Key Points
Question
Does infarct growth depend on the type of anesthesia used during endovascular therapy for stroke?
Findings
In this randomized, open-label clinical trial including 128 patients, no difference in infarct growth was found between patients randomized to the general anesthesia group and those randomized to the conscious sedation group.
Meaning
General anesthesia does not result in more infarct growth compared with conscious sedation during endovascular therapy for stroke.
Abstract
Importance
Endovascular therapy (EVT) is the standard of care for select patients who had a stroke caused by a large vessel occlusion in the anterior circulation, but there is uncertainty regarding the optimal anesthetic approach during EVT. Observational studies suggest that general anesthesia (GA) is associated with worse outcomes compared with conscious sedation (CS).
Objective
To examine the effect of type of anesthesia during EVT on infarct growth and clinical outcome.
Design, Setting, and Participants
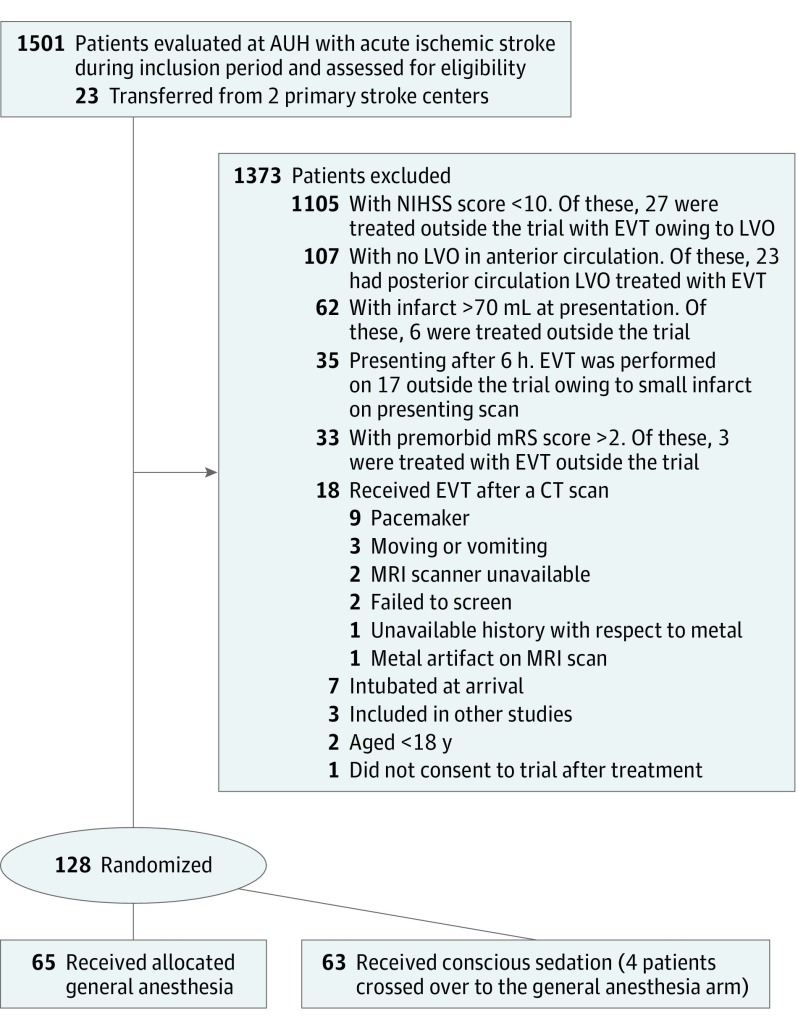
The General or Local Anesthesia in Intra Arterial Therapy (GOLIATH) trial was a single-center prospective, randomized, open-label, blinded end-point evaluation that enrolled patients from March 12, 2015, to February 2, 2017. Although the trial screened 1501 patients, it included 128 consecutive patients with acute ischemic stroke caused by large vessel occlusions in the anterior circulation within 6 hours of onset; 1372 patients who did not fulfill inclusion criteria and 1 who did not provide consent were excluded. Primary analysis was unadjusted and according to the intention-to-treat principle.
Interventions
Patients were randomized to either the GA group or the CS group (1:1 allocation) before EVT.
Main Outcomes and Measures
The primary end point was infarct growth between magnetic resonance imaging scans performed before EVT and 48 to 72 hours after EVT. The hypothesis formulated before data collection was that patients who were under CS would have less infarct growth.
Results
Of 128 patients included in the trial, 65 were randomized to GA, and 63 were randomized to CS. For the entire cohort, the mean (SD) age was 71.4 (11.4) years, and 62 (48.4%) were women. Baseline demographic and clinical variables were balanced between the GA and CS treatment arms. The median National Institutes of Health Stroke Scale score was 18 (interquartile range [IQR], 14-21). Four patients (6.3%) in the CS group were converted to the GA group. Successful reperfusion was significantly higher in the GA arm than in the CS arm (76.9% vs 60.3%; P = .04). The difference in the volume of infarct growth among patients treated under GA or CS did not reach statistical significance (median [IQR] growth, 8.2 [2.2-38.6] mL vs 19.4 [2.4-79.0] mL; P = .10). There were better clinical outcomes in the GA group, with an odds ratio for a shift to a lower modified Rankin Scale score of 1.91 (95% CI, 1.03-3.56).
