Abstract
Importance
Myelofibrosis is a hematologic malignancy characterized by splenomegaly and debilitating symptoms. Thrombocytopenia is a poor prognostic feature and limits use of Janus kinase 1 (JAK1)/Janus kinase 2 (JAK2) inhibitor ruxolitinib.
Objective
To compare the efficacy and safety of JAK2 inhibitor pacritinib with that of best available therapy (BAT), including ruxolitinib, in patients with myelofibrosis and thrombocytopenia.
Design, Setting, and Participants
For this phase 3 randomized international multicenter study—the PERSIST-2 study—of pacritinib vs BAT, 311 patients with myelofibrosis and platelet count 100 × 109/L or less were recruited for analysis. Crossover from BAT was allowed after week 24 or for progression of splenomegaly.
Interventions
Patients were randomized 1:1:1 to pacritinib 400 mg once daily, pacritinib 200 mg twice daily, or BAT.
Main Outcomes and Measures
Coprimary end points were rates of patients achieving 35% or more spleen volume reduction (SVR) and 50% or more reduction in total symptom score (TSS) at week 24. Efficacy analyses were performed on the intention-to-treat efficacy population, comprising all patients with a randomization date allowing for week 24 data.
Results
Overall, 311 patients (mean [SD] age, 63.70 [9.08] years; 171 men [55%] and 140 women [45%]) were included in the study; 149 patients (48%) had prior ruxolitinib. The most common BAT was ruxolitinib (44 patients [45%]); 19 patients (19%) received watchful-waiting only. The intention-to-treat efficacy population included 75 patients randomized to pacritinib once daily; 74, pacritinib twice daily, and 72, BAT. Pacritinib (arms combined) was more effective than BAT for 35% or more SVR (27 patients [18%] vs 2 patients [3%]; P = .001) and had a nonsignificantly greater rate of 50% or more reduction in TSS (37 patients [25%] vs 10 patients [14%]; P = .08). Pacritinib twice daily led to significant improvements in both end points over BAT (≥35% SVR: 16 patients [22%] vs 2 patients [3%]; P = .001; ≥50% reduction in TSS: 24 patients [32%] vs 10 patients [14%]; P = .01). Clinical improvement in hemoglobin and reduction in transfusion burden were greatest with pacritinib twice daily. For pacritinib once daily, pacritinib twice daily, and BAT, the most common (>10%) grade 3 or 4 adverse events were thrombocytopenia (32 patients [31%], 34 patients [32%], 18 patients [18%]), and anemia (28 patients [27%], 23 patients [22%], 14 patients [14%]). In the pacritinib once daily, twice daily, and BAT arms, discontinuation owing to adverse events occurred in 15 patients (14%), 10 patients (9%), and 4 patients (4%).
Conclusions and Relevance
In patients with myelofibrosis and thrombocytopenia, including those with prior anti-JAK therapy, pacritinib twice daily was more effective than BAT, including ruxolitinib, for reducing splenomegaly and symptoms.
Trial Registration
clinicaltrials.gov Identifier: NCT02055781
This phase 3 randomized clinical trial compares the efficacy and safety of Janus kinase 2 inhibitor pacritinib with that of best available therapy, including ruxolitinib, in patients with myelofibrosis and thrombocytopenia.
Key Points
Question
Is pacritinib (400 mg once daily or 200 mg twice daily) superior to best available therapy for patients with myelofibrosis and thrombocytopenia?
Findings
In this randomized clinical trial of 311 patients, pacritinib 200 mg twice daily was significantly more effective than best available therapy, including ruxolitinib, for reducing splenomegaly and symptoms in patients with myelofibrosis and thrombocytopenia, including those with prior ruxolitinib.
Meaning
Pacritinib can provide a treatment option for patients with myelofibrosis and baseline thrombocytopenia, for whom current treatment options are limited.
