Key Points
Question
Does a combination of sulindac and erlotinib inhibit colorectal adenoma formation in patients with familial adenomatous polyposis (FAP)?
Findings
In this secondary analysis of the FAP Erlotinib-Sulindac Trial, a phase 2 randomized placebo-controlled trial of sulindac and erlotinib in 82 patients with FAP, treatment with sulindac and erlotinib was associated with a 69% reduction in colorectal polyp burden in patients with FAP.
Meaning
This study lays an important foundation for effective and feasible chemoprevention of colorectal adenomas in patients with familial adenomatous polyposis, which has the potential to decrease the need for endoscopic treatment and surgical intervention.
This secondary analysis of data from the the FAP Erlotinib-Sulindac Trial (FAPEST) trial examines the effect of sulindac and erlotinib on colorectal adenoma formation and regression in patients with familial adenomatous polyposis.
Abstract
Importance
Patients with familial adenomatous polyposis (FAP) are at markedly increased risk for colorectal polyps and cancer. A combination of sulindac and erlotinib led to a 71% reduction in duodenal polyp burden in a phase 2 trial.
Objective
To evaluate effect of sulindac and erlotinib on colorectal adenoma regression in patients with FAP.
Design, Setting, and Participants
Prespecified secondary analysis for colorectal adenoma regression was carried out using data from a double-blind, randomized, placebo-controlled trial, enrolling 92 patients with FAP, conducted from July 2010 to June 2014 in Salt Lake City, Utah.
Interventions
Patients were randomized to sulindac, 150 mg twice daily, and erlotinib, 75 mg daily (n = 46), vs placebo (n = 46) for 6 months.
Main Outcomes and Measurements
The total number of polyps in the intact colorectum, ileal pouch anal anastomosis, or ileo-rectum were recorded at baseline and 6 months. The primary outcomes were change in total colorectal polyp count and percentage change in colorectal polyps, following 6 months of treatment.
Results
Eighty-two randomized patients (mean [SD] age, 40 [13] years; 49 [60%] women) had colorectal polyp count data available for this secondary analysis: 22 with intact colon, 44 with ileal pouch anal anastomosis and 16 with ileo-rectal anastomosis; 41 patients received sulindac/erlotinib and 41 placebo. The total colorectal polyp count was significantly different between the placebo and sulindac-erlotinib group at 6 months in patients with net percentage change of 69.4% in those with an intact colorectum compared with placebo (95% CI, 28.8%-109.2%; P = .009).
Conclusion and Relevance
In this double-blind, placebo-controlled, randomized trial we showed that combination treatment with sulindac and erlotinib compared with placebo resulted in significantly lower colorectal polyp burden after 6 months of treatment. There was a reduction in polyp burden in both those with an entire colorectum and those with only a rectal pouch or rectum.
Trial Registration
clinicaltrials.gov Identifier: NCT01187901
Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominant genetic disorder caused by germline mutations in the APC (adenomatous polyposis coli) gene.1 Patients with FAP have a nearly 100% risk of colorectal cancer if left untreated owing to the formation of hundreds of adenomatous polyps in the colorectum.2 Once the extent of colorectal polyposis is beyond endoscopic control, prophylactic colectomy has become the standard of care.
Multiple studies have shown that cyclooxygenase (COX) inhibitors, such as sulindac or celecoxib, significantly inhibit colorectal adenomatous polyps in patients with FAP.3,4,5,6 Celecoxib is no longer US Food and Drug Administration (FDA)-approved for this indication owing to lack of complete follow-up studies.7
Studies have suggested that APC inactivation and epidermal growth factor receptor (EGFR) signaling promote COX-2 expression and the subsequent development of intestinal neoplasia.8,9 Mouse models of FAP have demonstrated the convergence between the wingless-type and EGFR signaling pathways and COX-2 activity, in which a combination of sulindac and an EGFR inhibitor diminished small intestinal adenoma development by 87%.10 The results of the phase 2 double-blind placebo-controlled randomized FAP Erlotinib-Sulindac Trial (FAPEST) (NCT01187901) showed that combination of COX and EGFR inhibition with sulindac and erlotinib resulted in a profound 71% reduction in duodenal polyp burden after only 6 months of treatment.11 The objective of this secondary analysis of data from the FAPEST trial was to assess the effect of sulindac and erlotinib on colorectal adenoma formation and regression in patients with familial adenomatous polyposis.