Conclusions and Relevance
For patients who underwent thrombectomy for acute ischemic stroke caused by large vessel occlusions in the anterior circulation, GA did not result in worse tissue or clinical outcomes compared with CS.
Trial Registration
clinicaltrials.gov Identifier: NCT02317237
This randomized clinical trial analyzes the effect of general anesthesia and conscious sedation on infarct growth and clinical outcome of patients who underwent endovascular therapy for acute ischemic stroke.
Introduction
Endovascular therapy (EVT) is a standard-of-care treatment for selected patients with acute ischemic stroke (AIS) who harbor large vessel occlusions in the anterior circulation and present less than 6 hours from symptom onset. However, numerous questions remain regarding best practices for EVT, including which anesthetic strategy results in the best clinical outcomes.
Most observational studies report worse outcomes from general anesthesia (GA) than from conscious sedation (CS) during EVT, but these results may be confounded by selection bias given that patients with increased stroke severity are more likely to be treated under GA. In addition, retrospective studies do not report specific anesthesia protocols, and only few report details concerning hemodynamic data. Physiological and procedural considerations may potentially favor one approach over another. Performing GA will likely delay procedure initiation due to intubation. Furthermore, GA is often associated with a drop in blood pressure, with the potential for worsening cerebral ischemia. On the other hand, patient motion during CS might impede revascularization and promote procedural complications.
Two randomized clinical trials comparing GA and CS during EVT have shown conflicting results. The first trial did not show a difference in the primary end point (with a National Institutes of Health Stroke Scale [NIHSS] score improving on day 2), although the proportion of patients achieving functional independence was higher in the GA arm after 90 days. The second trial found no difference in the 90-day modified Rankin Scale (mRS) score. In light of the discrepancy between these trials and the observational studies, further data are warranted. In the present randomized clinical trial—General or Local Anesthesia in Intra Arterial Therapy (GOLIATH)—we aimed to test whether CS or GA reduces infarct growth in patients undergoing EVT for AIS.
Methods
Trial Design
The GOLIATH trial was an investigator-initiated, single-center prospective, randomized, open-label, blinded end-point (or PROBE) evaluation that enrolled patients from March 12, 2015, to February 2, 2017. Patients were randomized to GA or CS in a 1:1 fashion; the flowchart (Figure 1) displays the number of patients screened and included. The trial protocol has been published previously, and the original version is attached as Supplement 1. The ethics committee of the Central Denmark Region approved the study and accepted a waiver of consent before randomization because eligible patients typically were not able to give informed consent and treatment was time critical. Patients or their next of kin were later required to give written informed consent to remain in the trial, and only 1 patient refused to give postrandomization consent because he did not want to undergo repeated magnetic resonance imaging (MRI) scans. No data monitoring board was involved.
Figure 1. Flowchart of Patients Screened.
The figure includes all the patients evaluated for acute ischemic stroke, the reasons for not performing endovascular therapy, and the patients who were treated but excluded from the trial. AUH indicates Aarhus University Hospital; CT, computed tomography; EVT, endovascular therapy; LVO, large vessel occlusion; mRS, modified Rankin Scale; MRI, magnetic resonance imaging; and NIHSS, National Institutes of Health Stroke Scale.
Patients and Randomization
We screened all patients presenting to Aarhus University Hospital with symptoms suggestive of AIS as well as patients referred for EVT to the center by 2 primary stroke centers. We included all adult patients (18 years of age or older) who presented with large vessel occlusions in the anterior circulation and in whom groin puncture could be performed within 6 hours from symptom onset or when last seen well. We excluded patients who were intubated at presentation or with a Glasgow Coma Scale score (score range: 3-15, with a lower score indicating lower levels of consciousness) lower than 9 as well as those who were not living independently and had a premorbid mRS score (score range: 0-6, with a lower score indicating independent living) of more than 2. Because the primary trial end point was infarct growth, we required a diffusion-weighted imaging (DWI) MRI scan to establish a baseline (pre-EVT) infarct volume. Therefore, patients with a contraindication to MRI were excluded. In addition to the DWI scan, the imaging protocol consisted of a T2*—a T2 fluid attenuated inversion recovery—and an angiography sequence. Imaging time was 11 minutes. Patients with baseline infarcts greater than 70 mL were excluded, given their reduced likelihood for achieving good clinical outcomes. Movement or agitation was not a contraindication for the study.