Introduction
Myelofibrosis is a myeloproliferative neoplasm characterized by dysregulated Janus kinase/signal transducers and activators of transcription signaling and excessive production of inflammatory cytokines.1 Advanced disease is characterized by progressive bone marrow fibrosis, cytopenias frequently requiring red blood cell (RBC) and/or platelet transfusions, splenomegaly, and debilitating systemic symptoms.2,3,4 Median survival is approximately 6 years.5 Thrombocytopenia is an adverse prognostic variable that increases in prevalence with disease progression and is associated with higher symptom burden and shorter survival.4,6,7,8 The Janus kinase 1 (JAK1)/Janus kinase 2 (JAK2) inhibitor ruxolitinib is approved by the US Food and Drug Administration (FDA) for patients with intermediate-risk or high-risk myelofibrosis and a baseline platelet count of 50 × 109/L or more. Despite spleen volume reduction (SVR) and symptom improvement with ruxolitinib, an unmet clinical need exists for patients with baseline or treatment-emergent thrombocytopenia, and those relapsed after or refractory to ruxolitinib. Pacritinib, is a JAK2/tyrosine kinase 3 inhibitor with negligible activity against JAK1 that also suppresses the interleukin-1 directed inflammatory pathway via inhibition of interleukin 1 receptor associated kinase 1.9,10 Pacritinib was shown to be clinically active in patients with myelofibrosis with minimal myelosuppression.11 In the phase 3 PERSIST-1 trial (NCT01773187) comparing pacritinib once daily with best available therapy (BAT) excluding ruxolitinib, pacritinib demonstrated significant and durable SVR and symptom control irrespective of baseline platelet count.12 A full clinical hold was placed on pacritinib by the FDA on February 8, 2016, due to concerns over bleeding and cardiovascular events and deaths on PERSIST-1. The hold caused early termination of PERSIST-2 resulting in incomplete data. The FDA requested an additional study to determine if a lower dose of pacritinib would be safer with maintained efficacy. After review of mature PERSIST-1 data and complete PERSIST-2 data (NCT02055781), as well as submission of a study protocol comparing 3 doses of pacritinib (NCT03165734), the hold was removed on January 5, 2017. The final results (database lock August 19, 2016) of the PERSIST-2 trial comparing pacritinib with BAT (including ruxolitinib) in patients with intermediate/high-risk myelofibrosis and baseline platelet count less than or equal to 100 × 109/L are reported herein. Population pharmacokinetic modeling predicted higher steady state exposure and lower maximum concentrations with twice daily vs once daily pacritinib (early-phase studies)13; thus PERSIST-2 evaluated both schedules.
Methods
Patients
Adult patients with primary or secondary myelofibrosis were eligible if they had intermediate-1, intermediate-2, or high-risk disease by the Dynamic International Prognostic Scoring System (DIPSS), platelet count less than or equal to 100 × 109/L, and palpable splenomegaly 5 cm or larger below the left costal margin. Additional eligibility criteria included: total symptom score (TSS) greater than or equal to 13 on the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS 2.0); Eastern Cooperative Oncology Group performance status 0 to 3 (scale from 0 to 5, with 0 indicating no symptoms and higher numbers indicating increasing disability); peripheral blood blast count less than 10%; absolute neutrophil count greater than 0.5 × 109/L; adequate liver and renal function; and life expectancy 6 months or longer. Prior treatment with 1 or 2 other JAK inhibitors was allowed. Patients with active bleeding requiring hospitalization during screening or significant cardiac abnormalities (eg, recent history of myocardial infarction, severe/unstable angina, symptomatic congestive heart failure, ongoing grade ≥3 dysrhythmias, prolonged QTc) were excluded (eMethods in Supplement 1). The study was approved by the institutional review boards at each participating institution and conducted in accordance with the principles outlined in the Declaration of Helsinki. All patients provided written informed consent, and the trial protocol is available in Supplement 2.
Randomization and Treatment
Patients were centrally randomized 1:1:1 via interactive web or voice response system to pacritinib 400 mg once daily, pacritinib 200 mg twice daily, or BAT; BAT included any physician-selected treatment for myelofibrosis, symptom-directed treatment, or watch-and-wait. Randomization was stratified by geographic region, risk category, and rebound platelet count. Note, “rebound” refers to recovery of platelet count between informed consent and randomization, indicating likelihood of drug-induced thrombocytopenia rather than thrombocytopenia associated with myelofibrosis (eMethods in Supplement 1). Permuted blocks within strata were used to restrict treatment allocation. A patient’s treatment assignment was known to the investigator, site personnel, the patient, clinical monitors, and pharmacovigilance personnel. The sponsor remained blinded until database lock for the primary analysis and independent radiographic assessors remained blinded throughout the study. Patients in all arms were to be treated until progressive disease, unacceptable toxic effects, or the patient no longer derived benefit from treatment (eMethods in Supplement 1). Patients randomized to BAT could cross over to pacritinib for progression of splenomegaly or after 24 weeks of treatment with or without progression.
