Key Points
Question
What are the advantages of total neoadjuvant therapy (preoperative systemic chemotherapy in combination with chemoratiation [TNT]) compared with the traditional approach of preoperative chemoradiation and postoperative adjuvant chemotherapy in patients with locally advanced (T3/4 or node-positive) rectal cancer?
Findings
In this retrospective cohort analysis, 308 patients treated with TNT were compared with 320 patients treated with chemoRT with planned adjuvant chemotherapy. Patients in the TNT cohort received greater percentages of the planned systemic chemotherapy, had higher rates of complete response (pathologic and sustained clinical), and were more likely to have temporary ileostomy reversed within 15 weeks of proctectomy.
Meaning
Total neoadjuvant therapy appears to have short-term advantages over the traditional chemoRT and adjuvant chemotherapy regimen for locally advanced rectal cancer; long-term follow-up will be required to determine if this translates into improved overall survival.
This study compares preoperative chemoradiation followed by postoperative adjuvant chemotherapy vs total neoadjuvant therapy for treatment of locally advanced rectal cancer.
Abstract
Importance
Treatment of locally advanced rectal (LARC) cancer involves chemoradiation, surgery, and chemotherapy. The concept of total neoadjuvant therapy (TNT), in which chemoradiation and chemotherapy are administered prior to surgery, has been developed to optimize delivery of effective systemic therapy aimed at micrometastases.
Objective
To compare the traditional approach of preoperative chemoradiation (chemoRT) followed by postoperative adjuvant chemotherapy with the more recent TNT approach for LARC.
Design, Setting, and Participants
A retrospective cohort analysis using Memorial Sloan Kettering Cancer Center (MSK) records from 2009 to 2015 was carried out. A total of 811 patients who presented with LARC (T3/4 or node-positive) were identified.
Exposures
Of the 811 patients, 320 received chemoRT with planned adjuvant chemotherapy and 308 received TNT (induction fluorouracil- and oxaliplatin-based chemotherapy followed by chemoRT).
Main Outcomes and Measures
Treatment and outcome data for the 2 cohorts were compared. Dosing and completion of prescribed chemotherapy were assessed on the subset of patients who received all therapy at MSK.
Results
Of the 628 patients overall, 373 (59%) were men and 255 (41%) were women, with a mean (SD) age of 56.7 (12.9) years. Of the 308 patients in the TNT cohort, 181 (49%) were men and 127 (49%) were women. Of the 320 patients in the chemoRT with planned adjuvant chemotherapy cohort, 192 (60%) were men and 128 (40%) were women. Patients in the TNT cohort received greater percentages of the planned oxaliplatin and fluorouracil prescribed dose than those in the chemoRT with planned adjuvant chemotherapy cohort. The complete response (CR) rate, including both pathologic CR (pCR) in those who underwent surgery and sustained clinical CR (cCR) for at least 12 months posttreatment in those who did not undergo surgery, was 36% in the TNT cohort compared with 21% in the chemoRT with planned adjuvant chemotherapy cohort.
Conclusions and Relevance
Our findings provide additional support for the National Comprehensive Cancer Network (NCCN) guidelines that categorize TNT as a viable treatment strategy for rectal cancer. Our data suggest that TNT facilitates delivery of planned systemic therapy. Long-term follow-up will determine if this finding translates into improved survival. In addition, given its high CR rate, TNT may facilitate nonoperative treatment strategies aimed at organ preservation.
Introduction
Treatment for clinical stage II or III locally advanced rectal cancer (LARC) (T3/4, N0, or node-positive) consists of preoperative chemoradiotherapy (chemoRT) followed by total mesorectal excision and postoperative adjuvant chemotherapy with fluorouracil and oxaliplatin. This approach confers excellent local control, with distant recurrence substantially more common than local recurrence.1
Induction or consolidation chemotherapy with chemoRT prior to surgery for LARC, referred to as total neoadjuvant therapy (TNT), has been reported by several centers2,3,4 with the following benefits: improved delivery of planned therapy, increased downstaging, earlier introduction of optimal systemic chemotherapy to address possible micrometastases, and in-vivo assessment of chemosensitivity. In addition, delivery of all chemotherapy preoperatively obviates the need for postoperative therapy, reducing duration with a diverting ileostomy and alleviating the need for patients to undergo chemotherapy with a stoma.
A study of 61 patients treated at Memorial Sloan Kettering Cancer Center (MSK) with induction FOLFOX (folinic acid, fluorouracil, oxaliplatin) prior to chemoRT found high tolerance of therapy and improved treatment responses: 22 (36%) of 61 patients had either a pathologic complete response (pCR) or clinical complete response (cCR).3 Those results, along with others,2,4 led the National Comprehensive Cancer Network (NCCN) to categorize TNT as an acceptable treatment strategy for stage II/III LARC.5
In this study, we describe the results of the adoption of TNT as the most common treatment paradigm for LARC at a comprehensive cancer center. We compared tolerance of prescribed chemotherapy, tumor response, and short-term oncologic outcomes in 2 patient cohorts: TNT (specifically induction chemotherapy followed by chemoRT) vs preoperative chemoRT and planned adjuvant chemotherapy.