Methods
Study Design and Patient Population
The FAPEST (NCT01187901) study design has been described previously.11 Briefly, FAPEST was a double-blind, randomized, placebo-controlled trial of patients with FAP conducted at a single cancer center from July 2010 to June 2014 (Figure 1). Patients were identified and recruited from institutional research registries. Patients provided written informed consent to participate in the study, and ethical approval was obtained from the University of Utah institutional review board. The study protocol is available in Supplement 1. Eligible patients were 18 to 69 years at time of enrollment and were either confirmed carriers of a pathogenic mutation of the APC gene (“genetic diagnosis”) or had more than 100 adenomas in the colorectum and were members of a family with FAP (“clinical diagnosis”) and had a minimum duodenal polyp burden of 5 mm (primary end point of FAPEST) at baseline upper endoscopy. Patients with attenuated FAP and an APC genetic diagnosis were included. Exclusion criteria were described previously.11
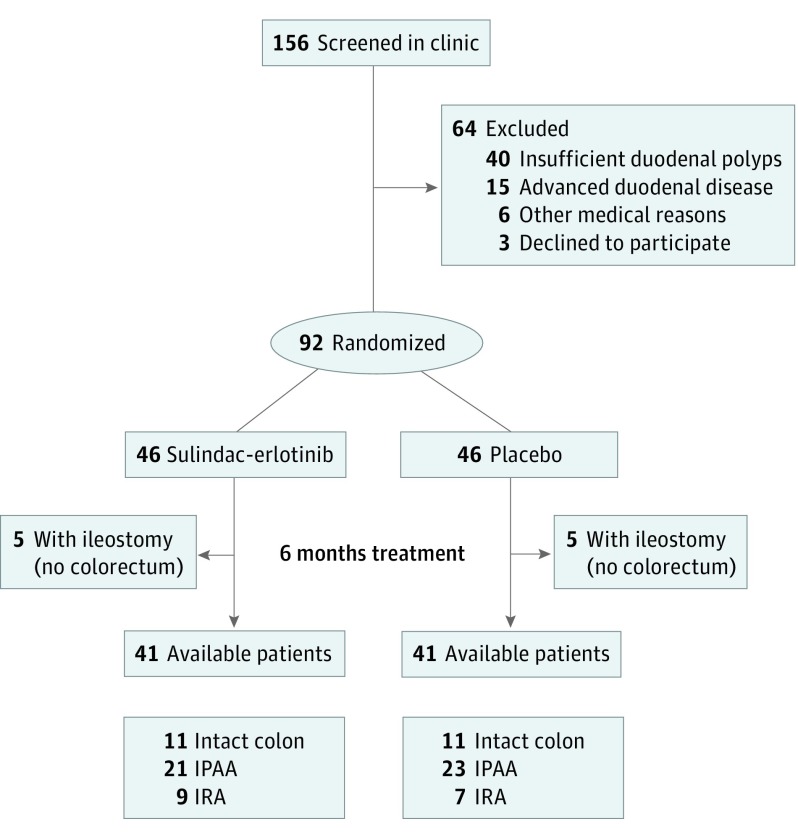
Figure 1. Study Flow Diagram.
IPAA indicates ileal pouch anal anastomosis; IRA, ileo-rectum.
Randomization and Study Intervention
Participants were randomly assigned with an equal probability in an uniform 1:1 allocation ratio to treatment with erlotinib and sulindac or matching placebo. Using a computer program, separate randomization tables were created for participants with classic and attenuated FAP. The randomization was done in blocks of 2 or 4. Patients were randomly assigned to receive combination therapy with sulindac at a dose of 150 mg twice daily and erlotinib at a dose of 75 mg per day or identically appearing placebo for 6 months. Erlotinib (FDA investigational new drug exemption 108086) and identically appearing placebo tablets were provided via the National Cancer Institute through a contract with the drug manufacturer. The Huntsman Cancer Institute Investigational Pharmacy provided encapsulated sulindac and identically appearing placebo capsules. The investigators and patients were blinded to study-group assignments. Upper and lower endoscopic examination at study entry were performed to determine eligibility. Study drugs were provided to participants and refilled at 1 to 3 month intervals based on scheduled trial visits. Drug compliance was assessed by pill count review of patient diaries.
