Key Points
Question
Is the benefit of concurrent chemotherapy with optimal radiotherapy conclusively proven in women with International Federation of Gynecology and Obstetrics (FIGO) stage IIIB squamous cell carcinoma of the uterine cervix?
Findings
In this randomized clinical trial of concurrent weekly cisplatin with optimal radiotherapy doses, data from 424 women in the chemoradiotherapy arm and 426 women in the radiotherapy-alone arm were examined for improvement in disease-free survival. Five-year disease-free and overall survival were significantly higher for women who received chemoradiotherapy vs those who received radiotherapy alone.
Meaning
This trial provides evidence in favor of concurrent weekly cisplatin chemotherapy for women with FIGO stage IIIB squamous cell carcinoma of the cervix in this setting.
Abstract
Importance
The evidence for concurrent chemoradiotherapy (CT-RT) in International Federation of Gynecology and Obstetrics (FIGO) stage IIIB squamous cell carcinoma of the uterine cervix is not robust. This study reports the final results of a randomized clinical trial of concurrent cisplatin-based CT-RT and radiotherapy alone (RT) in women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix.
Objective
To investigate the benefit of concurrent CT-RT in FIGO stage IIIB squamous cell carcinoma of the uterine cervix.
Design, Setting, and Participants
This phase 3 open-label randomized clinical trial accrued 850 women in Mumbai, India, between July 7, 2003, and September 22, 2011. Of 2121 screened, 850 women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix suitable for concurrent cisplatin chemotherapy were randomly assigned to CT-RT and RT using block randomization (1:1). The data were updated for a minimum follow-up period of 5 years until December 2016. The final analyses were performed in February and March 2017. This single-institution study was conducted at a tertiary cancer center setting.
Interventions
Randomization to receive RT (RT arm), comprising a combination of external beam RT (50 Gy in 25 fractions over 5 weeks) and brachytherapy, or to receive in addition to the same RT concurrent weekly cisplatin chemotherapy (40 mg/m2 per week) (CT-RT arm).
Main Outcomes and Measures
The primary end point was 5-year disease-free survival (DFS), defined as the time between the date of randomization and the date of any recurrence or death (whichever occurred first) in the intent-to-treat population.
Results
This trial included 424 women assigned to CT-RT (mean [SD] age, 49.4 [7.9] years) and 426 women assigned to RT (mean [SD] age, 49.3 [7.9] years). At a median follow-up of 88 months (interquartile range, 61.3-113.1 months), there were 222 recurrences and 213 deaths in the CT-RT arm and 252 recurrences and 243 deaths in the RT arm. The 5-year DFS was significantly higher in the CT-RT arm (52.3%; 95% CI, 52.2%-52.4%) compared with the RT arm (43.8%; 95% CI, 43.7%-43.9%), with a hazard ratio for relapse or death of 0.81 (95% CI, 0.68-0.98) (P = .03). Similarly, the 5-year overall survival (OS) was significantly higher in the CT-RT arm (54.0%; 95% CI, 53.9%-54.1%) compared with the RT arm (46.0%; 95% CI, 45.9%-46.1%), with a hazard ratio for death of 0.82 (95% CI, 0.68-0.98; P = .04). After adjusting for prognostic factors, CT-RT continued to be significantly superior to RT for DFS and OS. There was a higher incidence of acute hematological adverse effects in the CT-RT arm.
Conclusions and Relevance
Chemoradiotherapy using weekly cisplatin results in significantly better DFS and OS compared with RT in women with stage IIIB squamous cell carcinoma of the uterine cervix. This study provides level 1 evidence in the largest clinical trial reported so far in favor of concurrent weekly cisplatin chemotherapy in this setting.
Trial Registration
clinicaltrials.gov Identifier: NCT00193791
This randomized clinical trial investigates the benefit of concurrent chemoradiotherapy vs radiotherapy alone in patients with International Federation of Gynecology and Obstetrics (FIGO) stage IIIB squamous cell carcinoma of the uterine cervix.
Introduction
Cervical cancer is one of the most common cancers and a leading cause of cancer death in women. In India, approximately two-thirds of all locally advanced cervical cancer, which is defined as International Federation of Gynecology and Obstetrics (FIGO) stage IB to IVA, are seen as FIGO stage III/IV. Until 1999, the mainstay of treatment was radical radiotherapy alone (RT), and survival has been approximately 30% to 40% at 5 years.