Methods
Patients
After obtaining approval with a waiver of informed consent from the institutional review board at MSK, we identified LARC patients attending the MSK colorectal surgical oncology clinic from June 1, 2009, to March 1, 2015. We defined LARC as adenocarcinoma with distal margin of 15 cm or less from the anal verge on endoscopy, staged with endorectal ultrasound (ERUS) or magnetic resonance imaging (MRI) as cT3/cT4 N0 or cT(any) cN1/2, in line with NCCN guidelines.6 Disease was staged with computed tomography (CT) of the chest, abdomen, and pelvis pretreatment. Patients were excluded if they had recurrent or metastatic disease, previous surgical treatment for rectal cancer, or concurrent fistulizing inflammatory bowel disease of the rectum.
Neoadjuvant Regimens
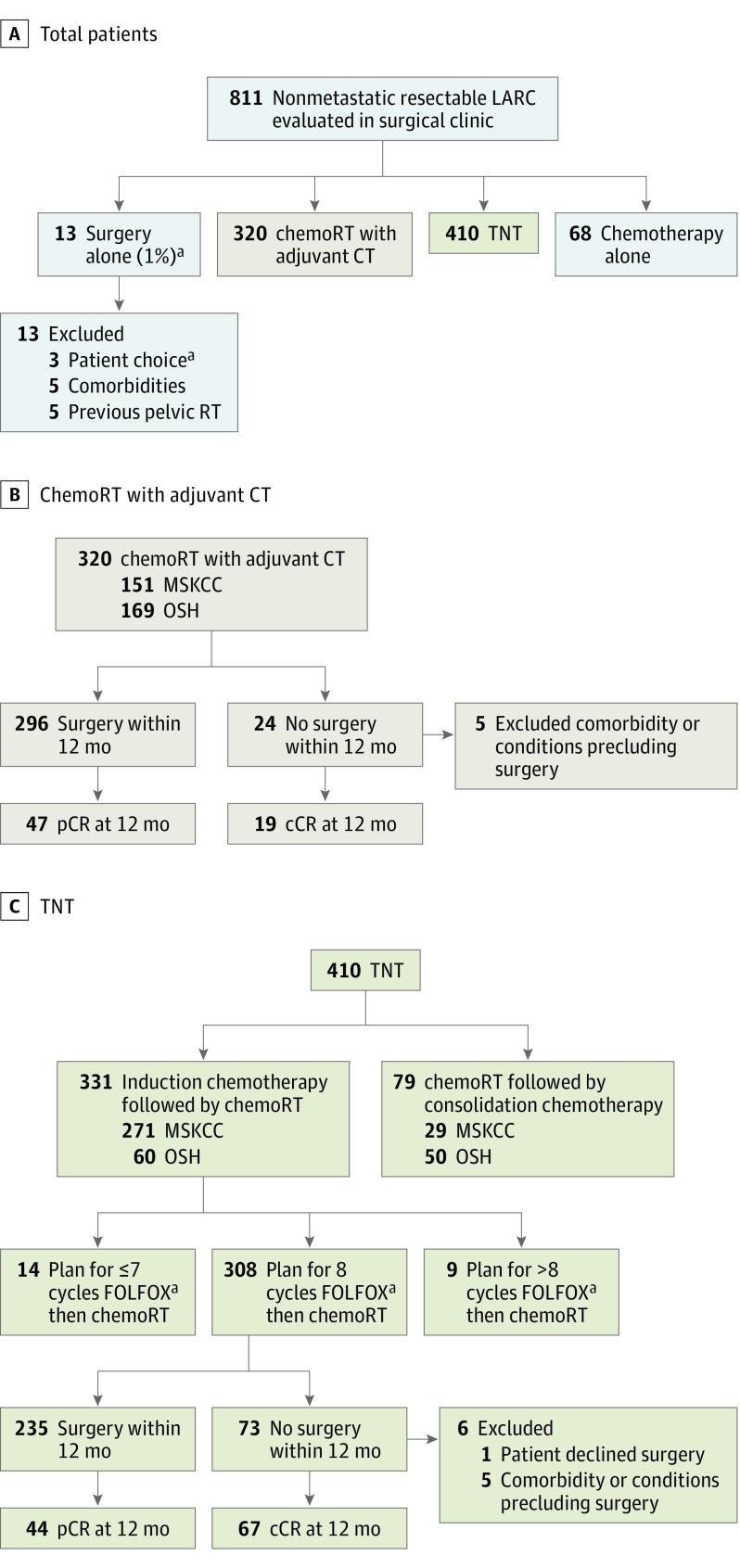
Total neoadjuvant therapy was defined as induction chemotherapy in the form of mFOLFOX6 for 8 cycles, CAPOX (capecitabine and oxaliplatin) for 5 cycles,7,8,9 or FLOX (weekly fluorouracil/leucovorin and biweekly oxaliplatin)10 prior to chemoRT (Figure). Consolidative or alternative chemotherapy regimens were excluded (Figure, C). ChemoRT commenced 2 to 3 weeks after completing induction chemotherapy and included 25 to 28 radiotherapy fractions with concurrent infusional fluorouracil at 225 mg/m2 or oral capecitabine at 825 mg/m2 twice daily. Patients underwent 3-dimensional conformal radiotherapy (3-D CRT) or intensity-modulated radiotherapy (IMRT) receiving 45 Gy in 1.8 Gy fractions to the pelvis, with a 5.4 Gy boost to the tumor. In 3-D CRT patients, the boost involved 3 additional 1.8 Gy fractions (total 50.4 Gy in 28 fractions), whereas in IMRT patients the boost involved dose-painting to 2.0 Gy per fraction (total 50 Gy in 25 fractions).
