Key Points
Question
What is the most efficient way to diagnose Powassan encephalitis in an immunocompromised patient?
Findings
In this case report of a patient with fatal Powassan encephalitis with negative serology, diagnosis was made by Powassan virus immunohistochemistry, targeted real-time polymerase chain reaction, and metagenomic next-generation sequencing.
Meaning
In immunosuppressed patients, many tools can be used to make a diagnosis when routine testing results are negative, including unbiased sequencing assays.
Abstract
Importance
Powassan virus is a rare but increasingly recognized cause of severe neurological disease.
Objective
To highlight the diagnostic challenges and neuropathological findings in a fatal case of Powassan encephalitis caused by deer tick virus (lineage II) in a patient with follicular lymphoma receiving rituximab, with nonspecific anti-GAD65 antibodies, who was initially seen with fever and orchiepididymitis.
Design, Setting, and Participants
Comparison of clinical, radiological, histological, and laboratory findings, including immunohistochemistry, real-time polymerase chain reaction, antibody detection, and unbiased sequencing assays, in a single case report (first seen in December 2016) at an academic medical center.
Exposure
Infection with Powassan virus.
Main Outcomes and Measures
Results of individual assays compared retrospectively.
Results
In a 63-year-old man with fatal Powassan encephalitis, serum and cerebrospinal fluid IgM antibodies were not detected via standard methods, likely because of rituximab exposure. Neuropathological findings were extensive, including diffuse leptomeningeal and parenchymal lymphohistiocytic infiltration, microglial proliferation, marked neuronal loss, and white matter microinfarctions most severely involving the cerebellum, thalamus, and basal ganglia. Diagnosis was made after death by 3 independent methods, including demonstration of Powassan virus antigen in brain biopsy and autopsy tissue, detection of viral RNA in serum and cerebrospinal fluid by targeted real-time polymerase chain reaction, and detection of viral RNA in cerebrospinal fluid by unbiased sequencing. Extensive testing for other etiologies yielded negative results, including mumps virus owing to prodromal orchiepididymitis. Low-titer anti-GAD65 antibodies identified in serum, suggestive of limbic encephalitis, were not detected in cerebrospinal fluid.
Conclusions and Relevance
Owing to the rarity of Powassan encephalitis, a high degree of suspicion is required to make the diagnosis, particularly in an immunocompromised patient, in whom antibody-based assays may be falsely negative. Unbiased sequencing assays have the potential to detect uncommon infectious agents and may prove useful in similar scenarios.
This case report highlights the diagnostic challenges and neuropathological findings in a fatal case of Powassan encephalitis caused by deer tick virus (lineage II) in a patient with follicular lymphoma receiving rituximab.
Introduction
Powassan virus (POWV), first isolated in Powassan, Ontario, Canada, in 1958, is a rare but increasingly recognized tick-borne flavivirus that can cause life-threatening neuroinvasive disease.1,2 An average of 7 cases per year are reported in the United States, predominantly in the spring and summer months, from the Northeast and the Great Lakes regions. The following 2 serologically indistinguishable lineages have been described: (1) POWV (lineage I, transmitted by Ixodes cookei) and (2) deer tick virus (DTV) (lineage II, transmitted by Ixodes scapularis). Approximately 10% of neuroinvasive cases are fatal, and half of the nonfatal cases develop severe neurological sequelae. No vaccines or specific treatments are currently available. Magnetic resonance imaging (MRI) of the brain often demonstrates basal ganglia T2-weighted hyperintensity, but abnormalities can also be seen within the cerebral cortex, thalami, brainstem, and cerebellum.3 Cerebrospinal fluid (CSF) typically reveals a lymphocytic pleocytosis but may be polymorphonuclear predominant early in the disease, with normal or slightly elevated protein level and normal glucose level.1 Laboratory confirmation of POWV is typically made by detection of IgM antibodies in serum or CSF. Neuropathological findings are similar to other arbovirus infections, including marked edema, necrosis, microglial nodules, neuronophagia, and perivascular, parenchymal, and leptomeningeal inflammation.2,4 We present the clinical, radiological, and pathological findings in a diagnostically challenging and ultimately fatal case of Powassan encephalitis because of DTV (lineage II).
