurred in 89 of 98 patients (91%), 77 of 96 patients (80%), and 75 of 98 patients (77%) in the daily, part-daily, and intermittent regimens, respectively. The difference in favorable response between the daily and the intermittent regimen (14%; 95% CI, 3.9%-25%) attained statistical significance at the second interim analysis stage, crossing the O’Brien-Fleming group sequential boundaries (calculated χ2 = 7.32 vs required χ2 = 5.67; P = .006; with Bonferroni correction, P = .009), whereas the difference between part-daily and intermittent remained insignificant (difference, 9.2%; 95% CI, 0.6%-18.3%; P = .53). Similarly, favorable responses in the per-protocol analysis occurred in 86 of 90 patients (95%), 76 of 88 patients (86%), and 74 of 87 patients (85%) in the daily, part-daily, and intermittent regimens, respectively. Kaplan-Meier estimates are provided in Figure 2.
Figure 2. Mean Survival Estimates for Time to an Unfavorable Event Among Antituberculosis Therapy Regimens.
The numbers given within parentheses reflect the number of individuals who experienced the event at that particular week after randomization (censored at 24 weeks after randomization). Event-free survival was 22.76 (95% CI, 21.91-23.61) in the daily regimen, 21.34 (95% CI, 20.11-22.57) in the part-daily regimen, and 21.07 (95% CI, 19.91-22.22) in the intermittent regimen, results that were significant according to the log-rank test (P = .03).
Results of the Cox proportional hazards regression analysis are provided in Table 2. The γ frailty model incorporating the potential confounders showed no heterogeneity among the regimens with respect to baseline parameters. A lower CD4 lymphocyte count (<150 cells/μL compared with ≥150 cells/μL) at 2 months rather than at baseline forecasted an unfavorable outcome (eFigure 2 in Supplement 2). Trends in various laboratory parameters during the treatment period are provided in eFigure 3 in Supplement 2.
Table 2. Cox Proportional Hazards Regression Analysis Evaluating the Risk of an Unfavorable Event During Treatment Period.
| Baseline Parameter | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Daily regimena | 0.310 (0.131-0.733) | .008 |
| Part-daily regimen | 0.834 (0.440-1.578) | .58 |
| Baseline CD4 lymphocyte count <100 cells/μL | 0.903 (0.496-1.644) | .74 |
| Sputum culture grade >2+ | 1.476 (0.817-2.667) | .20 |
| Isoniazid resistance at baselineb | 2.340 (1.107-4.947) | .03 |
| Baseline weight, kg | 0.961 (0.924-1.000) | .05 |
| Baseline viral load, log10 copies/mL | 1.363 (0.995-1.866) | .05 |
| Radiographic involvement >3 zones | 0.906 (0.479-1.714) | .76 |
| Receiving ART at initiation of ATT | 1.113 (0.530-2.340) | .78 |
Abbreviations: ART, antiretroviral therapy; ATT, antituberculosis therapy.
The hazard of an unfavorable response was reduced to one-third when a daily regimen replaced an intermittent regimen.
The hazard of an unfavorable response increased 2.3 times when there was isoniazid resistance at baseline.
Failures and ARR
In the intermittent regimen, 6 patients experienced bacteriological failures of treatment, with 4 of these patients developing ARR. Two patients experienced clinical failures (1 persistent bronchopleural fistula and 1 TB pontine abscess). Treatment failed in 3 patients in the part-daily regimen, but the patients did not develop ARR. The daily regimen had no failures (eTable 1 in Supplement 2). While none of the 16 patients with pretreatment isoniazid resistance (eTable 1 in Supplement 2) treated with a daily intensive phase developed ARR, 3 of 11 patients in the intermittent group developed ARR, and treatment failed subsequently.
Deaths
Overall, 18 deaths occurred during therapy (daily group, 4; part-daily group, 9; and intermittent group, 5). The baseline median (IQR) CD4 lymphocyte count of patients who died was 58 (45-136) cells/μL compared with 145 (78-259) cells/μL among patients who survived (P = .04) (eTable 2 in Supplement 2).