End Points
The primary objective was to compare the efficacy of the pooled pacritinib arms with BAT. The efficacy coprimary end points were the proportions of patients achieving 35% or more SVR (computed tomography/magnetic resonance imaging [CT/MRI]) and 50% or more reduction in TSS (MPN-SAF TSS 2.0) from baseline to week 24. A secondary objective was to separately compare efficacy of pacritinib once daily or twice daily vs BAT. Exploratory end points included overall survival, changes in hematologic parameters, and additional patient-reported outcomes. Safety and tolerability were assessed from time of informed consent through last day of study participation. Pharmacokinetics of pacritinib were characterized to assess exposure and exposure-response relationships (safety and efficacy).
Assessments
Spleen volume by CT/MRI was reviewed centrally by a blinded, independent radiology facility at baseline and every 12 weeks through 48 weeks until progressive disease or withdrawal from study treatment. Patient-reported symptoms were recorded daily (MPN-SAF TSS 2.0) through 48 weeks or until end of study treatment, whichever occurred first. Scores for Patient Global Impression Assessment were assessed every 8 weeks through week 24, then every 12 weeks until discontinuation. Adverse events were classified and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.03.
Statistical Analysis
A sample size of 300 patients was planned to provide greater than 99% power in detecting treatment differences (pacritinib vs BAT) in SVR and TSS reduction at α level (2-sided) of .05. This sample size also provided 96% and greater than 99% power to detect treatment differences (pacritinib once daily or pacritinib twice daily vs BAT) in SVR and TSS reduction, respectively, at α level (2-sided) of .025. A 20% and 45% difference in the proportion of pacritinib vs BAT patients with 35% or more SVR and 50% ore more reduction in TSS at week 24, respectively, was assumed for power calculation. Efficacy analyses were performed on the intention-to-treat efficacy population, comprising all patients with a randomization date allowing for week 24 data (≥22 weeks prior to clinical hold). Treatment differences in proportion of patients achieving SVR or TSS reduction were tested using the Fisher exact test, and confidence intervals were based on the Agresti-Caffo method. Time-to-event analyses were censored on the date of clinical hold. The safety population included all patients who received 1 or more dose of study treatment. Final end-of-treatment data were used.
Results
Patients and Study Treatment
From July 2014 to February 2016, 311 patients were randomized to pacritinib once daily (n = 104), pacritinib twice daily (n = 107), or BAT (n = 100; Figure 1). The most commonly used active single agents in the BAT arm were ruxolitinib (n = 44 [45%]), hydroxyurea (n = 19 [19%]), and prednisone and/or prednisolone (n = 13 [13%]); 19 patients (19%) received watchful-waiting only (eTable 1 in Supplement 1). One patient randomized to pacritinib twice daily and 2 patients randomized to BAT withdrew before receiving treatment and were excluded from the safety population. The intention-to-treat efficacy population included 75, 74, and 72 patients randomized to pacritinib once daily, twice daily, and BAT, respectively. Baseline demographics and disease characteristics were balanced across arms (eTable 2 in Supplement 1). One-hundred fifteen patients (52%) had an intermediate-2 DIPSS score, and 66 (30%) had high-risk DIPSS scores. In the pacritinib once daily, twice daily, and BAT arms, 38 patients (51%), 31 patients (42%), and 32 patients (44%) had baseline platelet count less than 50 × 109/L, respectively.
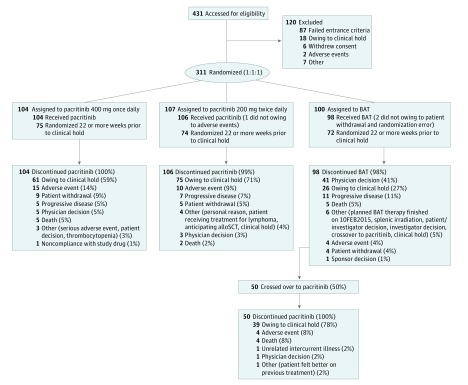
Figure 1. CONSORT Diagram.