Figure. Treatment Pathways.
Abbreviations: ChemoRT, chemoradiation; CT, chemotherapy; OSH, outside hospital; TNT, total neoadjuvant therapy. A, Treatment plans for patients with nonmetastatic LARC newly diagnosed at the MSK colorectal surgery clinic from June 1, 2009, to March 1, 2015. B, Treatment pathways and outcomes for patients who received chemoRT with planned adjuvant chemotherapy. C, Treatment pathways and outcomes for patients who received TNT.
aFour months folinic acid, fluorouracil, oxaliplatin (FOLFOX); capecitabine and oxaliplatin (CAPOX), or weekly fluorouracil/leucovorin and biweekly oxaliplatin (FLOX).
Patients were included in the chemoRT with planned adjuvant chemotherapy cohort if they received chemoRT as initial treatment with planned 4-month course of postoperative FOLFOX, CAPOX, or FLOX. Data on scheduled treatment, number of cycles administered, and dose reductions were collected from electronic records. Analyses comparing preoperative vs postoperative chemotherapy dosing were limited to FOLFOX because this was most commonly prescribed with standardized scheduling.
Resection
Following neoadjuvant therapy, patients underwent repeat proctoscopy, CT, and MRI restaging. Surgery was performed in accord with principles of anatomic, total mesorectal excision. The possibility of pCR was discussed with patients who demonstrated cCR (absence of viable tumor on proctoscopy/MRI), and patients who chose to forgo rectal resection, electing nonoperative treatment, were placed under close observation.11 Delayed resection was performed when there was clinical concern for tumor regrowth or patient choice to move off a nonoperative approach.
pCR and cCR
We defined pCR as absence of viable tumor cells in the resection specimen, as previously described.12,13 Throughout the study period, assessment for sustained cCR was based on previously described criteria.14,15,16 We used the term complete response (CR) to define the proportion of patients who either have pCR determined after surgery or sustained cCR for 12 months or longer while under nonoperative surveillance. The 12-month mark was chosen because most local regrowths after apparent cCR occur within this period; therefore, cCRs beyond this time are likely to be sustained.11
Statistical Analysis
Data are presented as medians with interquartile range (IQR) unless otherwise stated. Groups were compared using either χ2 or Kruskal-Wallis tests. Associations between individual variables and CR were evaluated with binary logistic regression. The significance level was P < .05. Analyses were performed using R17 (version 3.2.5, R Foundation) and SAS statistical software (version 9.3; SAS Institute, Inc).
Results
Treatment plans for 811 patients who met the inclusion criteria are shown in the Figure, A. In total, 320 patients were treated with the chemoRT with planned adjuvant chemotherapy paradigm and 410 patients were treated with TNT. Patients who underwent surgery alone (n = 13) or chemotherapy alone (n = 68) were excluded.
In the chemoRT with planned adjuvant chemotherapy cohort, 296 of the 320 patients underwent surgery within 12 months after completing neoadjuvant therapy; 49 (17%) had a pCR. The remaining 24 (8%) of the 320 patients did not undergo surgery within 12 months; 19 of those 24 (79%) had a sustained cCR with nonoperative treatment. Five patients had medical comorbidities precluding surgery (Figure, B).
In the TNT group, 410 patients were treated with chemoRT and systemic chemotherapy. Of those, 102 patients received chemoRT with consolidative or split-course chemotherapy and were excluded. The TNT cohort therefore includes 308 patients treated with induction FOLFOX (n = 288), CAPOX (n = 17), or FLOX (n = 3). Of those, 235 (76%) underwent surgery within 12 months after completing TNT; 43 (18%) had a pCR. The remaining 73 (24%) did not undergo surgery within 12 months; 67 (92%) had a sustained cCR and elected nonoperative treatment. One patient declined surgery, and 5 patients had medical comorbidities precluding surgery (Figure, C).