Treatment-Emergent Adverse Drug Reactions
Adverse reactions related to ATT occurred in 30 of 111 patients (27%), 23 of 110 patients (21%), and 19 of 110 patients (17%) in the daily, part-daily, and intermittent regimens, respectively (difference, 9.8%; 95% CI, −1.2% to −20.5%; P = .08), with no drug-induced deaths. Four patients (1 with jaundice; 2, thrombocytopenia; and 1, Stevens-Johnson syndrome) had their study regimen permanently modified (Table 3). Jaundice occurred in 21 patients (daily group, 11; part-daily group, 8; and intermittent group, 2), with 16 of 21 events occurring while the patient was receiving concomitant ART (eFigure 4 in Supplement 2). Seven patients developed jaundice as part of the immune reconstitution inflammatory syndrome (IRIS) spectrum. Hepatotoxic effects were experienced by 19 of 221 patients (9%) receiving treatment daily vs 2 of 110 patients (2%) receiving intermittent treatment during the intensive phase. Median (range) time of occurrence of jaundice was 19 (6-80), 27 (9-56), and 20 (18-23) days after ATT. All cases of jaundice resolved by 28 days in the daily and part-daily regimens, and by 20 days in the intermittent regimen. These 21 patients with jaundice, however, attained bacteriological quiescence at the end of 6 months.
Table 3. Treatment-Emergent Adverse Drug Reactions Among HIV-Positive Patients With Tuberculosis Receiving 3 Antituberculosis Therapy Regimens.
| Toxic Effect Type | Grade of Toxic Effects | Regimena | ||
|---|---|---|---|---|
| Daily (n = 111) | Part-Daily (n = 110) | Intermittent (n = 110) | ||
| Gastrointestinal and hepatic | 1 and 2 | 5 | 1 | 8 |
| 3 and 4 | 11 | 11b | 2 | |
| Neurological | 1 and 2 | 11 | 8 | 8 |
| 3 and 4 | 1 | 1 | 0 | |
| Cutaneous | 1 and 2 | 1 | 0 | 1 |
| 3 and 4 | 1c | 0 | 0 | |
| Others | 1 and 2 | 0 | 0 | 0 |
| 3 and 4 | 0 | 2d | 0 | |
| Overall adverse drug reactions, No. (%) | 30 (27) | 23 (21) | 19 (17) | |
Four patients had permanent discontinuation or modification of antituberculosis therapy.
Two patients had jaundice.
One patient had Stevens-Johnson syndrome.
One patient had thrombocytopenia.
Recurrences
Six patients (7.21/100 person-years), 4 patients (5.33/100 person-years), and 6 patients (9.01/100 person-years) in the daily, part-daily, and intermittent regimens, respectively, had TB recurrence (Figure 1). There was no statistically significant difference in recurrences when the daily regimen (1.6/100 person-years; 95% CI, −0.7 to −10.2; P = .71) or part-daily regimen (0.8/100 person-years; 95% CI, −8.2 to −9.9; P = .42) were compared with the intermittent regimen.
Immune Reconstitution Inflammatory Syndrome
Overall, there were 82 cases of IRIS (27 of 98 patients [28%] in the daily regimen, 21 of 96 patients [22%] in the part-daily regimen, and 34 of 98 patients [35%] in the intermittent regimen). The median (IQR) interval between administration of ATT and that of ART was 17 (4-39) days and the median (IQR) time of IRIS occurrence was 15 (7-22) days after ART. Immune reconstitution inflammatory syndrome lesions were pulmonary in 26 of 82 patients (32%) and extrapulmonary in 56 of 82 patients (68%), requiring steroids for amelioration of symptoms in 59 of 82 patients (72%). Antiretroviral therapy was temporarily withheld in 4 cases (2 with brain tuberculomas and 2 with obstructive jaundice).
Discussion
This study addresses the vital issue of optimal frequency of anti-TB drug administration among HIV-positive patients with TB by comparing the efficacy of daily, part-daily, and intermittent ATT regimens in a pure group of patients newly diagnosed with culture-positive, rifampicin-sensitive, pulmonary TB. Not only did the daily regimen prove superior to a 6-month intermittent regimen, but it also prevented ARR, prompting the data safety monitoring committee to halt the study at the stage of second interim analysis. The per-protocol analysis yielded similar results. In the Cox proportional hazards model, significant risk factors leading to an unfavorable response were intermittent administration of ATT and pretreatment isoniazid resistance, confirming the hypotheses raised by previous analyses of observational cohorts. The meta-analysis by Khan et al among HIV-positive patients with TB showed that the odds of treatment failure increased by 2.0 times (95% CI, 0.8-5) and the odds of ARR increased by 3.7 times (95% CI, 0.7-18.9) when an intermittent rather than a daily intensive phase of ATT was used. In an observational cohort study among 1460 HIV-positive patients with TB from south India, higher mortality was noted with supervised intermittent ATT compared with unsupervised daily ATT, with similar default rates in both groups. This study by Alvarez-Uria et al had its own limitation, which was that only one-third of patients had access to ART.