Primary and Secondary Outcomes
The primary efficacy endpoint for FAPEST was a change in duodenal polyp number after 6 months of therapy between the placebo and sulindac-erlotinib group and is previously reported.11 A prespecified secondary end point to assess change in colorectal adenomatous polyp burden was performed after the end of the trial.
The burden of colorectal adenomatous polyps was assessed by endoscopy with flexible video colonoscopes. Endoscopic evaluations were performed before treatment initiation with sulindac-erlotinib or placebo was begun (within 30 days) (month 0) and 6 months after treatment was initiated (month 6). At each examination, 1 of 4 experienced endoscopists counted the total number and size of polyps in the lower intestine. The end point was a change in colorectal polyp number, and the length of lower intestine remaining was stratified into 3 groups: colorectum, ileal pouch anal anastomosis (IPAA), or rectal remnant in those with an ileo-rectal anastomosis (IRA). Other secondary end points included percentage change from baseline in colorectal polyp number.
Statistical Analysis
The Wilcoxon (Mann-Whitney) test was used to compare the groups according to an intention to treat (ITT) principle for the colorectal end points. An analysis was also performed per-protocol and included all patients who had a colonoscopy at baseline and at 6 months after initiating treatment.
Bootstrap sampling was used to create multiple imputation estimates for the 19 participants missing end point colorectal polyp count (9 missing in the treatment group and 10 missing in the placebo group). For each bootstrap sample, missing values were imputed based on linear regression prediction adjusted for randomized treatment group, baseline demographics (sex, age, height, weight, classic or attenuated FAP status), and baseline endoscopy results. Hodges-Lehman estimates of net difference and Wilcoxon U statistics were calculated for each sample. Percentile bootstrap confidence intervals were calculated for the Hodges Lehmann estimator. The bootstrap Wilcoxon statistics, adjusted to have mean zero under the null hypothesis, and bootstrap standard error were used to compute a z statistic. Separate bootstrap samples were run for each subgroup to create equal treatment groups and subgroups that had the same balance as the randomized group. Stratified Wilcoxon rank sum tests were used in the analysis across the 3 subgroups using Rosner-Glynn-Lee method. This method estimates a test statistic for which we calculated a bootstrap percentile confidence interval. The null value used for comparison against the confidence interval was zero.
An extreme case sensitivity analysis was also completed, in which the minimum and maximum differences within strata with the smallest difference (increase of 29) were imputed for the 9 missing treatment values and the largest difference (decrease of 286) were imputed for the 9 missing placebo values.
Descriptive statistics were used for study variables (including age, sex, months in the study) with frequency tabulations for categorical variables and summary statistics (mean and range) for continuously distributed variables. Safety was assessed in patients completing the study using descriptive statistics. Statistical analysis was performed using R statistical software (version 3.2.1, R Foundation). The box and whiskers plot was created using SAS statistical software (version 9.4, SAS Institute Inc).
Results
Demographic Characteristics of Patients
Between July 2010 and June 2014, 156 patients were assessed for eligibility (Figure 1). Sixty-four patients were excluded because they did not meet the inclusion criteria or declined to participate, leaving 92 patients to be randomized. The FAPEST trial was stopped after the second interim analysis of 67 patients by the Data Safety Monitoring Board, because the prespecified stopping rule for the primary duodenal polyp burden end point was met. All patients and investigators remained blinded to randomization status until the final study patient completed their end point endoscopies.
At the time of the decision to stop the study, 92 patients had been recruited and randomized into the study. Ten patients had an end ileostomy and thus had no colorectum available for polyp counts, leaving 82 patients with available or imputed baseline and end point data for colorectal polyp counts: 22 with intact colon, 44 with IPAA, and 16 with ileorectal anastomosis were included in the intention-to-treat analysis. Of the 82 patients, 41 received sulindac-erlotinib and 41 received placebo. A secondary per-protocol analysis using only patients who completed the trial with known baseline and end point colorectal polyp counts was also completed (n = 64).
Demographic characteristics between the treatment and placebo groups stratified by colorectal status are shown in eTable 1 in Supplement 2. Sex was similarly distributed in the IPAA and IRA groups, though there was a larger number of men with intact colorectum in the placebo group. Attenuated FAP represented 17 patients (77.3%) in the intact colorectum group. A germline APC mutation was confirmed in 77 patients (94%), including all patients with attenuated FAP.