Table 1 details clinical and pathological characteristics of patients in the 2 cohorts. Patients in the chemoRT with planned adjuvant chemotherapy group were slightly older and less likely to have clinical stage III disease than the TNT group. Patients in the chemoRT with planned adjuvant chemotherapy group were more likely to be treated earlier in the study period. Between 2009 and 2011, 217 (89%) of 245 received chemoRT with planned adjuvant chemotherapy, compared with 38 (20%) of 125 in 2014 to 2015 (absolute difference, 68%; P < .001), reflecting institutional change in treatment strategy (eFigure in the Supplement).
Table 1. Patient and Treatment Characteristics.
| Characteristic | Patients, No. (%) | P Value | |
|---|---|---|---|
| ChemoRT With Planned Adjuvant Chemotherapy (n = 320) | TNT (n = 308) | ||
| Age, y | |||
| <55 | 128 (40.0) | 161 (52.3) | <.001 |
| 55-75 | 152 (47.5) | 127 (41.2) | |
| >75 | 40 (12.5) | 20 (6.5) | |
| Sex | |||
| Male | 192 (60.0) | 181 (58.8) | .81 |
| Female | 128 (40.0) | 127 (41.2) | |
| Period | |||
| 2009-2011 | 217 (67.8) | 28 (9.1) | <.001 |
| 2012-2013 | 65 (20.3) | 133 (43.2) | |
| 2014-2015 | 38 (11.9) | 147 (47.7) | |
| Tumor height (cm) above anal verge | |||
| <5 | 98 (30.6) | 102 (33.1) | .55 |
| 5-10 | 175 (54.7) | 143 (46.4) | |
| >10 | 47 (14.7) | 63 (20.5) | |
| Imaging for pretreatment staginga | |||
| ERUS | 85 (34.7) | 12 (4.1) | <.001 |
| MRI | 84 (34.3) | 165 (56.1) | |
| ERUS and MRI | 76 (31.0) | 117 (39.8) | |
| cT stage | |||
| cT1 | 3 (0.9) | 2 (0.6) | .06 |
| cT2 | 20 (6.3) | 19 (6.2) | |
| cT3 | 277 (86.6) | 251 (81.5) | |
| cT4 | 20 (6.2) | 36 (11.7) | |
| cN stage | |||
| cN0 | 94 (29.4) | 43 (14.0) | <.001 |
| cN positive | 226 (70.6) | 265 (86.0) | |
| Surgery within 12 mo | |||
| Yes | 296 (92.5) | 235 (76.3) | <.001 |
| No | 24 (7.5) | 73 (23.7) | |
| Type of surgery | |||
| Open | 156 (52.7) | 65 (27.8) | <.001 |
| Minimally invasive | 140 (47.3) | 169 (72.2) | |
| Days to surgery, median (IQR)b | 56 (48-71) | 63 (52-75) | .002 |
| Weeks to surgeryb,c | |||
| <8 | 153 (51.7) | 82 (34.9) | .001 |
| 8-12 | 100 (33.8) | 108 (46.0) | |
| 12-26 | 41 (13.9) | 36 (15.3) | |
| >26 | 2 (0.6) | 9 (3.8) | |
| Postoperative chemotherapyd | |||
| No | 63 (21.5) | 214 (94.7) | <.001 |
| Yes | 230 (78.5) | 12 (5.3) | |
| Ileostomy after low anterior resection | |||
| No | 33 (14.5) | 23 (12.5) | .56 |
| Yes | 195 (85.5) | 161 (87.5) | |
| Days to ileostomy closure, median (IQR) | 192 (166-243) | 89 (71-107) | <.001 |
| Ileostomy closure within 15 weekse | |||
| No | 176 (91.2) | 44 (28.0) | <.001 |
| Yes | 17 (8.8) | 113 (72.0) | |
| Months of follow-up, median (range) | 40 (6-92) | 23 (6-71) | <.001 |
Abbreviations: ChemoRT, chemoradiation; ERUS, endorectal ultrasound; MRI, magnetic resonance imaging; TNT, total neoadjuvant therapy.
Data were available for 245 patients in the chemoRT with planned adjuvant chemotherapy cohort and 294 patients in the TNT cohort.
After completion of neoadjuvant treatment.
Excluding patients in nonoperative protocols, ie, patients who did not undergo surgery within 12 months after completion of neoadjuvant therapy.
In patients who underwent surgery within 12 months.
In patients who underwent LAR with diverting ileostomy within 12 months.