The recommendation for the use of concurrent chemotherapy with RT was based on the results of 5 randomized clinical trials that in aggregate showed a disease-free survival (DFS) and overall survival (OS) benefit in favor of the intervention. However, the benefit of concurrent chemotherapy was not conclusively proven in women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix for the following reasons. First, 3 of 5 trials that prompted the initial National Cancer Institute alert delivered chemoradiotherapy (CT-RT) for locally advanced (FIGO stages IB and IIB predominantly) cervical cancer as a definitive treatment and in postoperative high-risk settings. Second, the fraction of patients with FIGO stage IIIB squamous cell carcinoma of the uterine cervix was less than 30%, and a subsequent meta-analysis of individual patient data showed a much smaller survival benefit, with the 95% CIs of the hazard ratios (HRs) being not significant. Third, a subsequent randomized clinical trial from Canada reported no benefit in DFS or OS with concurrent chemoradiotherapy. Fourth, the chemotherapy regimens and schedules were heterogeneous in the previously reported trials, and a comparison with pelvic RT alone (optimal doses) was lacking except for Radiation Therapy Oncology Group 90-01 as the standard arm.
Also, a major challenge in women with advanced disease includes presence of comorbidities and malnutrition, increasing the need for supportive care and treatment compliance with aggressive protocols. Approximately 50% compliance with concurrent CT-RT has been reported, especially in developing countries. With an aim to investigate the benefit of concurrent CT-RT in FIGO stage IIIB squamous cell carcinoma of the uterine cervix, we undertook a phase 3 randomized clinical trial in 2003. We completed the trial and present herein the results of the Concomitant Chemoradiation in Advanced Stage Carcinoma Cervix (CRACx) trial of concurrent weekly cisplatin with optimal RT doses in women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix.
Methods
Study Population
We performed a phase 3 open-label randomized clinical trial at Tata Memorial Hospital, Mumbai, India. Women with biopsy-proven squamous cell carcinoma of the uterine cervix and FIGO stage IIIB disease were invited to participate in this study. Eligibility for the study included women aged 18 to 65 years, World Health Organization performance index of 0 or 1, hemoglobin level of at least 10 g/dL, normal leukocyte and platelet counts, and normal renal functions (to convert hemoglobin level to grams per liter, multiply by 10.0). Women were excluded if they were HIV positive or had medical renal disease, bilateral hydronephrosis, or gross significant para-aortic nodes on imaging. All women were evaluated with a complete history and physical examination, including pelvic examination (using anesthesia if needed), clinical staging by at least 2 physicians involved in the conduct of the study, and sigmoidoscopy and cystoscopy if clinically indicated. Metastatic workup included chest radiograph, ultrasonography, or computed tomographic scan of the abdomen and pelvis. None of the patients underwent positron emission tomographic scan screening in this study. The Institutional Ethics Committee of Tata Memorial Hospital approved the study. Written informed consent was obtained from participants.
Study Design and Treatment
After providing their written informed consent, eligible women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix were randomized to the study arm of cisplatin-based concurrent CT-RT or the standard arm of definitive RT. The Gynecologic Disease Management Group, the Department of Radiation Oncology, and the Department of Clinical Research Secretariat at Tata Memorial Hospital coordinated and managed the trial. We randomly allocated patients to either concurrent CT-RT or definitive RT arms using block randomization (1:1). Women were randomly assigned to receive RT alone (RT arm), comprising a combination of external beam RT (50 Gy in 25 fractions over 5 weeks [to convert grays to rads, multiply by 100]) and brachytherapy, or to receive in addition to the same RT concurrent weekly cisplatin chemotherapy (40 mg/m2 per week) (CT-RT arm). Patient randomization was performed by a telephone call to the Department of Clinical Research Secretariat (a centrally available facility for research) at Tata Memorial Hospital. Patients, treating physicians, and investigators were all aware of the treatment allocated. The trial protocol is available in Supplement 1.