Outcomes
The change in total colorectal polyp number, accounting for extent of remaining bowel, was significantly different between the placebo and sulindac-erlotinib groups at 6 months (change in polyp number, −5.1; 95% CI, −6.4 to −3.5; P < .001).
In an intention-to-treat analysis, there was a median decrease of 27 polyps from baseline in the sulindac erlotinib group and a 2-polyp decrease from baseline in the placebo group (group difference, −27.5 polyps; 95% CI, 9.6-106.5; P = .09) (Table). This is also presented as percentage change in colorectal polyp number, showing a net decrease of 69.4% in colorectal polyp number from baseline in the sulindac-erlotinib group compared with the placebo group (95% CI, 28.8%-109.2%; P = .04).
Table. Change in Colorectal Polyp Number From Baseline for Intention-to-Treat Analysis.
| Intention-to-Treat | Colorectal Polyp Number | |||||||
|---|---|---|---|---|---|---|---|---|
| Participants, No. | Baseline Median (IQR) | 6-mo Follow-up, Median (IQR) | Change (6-mo Follow-up-Baseline) Median (IQR) | Net Between-Group Differences (95% CI) | P Value | Net % Change (95% CI) | ||
| Median Change | Median Change, % | |||||||
| Intact colon (colorectal) | ||||||||
| Sulindac and erlotinib | 11 | 39 (19 to 81) | 2 (1 to 2) | −27 (−34 to −26) | −96.3 (−96.3 to −85) | −27.5 (−106.5 to −9.6) | .009 | −69.4 (−109.2 to −28.8) |
| Placebo | 11 | 16 (4 to 26) | 14 (9 to 17) | −2 (−3 to −0.8) | −11.1 (−20.5 to −2.8) | |||
| IPAA | ||||||||
| Sulindac and erlotinib | 21 | 5 (2 to 17) | 0 (0 to 1) | −4 (−5.1 to −3) | −83 (−100 to −71.8) | −14.5 (−28.1 to −3.5) | .003 | −121.7 (−280 to −71.6) |
| Placebo | 23 | 6 (0 to 22) | 22 (8 to 28) | 1 (0 to 3) | 21.7 (0 to 120) | |||
| Rectum (IRA) | ||||||||
| Sulindac and erlotinib | 9 | 7 (4 to 15) | 6 (2 to 15) | −1 (−5 to 5.9) | −60 (−71.4 to 93.9) | −13 (−30.5 to 3.9) | .24 | −175.5 (−1087.3 to 52.5) |
| Placebo | 7 | 3 (2 to 12) | 18.3 (17 to 30) | 11.4 (8 to 16) | 119.3 (114.3 to 133.3) | |||
Abbreviations: IPAA, ileal pouch anal anastomosis; IQR, interquartile range; IRA, ileo-rectum.
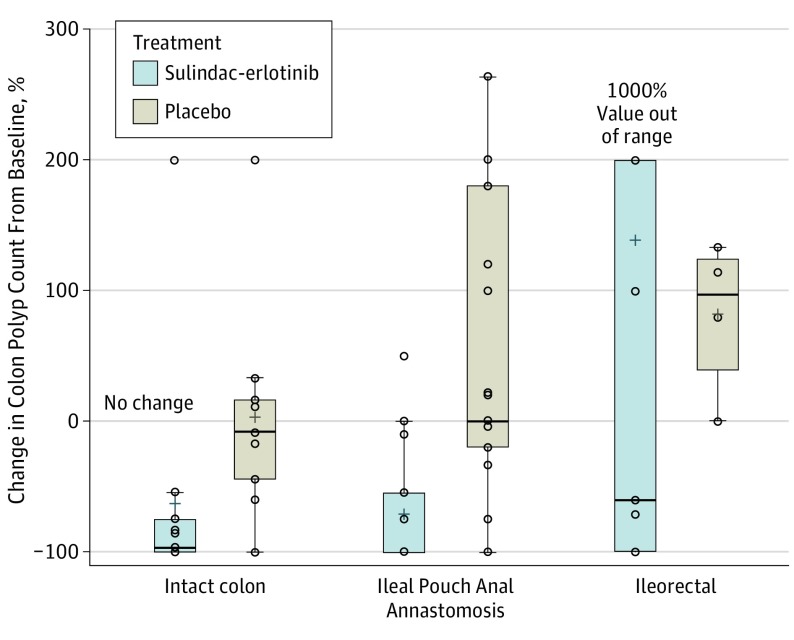
Similarly, in those with an IPAA, there was a median decrease of 4 polyps from baseline in the sulindac-erlotinib group and a 1-polyp increase from baseline in the placebo group (group difference,−14.5 polyps; 95% CI, 3.5-28.1; P = .003). For those with a retained rectum only (IRA) there was a trend toward a decrease in polyp count associated with sulindac-erlotinib therapy but this did not reach statistical significance (Table). This is also shown in the box and whisker plot in Figure 2.