Outcomes for all randomized patients are shown. BAT indicates best available therapy; alloSCT, allogeneic stem cell transplant.
After 23, 25, and 21 median weeks on pacritinib once daily, twice daily, and BAT, respectively, all 308 treated patients had discontinued therapy (eTable 3 in Supplement 1); of the patients that had discontinued therapy 62 of 104 patients treated with pacritinib once daily, 75 of 106 patients treated with pacritinib twice daily, and 27 of 98 patients treated with BAT were discontinued due to institution of the clinical hold. The majority of BAT discontinuations were owing to crossover to pacritinib; 50 of 98 patients (51%) treated with BAT crossed over primarily (n = 43 [86%]) at or after week 24. Of these patients, 39 (78%) continued on pacritinib until the clinical hold. Prior to the hold, discontinuations owing to adverse events trended higher with pacritinib once daily (15 patients [14%]) vs other arms (10 patients [9%] with pacritinib twice daily, 4 patients [4%] with BAT), and discontinuation due to progressive disease trended higher with BAT (10 patients [10%]) vs pacritinib (once daily, 5 patients [5%]; twice daily, 7 patients [7%]). At the time of the clinical hold, 76 patients (71%) randomized to pacritinib twice daily vs 61 patients (59%) randomized to pacritinib once daily were on study treatment. Dose interruptions due to adverse events were higher with pacritinib (once daily, 39 patients [38%]; twice daily, 29 patients [27%]) vs BAT (10 patients [10%]) and dose reductions due to adverse events were higher with pacritinib once daily (21 patients [20%]) vs other arms (13 patients [12%] with pacritinib twice daily; 7 patients [7%] with BAT) (eTable 3 in Supplement 1).
Spleen Volume Reduction
At week 24, 27 patients (18%) on pacritinib arms (11 [15%], pacritinib once daily; 16 [22%], pacritinib twice daily) achieved SVR 35% or more compared with 2 patients (3%) on BAT (P = .001, P = .02, P = .001, respectively (Table) (Figure 2). Only 1 of 32 patients (3%) on the BAT arm who received ruxolitinib achieved SVR 35% or more. Of note, the majority of ruxolitinib-treated patients began with 5 mg twice daily dosing (at discretion of treating physician). With pacritinib once daily and twice daily, SVR 35% or more was achieved in 2 patients (6%) and 4 patients (13%) with prior ruxolitinib (Table). Additional subgroup analyses demonstrated pacritinib patients achieved SVR 35% or more regardless of subgroup, including as defined by sex, age, JAK2 V617F mutation status, prior treatment with JAK2 inhibitors, and baseline cytopenias (eFigure 1 in Supplement 1).
Table. Efficacy Summary of the Intention-to-Treat Efficacy Population.
| Reductions From Baseline to Week 24 | Pacritinib 400 mg Once Daily | Pacritinib 200 mg Twice Daily | BAT |
|---|---|---|---|
| Overall | |||
| Patients with ≥35% SVR | |||
| Overall population, No. | 75 | 74 | 72 |
| Achieved end point, No. (%) | 11 (15) | 16 (22) | 2 (3) |
| 95% CI for the %a | 7.6-24.7 | 12.9-32.7 | 0.3-9.7 |
| P value vs BAT | .02 | .001 | NA |
| Patients with ≥50% reduction in TSS | |||
| Overall population, No. | 75 | 74 | 72 |
| Achieved end point, No. (%) | 13 (17) | 24 (32) | 10 (14) |
| 95% CI for the %a | 9.6-27.8 | 22.0-44.3 | 6.9-24.1 |
| P value vs BAT | .65 | .01 | NA |
| Patients With Prior Ruxolitinib | |||
| Patients with ≥35% SVR | |||
| Overall population, No. | 31 | 31 | 33 |
| Achieved end point, No. (%) | 2 (6) | 4 (13) | 1 (3) |
| 95% CI for the %a | 0.8-21.4 | 3.6-29.8 | 0.1-15.8 |
| Patients with ≥50% reduction in TSS | |||
| Overall population, No. | 31 | 31 | 33 |
| Achieved end point, No. (%) | 3 (10) | 10 (32) | 5 (15) |
| 95% CI for the %a | 2.0-25.8 | 16.7-51.4 | 5.1-31.9 |
| Patients With Baseline Platelets <50 × 109/L | |||
| Patients with ≥35% SVR from baseline to week 24 | |||
| Overall population, No. | 38 | 31 | 32 |
| Achieved end point, No. (%) | 7 (18) | 9 (29) | 1 (3) |
| 95% CI for the %a | 7.7-34.3 | 14.2-48.0 | 0.1-16.2 |
| Patients with ≥50% reduction in TSS | |||
| Overall population, No. | 38 | 31 | 32 |
| Achieved end point, No. (%) | 6 (16) | 7 (23) | 4 (13) |
| 95% CI for the %a | 6.0-31.3 | 9.6-41.1 | 3.5-29.0 |
Abbreviations: BAT, best available therapy; NA, not applicable; SVR, spleen volume reduction; TSS, total symptom score.