Of the 539 patients in whom staging was performed at MSK, data were available for 245 patients in the ChemoRT with planned adjuvant therapy cohort and 294 patients in the TNT cohort. Staging modality evolved from ERUS to MRI during the period. Between 2009 and 2011, 67 (35%) of 190 patients were staged exclusively with ERUS and 123 (65%) with MRI compared with 4 (2%) of 172 with ERUS alone and 168 (98%) with MRI in 2014 to 2015. Therefore MRI was used in 442 of 539 patients: 160 (65%) of 245 patients in the ChemoRt with planned adjuvant therapy cohort and 282 (96%) of 294 patients in the TNT cohort (absolute difference, 31%; P < .001) (Table 1). In patients who had surgery within 12 months, median time to surgery following neoadjuvant therapy was longer in the TNT group (56 [IQR, 48-71] days vs 63 [IQR, 52-75] days, P < .002). Nonoperative treatment was more common in the TNT cohort: a higher proportion of TNT cohort patients did not undergo surgery within 12 months (73 [24%] of 308 vs 24 [8%] of 320; absolute difference, 16%; P < .001). Minimally invasive surgery was more common in the TNT cohort (169 [72%] of 265 vs 140 [47%] of 296; absolute difference 25%; P < .001). Diverting ileostomy rates following low anterior resection were comparable (chemoRT with planned adjuvant chemotherapy cohort ileostomy rate 195 [85.5%] of 228, vs TNT cohort ileostomy rate 161 [87.5%] of 184; absolute difference, 2%; P = .56), but stoma closure was earlier in the TNT group (closure within 15 weeks, 113 [72%] of 157 vs 176 [9%] of 193; absolute difference, 63%; P < .001).
FOLFOX Dosing and Tolerance
For analysis of prescribed and received dosing of FOLFOX, we included 101 of the total 230 patients in the chemoRT and planned adjuvant chemotherapy cohort and 249 patients in the TNT cohort because they received all treatment at MSK with dosage information available in the electronic medical record. Patients were treated according to NCCN guidelines, which include 4-month FOLFOX. eTable 1 in the Supplement lists the prescribed and received doses of fluorouracil and oxaliplatin. Analysis of fluorouracil showed that the TNT group had higher average doses received (96% [IQR, 93%-99%] vs 88% [IQR, 86%-90%], P = .003), fewer dose reductions (51 [50% ] of 101 vs 64 [26%] of 249; absolute difference, 25%; P < .001), greater proportions receiving more than 75% (231 [93%] of 249 vs 80 [79%] of 101; absolute difference, 14%; P < .001), and more than 90% (209 [84%] of 249 vs 56 [55%] of 101; absolute difference, 29%; P < .001) of planned dose, and a higher proportion receiving more than 6 cycles (236 [95%] of 249 vs 84 [83%] of 101; absolute difference, 22%; P < .001) compared with chemoRT and planned adjuvant chemotherapy. Similarly for oxaliplatin, the TNT group had higher average doses received (90% [IQR, 89%-91%] vs 73% [IQR, 69%-77%]; P < .001), fewer dose reductions (134 [54%] of 249 vs 77 [76%] of 101; absolute difference, 22%; P < .001), greater proportions of patients receiving more than 75% (210 [84%] of 249 vs 60 [60%] of 101; absolute difference, 24%; P < .001) and more than 90% (152 [61%] of 249 vs 28 [28%] of 101; absolute difference, 33%; P < .001) of planned dose, with a higher proportion receiving more than 6 cycles (214 [86%] of 249 vs 64 [63%] of 101; absolute difference, 23%; P < .001).
Clinical and Pathological Responses
Table 2 details CR data, including pCR and sustained cCR. In the chemoRT with planned adjuvant chemotherapy cohort, 49 [17%] of the 296 patients who underwent surgery within 12 months of preoperative therapy had a pCR, whereas 19 (6%) of 320 did not have surgery and had a sustained cCR at 12 months. The CR rate at 12 months after completing neoadjuvant chemoRT (combining pCR and cCR) was 21%. For 94 patients with clinical stage II disease and 226 with clinical stage III disease, CR rates were 25% and 20%, respectively.
Table 2. Responses to Treatment.
| Treatment Groupa | All Patients, No. | All Patients, Sustained cCR, No. (%)b | Surgery Within 12 Months, No. | Surgery Within 12 Months, pCR, No. (%)b | Complete Response (pCR and Sustained cCR) at 12 Months, No. (%) |
|---|---|---|---|---|---|
| ChemoRT with planned adjuvant chemotherapy | |||||
| Stage II | 94 | 9 (9.6) | 82 | 14 (17.1) | 23 (24.5) |
| Stage III | 226 | 10 (4.4) | 214 | 35 (16.4) | 45 (19.9) |
| Total | 320 | 19 (5.9) | 296 | 49 (16.6) | 68 (21.3) |
| TNT | |||||
| Stage II | 43 | 23 (53.5) | 20 | 0 | 23 (53.5) |
| Stage III | 265 | 44 (16.6) | 215 | 43 (20.0) | 87 (32.8) |
| Total | 308 | 67 (21.8) | 235 | 43 (18.3) | 110 (35.7) |
Abbreviations: cCR, clinical complete response; pCR, pathologic complete response; TNT, total neoadjuvant thearpy.