Methods
Serum and CSF real-time polymerase chain reaction (RT-PCR) (targeting POWV genome nucleotide genome position 10 617 to 10 668) and IgM-capture enzyme-linked immunoabsorbent assay (with inactivated virus) testing was performed by the Centers for Disease Control and Prevention Division of Vector-Borne Diseases (Fort Collins, Colorado). Immunohistochemistry (using mouse ascitic fluid generated against POWV) and RT-PCR of brain tissue with sequencing of the flavivirus nonstructural protein 5 gene (NS5) were performed by the Centers for Disease Control and Prevention Infectious Diseases Pathology Branch (Atlanta, Georgia).5 Unbiased metagenomic next-generation sequencing (mNGS) of total RNA extracted from CSF was performed, and the genomic data were analyzed using a previously described bioinformatics pipeline under research protocol 13-12236 at the University of California, San Francisco.6
Results
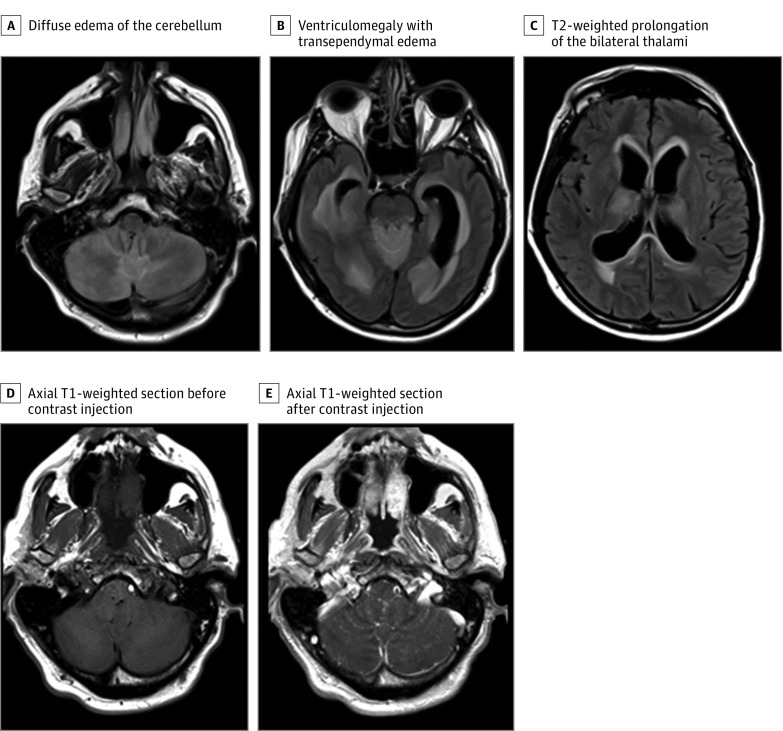
A man in his early 60s from Cape Cod, Massachusetts, with follicular lymphoma receiving maintenance rituximab therapy (the last dose had been administered 2 months prior) was first seen at a community hospital emergency department in December 2016, with 1 week of testicular pain and fever. Testicular ultrasonography demonstrated orchiepididymitis, and he was discharged on a regimen of levofloxacin. Three days later, he developed dysarthria and gait instability and was admitted. Physical examination revealed low-grade fever, dysarthria, meningismus, and bilateral upper extremity dysmetria. At admission, peripheral blood contained 0% CD20+ lymphocytes, and lumbar puncture demonstrated 10 nucleated cells per microliter (60% lymphocytes and 40% neutrophils), glucose level of 62 mg/dL, and total protein level of 83 g/dL (to convert glucose level to millimoles per liter, multiply by 0.0555; to convert protein level to grams per liter, multiply by 10.0). The patient was treated with vancomycin hydrochloride, ceftriaxone sodium, ampicillin sodium, and acyclovir sodium for presumed meningoencephalitis. Head computed tomography without contrast showed no hemorrhage, large territorial infarction, or mass effect. Six days after initial presentation, the patient became obtunded, prompting intubation and transfer to Brigham and Women’s Hospital. Examination off sedation was notable for Glasgow Coma Scale score of 4T for lack of eye opening, absent response to pain, and intubation status. Brainstem reflexes were present, deep tendon reflexes were symmetric, and toes were plantar bilaterally. Magnetic resonance imaging of the brain showed diffuse cerebellar edema, obstructive hydrocephalus, diffuse leptomeningeal enhancement, and periventricular, thalamo-mesencephalic, and basal ganglia T2-weighted signal abnormality (Figure 1). He received hyperosmolar therapy and underwent emergent extraventricular drain placement, followed by decompressive suboccipital craniotomy and cerebellar biopsy. Despite aggressive intervention, the patient’s neurological examination findings remained poor, and he was treated empirically with intravenous immunoglobulin and corticosteroids, without improvement.