After the qualifying scan, an intravenous tissue plasminogen activator was administered in the absence of a contraindication. Randomization was achieved by a web-based program. Patients were stratified according to age (18-65 years or ≥66 years) and NIHSS score (10-16 or ≥17 points; NIHSS score range: 0-42, with higher scores indicating more severe deficits). Block randomization (with sizes 4, 6, and 8) was performed after stratification. Allocation of block size was also random. The allocation to either GA or CS could not be blinded but was unknown by the imaging core laboratory that evaluated the primary outcome and by the nurse who evaluated the 90-day mRS score.
Anesthesia Protocol
The anesthesia protocol is provided in the eAppendix of Supplement 2.
Thrombectomy Procedure
All procedures were performed by 1 of 2 neurointerventionists with 8 and 13 years of experience. Use of stent retriever, direct thrombus aspiration, or intra-arterial thrombolysis alone or in combination was at the discretion of the neurointerventionist. In case of a cervical internal carotid artery stenosis or occlusion, stenting was performed when possible. Reperfusion was graded by an independent imaging core laboratory according to the modified Thrombolysis in Cerebral Ischemia (mTICI) scale score (range: 0—no flow beyond the occlusion, 1—minimal reperfusion, 2a—less than 50% of the affected vascular territory reperfused, 2b—greater than 50% reperfusion, and 3—complete reperfusion). Successful reperfusion was defined as mTICI 2b or 3.
Outcomes and Imaging Analysis
The primary outcome was infarct growth, measured in milliliters. Secondary outcome measures were mRS scores after 90 days, time and blood pressure levels, and safety end points. The mRS score was evaluated at 90 days (80-100 days) after the stroke over the telephone by a certified study nurse who was blinded to randomization.
Infarct size before and after the procedure, mTICI score, and procedural safety measures (dissection, perforation, and clot migration) were evaluated by an independent core imaging laboratory to ensure the unbiased assessment of the primary outcome. Baseline infarct size was determined on DWI or apparent diffusion coefficient imaging. Follow-up scan (preferably MRI) was obtained 48 to 72 hours after symptom onset to avoid false DWI reversal and to minimize early edema. Final infarct size measurement was performed, using a T2 fluid attenuated inversion recovery sequence with additional reference to the DWI or apparent diffusion coefficient imaging, and included regions of hemorrhagic conversion.
Safety outcomes were symptomatic intracranial hemorrhage, 90-day mortality, vessel injury, and clot migration to a previous unaffected territory; all of these outcomes were evaluated by the independent imaging core laboratory. Intracranial hemorrhage was graded on gradient echo imaging with additional reference to the T2 fluid attenuated inversion recovery scan. Given the increased sensitivity of gradient echo imaging for blood products, symptomatic intracranial hemorrhage was defined as type 2 parenchymal hematoma with an associated NIHSS score worsening of 4 or more points (Safe Implementation of Thrombolysis in Stroke-Monitoring Study classification). Type 2 parenchymal hematoma is larger than 30% of the infarcted area with an associated mass effect. Owing to poor medical condition, 3 patients underwent noncontrast computed tomography scan at 48 to 72 hours for determination of final infarct volume and the presence of intracranial hemorrhage. These infarcts were all large and clearly demarcated.
Statistical Analysis
A difference in infarct growth of 10 mL was deemed clinically meaningful and was used for sample size calculation. Based on the assumption of an SD of 20 mL, the planned sample size at the start of the trial was 128 patients. The protocol, which was published after trial initiation, contained 2 errors. First, the sample size calculation used an SD of 25 mL, which yielded 98 patients per arm. Second, these 98 patients were mistakenly taken as the entire sample size. Thus, to preserve the protocol with the original sample size of 128 patients, a 30% attrition rate was added in the protocol. These 2 errors were identified after trial completion. Nevertheless, the originally planned sample size was attained at the end of the trial. Analyses were performed using Stata, version 12.0 (StataCorp LLC) and MedCalc software, version 14.12.0 (MedCalc).
Primary analysis was unadjusted and according to the intention-to-treat principle. Categorical variables were compared using the χ2 test or Fisher exact test where appropriate, and continuous variables were compared with either the unpaired, 2-tailed t test or Mann-Whitney test, where appropriate. We also evaluated outcomes by patients allocated in a per-protocol (as treated) fashion. Statistical significance was defined as a 2-tailed P < .05.
Results
The first patient was enrolled on March 12, 2015, and the last patient was enrolled on February 2, 2017. In that period, 1501 patients were evaluated for suspected AIS, and EVT was performed on 235 patients. A total of 128 patients were included in the trial. For the entire cohort, the mean (SD) age was 71.4 (11.4) years, and 62 (48.4%) were women and 66 (51.6%) were men. The median NIHSS score was 18 (interquartile range [IQR], 14-21). All patients received the intended treatment except for 4 patients of the 63 allocated to CS (6.3%) who crossed over from the CS to the GA arm but remained in the CS group for intention-to-treat analysis. Sixty-five patients (50.8%) were randomized to GA, and 63 (49.2%) were randomized to CS. The 2 groups were balanced regarding age, sex, NIHSS score, stroke risk factors, time to qualifying scan, level of occlusion, rate of intravenous tissue plasminogen activator pretreatment, and EVT technical approach (Table 1). Initial infarct size was also comparable between GA and CS (median [IQR], 10.5 [2.4-23.6] mL vs 13.3 [5.2-31.1] mL; P = .26) (Table 2).