Standard external beam RT and brachytherapy were delivered to all patients. The external beam RT planning was done using a conventional or computed tomography–based simulator, and treatment was delivered to the whole pelvis using 10-mV photons or cobalt 60 gamma rays with either box field or anteroposterior technique to a dose of 50 Gy in 25 fractions at 2 Gy per fraction over 5 weeks (with midline shield for the last 10 Gy), followed by intracavitary brachytherapy, which was interdigitated with external beam RT. Patients who underwent midline shield for the last 10 Gy of external beam RT received either a 30-Gy low-dose rate (LDR) in 1 fraction or a 7-Gy high-dose rate (HDR) to point A clinical staging by at least 2 physicians involved in the conduct of the study for 3 fractions of brachytherapy once weekly starting from the fifth week of RT onward. Patients with involved pelvic nodes at diagnosis or significant parametrial disease at the time of brachytherapy received 50 Gy in 25 fractions without midline shield, followed by brachytherapy of either 25-Gy LDR for 1 fraction or 7-Gy HDR to point A for 2 fractions once weekly. The aim was to deliver 75 to 80 Gy (LDR equivalent) to point A. None of the patients was planned for additional parametrial or pelvic nodal boost or image-based brachytherapy. The total doses delivered were reported as dose equivalent of 2 Gy per fraction (EQD2). The RT details are described in the eMethods in Supplement 2.
Women randomized to the CT-RT arm received injection cisplatin at 40 mg/m2 once per week for at least 5 weeks during the course of external beam RT. Appropriate hydration and antiemetics were given before and after cisplatin administration. Cisplatin doses were modified according to the complete blood cell counts and renal functions as described in the eMethods in Supplement 2. Patients received blood transfusions to maintain a hemoglobin level of at least 10 g/dL during the active treatment. Complete blood cell counts and renal functions were monitored once every week during the active treatment. Follow-up assessment consisted of physical examination, including pelvic examination, assessment of late adverse effects, and relevant investigations depending on patient symptoms. After completion of treatment, all women were evaluated at 3 months for response and once every 3 months for the next 2 years, as well as every 6 months thereafter for the next 5 years. Response assessment at 3 months after treatment duration was done according to World Health Organization criteria. Acute adverse effects were scored according to the Common Toxicity Criteria (version 2.0) and late adverse effects were assessed according to the Late Effects Normal Tissue–Subjective, Objective, Management and Analytic (LENT-SOMA) scoring criteria.
Statistical Analysis
The primary end point was 5-year DFS, which was defined as the time between the date of randomization and the date of any recurrence or death (whichever occurred first) in the intent-to-treat population, which was all patients assigned to a treatment. Secondary end points were OS and relapse rates, including distant metastasis and adverse effects. Overall survival was defined as the time between the date of randomization and the date of death from any cause. All patients were treated as censored at the time of dropout and were not excluded from the analysis.
The trial was planned based on a 5-year rate of DFS of 35% in the definitive RT arm, with an absolute increase in the rate of DFS of 10 percentage points in the CT-RT arm at an α level of .05 and a statistical power of 80%. The calculated sample size after accounting for 10% attrition in each arm was approximately 850 patients. This calculation accounted for a planned interim analysis after the occurrence of 340 events (50% of expected events) with an α level of less than .001 in favor of the CT-RT arm as the predefined stopping boundary. However, no planned interim analysis was performed because the accrual was complete before we achieved the desired events. The primary and secondary end points were assessed on an intent-to-treat basis and were tested by means of 2-sided log-rank tests. We used the Kaplan-Meier method to estimate DFS and OS. Analyses of DFS and OS were performed in the subgroups with the use of univariate Cox proportional hazards regression model analysis. In addition, we performed post hoc subgroup analyses to evaluate potential heterogeneity of study intervention effect. A Cox proportional hazards regression model was used to perform multivariate analysis of various factors affecting DFS and OS, including the study intervention. The analysis was also carried out on a per protocol basis (eTables 1-4 in Supplement 2). All analyses were performed with the use of statistical software (SPSS Statistics for Windows, version 20.0; IBM Corporation).