Stages are clinical.
pCR rates are percentages of patients among those who underwent resection within 12 months after completion of neoadjuvant therapy. cCR rates are percentages of patients among all patients in each cohort.
Among the 308 patients in the TNT cohort, 43 (18%) of the patients who underwent surgery within 12 months had a pCR, whereas 67 (22%) of 308 did not have surgery and had a sustained cCR at 12 months. The combined CR rate (pCR and cCR) at 12 months was 36%. For 43 patients with clinical stage II disease and 265 with clinical stage III disease, CR rates were 54% and 33%, respectively. To eliminate time to surgery as a confounder, a further analysis was performed excluding patients having surgery 8 weeks or less after neoadjuvant therapy. The CR rate remained higher at 41% in the TNT cohort (93 of 226) vs 27% in the chemoRT with planned adjuvant chemotherapy (45 of 166) cohort (absolute difference, 14%; P = .004).
Regression analyses identified age (using <65 years as reference category: 65-75 years; OR, 0.98; 95% CI, 0.63-1.36; P = .70; >75 years; OR, 3.28; 95% CI, 1.81-5.95; P < .001), recency of diagnosis (using 2009-2011 as reference category: 2012-2013; OR, 1.84; 95% CI, 1.20-2.83; P < .005; 2014-2015; OR, 1.72; 95% CI, 1.10-2.67; P = .02), lower clinical T stage (using cT4 as reference category: cT3; OR, 1.86; 95% CI, 0.89-3.91; P = .10; cT1-2; OR, 4.07; 95% CI, 1.61-10.33; P = .003), and TNT (OR, 2.06; 95% CI, 1.44-2.96; P < .001) to be associated with CR (pCR or cCR) at 12 months (eTable 2 in the Supplement).
Patients Followed With cCR
By definition for this study, all patients considered to have sustained cCR had no evidence of tumor regrowth for at least 12 months. In addition, no patients with sustained cCR developed distant recurrence within 12 months. In the chemoRT with planned adjuvant chemotherapy group, 19 (6%) of the total 320 patients with sustained cCR were treated nonoperatively beyond 12 months. and 10 (91%) of 11 patients with 24-month follow-up had a durable cCR. In the TNT group, 67 (22%) of the 308 patients had a sustained cCR and were treated nonoperatively beyond 12 months. Of 31 patients with 24-month follow-up, 27 (87%) had a durable cCR.
Discussion
To our knowledge, this single-institution study represents the largest published series of patients with LARC treated with neoadjuvant chemotherapy followed by chemoRT. Compared with chemoRT with planned adjuvant chemotherapy, patients receiving TNT were more likely to complete planned chemotherapy with fewer dose reductions. Patients receiving TNT also had greater treatment response, achieving higher CR rates. Our findings are consistent with previous studies demonstrating higher compliance and lower toxic event rates for TNT-based regimens, although response rates in those studies varied, with pCR rates ranging from 14% to 36%.2,18,19
In some patients, TNT facilitates early ileostomy closure after sphincter-sparing low anterior resection, and in other patients it can facilitate a nonoperative approach. Assessing response to neoadjuvant therapy requires accounting for both pCR in those undergoing surgery, as well as sustained cCR in those electing nonoperative treatment. We used the term CR to include pCR and sustained CR for longer than 12 months. In this TNT cohort, 43 (18%) of 235 patients who underwent surgery had a pCR, and an additional 67 (22%) of all 308 patients elected nonoperative therapy and had a sustained cCR for at least 12 months. This resulted in a combined CR (either pCR or cCR at 12 months) being observed in 110 (36%) of 308 patients. A pCR of 18% is lower than reported in other studies but reflects the fact that a high proportion of patients achieve a sustained cCR and opt for nonsurgical treatment. Patients with clinical stage II tumors had a higher CR rate than patients with clinical stage III tumors (23 [54%] of 43 vs 87 [33%] of 265, respectively). Pathological CR rates in other TNT studies range from 14% to 36%, although direct comparison is limited by differences in inclusion criteria (some studies included stage I disease), staging modalities and variable treatment of cCRs.18,19,20,21 The CR rate in the chemoRT with planned adjuvant chemotherapy cohort was 68 (21%) of 320, comparable to rates obtained in randomized clinical trials.22,23,24
We used a 12-month threshold for determining sustained cCR, which is supported in the literature, because over 70% of local regrowth will occur within 12 months.6,11,25,26,27 Of the 531 patients undergoing surgery within 12 months, 443 (83%) had resections within 12 weeks; 78 (18%) had pCRs. Seventy-seven (11%) had surgery between 12 and 26 weeks; 7 (9%) had pCRs. Eleven (2%) had surgery between 26 and 52 weeks; 5 (45%) had pCRs. Beyond 26 weeks, most patients had surgery as a result of clinical or radiological concern for tumor regrowth, but because almost half of these patients had a pCR, it is apparent that accurate identification of durable complete responses remains a challenge.