Figure 2. Box Plot of Percent Change in Polyps From Baseline to 6 Months.
Circles indicate observed data points. The box plot lines correspond from bottom of box to top: 25th percentile, median percentile, 75th percentile. The whiskers extend to the minimum and maximum values.
A per-protocol analysis (n = 64) showed consistent results with treatment associated with a significant reduction in colorectal polyp number compared with placebo in the groups with an intact colorectum or IPAA (group difference,−28 polyps; 95% CI, −107 to −11; P = .001 for intact colorectum and group difference,−5.5 polyps; 95% CI, −18 to −1; P = .003 for IPAA) (eTable 2 in Supplement 2). The results of a logistic regression model to identify covariates associated with the probability of missingness and an extreme case sensitivity analysis were also completed, and the results were largely unchanged (eResults and eTable 3 in Supplement 2).
Study Safety
Treatment with sulindac-erlotinib for a 6 month period was generally well tolerated. Adverse events were reported in 68 individuals (83%), though no grade 4 events were reported, 27 individuals (33%) had grade 2 or 3 adverse events (eTable 4 in Supplement 2). The most common adverse event was an erlotinib-induced acneiform-like cutaneous eruption, which occurred in 28 patients in the treatment group (68.3%) and 9 in the placebo group (22%) (95% CI, 27.2-65.4; P < .001). A combination of topical cortisone and/or clindamycin was used to treat the cutaneous eruption. Additional adverse events commonly increased in the treatment group included oral mucositis (13 [32%]), diarrhea (10 [24%]), and nausea (10 [24%]). Grade 2 or 3 adverse events were more common in the treatment group (18 [44%]) vs placebo group (9 [22%]). Nineteen participants (10 in the placebo group and 9 in the treatment group) withdrew from the study; 5 owing to early study halt, 5 owing to drug-induced adverse events or possible allergic reaction, 3 owing to unrelated health reasons, 3 were lost to follow-up, 1 was noncompliant, and 2 for pregnancy beginning during study course (both of whom were receiving placebo).
Erlotinib dose reductions were performed on 11 patients in the placebo group (28%) and 30 patients in the treatment group (73%). Sulindac dose reductions were performed on 11 patients in the placebo group (28%) and 22 patients (54%) in the treatment group. Erlotinib dose reductions included 16 cases of grade 1 to 3 cutaneous eruption that were found to be intolerable by the patient. In addition, there were 11 cases in which study drugs were temporarily discontinued owing to concern for gastrointestinal tract bleeding (n = 6), elevated blood pressure (n = 1), ocular pain or change in vision (n = 2), elevated alanine aminotransferase levels (n = 1), and tonsillitis (n = 1). Three patients had their erlotinib dose reduced to 50 mg per day, 13 patients had a reduction to 25 mg per day, and 11 patients had a temporary discontinuation of both study drugs. Erlotinib and sulindac were reescalated as tolerated when symptoms improved. The median administered dose of sulindac was 287.4 mg per day (range, 131.7 mg/d to 300.0 mg/d) and erlotinib was 48.7 mg per day (range, 23.3 mg/d to 75.0 mg/d). The overall compliance of participants in the trial was very high, with over 88% of patients being at least 80% compliant with their study medication. There was no correlation between total drug consumed and response, indicating that the study was conducted within the range of efficacy, even when participants were dose-reduced.
All statistics in the Table; and eTables 1, 3, and 4 in Supplement 2 represent all those randomized with a baseline examination, which is the intent to treat population. The Table has imputed end point values for colorectal polyp number. eTable 2 in Supplement 2 is the per-protocol analysis limited to participants who completed baseline and end point examinations.
Discussion
In this double-blind, placebo-controlled, randomized trial, sulindac in combination with erlotinib effectively reduced the total colorectal polyp number in patients with familial adenomatous polyposis compared with placebo. This effect was significant after 6 months of therapy and was observed in those with an entire colorectum and in those with an ileal pouch anal anastamosis.