Table 1. Baseline Demographic, Clinical, and Treatment Dataa.
| Variable | General Anesthesia (n = 65) | Conscious Sedation (n = 63) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 71.0 (10.0) | 71.8 (12.8) | .68 |
| Women, No. (%) | 29 (44.6) | 33 (52.4) | .38 |
| Premorbid mRS score, No. (%) | |||
| 0 | 50 (76.9) | 51 (81.0) | .44 |
| 1 | 9 (13.8) | 10 (15.9) | |
| 2 | 4 (6.2) | 2 (3.2) | |
| 3 | 2 (3.1) | 0 | |
| Left-side stroke, No. (%) | 39 (60.0) | 32 (50.8) | .30 |
| Admission NIHSS score, median (IQR) | 18 (13-21) | 17 (15-21) | .84 |
| Hypertension, No. (%) | 39 (60.0) | 32 (50.8) | .34 |
| Atrial fibrillation, No. (%) | 24 (36.9) | 27 (42.9) | .45 |
| Diabetes, No. (%) | 9 (13.8) | 9 (14.3) | .91 |
| Smokers, No. (%) | 20 (30.8) | 20 (31.7) | .81 |
| Time from onset to qualifying MRI, median (IQR), min | 118 (77.5-203.8) | 112 (68.5-185.0) | .49 |
| Lesion location, No. (%) | |||
| ICA-neck (isolated) | 6 (9.2) | 2 (3.2) | .10 |
| ICA-T | 8 (12.3) | 11 (17.4) | |
| M1 | 21 (32.3) | 32 (50.8) | |
| M2 | 12 (18.5) | 7 (11.1) | |
| Tandem | 18 (27.7) | 11 (17.5) | |
| IV tPA pretreated, No. (%) | 50 (76.9) | 46 (73.0) | .61 |
| Spontaneous reperfusion (to mTICI 2b-3), No. (%) | 4 (6.2) | 5 (7.9) | .69 |
| Thrombectomy approach, No. (%) | |||
| Stent retriever | 14 (21.5) | 12 (19.0) | .96 |
| Penumbra (ADAPT technique) | 25 (38.5) | 24 (38.1) | |
| Both | 11 (16.9) | 10 (15.9) | |
| Intra-arterial tPA, No. (%) | 9 (13.8) | 8 (12.7) | .81 |
| Cervical angioplasty with or without stent, No. (%) | 18 (27.7) | 12 (19.0) | .27 |
| Systolic BP at induction, mean (SD), mm Hg | 164 (24.7) | 163 (27.1) | .83 |
| MAP at induction, mean (SD), mm Hg | 110 (17.0) | 108 (18.4) | .48 |
| Systolic BP at groin puncture, median (IQR), mm Hg | 131 (120-147) | 156 (136-172) | <.001 |
| MAP at groin puncture, median (IQR), mm Hg | 90 (82-99) | 102 (88-111) | <.001 |
| Heart rate at groin puncture, median (IQR), beats per min | 62 (55-75) | 72 (64-82) | <.001 |
| Maximum systolic BP during the procedure, mean (SD), mm Hg | 178 (24) | 177 (23) | .95 |
| Minimum systolic BP during the procedure, mean (SD), mm Hg | 107 (21) | 132 (23) | <.001 |
| Maximum MAP during the procedure, mean (SD), mm Hg | 120 (16) | 117 (19) | .21 |
| Minimum MAP during the procedure, mean (SD), mm Hg | 72 (13) | 86 (16) | <.001 |
| Referrals, No. (%) | 13 (20.0) | 10 (15.9) | .54 |
Abbreviations: ADAPT, A Direct Aspiration First Pass Technique; BP, blood pressure; ICA, internal carotid artery; IQR, interquartile range; IV, intravenous; MAP, mean arterial pressure; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; mTICI, modified Thrombolysis in Cerebral Ischemia; NIHSS, National Institute of Health Stroke Scale; tPA, tissue plasminogen activator.
Age, systolic BP, and MAP at induction and during the procedure were normally distributed. NIHSS scores, time metrics, BPs, and heart rate at groin puncture were not normally distributed.