We also evaluated durable 24-month sustained cCR rates for patients with adequate follow-up. One (9%) of 11 patients and 4 (13%) of 31 patients with sustained cCR at 12 months had local tumor regrowth within 24 months in the chemoRT with planned adjuvant chemotherapy and TNT groups, respectively. These data support the use of the 12-month threshold to identify most durable cCRs. However, with further follow-up, this threshold may change.
The current study defined TNT as induction chemotherapy followed by chemoRT but consolidation chemotherapy regimens (delivered after chemoRT and prior to surgery) are another form of TNT (Figure, C) that is being investigated. Optimal scheduling of chemotherapy in relation to chemoRT is not known and is subject to an ongoing randomized clinical trial.28
During the study period, staging and treatment of LARC at MSK evolved with adoption of MRI (replacing ERUS) for local staging, increased use of TNT, and lengthening of the interval between chemoradiation and surgery. The increased use of MRI in later years of the study may have implications for interpretation of these results. For example, increased use of MRI could potentially explain the higher rate of clinically node-positive disease in the TNT group. However, we hypothesize that this finding was related to a tendency for clinicians to prescribe TNT early in the study period for more advanced tumors. Repeated analysis of MRI-staged patients confirms higher rates of clinically node-positive and cT4 tumors in the TNT cohort. This variation between cohorts was no longer apparent in 2014 to 2015 (n = 185).
Time from completing neoadjuvant therapy to surgery also lengthened during the study period, based on growing evidence that operating 12 weeks after radiotherapy is safe with improved treatment response.29,30 Time from neoadjuvant therapy to surgery was longer in the TNT cohort because this was the dominant treatment in recent years. To eliminate time to surgery as a confounder, we repeated the analysis excluding patients having surgery 8 weeks or less after radiotherapy. The higher CR rate (pCR and cCR combined) in the TNT group persisted.
Nonoperative treatment increased during the study period. A greater proportion receiving TNT chose a nonoperative approach compared with chemoRT and planned adjuvant chemotherapy. In this nonrandomized study, we cannot definitively attribute this finding to TNT. Nonetheless, TNT may optimize potential for cCRs, thereby potentially expanding the role of nonoperative treatment in LARC.
Limitations
Our study is limited by its retrospective design. There are potentially important differences between the groups that may have confounded results. For example the higher proportion of cT4 and clinically node-positive patients in the TNT cohort underestimated treatment effect in the TNT group. Conversely, longer time from completing treatment to surgery, along with recency of diagnosis, may be associated with greater treatment responses in the TNT cohort. Analyses of treatment effects may be limited by temporal trends, other baseline differences including differences in staging modality between the 2 cohorts (Table 1), and unrecognized changes in practice during the study period.
With limited follow-up, it is not known whether TNT, which optimizes dosing of systemic chemotherapy, improves disease-free survival. Further investigation with mature follow-up data are warranted. However the association between TNT and CR is encouraging because tumor response is associated with long-term outcome.31 Although not assessed routinely in these patients, molecular characteristics have potential to influence response to FOLFOX and CRT. Future work with the aim of determining the role of pretreatment molecular profiling including MSI status in LARC represents an important research priority.
Although definitive toxic effects data are not available, the higher chemotherapy doses prescribed in the TNT cohort may reflect better tolerance, consistent with other studies2,18 as well as a randomized clinical trial,19 where lower rates of treatment-related grade 3 to 4 events were reported for preoperative vs postoperative chemotherapy in LARC. Although a prospective randomized clinical trial would be definitive, TNT has already been adopted by the NCCN guidelines5 and incorporated into LARC trials. For example, TNT is the backbone for NCT02921256, a multiarm randomized phase II clinical trial in which patients receive induction FOLFOX before randomization to standard chemoradiation or a variety of sensitizers and radiation.
An expected criticism of the approach is that it does not allow for adaptive treatment strategies based on pathologic findings after chemoradiation. This could lead to potential overtreatment of LARC with systemic chemotherapy, especially for clinical stage II disease. A subset of 43 patients were clinically stage II, but notably, this cohort had the greatest response, supporting the use of TNT as part of an organ-preserving strategy in LARC. Nonetheless, use of systemic chemotherapy in clinical stage II disease may expose patients to unnecessary treatment-related toxic effects including oxaliplatin-related neuropathy and even hospitalization. Use of systemic chemotherapy for LARC is in keeping with the consensus approach per the NCCN guidelines, which are accepted and followed at our institution and in which adjuvant systemic therapy is based on the clinical stage and not the final pathologic stage. Thus, we report differences in response to and tolerance of neoadjuvant vs adjuvant treatment, not vs omission of chemotherapy.