This preliminary trial lays the groundwork for future chemoprevention efforts. Nonetheless, our 6-month study leaves many important questions unanswered. These include whether prolonged treatment with these medications can replace, delay the need for, or limit the anatomical extent of proctocolectomy and whether such treatment can inhibit progression to cancer. Our findings do suggest, however, that erlotinib and sulindac in combination could serve as an adjunct to current treatment by suppressing polyp formation in patients with an intact colon who are awaiting colectomy or have a more modest polyp load as in attenuated FAP or by suppression of polyp formation in patients with a residual rectum or J-pouch after colectomy preventing the need for a revision surgery.
Several investigators have described regression of colorectal adenomatous polyps in patients with familial adenomatous polyposis who received sulindac or celecoxib alone. Regression of rectal adenomas following therapy with sulindac was demonstrated in 2 placebo-controlled studies by Giardiello et al3 and Nugent et al.12 In the former study,3 12 patients treated with sulindac showed improvement in rectal polyp burden by month 6; however, no patient had complete remission, and rapid recurrence was also observed 3 to 4 months after discontinuation of sulindac. In a similar study using a high dose of celecoxib, the investigators found a nearly 30% reduction in colorectal polyps after 6 months of therapy.6 Long-term studies, as well as direct comparisons of sulindac alone vs erlotinib alone are needed and could further define the relative clinical benefits of these drugs. The incomplete efficacy of sulindac and erlotinib in some patients necessitates continued endoscopic surveillance and surgery for increasing colorectal polyp burden or development of advanced colorectal neoplasia.
Preclinical data suggested a beneficial role for EGFR inhibition in familial adenomatous polyposis. These studies showed a greater than 85% decrease in the progression of intestinal microadenomas through genetic or biochemical inhibition of EGFR tyrosine kinase activity in the ApcMin/+ mouse model of familial adenomatous polyposis.10,13 Epidermal growth factor receptor inhibitors are successfully used in the current treatment of non–small cell lung cancer lacking oncogenic KRAS mutation.14,15,16 Our trial suggests the effects of COX and EGFR inhibition observed in the murine models may be observed in the large intestine of patients with familial adenomatous polyposis as well.
There was, however, a high rate of adverse events in our study, the most notable being an acneiform cutaneous eruption in 28 patients (68%) and oral mucositis in 13 patients (32%) in the treatment group. Whereas the dosing of sulindac was based on prior chemoprevention studies,3,4 the dosing of erlotinib was estimated from cancer treatment and lung cancer chemotherapy trials.17,18 Dose ranging studies will be needed to determine if lower and/or less frequent dosing of erlotinib could diminish these adverse effects but retain efficacy.
This study lays an important foundation for effective and feasible chemoprevention of colorectal adenomas in patients with familial adenomatous polyposis, which has the potential to decrease the need for endoscopic surveillance and surgical intervention.
Limitations
This study has some limitations. Because the study measured polyp regression, it is unknown if sulindac and erlotinib would be effective in preventing the emergence of new colorectal adenomas. This issue arose in a pediatric familial adenomatous polyposis trial that suggested sulindac may be ineffective in preventing the emergence of colonic adenomas in children with familial adenomatous polyposis.19 Without long-term follow-up data, the durability of the effect of sulindac and erlotinib, the potential to develop resistance to either drug, and whether patients ultimately undergo fewer surveillance endoscopies and/or surgery, or develop fewer cancers are unknown. Both sulindac and erlotinib can be associated with rare and serious adverse effects, such as cardiotoxic effects20 and interstitial lung disease,21,22 respectively, though no such effects were encountered in the present study. This cohort was not sufficient in size to study the effects of erlotinib or sulindac alone. Studies that are terminated early for efficacy may overestimate the true effect size. Although the tumorigenesis pathway in FAP mirrors most sporadic colorectal neoplasia, the results from this study cannot be generalized to this broader population without appropriately designed clinical trials in those cohorts, and the adverse effect profile (especially with erlotinib) would likely outweigh any benefit when applied to an average-risk population.
Conclusions
Among patients with familial adenomatous polyposis, the use of sulindac and erlotinib compared with placebo resulted in a lower colorectal polyp burden after 6 months. However, the frequency of adverse events may limit the use of these medications at the doses used in this study. Further research is necessary to evaluate these preliminary findings in a larger study population with longer follow-up to determine whether the observed effects will result in improved clinical outcomes.