Clopper-Pearson method.
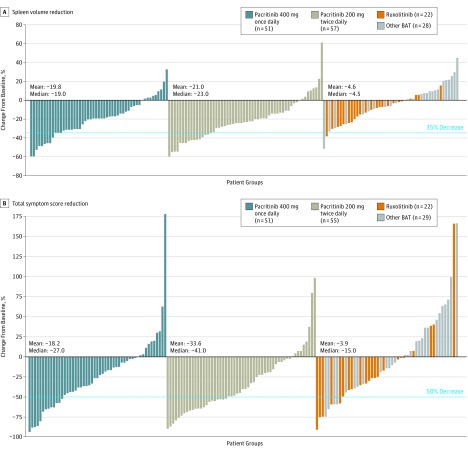
Figure 2. Spleen Volume Reduction and Reduction in Total Symptom Score in Evaluable Patients.
Waterfall plots for spleen volume reduction (A) and total symptom score reduction (B) from baseline for evaluable patients are shown. Mean and median percent decreases from baseline were greater with pacritinib vs BAT. Of note, SVR≥10% was achieved in 72.5%, 78.9%, and 36.0% of evaluable patients with pacritinib once daily, pacritinib twice daily, and best available therapy, respectively. BAT indicates best available therapy. Each bar represents a single patient.
Symptom Reduction
At week 24, 37 patients (25%) on pacritinib arms (13 [17%], once daily; 24 [32%], twice daily) achieved reduction in TSS 50% or more, compared with 10 patients (14%) on BAT (P = .08, P = .65, and P = .01, respectively) (Table) (Figure 2). Six of 32 patients (19%) on BAT who received ruxolitinib achieved reduction in TSS 50% or more. With pacritinib once daily and twice daily, TSS reduction greater than or equal to 50% was achieved in 3 patients (10%) and 10 patients (32%) with prior ruxolitinib (Table). Median percent change in TSS at week 24 was −27%, −41%, and −15% with pacritinib once daily, twice daily, and BAT, respectively. Median percent change in individual MPN-SAF TSS 2.0 symptom scores also showed greater improvements with pacritinib once daily and twice daily in 7 of 8 and 8 of 8 symptoms, respectively, vs BAT (eFigure 2 in Supplement 1). Additionally, the number of patients with much-improved or very much–improved Patient Global Impression Assessment scores were 16 of 45 (36%) and 26 of 46 (57%) with pacritinib once daily and twice daily vs 11 of 40 (28%) with BAT (eFigure 3 in Supplement 1).
Overall Survival
Overall survival curves from randomization dates to clinical hold are shown in eFigure 4 in Supplement 1. Differences between the 3 arms did not reach significance (hazard ratios vs BAT for pacritinib once daily and twice daily: 1.18; 95% CI, 0.57-2.44 and 0.68; 95% CI, 0.30-1.53). For patients on the BAT arm, the overall death rate was lower for those who crossed over to pacritinib (4 patients [8%]) vs those who did not (10 patients [20%]). Subgroup analyses for survival by risk factors showed that hazard ratios for pacritinib twice daily were less than 1 with the exception of patients who were more than 1.5 years from diagnosis or secondary myelofibrosis (eFigure 5A in Supplement 1). Similar analyses of pacritinib once daily vs BAT are shown in eFigure 5B in Supplement 1, where hazard ratios were more disparate across arms.