Conclusions
Total neoadjuvant therapy was associated with improved delivery of systemic therapy and increased response to treatment, and it provides a promising platform for nonoperative watch-and-wait protocols. Long-term follow-up is necessary to determine if early systemic chemotherapy improves overall outcome.
eTable 1. FOLFOX chemotherapy doses received
eTable 2. Binary logistic regression analysis of CR at 12 months (pCR or cCR) in relation to patient and tumor characteristics (N=628)
eFigure. Trends in the delivery of neoadjuvant therapy for LARC at MSK
References
- 1.Cunningham D, Atkin W, Lenz HJ, et al. . Colorectal cancer. Lancet. 2010;375(9719):-. [DOI] [PubMed] [Google Scholar]
- 2.Chau I, Brown G, Cunningham D, et al. . Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24(4):668-674. [DOI] [PubMed] [Google Scholar]
- 3.Cercek A, Goodman KA, Hajj C, et al. . Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12(4):513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. . Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26(8):1722-1728. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network NCCN guidelines: rectal cancer. http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed February 2, 2017. 2016.
- 6.Appelt AL, Pløen J, Harling H, et al. . High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919-927. [DOI] [PubMed] [Google Scholar]
- 7.Haller DG, Tabernero J, Maroun J, et al. . Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465-1471. [DOI] [PubMed] [Google Scholar]
- 8.Schmoll HJ, Tabernero J, Maroun J, et al. . Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol. 2015;33(32):3733-3740. [DOI] [PubMed] [Google Scholar]
- 9.Schmoll HJ, Twelves C, Sun W, et al. . Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 2014;15(13):1481-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yothers G, O’Connell MJ, Allegra CJ, et al. . Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JJ, Chow OS, Eaton A, et al. . Organ preservation in patients with rectal cancer with clinical complete response after neoadjuvant therapy. J Clin Oncol. 2015;33(Suppl. 3):abstr. 509. [Google Scholar]
- 12.Trakarnsanga A, Gönen M, Shia J, et al. . Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106(10):dju248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washington MK, Berlin J, Branton P, et al. ; Members of the Cancer Committee, College of American Pathologists . Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133(10):1539-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habr-Gama A, Perez RO, Nadalin W, et al. . Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692-1698. [DOI] [PubMed] [Google Scholar]
- 16.Maas M, Beets-Tan RG, Lambregts DM, et al. . Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633-4640. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team R: a language and environment for statistical computing, 2014. http://www.R-project.org. Accessed July 5, 2017.
- 18.Nogué M, Salud A, Vicente P, et al. ; AVACROSS Study Group . Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16(5):614-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Martos C, Pericay C, Aparicio J, et al. . Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28(5):859-865. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Aguilar J, Chow OS, Smith DD, et al. ; Timing of Rectal Cancer Response to Chemoradiation Consortium . Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua YJ, Barbachano Y, Cunningham D, et al. . Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(3):241-248. [DOI] [PubMed] [Google Scholar]
- 22.Aschele C, Cionini L, Lonardi S, et al. . Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773-2780. [DOI] [PubMed] [Google Scholar]
- 23.Gérard JP, Azria D, Gourgou-Bourgade S, et al. . Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30(36):4558-4565. [DOI] [PubMed] [Google Scholar]
- 24.Rödel C, Graeven U, Fietkau R, et al. ; German Rectal Cancer Study Group . Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979-989. [DOI] [PubMed] [Google Scholar]
- 25.Smith JD, Ruby JA, Goodman KA, et al. . Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256(6):965-972. [DOI] [PubMed] [Google Scholar]
- 26.Renehan AG, Malcomson L, Emsley R, et al. . Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174-183. [DOI] [PubMed] [Google Scholar]
- 27.Habr-Gama A, Perez RO, Proscurshim I, et al. . Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10(10):1319-1328. [DOI] [PubMed] [Google Scholar]
- 28.Smith JJ, Chow OS, Gollub MJ, et al. ; Rectal Cancer Consortium . Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habr-Gama A, Perez RO, Proscurshim I, et al. . Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71(4):1181-1188. [DOI] [PubMed] [Google Scholar]
- 30.Kalady MF, de Campos-Lobato LF, Stocchi L, et al. . Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(4):582-589. [DOI] [PubMed] [Google Scholar]
- 31.Quah HM, Chou JF, Gonen M, et al. . Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer. 2008;113(1):57-64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. FOLFOX chemotherapy doses received
eTable 2. Binary logistic regression analysis of CR at 12 months (pCR or cCR) in relation to patient and tumor characteristics (N=628)
eFigure. Trends in the delivery of neoadjuvant therapy for LARC at MSK