Results
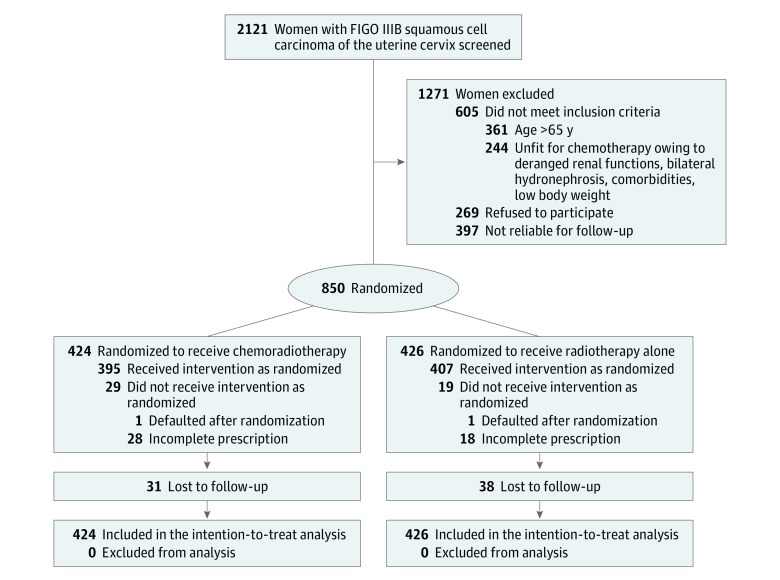
Between July 7, 2003, and September 22, 2011, of 2121 women screened, 850 women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix suitable for concurrent cisplatin chemotherapy were randomly assigned to CT-RT and RT alone. The data were updated for a minimum follow-up period of 5 years until December 2016. The final analyses were performed in February and March 2017. This single-institution study was conducted at a tertiary cancer center setting. The reasons for nonrandomization are shown in Figure 1. Four hundred twenty-four women were randomized to the CT-RT arm and 426 patients to definitive RT. Of 850 patients, 48 women (19 [4.5%] in the CT-RT arm and 29 [6.8%] in the RT arm) defaulted during the treatment. After completion of treatment, the number of women who had been lost to follow-up because of incorrect contact details or relocation was similar in the 2 arms (31 [7.3%] in the CT-RT arm and 38 [8.9%] in the RT arm). The median duration of follow-up was 88 months (interquartile range, 61.3-113.1 months) among surviving women. There were 222 recurrences and 213 deaths in the CT-RT arm and 252 recurrences and 243 deaths in the RT arm. The 2 arms were balanced with respect to baseline characteristics (Table 1). Slightly higher percentages of women had larger clinical tumor size and bilateral parametrial invasion in the definitive RT arm, while the mean age and pretreatment hemoglobin level were comparable in both arms. Most women in both arms (≥90%) received planned doses of RT (combined external beam and brachytherapy). In the CT-RT arm, 293 women (69.1%) received at least 5 cycles (9 women [2.1%] received 6 cycles) of cisplatin chemotherapy during external beam RT.
Figure 1. CONSORT Flow Diagram.
CONSORT indicates Consolidated Standards of Reporting Trials; FIGO, International Federation of Gynecology and Obstetrics.
Table 1. Characteristics of Women and Their Treatment.
| Variable | Chemoradiotherapy (n = 424) |
Radiotherapy Alone (n = 426) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 49.4 (7.9) | 49.3 (7.9) |
| Median (IQR) | 50 (45-55) | 50 (45-55) |
| Clinical tumor size, cm, No. (%) | ||
| ≤4 | 194 (45.7) | 185 (43.4) |
| >4 | 230 (54.3) | 241 (56.6) |
| Parametrial invasion, No. (%) | ||
| Unilateral | 176 (41.5) | 150 (35.2) |
| Bilateral | 248 (58.5) | 276 (64.8) |
| Pretreatment hemoglobin level, g/dL | ||
| Mean (SD) | 11.1 (1.3) | 11.0 (1.3) |
| Median (IQR) | 11 (10-12) | 11 (10-12) |
| External beam radiotherapy dose | ||
| Mean (SD), Gy | 48.3 (7.9) | 48.7 (6.4) |
| Median (IQR), Gy | 50 (50-50) | 50 (50-50) |
| Range, Gy | 4-66 | 2-66 |
| ≥45 Gy, No. (%) | 398 (93.9) | 402 (94.4) |
| <45 Gy, No. (%) | 26 (6.1) | 24 (5.6) |
| Brachytherapy, No. (%) | ||
| Low-dose rate | 62 (14.6) | 68 (16.0) |
| High-dose rate | 333 (78.5) | 337 (79.1) |
| Defaulted | 29 (6.8) | 21 (4.9) |
| Point A EQD2 total dose | ||
| Median (IQR), Gy | 69.7 (69.7-69.8) | 69.7 (69.7-69.8) |
| Mean (SD), Gy | 67.4 (14.6) | 68.8 (12.3) |
| ≥68 Gy, No. (%) | 351 (82.8) | 368 (86.4) |
| <68 Gy, No. (%) | 73 (17.2) | 58 (13.6) |