Figure 1. Magnetic Resonance Imaging Findings.
A through C, Axial T2-weighted/fluid-attenuated inversion recovery sections from hospital day 5 are shown. D and E, Diffuse leptomeningeal enhancement overlying the cerebellar folia is shown.
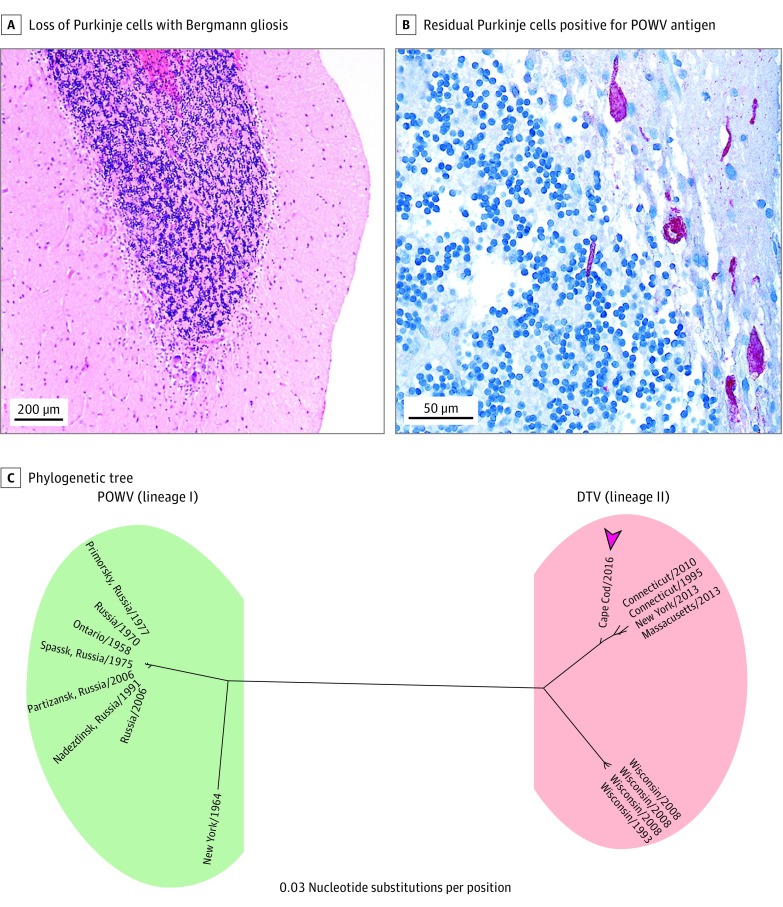
The patient’s broad-spectrum antibiotics were eventually narrowed to ceftriaxone and doxycycline hyclate after his family reported a recent tick bite. Extensive infectious workup was negative for bacterial, fungal, and parasitic infections, including Lyme, syphilis, Cryptococcus, and Toxoplasma. Viral serum and CSF studies were negative for Herpesviridae, HIV-1, JC virus, mumps, lymphocytic choriomeningitis virus, eastern equine encephalitis virus, western equine encephalitis virus, La Crosse encephalitis virus, Saint Louis encephalitis virus, and West Nile virus (WNV). Cerebrospinal fluid flow cytometry and cytology showed no evidence of malignant cells. A serum paraneoplastic panel demonstrated borderline GAD65 antibody positivity (0.12 nmol/L; reference range, ≤0.02 nmol/L); however, CSF GAD65 was negative. Powassan virus RNA was detected by RT-PCR in serum and CSF (on hospital day 14), while IgM-capture enzyme-linked immunoabsorbent assay was negative in both samples; these results were reported several weeks after death. Cerebrospinal fluid mNGS generated 23 812 828 paired-end sequences. After multiple rounds of filtering out low-quality, redundant, and human sequences, 182 992 unique, nonhuman read pairs (0.8%) remained. Four of these sequence pairs aligned with 98.4% similarity to the polyprotein of DTV lineage II (GenBank HM440559) and only 88.9% similarity to the POWV lineage I (GenBank HM440563.1). The remaining sequences aligned to common laboratory contaminants. Sanger sequencing of the amplicon generated from subsequent RT-PCR on the CSF sample (targeting POWV nucleotide genome position 9091 to 10 123) was also consistent with DTV lineage II (Figure 2C) (GenBank MG196295). Cerebellar biopsy results revealed a prominent T-cell infiltrate involving the leptomeninges and cerebellar cortex with Purkinje cell loss and Bergmann gliosis (Figure 2A and B).