Table 2. Primary and Secondary Imaging and Clinical Outcomes.
| Outcome | General Anesthesia (n = 65) | Conscious Sedation (n = 63) | P Value |
|---|---|---|---|
| Successful reperfusion (mTICI 2b-3), No. (%) | 50 (76.9) | 38 (60.3) | .04 |
| Acute infarct volume, median (IQR), mL | 10.5 (2.4-23.6) | 13.3 (5.2-31.1) | .26 |
| Final infarct volume, median (IQR), mL | 22.3 (8.1-64.5) | 38.0 (16.7-128.0) | .04 |
| Infarct volume growth, median (IQR), mL | 8.2 (2.2-38.6) | 19.4 (2.4-79.0) | .10 |
| 90-d mRS score, median (IQR) | 2 (1-3) | 2 (1-4) | .04 |
| NIHSS score in 24 h, median (IQR) | 6 (3-14) | 10 (2-19) | .19 |
| Change in NIHSS score after 24 h, median (IQR) | −10 (−14 to −5) | −7 (−13 to 0) | .11 |
Abbreviations: IQR, interquartile range; mRS, modified Rankin Scale; mTICI, modified Thrombolysis in Cerebral Ischemia; NIHSS, National Institute of Health Stroke Scale.
Primary End Point
Final infarct volume was smaller in the GA group (Table 2), but no statistically significant difference in the primary end point of infarct growth was found between the GA and CS arms (median [IQR], 8.2 [2.2-38.6] mL vs 19.4 [2.4-79.0] mL; P = .10). Assuming a normal distribution, the mean infarct growth for CS was 57.4 mL and for GA was 34.1 mL (difference, 23.2 mL; 95% CI, −6.4 to 52.9).
Secondary Angiographic and Clinical End Points
Successful reperfusion was higher in the GA group than in the CS group (76.9% vs 60.3%; P = .04). Early neurological outcomes as measured by the median (IQR) 24-hour NIHSS score (6 [3-14] vs 10 [2-19]; P = .19) and 24-hour change in NIHSS score (−10 [−14 to −5] vs –7 [–13 to 0]; P = .11) favored GA over CS but were not statistically significant (Table 2).
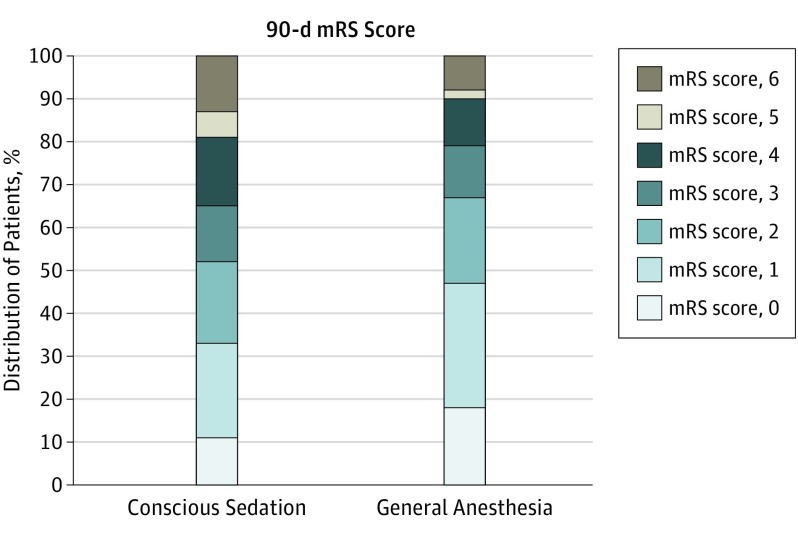
At 90 days, there was a shift to lower mRS scores in the GA group (Figure 2). The odds ratio (OR) for a shift to lower mRS scores was 1.91 (95% CI, 1.03-3.56). Functional independence—90-day mRS score of 0 to 2—was nominally more frequent with GA than with CS (OR, 1.90; 95% CI, 0.93-3.90).
Figure 2. Modified Rankin Scale (mRS) Score Distribution of Patients Treated Under General Anesthesia and Conscious Sedation.
The shift toward better outcome in the general anesthesia group was significant. The odds ratio for a better outcome was 1.91 (95% CI, 1.03-3.56).
Secondary Time Metrics and Blood Pressure End Points
The only significant difference in time metrics was the median (IQR) time from arrival at the neurointerventional suite to groin puncture (24 [20-27] minutes for the GA group vs 15 [12-20] minutes for the CS group; P < .001). There were no differences between the GA and CS groups in the mean (SD) time from symptom onset to groin puncture (202 [70.9] minutes vs 186 [72.2] minutes; P = .22) or median (IQR) time from symptom onset to reperfusion (212 [180-288] minutes vs 216 [162-285] minutes; P = .63) (Table 3).