Changes in Hematologic Parameters
For patients who received 1 or more RBC unit on study, transfusion requirements at weeks 12 and 24 were lower in each pacritinib arm vs BAT (eFigure 6 in Supplement 1). For patients not RBC transfusion-independent at baseline (Gale criteria14), the proportion of patients with reduced RBC transfusion burden at week 24 was higher with pacritinib once daily (7 of 37 patients [19%]) and pacritinib twice daily (8 of 36 patients [22%]) vs BAT (3 of 35 patients [9%]). Transfusion independence (Gale criteria14) was achieved in 2 patients, 1 each with pacritinib once daily and twice daily. For patients with hemoglobin less than 10 g/dL at baseline, the proportion of patients with clinical improvement in hemoglobin at week 24 (International Working Group response criteria, increase of ≥2.0 g/dL or RBC transfusion independence for ≥8 weeks prior) was greatest with pacritinib twice daily (11 of 44 patients [25%]) vs pacritinib once daily (6 of 45 patients [13%]) or BAT (5 of 41 patients [12%]). For patients with baseline platelet count less than 50 × 109/L, median percent changes in platelets through week 24 are shown in eFigure 7 in Supplement 1. No evidence for increasing thrombocytopenia or neutropenia with pacritinib or BAT was noted.
Pharmacokinetics
As predicted by pharmacokinetic modeling and simulation data, in 144 pacritinib-treated patients with up to 24-week postdose samples, pacritinib twice daily was associated with higher systemic exposure (ie, steady state minimum serum concentration [Cmin]) relative to pacritinib once daily (eTable 4 in Supplement 1). In an exposure-response analysis, no trends were detected for a relationship between observed steady state Cmin and death, cardiovascular death, hemorrhagic death, cardiac events (grade ≥2 or ≥3), hemorrhagic events, thrombocytopenia (grade ≥2 or ≥3), anemia (grade ≥2 or ≥3), gastrointestinal events (any grade, grade ≥2, or ≥3), or maximum QTcF greater than 450 ms. Treatment with pacritinib twice daily but not once daily was associated with a trend for increased SVR and TSS reduction with increased Cmin.
Safety
The most commonly reported (≥15%) nonhematologic adverse events with pacritinib were gastrointestinal adverse events, fatigue, peripheral edema, and dizziness; with BAT (including 19 patients with watchful-waiting only) were abdominal pain, fatigue, diarrhea, and peripheral edema (eTable 3 in Supplement 1); the majority of common nonhematologic adverse events were grade 1 or 2 in severity. Diarrhea was the most frequently observed adverse event with pacritinib (112 instances [53%], grade 1/2; 9 instances [4%], grade 3), most often occurring during weeks 1 to 8. Incidence of diarrhea was lower with pacritinib twice daily vs once daily (51 instances [48%] vs 70 instances [67%]) (eTable 5 in Supplement 1). Diarrhea was manageable with standard antidiarrheal agents (eg, loperamide) and generally resolved within 1 to 2 weeks (eTable 5 in Supplement 1). The most common adverse event leading to discontinuation was thrombocytopenia with pacritinib once daily (4 instances [4%]) and BAT (2 instances [2%]) and anemia with pacritinib twice daily (3 instances [3%] (eTable 6 in Supplement 1). Rate of on-study death was lowest with pacritinib twice daily (6 instances [6%]) vs BAT (9 instances [9%]) or pacritinib once daily (14 instances [14%] (eTable 7 in Supplement 1).