Figure 2. Histological Findings From Cerebellar Biopsy and Phylogenetic Tree.
A and B, Biopsy specimens of cerebellar cortex reveal marked loss of Purkinje cells with Bergmann gliosis (hematoxylin-eosin, original magnification x4) (A) and residual Purkinje cells positive for Powassan virus (POWV) antigen (red) (anti-POWV antibody immunohistochemistry, original magnification x40) (B). C, The 1032-nucleotide segment (nucleotide genome position 9091 to 10 123) of the deer tick virus (DTV) (lineage II) nonstructural protein 5 gene amplified from the patient’s cerebrospinal fluid (Cape Cod/2016 [arrowhead]) was compared against 18 POWV (lineage I) and DTV whole genomes using the neighbor-joining method, demonstrating that this patient’s strain is temporally and geographically most proximate to the DTVs circulating in the northeastern United States over the past 20 years. The phylogenetic tree was produced using a software program (Geneious, version 10.1.3; Biomatters Limited). GenBank accession numbers for the viruses included in the phylogenetic tree are listed in the eTable in the Supplement.
Owing to continued clinical deterioration, the patient was transitioned to comfort care and died on hospital day 14 (19 days after initial presentation). Full autopsy was performed and did not reveal evidence of active follicular lymphoma. The left testicle contained nonspecific chronic inflammation and positive staining for POWV antigen (eFigure, G and H in the Supplement). Examination of the brain (1610 g; normal range, 1250-1400 g) revealed significant edema. Microscopic findings were most prominent in the cerebellum and consisted of diffuse severe Purkinje cell loss, gliosis, microglial nodules, multifocal infiltration of macrophages and T cells, numerous cerebellar white matter microinfarctions with diffuse axonal loss, and severe loss of neurons in the dentate nucleus (eFigure, A-F in the Supplement). Other brain structures were diffusely involved, including the brainstem, hippocampus, thalamus, basal ganglia, deep white matter, and cerebral cortex, characterized by microglial nodules, scattered clusters of macrophages, and focal necrosis. Severe neuronal loss was present in the inferior olives, and mild pyramidal cell loss was present in the hippocampus. The leptomeninges were involved by a lymphohistiocytic infiltrate. Powassan virus immunohistochemistry revealed numerous infected neurons, including residual Purkinje cells. Powassan virus RNA was detected from formalin-fixed paraffin-embedded brain tissue by RT-PCR and sequencing of the flavivirus NS5 gene.
Discussion
We present a diagnostically challenging, fatal case of Powassan encephalitis in an immunocompromised patient who was initially seen in December 2016, outside of the typical tick-borne disease season. The combination of encephalitis and orchiepididymitis is most commonly associated with mumps but has been reported with other neurotropic viruses, including WNV, lymphocytic choriomeningitis virus, and Toscana virus.7,8,9 It remains unclear whether the testicular symptoms and POWV immunoreactivity represent local viral spread from a tick bite or an early sign of hematogenous spread. A striking feature of this case was the extent of cerebellar involvement, previously identified as a poor prognostic feature.3,10 Serum GAD65 positivity raised the possibility of paraneoplastic cerebellitis; however, the antibody is nonspecific, particularly at low titers, and was not detected in the CSF.11 Similar to the 3 previously reported Powassan encephalitis autopsy cases,2,4,10 edema, lymphocytic infiltration, gliosis, and microgliosis were diffusely present throughout the brain in our patient. The virus exhibited strong neuronotropism, evidenced by severe loss of neurons in multiple brain regions and by detection of viral antigens in residual neurons.