Table 3. Secondary Outcomes Associated With Time and Blood Pressurea.
| Time Intervalb | General Anesthesia (n = 65) | Conscious Sedation (n = 63) | P Value |
|---|---|---|---|
| Time from symptom onset to arrival at neurointerventional suite, median (IQR), min | 159 (122-230) | 145 (113-231) | .55 |
| Time from arrival at neurointerventional suite to groin puncture, median (IQR), min | 24 (20-27) | 15 (12-20) | <.001 |
| Time from onset to groin puncture, mean (SD), min | 202 (71) | 186 (72) | .22 |
| Time from imaging to groin puncture, median (IQR), min | 61 (48-73) | 54 (40-75) | .13 |
| Time from groin puncture to reperfusion, median (IQR), min | 34 (21-51)c | 29 (16-51)d | .27 |
| Time from onset to reperfusion, median (IQR), min | 212 (180-288)c | 216 (162-285)d | .63 |
| Patients with 20% MAP decrease, No. (%) | 57 (87.7) | 22 (34.9) | <.001 |
| Patients with MAP <70 mm Hg | |||
| No. (%) | 23 (35.4) | 10 (15.9) | .01 |
| Median time (IQR), min | 2 (1-5.5) | 6.5 (2-13) | .09 |
| Phenylephrine hydrochloride, median (IQR), mg | 2.2 (1.2-3.0) | 0.2 (0.0-1.0) | <.001 |
| Ephedrine sulfate, median (IQR), mg | 10 (0-10) | 0 | <.001 |
Abbreviations: IQR, interquartile range; MAP, mean arterial pressure.
Decreases in blood pressure levels were treated with vasopressors (ephedrine and phenylephrine) to maintain the levels within recommended limits (systolic blood pressure >140 mm Hg; MAP >70 mm Hg).
Time from onset to groin puncture was normally distributed. Other time intervals were not normally distributed.
For the 50 reperfused patients.
For the 38 reperfused patients.
Significantly more patients in the GA group than in the CS group experienced a decrease of greater than 20% in mean arterial pressure (MAP) (57 patients [87.7%] vs 22 patients [34.9%]; P = .001) (Table 3). However, when MAP dropped below 70 mm Hg, the duration was nonsignificantly longer for CS patients than for GA patients (6.5 [2-13] minutes vs 2 [1-5.5] minutes; P = .09).
Safety End Points
Four patients (6.3%) in the CS group converted to the GA group due to movement. Two of these patients also vomited, and 1 experienced desaturation due to aspiration.
In the GA group, 4 patients (6.2%) had type 2 parenchymal hematoma hemorrhage and 2 patients (3.1%) were symptomatic. In the CS group, 3 patients (4.8%) had type 2 parenchymal hematoma hemorrhage, of which 1 (1.6%) was associated with clinical deterioration. Mortality rate at 90 days was not significantly different in the GA group than the CS group (7.7% vs 12.7%; P = .35) (eTable in Supplement 2).
Supplementary Analyses
In the per-protocol analysis, in which the 4 patients who crossed over from the CS to the GA group were included in the GA group and the 2 patients with mRS scores greater than 2 before inclusion were excluded, successful reperfusion was no longer significantly higher in the GA group than the CS group (73.1% vs 62.7%; P = .21). Similarly, the final infarct size (median [IQR], 23.2 [8.3-68.1] mL vs 33.3 [16.0-119.9] mL; P = .15) and the 90-day mRS score (OR, 1.54 [95% CI, 0.83-2.87]) were no longer different between the GA and CS groups.
Discussion
In this single-center randomized clinical trial, the primary outcome of infarct growth during EVT was not significantly different between the GA and CS arms. Nevertheless, at 90 days, improved functional outcomes were seen among patients in the GA group. No clinically meaningful differences in safety end points were seen between the 2 arms. These findings support GA as a viable anesthetic approach during EVT.
Contrary to numerous nonrandomized studies that have reported better outcomes with CS, the GOLIATH trial shows signals in favor of GA for multiple end points. In addition to the lower 90-day mRS scores, the GA arm had numerically smaller infarct growth and larger reductions in NIHSS score between baseline and 24 hours. This result is most likely due to the higher rate of successful reperfusion (mTICI 2b to 3) among patients in the GA group. Similarly, in the Sedation vs Intubation for Endovascular Stroke Treatment (SIESTA) study, the GA arm had a higher rate of functional independence at 90 days and a higher, albeit nonsignificant, rate of mTICI 2b to 3 (an absolute difference of 8.5%). These data seem to support the idea that EVT might be performed with greater technical success when patients are under GA and not moving, but no such benefits in terms of reperfusion and functional outcome were seen in the GA arm of the AnStroke trial. In addition, the nonrandomized studies that have reported these data generally found no difference in EVT performance such as procedure duration or rate of reperfusion under different anesthesia regimens. These variable results may be associated with the differences in institutional or operator experience with performing thrombectomy using CS. At Aarhus University Hospital, prior to the trial, thrombectomy was routinely performed using CS; thus, operator inexperience is unlikely to account for the differences in reperfusion between the trial arms. Taken together, the recent randomized trials of anesthesia for EVT demonstrate that GA does not necessarily lead to worse outcomes after EVT.