Clinical trials among HIV-negative patients with TB have not shown any significant difference in treatment responses between daily and intermittent regimens. The meta-analysis by Kasozi et al of HIV-negative patients newly diagnosed with drug-susceptible TB revealed similar cure rates with intermittent (88%) and daily (90%) ATT. Additionally, the meta-analysis by Menzies et al done in a predominantly HIV-negative population of patients with TB revealed an increased adjusted risk of ARR with a thrice-weekly intensive phase with no difference in relapse or failure.
Interestingly, in our study a lower CD4 lymphocyte count at the end of the intensive phase, rather than at baseline, was associated with an unfavorable outcome. Even among patients with advanced immunodeficiency that persisted (CD4 lymphocyte count <150 cells/μL at the end of 2 months), patients receiving the daily regimen exhibited better survival compared with those who received the part-daily or intermittent regimens (eFigure 2 in Supplement 2). Three of 4 patients who developed ARR in the intermittent group had lower CD4 lymphocyte counts repeatedly, despite timely ART and effective viral suppression (eTable 1 in Supplement 2). Furthermore, the part-daily regimen conferred no added advantage over the intermittent regimen in terms of efficacy or toxic effects, but prevented ARR. Even though there seem to be more patients with isoniazid -resistant disease or advanced immunodeficiency at baseline in the part-daily regimen group, this was not statistically different, and the γ frailty model failed to reveal any heterogeneity among regimens with respect to these baseline parameters. Comparing previous trials conducted at NIRT, ART initiation within the intensive phase of ATT augmented cure rates, reduced mortality, and prevented ARR but contributed to higher incidence of toxic effects and IRIS. Significant increases in weight, CD4 lymphocyte count, hemoglobin, red blood cells, and hematocrit and decline in HIV viral load that were uniformly observed across all 3 groups confirmed the advantage of early ART initiation and good clinical management of HIV (eFigure 3 in Supplement 2).
Overall toxic effects were statistically similar among regimens. However, patients receiving the daily intensive phase of ATT with coadministered ART had a higher incidence of hepatotoxic effects despite normal baseline liver function. These effects were manageable under trial conditions. Studies of HIV-positive patients with TB have reported hepatotoxic effects ranging from 5% to 33%. Except in 2 cases that mandated permanent modification of the regimen because of hepatotoxic effects, the study regimen was successfully reinstituted and the patients attained bacteriological quiescence at treatment completion. Factors possibly contributing to higher incidence of hepatotoxic effects among patients in our trial were prior alcohol intake, extrapulmonary site of involvement, advanced immunodeficiency, and a shorter interval between administration of ATT and ART. Yimer et al similarly reported the risk of hepatotoxic effects to be 3.6 times greater (95% CI, 1.5-8.5) with HIV, 2.7 times greater (95% CI, 1.2-5.8) with concomitant drug intake, and 5.2 times greater (95% CI, 0.6-46) when CD4 lymphocyte count was lower than 100 cells/μL. Furthermore, early hepatic injury was more common in patients with HIV-TB coinfection than in those with TB alone (33% vs 7% [P = .004]), indicating the need for close monitoring.
Recurrences were similar in the 3 groups during the 1-year follow-up period. Dosing schedule was not associated with recurrences in our study, probably because of effective and early highly active ART therapy. We believe that these results may be sustainable even over a longer follow-up period, as three-fourths of recurrences occur within the first year. Analogous to toxic effects, shorter interval between administration of ATT and ART, advanced immunodeficiency, and extrapulmonary involvement coupled with active surveillance for IRIS resulted in a higher IRIS incidence reported. Steroids were required in most of the cases.
Limitations
The strengths of this study included a pure group of study participants (culture-confirmed, rifampicin-sensitive, ATT-naive, HIV-positive patients with TB) treated with quality-assured, assayed drugs and fully supervised drug intake. The bacteriology department was blinded to each patient’s clinical condition and regimen. The outcomes were ratified by an independent end point review committee, reducing the bias arising out of the open-label design. The main limitations of the study were the slow recruitment rate due to stringent selection criteria and failure to stratify for isoniazid resistance at baseline, as rapid tests for resistance detection were unavailable at that time and we wanted to replicate programmatic conditions.