Conclusions
The optimal method for diagnosis of POWV infection has not been well established because of the rarity of the disease and lack of widely available testing options. Serology is often the preferred method for detection because of the typically narrow window of viremia. In this case, the patient’s negative POWV antibody testing and prolonged viremia were likely because of rituximab exposure, and diagnosis depended on detection of virus nucleic acid. Similar seronegative cases6,12,13,14 have been reported for WNV, eastern equine encephalitis virus, and tick-borne encephalitis virus. In the present case, mNGS not only detected viral nucleic acid in CSF but also was able to subclassify the virus as DTV lineage II, a virus with an enzootic cycle distinct from POWV. These results support the utility of unbiased pathogen detection assays capable of detecting a wide variety of infectious agents in cases of encephalitis in which no causative etiology has been identified.
eTable. GenBank Accession Numbers for the Viruses Included in the Phylogenetic Tree (Figure 2C)
eFigure. Histological Findings From Postmortem Examination
References
- 1.Doughty CT, Yawetz S, Lyons J. Emerging causes of arbovirus encephalitis in North America: Powassan, Chikungunya, and Zika viruses. Curr Neurol Neurosci Rep. 2017;17(2):12. [DOI] [PubMed] [Google Scholar]
- 2.McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. CMAJ. 1959;80(9):708-711. [PMC free article] [PubMed] [Google Scholar]
- 3.Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62(6):707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gholam BI, Puksa S, Provias JP. Powassan encephalitis: a case report with neuropathology and literature review. CMAJ. 1999;161(11):1419-1422. [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatnagar J, Blau DM, Shieh WJ, et al. Molecular detection and typing of dengue viruses from archived tissues of fatal cases by rt-PCR and sequencing: diagnostic and epidemiologic implications. Am J Trop Med Hyg. 2012;86(2):335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MR, Zimmermann LL, Crawford ED, et al. Acute West Nile virus meningoencephalitis diagnosed via metagenomic deep sequencing of cerebrospinal fluid in a renal transplant patient. Am J Transplant. 2017;17(3):803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith RD, Konoplev S, DeCourten-Myers G, Brown T. West Nile virus encephalitis with myositis and orchitis. Hum Pathol. 2004;35(2):254-258. [DOI] [PubMed] [Google Scholar]
- 8.Baldelli F, Ciufolini MG, Francisci D, et al. Unusual presentation of life-threatening Toscana virus meningoencephalitis. Clin Infect Dis. 2004;38(4):515-520. [DOI] [PubMed] [Google Scholar]
- 9.Lewis JM, Utz JP. Orchitis, parotitis and meningoencephalitis due to lymphocytic-choriomeningitis virus. N Engl J Med. 1961;265:776-780. [DOI] [PubMed] [Google Scholar]
- 10.Tavakoli NP, Wang H, Dupuis M, et al. Fatal case of deer tick virus encephalitis. N Engl J Med. 2009;360(20):2099-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vodopivec I, Loomis A, Baron J, Cho T, Bhattacharyya S Anti-GAD65 antibody testing at a tertiary care center (P1.407). Neurology 2017;88(16)(suppl):P1.407. http://n.neurology.org/content/88/16_Supplement/P1.407. Published April 17, 2017. Accessed January 6, 2018.
- 12.Huang C, Slater B, Rudd R, et al. First isolation of West Nile virus from a patient with encephalitis in the United States. Emerg Infect Dis. 2002;8(12):1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon IH, Ciarlini PD, Santagata S, et al. Fatal eastern equine encephalitis in a patient on maintenance rituximab: a case report. Open Forum Infect Dis. 2017;4(1):ofx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight A, Pauksens K, Nordmark G, Kumlien E. Fatal outcome of tick-borne encephalitis in two patients with rheumatic disease treated with rituximab. Rheumatology (Oxford). 2017;56(5):855-856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. GenBank Accession Numbers for the Viruses Included in the Phylogenetic Tree (Figure 2C)
eFigure. Histological Findings From Postmortem Examination