Trial Protocol
eTable 1. Baseline Demographic Characteristics of Participants
eTable 2. Change in colorectal polyp number from baseline for Per Protocol analysis
eTable 3. Extreme case sensitivity analysis
eTable 4. Observed adverse events in those with baseline colorectal polyp counts
References
- 1.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66(3):589-600. [DOI] [PubMed] [Google Scholar]
- 2.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313-1316. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello FM, Yang VW, Hylind LM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346(14):1054-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arber N, Eagle CJ, Spicak J, et al. ; PreSAP Trial Investigators . Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885-895. [DOI] [PubMed] [Google Scholar]
- 6.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946-1952. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen M. Withdrawal of approval of familial adenomatous polyposis indication for celebrex. 2012; The Food and Drug Administration (FDA) is withdrawing approval of the familial adenomatous polyposis (FAP) indication for CELEBREX (celecoxib) Capsules held by Pfizer, Inc. (Pfizer), 235 East 242nd St., New York, NY 10017-15755. Pfizer has voluntarily requested that approval of this indication be withdrawn, thereby waiving its opportunity for a hearing. https://www.federalregister.gov/articles/2012/06/08/2012-13900/pfizer-inc-withdrawal-of-approval-of-familial-adenomatous-polyposis-indication-for-celebrex. Accessed December 19, 2014.
- 8.Coffey RJ, Hawkey CJ, Damstrup L, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94(2):657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisinger AL, Nadauld LD, Shelton DN, et al. The adenomatous polyposis coli tumor suppressor gene regulates expression of cyclooxygenase-2 by a mechanism that involves retinoic acid. J Biol Chem. 2006;281(29):20474-20482. [DOI] [PubMed] [Google Scholar]
- 10.Roberts RB, Min L, Washington MK, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2002;99(3):1521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samadder NJ, Neklason DW, Boucher KM, et al. Effect of sulindac and erlotinib vs placebo on duodenal neoplasia in familial adenomatous polyposis: a randomized clinical trial. JAMA. 2016;315(12):1266-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80(12):1618-1619. [DOI] [PubMed] [Google Scholar]
- 13.Torrance CJ, Jackson PE, Montgomery E, et al. Combinatorial chemoprevention of intestinal neoplasia [see comments]. Nat Med. 2000;6(9):1024-1028. [DOI] [PubMed] [Google Scholar]
- 14.Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol. 2008;3(2):111-116. [DOI] [PubMed] [Google Scholar]
- 15.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sos ML, Zander T, Thomas RK, Staratschek-Jox A, Claasen J, Wolf J. Expression of signaling mediators downstream of EGF-receptor predict sensitivity to small molecule inhibitors directed against the EGF-receptor pathway. J Thorac Oncol. 2008;3(2):170-173. [DOI] [PubMed] [Google Scholar]
- 17.Lynch TJ, Fenton D, Hirsh V, et al. A randomized phase 2 study of erlotinib alone and in combination with bortezomib in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2009;4(8):1002-1009. [DOI] [PubMed] [Google Scholar]
- 18.Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA. Erlotinib for advanced non-small-cell lung cancer in the elderly. J Clin Oncol. 2008;26(14):2350-2357. [DOI] [PubMed] [Google Scholar]
- 19.Lynch PM, Ayers GD, Hawk E, et al. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am J Gastroenterol. 2010;105(6):1437-1443. [DOI] [PubMed] [Google Scholar]
- 20.Watson Laboratories I. Sulindac package insert and black box warnings. 2008. http://pi.actavis.com/data_stream.asp?product_group=1310&p=pi&language=E. Accessed December 14, 2017
- 21.Qi WX, Sun YJ, Shen Z, Yao Y. Risk of interstitial lung disease associated with EGFR-TKIs in advanced non-small-cell lung cancer. J Chemother. 2015;27(1):40-51. [DOI] [PubMed] [Google Scholar]
- 22.Shi L, Tang J, Tong L, Liu Z. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer. Lung Cancer. 2014;83(2):231-239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Demographic Characteristics of Participants
eTable 2. Change in colorectal polyp number from baseline for Per Protocol analysis
eTable 3. Extreme case sensitivity analysis
eTable 4. Observed adverse events in those with baseline colorectal polyp counts