The incidence of all hematologic adverse events was similar for patients with baseline platelet count less than 50 × 109/L vs 50 × 109/L or more with pacritinib once daily (27 patients [54%] vs 27 patients [52%]) and pacritinib twice daily (28 patients [60%] vs 33 patients [57%]), but was higher for patients with a baseline platelet count less than 50 × 109/L vs 50 × 109/L or more with BAT (22 patients [52%] vs 21 patients [38%]). Cardiac events (per standardized MedDRA query, see eMethods in Supplement 1) were reported at similar rates in all arms (67 patients [32%], pacritinib once daily or twice daily; 27 patients [28%], BAT) and were most commonly peripheral edema in all arms (13%-20%). Grade 3 or 4 cardiac events were reported in 13 patients (13%) treated with pacritinib once daily, 7 patients (7%) treated with pacritinib twice daily, and 9 patients (9%) treated with BAT (eTable 8 in Supplement 1). No cardiac event led to discontinuation of therapy in 2 or more patients (eTable 6 in Supplement 1). Bleeding events (per standardized MedDRA query, see eMethods in Supplement 1) were reported at similar rates in all arms (37 patients [36%], 45 patients [42%], and 40 patients [41%] treated with pacritinib once daily, twice daily, and BAT, respectively) and were most commonly epistaxis in all arms (11%-13%). Grade 3 or 4 bleeding events were reported in 7 patients (7%), 15 patients (14%), and 7 patients (7%) treated with pacritinib once daily, twice daily, and BAT, respectively (eTable 8 in Supplement 1). Epistaxis was the only bleeding event that led to discontinuation in 2 or more patients (1 pacritinib twice daily, 1 BAT) (eTable 6 in Supplement 1). Rates of grade 3 or 4 bleeding events were similar for patients with baseline platelet count less than 50 × 109/L vs 50 × 109/L or more with pacritinib twice daily (7 patients [15%] vs 8 patients [14%]) and higher for those with less than 50 × 109/L with pacritinib once daily (6 patients [12%] vs 1 patient [2%]) and BAT (5 patients [12%] vs 2 patients [4%]).
When the clinical hold was put in place and patients abruptly discontinued pacritinib, spleen size increased, and symptoms recurred rapidly and were difficult to control. While withdrawal syndrome is a potential safety concern, the longer elimination half-life of pacritinib (approximately 40 hours) may reduce exposure more gradually.
Discussion
Myelofibrosis is a chronic progressive myeloproliferative neoplasm with no proven therapeutic options for patients with thrombocytopenia,2 which increases in prevalence and severity with disease progression.6 Patients ineligible for, or who fail therapy with JAK1/JAK2 inhibitor ruxolitinib, lack therapeutic options and have extremely poor prognosis.15 In the primary analysis of the PERSIST-2 study at week 24, the combined pacritinib arms were significantly more effective than BAT for SVR and trended toward improved TSS reduction in patients with myelofibrosis and thrombocytopenia. The benefit of pacritinib in terms of SVR and TSS reduction was observed in patients with baseline platelet count less than 50 × 109/L and those with prior ruxolitinib treatment (comprising >40% of patients). The pacritinib twice daily arm met both coprimary SVR and TSS reduction end points. The pharmacokinetic profile of pacritinib twice daily also supports the use of this dose schedule.
Pacritinib was well tolerated and gastrointestinal toxic effects were generally low grade, less frequent with twice daily dosing, and rarely led to treatment discontinuation. Clinical improvement in hemoglobin and reduction in transfusion requirements were also more frequent in patients who received pacritinib, particularly with twice-daily dosing. A potential increased risk of grade 3 or 4 bleeding was noted with pacritinib twice daily but appeared to be independent of platelet count. Rate of grade 3 or 4 cardiac events was lowest with pacritinib twice daily vs other arms and no cardiac failure or deaths due to cardiac events occurred with pacritinib twice daily.
Limitations
Study truncation due to the clinical hold compromised the ability to evaluate the effect of treatment on week 24 end points (including coprimary end points) by reducing the effective sample size, and also compromised time-to-event end points (including overall survival) by reducing follow-up time for assessment that would enable analyses that follow the intention-to-treat principle.16 Time-to-event analyses were also confounded by patient crossover. Truncation of the study also limited the follow-up of patients for additional safety data. Despite these limitations, pacritinib 200 mg twice daily was more effective than BAT with a benefit-risk profile that compared favorably with pacritinib once daily and BAT.