Conclusions
A daily ATT regimen for 6 months in HIV-positive patients with TB receiving concomitant ART proved superior to an intermittent regimen in terms of efficacy and prevention of ARR. A slightly higher incidence of hepatotoxic effects for patients receiving the daily regimen demanded closer monitoring of liver function. The part-daily regimen did not confer any advantage over an intermittent regimen with regard to efficacy or tolerability but prevented ARR. A higher CD4 lymphocyte count (≥150 cells/μL ) at the end of the intensive phase, rather than at baseline, was associated with a successful outcome.
Trial Protocol
eAppendix. Methods elaborated
eFigure 1. Month-wise Sputum Smear (A) and Culture (B) negativity (%) Among the Three ATT Regimens
eFigure 2. Kaplan Meier Survival Estimates Based on CD4 cell Counts at Baseline (Panel A) and Second Month (Panel B) Censored at 24 Weeks post-ATT
eFigure 3. Trends in Lab Parameters at Baseline, End of Intensive and Continuation Phase of Three ATT regimens
eFigure 4. Days from Initiation of ATT to Onset of Jaundice
eTable 1. Comparison of Characteristics at Baseline (BL) and at Event among Failures to TB Therapy
eTable 2. Comparison of Characteristics at Baseline and Subsequent Time Point Nearest to Event among Deaths
References
- 1.World Health Organization Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 2.World Health Organization Treatment of Tuberculosis Guidelines. 4th ed. http://www.tbonline.info/guidelines/. Accessed July 8, 2017.
- 3.Central Tuberculosis Division, Directorate General of Health Services, Ministry of Health and Family Welfare TB India 2008-RNTCP Status Report. https://tbcindia.gov.in/index1.php?lang=1&level=1&sublinkid=4160&lid=2807. Accessed October 2, 2016.
- 4.Swaminathan S, Narendran G, Venkatesan P, et al. . Efficacy of a 6-month versus 9-month intermittent treatment regimen in HIV-infected patients with tuberculosis: a randomized clinical trial. Am J Respir Crit Care Med. 2010;181(7):743-751. [DOI] [PubMed] [Google Scholar]
- 5.Swaminathan S, Padmapriyadarsini C, Venkatesan P, et al. . Efficacy and safety of once-daily nevirapine- or efavirenz-based antiretroviral therapy in HIV-associated tuberculosis: a randomized clinical trial. Clin Infect Dis. 2011;53(7):716-724. [DOI] [PubMed] [Google Scholar]
- 6.Banu Rekha VV, Rajaram K, Kripasankar AS, et al. . Efficacy of the 6-month thrice-weekly regimen in the treatment of new sputum smear-positive pulmonary tuberculosis under clinical trial conditions. Natl Med J India. 2012;25(4):196-200. [PubMed] [Google Scholar]
- 7.Vashishtha R, Mohan K, Singh B, et al. . Efficacy and safety of thrice weekly DOTS in tuberculosis patients with and without HIV co-infection: an observational study. BMC Infect Dis. 2013;13:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernon A, Burman W, Benator D, Khan A, Bozeman L; Tuberculosis Trials Consortium . Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Lancet. 1999;353(9167):1843-1847. [DOI] [PubMed] [Google Scholar]
- 9.Nahid P, Gonzalez LC, Rudoy I, et al. . Treatment outcomes of patients with HIV and tuberculosis. Am J Respir Crit Care Med. 2007;175(11):1199-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burman W, Benator D, Vernon A, et al. ; Tuberculosis Trials Consortium . Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med. 2006;173(3):350-356. [DOI] [PubMed] [Google Scholar]
- 11.Tuberculosis Research Centre Low rate of emergence of drug resistance in sputum positive patients treated with short course chemotherapy. Int J Tuberc Lung Dis. 2001;5(1):40-45. [PubMed] [Google Scholar]
- 12.Narendran G, Menon PA, Venkatesan P, et al. . Acquired rifampicin resistance in thrice-weekly antituberculosis therapy: impact of HIV and antiretroviral therapy. Clin Infect Dis. 2014;59(12):1798-1804. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Munsiff SS, Driver CR, Sackoff J. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997-2000. Clin Infect Dis. 2005;41(1):83-91. [DOI] [PubMed] [Google Scholar]
- 14.Khan FA, Minion J, Pai M, et al. . Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50(9):1288-1299. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad Khan F, Minion J, Al-Motairi A, Benedetti A, Harries AD, Menzies D. An updated systematic review and meta-analysis on the treatment of active tuberculosis in patients with HIV infection. Clin Infect Dis. 2012;55(8):1154-1163. [DOI] [PubMed] [Google Scholar]
- 16.National AIDS Control Organisation Antiretroviral Therapy Guidelines for HIV-Infected Adults and Adolescents Including Post-exposure Prophylaxis. NACO; 2007. http://naco.gov.in/sites/default/files/1.%20Antiretroviral%20Therapy%20Guidelines%20for%20HIV-Infected%20Adults%20and%20Adolescents%20Including%20Post-exposure.pdf. Accessed January 23, 2017. [Google Scholar]
- 17.Allen BW, Baker FJ. Mycobacteria: Isolation, Identification and Sensitivity Testing. Oxford, UK: Butterworths; 1968. [Google Scholar]
- 18.Canetti G, Fox W, Khomenko A, et al. . Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41(1):21-43. [PMC free article] [PubMed] [Google Scholar]
- 19.Tortoli E, Bartoloni A. High-performance liquid chromatography and identification of mycobacteria. Rev Med Microbiol. 1996;7(4):207-220. [Google Scholar]
- 20.National AIDS Control Organisation Antiretroviral Therapy Guidelines for HIV-Infected Adults and Adolescents. 2013. http://naco.gov.in. Accessed January 24, 2017.