The marked discrepancy in findings between the randomized and nonrandomized studies highlights the problem of bias, in particular confounding by indication. Patients with AIS who have very poor presentation (eg, severe stroke, respiratory compromise) are not only more likely to have worse outcomes but also more likely to require GA, and these patients were certainly included in the GA cohort of retrospective studies. This finding is illustrated by comparing the intention-to-treat and per-protocol analyses in the GOLIATH trial. In the intention-to-treat analysis, final infarct volumes were larger and clinical outcomes were worse for the CS group. These differences were no longer significant in the per-protocol analysis. The 4 patients who crossed over from the CS to the GA arm had extensive final infarcts (median final infarct volume, 130 mL), supporting the idea that patients with a medical indication for GA are sicker and simply have worse outcomes. This observation was similarly seen in a post hoc analysis of the Interventional Management of Stroke (IMS) III trial, wherein the worse outcomes in the GA group were driven largely by those who had a medical indication for GA. Previous nonrandomized studies tried to adjust for baseline differences in patients under GA and those under CS, but it is difficult to remove residual confounding due to unmeasured sources of bias.
Possible improvements in GA administration in recent randomized clinical trials might also explain the better outcomes achieved with GA compared with outcomes reported in previous literature. Four main factors have been proposed to explain the association between anesthesia and outcomes after EVT: blood pressure, treatment delay, neuroprotection, and ventilation status.
Blood pressure decreases are more frequent with GA and have been associated with worse EVT outcomes, although optimal blood pressure targets remain unknown. Small changes (10%) in MAP have been associated with poor outcome, and others have reported that more dramatic MAP changes (>40%) lead to worse outcomes. This association has also been characterized in a post hoc analysis of the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) trial. Notably, the blood pressure levels in the MR CLEAN study were generally below recommended values; more than 75% of the patients included in the analysis had a systolic blood pressure lower than 140 mm Hg. All 3 recent anesthesia trials established a goal of intraprocedural systolic blood pressure greater than or equal to 140 mm Hg, which is consistent with recent consensus recommendations. Blood pressure was lower in the GA arm in both the AnStroke and GOLIATH trials (no difference was seen in the SIESTA trial), but it is conceivable that greater attention to preventing hypotension promoted better neurological outcomes within the trials.
A longer delay for patients in the GA group was observed from arrival at the neurointerventional suite to groin puncture. However, the median difference was only 9 minutes. This time delay for induction and intubation is acceptable in the context of the much longer overall time from stroke onset to treatment and from stroke onset to reperfusion, which was not significantly different between the competing arms. This finding was remarkably consistent across the 3 trials, demonstrating the feasibility of rapid anesthesia workflow for GA. Regarding our choice of MRI as a stroke imaging tool, the time delay from hospital admission to vessel puncture in this study was much shorter (median [IQR] for all patients, 68 [55-87] minutes) than in the Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment (SWIFT PRIME) trial (median [IQR], 90 [69-120] minutes), which largely employed computed tomography imaging.
There are no conclusive data on neuroprotective properties of anesthetic agents to help with recommending one anesthetic agent over another. In this study, propofol was used as both a general anesthetic and a sedative in the CS group to minimize the confounding effect of different drugs. In anesthetic doses, propofol reduces the cerebral metabolic rate of oxygen with the preservation of flow-metabolism coupling and may increase brain tolerance to ischemic insults. Numerous experimental studies have demonstrated the neuroprotective effects of propofol through different molecular pathways. However, this benefit has not been demonstrated in clinical studies. Volatile anesthetic agents have also been suggested as being of benefit.
Finally, the influence of ventilation on stroke outcomes is unknown. To our knowledge, no studies have demonstrated the effect of ventilatory status on outcome after thrombectomy. However, in this group of patients with poor cerebral blood flow, it can be argued that hypercapnia may potentially increase blood flow and oxygenation into critically hypoperfused areas. This matter requires further investigation.
Limitations
The primary limitation of the GOLIATH trial is that it was conducted at a single center, which may limit its generalizability to centers that use different approaches to anesthesia and neurointerventional treatment. However, we standardized as much as possible the anesthesia protocol used in this trial by adhering to the recent consensus recommendations of the Society for Neuroscience in Anesthesiology and Critical Care regarding respiratory and hemodynamic values. In addition, the primary end point was infarct growth, and consequently, no definitive conclusions can be drawn regarding clinical outcomes. As mentioned, the difference in reperfusion in favor of the GA arm may reflect the discomfort of local neurointerventionists with treatment using CS. The literature varies on this point, with most studies reporting reperfusion rates to be similar between GA and CS. However, the neurointerventionists at our center are experienced and had used a standard thrombectomy protocol that incorporated CS before the start of the trial. These factors were expected to maximize treatment success in both arms. Despite this limitation, all 3 single-center randomized anesthesia trials found that GA does not lead to worse outcomes. But it should be emphasized that these studies were performed at institutions with easy access to advanced anesthesia care, which might have contributed to the success of GA use.
Another limitation of the GOLIATH trial, and the other trials, is the relatively small sample size, which may potentially cause important differences between the treatment arms to be missed. Indeed, given the observed numerical difference in infarcts growth in favor of GA, our study may have been underpowered for the primary end point. Pooled individual patient data meta-analysis of the anesthesia trials is planned to address this issue.