Conclusions
The results of PERSIST-2 demonstrate the clinical benefit of pacritinib in patients with myelofibrosis and thrombocytopenia (platelet count ≤100 × 109/L), including those with prior JAK2 inhibitor therapy.
eAppendix. List of PERSIST-2 Study Investigators
eMethods.
eTable 1. Best Available Therapy Received in ≥1 Patient
eTable 2. Patient Demographics and Disease Characteristics (Intention-to-Treat Efficacy Population)
eTable 3. Treatment Outcomes and Treatment-Emergent Adverse Events (Safety Population)
eTable 4. Pacritinib Exposure
eTable 5. Time to Onset of First Diarrhea (Safety Population)
eTable 6. Discontinuations Due to Adverse Events (Safety Population)
eTable 7. Summary of On-Study Deaths (Intention-To-Treat Population)
eTable 8. Cardiac and Bleeding Events (Safety Population)
eFigure 1. Proportion of Patients with SVR≥35% by Subgroup
eFigure 2. Changes in Individual Symptom Scores per MPN-SAF TSS 2.0
eFigure 3. Patient Global Impression Assessment (Evaluable Population)
eFigure 4. Overall Survival (Intention-to-Treat, Censored at Date of Clinical Hold)
eFigure 5. Subgroup Analysis for Overall Survival (Intention-to-Treat, Censored at Date of Clinical Hold)
eFigure 6. RBC Transfusions Over Time (Intention-to-Treat)
eFigure 7. Change in Platelets Over Time in Patients with Platelets <50 × 109/L at Baseline
Trial Protocol.
References
- 1.Rampal R, Al-Shahrour F, Abdel-Wahab O, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123(22):e123-e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cervantes F. How I treat myelofibrosis. Blood. 2014;124(17):2635-2642. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392-397. [DOI] [PubMed] [Google Scholar]
- 5.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895-2901. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc. 2012;87(1):25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geyer H, Scherber R, Kosiorek H, et al. Symptom burden profile in myelofibrosis patients with thrombocytopenia: lessons and unmet needs. Blood. 2015:126.abstract 4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alhuraiji A, Masarova L, Bose P, et al. Clinical features and outcome of patients with poor-prognosis myelofibrosis based on platelet count <50 x 109/L: a single-cener experience in 1100 myelofibrosis patients. J Clin Oncol. 2016;34 suppl:abstract 7068. [Google Scholar]
- 9.Hatzimichael E, Tsolas E, Briasoulis E. Profile of pacritinib and its potential in the treatment of hematologic disorders. J Blood Med. 2014;5:143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer JW, Al-Fayoumi S, Ma H, Komrokji RS, Mesa R, Verstovsek S. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J Exp Pharmacol. 2016;8:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komrokji RS, Seymour JF, Roberts AW, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood. 2015;125(17):2649-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesa R, Vannucchi A, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225-e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Fayoumi S, Wang L, Li H, Wada R, Dean JP. Exposure-response analysis for pacritinib (SB1518), a novel oral JAK2/FLT3 inhibitor, in patients with myelofibrosis. Blood. 2013:122.abstract 4080. [Google Scholar]
- 14.Gale RP, Barosi G, Barbui T, et al. What are RBC-transfusion-dependence and -independence? Leuk Res. 2011;35(1):8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardanani A, Tefferi A. Definition and management of ruxolitinib treatment failure in myelofibrosis. Blood Cancer J. 2014;4:e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ. 2001;165(10):1339-1341. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. List of PERSIST-2 Study Investigators
eMethods.
eTable 1. Best Available Therapy Received in ≥1 Patient
eTable 2. Patient Demographics and Disease Characteristics (Intention-to-Treat Efficacy Population)
eTable 3. Treatment Outcomes and Treatment-Emergent Adverse Events (Safety Population)
eTable 4. Pacritinib Exposure
eTable 5. Time to Onset of First Diarrhea (Safety Population)
eTable 6. Discontinuations Due to Adverse Events (Safety Population)
eTable 7. Summary of On-Study Deaths (Intention-To-Treat Population)
eTable 8. Cardiac and Bleeding Events (Safety Population)
eFigure 1. Proportion of Patients with SVR≥35% by Subgroup
eFigure 2. Changes in Individual Symptom Scores per MPN-SAF TSS 2.0
eFigure 3. Patient Global Impression Assessment (Evaluable Population)
eFigure 4. Overall Survival (Intention-to-Treat, Censored at Date of Clinical Hold)
eFigure 5. Subgroup Analysis for Overall Survival (Intention-to-Treat, Censored at Date of Clinical Hold)
eFigure 6. RBC Transfusions Over Time (Intention-to-Treat)
eFigure 7. Change in Platelets Over Time in Patients with Platelets <50 × 109/L at Baseline
Trial Protocol.