- 21.Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services Division of AIDS table for grading the severity of adult and pediatric adverse events. 2009. http://rsc.tech-res.com/docs/default-source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf. Accessed February 14, 2017.
- 22.Narendran G, Ramesh Kumar S, Padmapriyadarsini C, et al. . Daily is better than thrice-weekly anti-tuberculosis therapy in HIV patients with culture confirmed pulmonary TB: a randomised controlled clinical trial from south India. Paper presented at: AIDS 2016 Conference; July 18-22, 2016; Durban, South Africa. http://programme.aids2016.org/Abstract/Abstract/1300. Accessed April 20, 2017.
- 23.Menzies D, Benedetti A, Paydar A, et al. . Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med. 2009;6(9):e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez-Uria G, Midde M, Pakam R, Naik PK. Directly-observed intermittent therapy versus unsupervised daily regimen during the intensive phase of antituberculosis therapy in HIV infected patients. Biomed Res Int. 2014;2014:937817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal PK, Mandal A, Bhattacharyya SK. Comparing the daily versus the intermittent regimens of the anti-tubercular chemotherapy in the initial intensive phase in non-HIV, sputum positive, pulmonary tuberculosis patients. J Clin Diagn Res. 2013;7(2):292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasozi S, Clark J, Doi SA. Intermittent versus daily pulmonary tuberculosis treatment regimens: a meta-analysis. Clin Med Res. 2015;13(3-4):117-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Treatment of Tuberculosis: Guidelines. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 28.Sharma SK, Singla R, Sarda P, et al. . Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis. 2010;50(6):833-839. [DOI] [PubMed] [Google Scholar]
- 29.Jong E, Conradie F, Black A, Menezes C, John MA, Meintjes G. Consensus statement: management of drug-induced liver injury in HIV-positive patients treated for TB: guideline. South Afr J HIV Med. 2013;14(3):113-119. [Google Scholar]
- 30.Yimer G, Aderaye G, Amogne W, et al. . Anti-tuberculosis therapy-induced hepatotoxicity among Ethiopian HIV-positive and negative patients. PLoS One. 2008;3(3):e1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singanayagam A, Sridhar S, Dhariwal J, et al. . A comparison between two strategies for monitoring hepatic function during antituberculous therapy. Am J Respir Crit Care Med. 2012;185(6):653-659. [DOI] [PubMed] [Google Scholar]
- 32.Narendran G, Andrade BB, Porter BO, et al. . Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One. 2013;8(5):e63541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopalan N, Andrade BB, Swaminathan S. Tuberculosis-immune reconstitution inflammatory syndrome in HIV: from pathogenesis to prediction. Expert Rev Clin Immunol. 2014;10(5):631-645. [DOI] [PubMed] [Google Scholar]
- 34.Meintjes G, Wilkinson RJ, Morroni C, et al. . Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(15):2381-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Methods elaborated
eFigure 1. Month-wise Sputum Smear (A) and Culture (B) negativity (%) Among the Three ATT Regimens
eFigure 2. Kaplan Meier Survival Estimates Based on CD4 cell Counts at Baseline (Panel A) and Second Month (Panel B) Censored at 24 Weeks post-ATT
eFigure 3. Trends in Lab Parameters at Baseline, End of Intensive and Continuation Phase of Three ATT regimens
eFigure 4. Days from Initiation of ATT to Onset of Jaundice
eTable 1. Comparison of Characteristics at Baseline (BL) and at Event among Failures to TB Therapy
eTable 2. Comparison of Characteristics at Baseline and Subsequent Time Point Nearest to Event among Deaths