Conclusions
Performing EVT under GA, compared with CS, does not result in worse tissue or clinical outcomes when using a GA protocol that limits the time delay for intubation (<10 minutes) and blood pressure level within recommended limits (systolic blood pressure >140 mm Hg and MAP >70 mm Hg).
Trial Protocol
eAppendix. Anesthesia Protocol
eTable. Safety Outcomes
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Derdeyn CP, Biller J, et al. ; American Heart Association Stroke Council . 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020-3035. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Chebl A, Lin R, Hussain MS, et al. . Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke. 2010;41(6):1175-1179. [DOI] [PubMed] [Google Scholar]
- 5.Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(3):525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou-Chebl A, Zaidat OO, Castonguay AC, et al. . North American SOLITAIRE Stent-Retriever Acute Stroke Registry: choice of anesthesia and outcomes. Stroke. 2014;45(5):1396-1401. [DOI] [PubMed] [Google Scholar]
- 7.Bekelis K, Missios S, MacKenzie TA, Tjoumakaris S, Jabbour P. Anesthesia technique and outcomes of mechanical thrombectomy in patients with acute ischemic stroke. Stroke. 2017;48(2):361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schönenberger S, Uhlmann L, Hacke W, et al. . Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986-1996. [DOI] [PubMed] [Google Scholar]
- 9.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. . General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke trial (anesthesia during stroke). Stroke. 2017;48(6):1601-1607. [DOI] [PubMed] [Google Scholar]
- 10.Simonsen CZ, Sørensen LH, Juul N, et al. . Anesthetic strategy during endovascular therapy: General anesthesia or conscious sedation? (GOLIATH–General or Local Anesthesia in Intra Arterial Therapy) a single-center randomized trial. Int J Stroke. 2016;11(9):1045-1052. [DOI] [PubMed] [Google Scholar]
- 11.Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization Working Group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundiyanapurath S, Schönenberger S, Rosales ML, et al. . Circulatory and respiratory parameters during acute endovascular stroke therapy in conscious sedation or general anesthesia. J Stroke Cerebrovasc Dis. 2015;24(6):1244-1249. [DOI] [PubMed] [Google Scholar]
- 13.Berkhemer OA, van den Berg LA, Fransen PS, et al. ; MR CLEAN investigators . The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. Neurology. 2016;87(7):656-664. [DOI] [PubMed] [Google Scholar]
- 14.Jumaa MA, Zhang F, Ruiz-Ares G, et al. . Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke. 2010;41(6):1180-1184. [DOI] [PubMed] [Google Scholar]
- 15.Abou-Chebl A, Yeatts SD, Yan B, et al. . Impact of general anesthesia on safety and outcomes in the endovascular arm of Interventional Management of Stroke (IMS) III trial. Stroke. 2015;46(8):2142-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald JS, Brinjikji W, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anaesthesia during mechanical thrombectomy for stroke: a propensity score analysis. J Neurointerv Surg. 2015;7(11):789-794. [DOI] [PubMed] [Google Scholar]
- 17.Davis MJ, Menon BK, Baghirzada LB, et al. ; Calgary Stroke Program . Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116(2):396-405. [DOI] [PubMed] [Google Scholar]
- 18.Treurniet KM, Berkhemer OA, Immink RV, et al. ; MR CLEAN investigators . A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia [published online April 12, 2017]. J Neurointerv Surg. doi: 10.1136/neurintsurg-2017-012988 [DOI] [PubMed] [Google Scholar]
- 19.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. . Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46(9):2678-2680. [DOI] [PubMed] [Google Scholar]
- 20.Whalin MK, Halenda KM, Haussen DC, et al. . Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR Am J Neuroradiol. 2017;38(2):294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talke PO, Sharma D, Heyer EJ, Bergese SD, Blackham KA, Stevens RD. Society for Neuroscience in Anesthesiology and Critical Care Expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke. J Neurosurg Anesthesiol. 2014;26(2):95-108. [DOI] [PubMed] [Google Scholar]
- 22.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. [DOI] [PubMed] [Google Scholar]
- 23.Oshima T, Karasawa F, Satoh T. Effects of propofol on cerebral blood flow and the metabolic rate of oxygen in humans. Acta Anaesthesiol Scand. 2002;46(7):831-835. [DOI] [PubMed] [Google Scholar]
- 24.Zwerus R, Absalom A. Update on anesthetic neuroprotection. Curr Opin Anaesthesiol. 2015;28(4):424-430. [DOI] [PubMed] [Google Scholar]
- 25.Sivasankar C, Stiefel M, Miano TA, et al. . Anesthetic variation and potential impact of anesthetics used during endovascular management of acute ischemic stroke. J Neurointerv Surg. 2016;8(11):1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Anesthesia Protocol
eTable. Safety Outcomes