| ICRU rectum dose to OAR, Gy | ||
| Median (IQR) | 61.6 (56.2-67.1) | 62.2 (56.0-67.6) |
| Mean (SD) | 59.2 (19.5) | 59.9 (17.2) |
| ICRU bladder dose to OAR, Gy | ||
| Median (IQR) | 62.1 (54.3-70.9) | 63.6 (56.2-71.7) |
| Mean (SD) | 60.7 (21.6) | 62.9 (20.9) |
| Radiotherapy, No. (%) | ||
| Complete | 395 (93.2) | 407 (95.5) |
| Incomplete | 29 (6.8) | 19 (4.5) |
| Overall treatment time | ||
| Mean (SD), d | 44.3 (11.2) | 44.2 (9.4) |
| Median (IQR), d | 44 (41-49) | 44 (40-48) |
| ≤56 d, No. (%) | 386 (91.0) | 397 (93.2) |
| >56 d, No. (%) | 38 (9.0) | 29 (6.8) |
| Chemoradiotherapy | ||
| Mean (SD), cycles | 4.4 (1.3) | NA |
| Median (IQR), cycles | 5 (4-5) | NA |
| <5 Cycles, No. (%) | 131 (30.9) | NA |
| ≥5 Cycles, No. (%) | 293 (69.1) | NA |
Abbreviations: EQD2, dose equivalent of 2 Gy per fraction; ICRU, International Commission on Radiation Units and Measurments; IQR, interquartile range; NA, not applicable; OAR, organ at risk.
SI conversion factors: To convert grays to rads, mulitply by 100; hemoglobin level to grams per liter, multiply by 10.0.
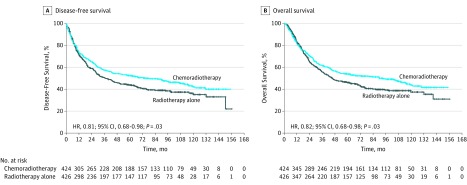
There were 180 recurrences in the CT-RT arm and 207 recurrences in the RT arm. At 5 years, the rates of DFS were 52.3% (95% CI, 52.2%-52.4%) in the CT-RT arm and 43.8% (95% CI, 43.7%-43.9%) in the RT arm, with an unadjusted HR for relapse or death of 0.81 (95% CI, 0.68-0.98) (P = .03) (Figure 2A). The rate of DFS was also significantly higher in the CT-RT arm after adjustment for covariates (adjusted HR, 0.81; 95% CI, 0.67-0.97) (Table 2).
Figure 2. Survival Plots for Disease-Free Survival and Overall Survival.
A, Disease-free survival. B, Overall survival. HR indicates hazard ratio.
Table 2. Multivariate Analyses for Disease-Free Survival and Overall Survivals.
| Variable | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Chemoradiotherapy vs radiotherapy alone | 0.81 (0.67-0.97) | .03 | 0.81 (0.67-0.97) | .02 |
| Age ≤50 vs >50 y | 0.97 (0.80-1.17) | .72 | 0.97 (0.81-1.18) | .78 |
| Clinical tumor size ≤4 vs >4 cm | 0.72 (0.60-0.87) | <.001 | 0.76 (0.63-0.91) | .004 |
| Parametrial invasion unilateral vs bilateral | 1.02 (0.84-1.24) | .81 | 1.04 (0.86-1.25) | .71 |
| Pretreatment hemoglobin level ≤11 vs >11 g/dL as a continuous variable | 0.91 (0.85-0.98) | .009 | 0.92 (0.85-0.98) | .001 |
| EQD2 ≥ 68 vs <68 Gy | 0.51 (0.40-0.64) | <.001 | 0.51 (0.41-0.65) | <.001 |
| Overall treatment time ≤56 vs >56 d | 0.95 (0.67-1.33) | .75 | 0.91 (0.67-1.27) | .59 |
Abbreviations: EQD2, dose equivalent of 2 Gy per fraction; HR, hazard ratio.
SI conversion factors: To convert grays to rads, multiply by 100; hemoglobin level to grams per liter, multiply by 10.0.
There were 213 deaths (50.2%) in the CT-RT arm and 243 deaths (57.0%) in the RT arm. At 5 years, the rates of OS were 54.0% (95% CI, 53.9%-54.1%) in the CT-RT arm and 46.0% (95% CI, 45.9%-46.1%) in the RT arm, with an unadjusted HR for death of 0.82 (95% CI, 0.68-0.98) (P = .03) (Figure 2B). The rate of OS was also significantly higher in the CT-RT arm after adjustment for covariates (adjusted HR, 0.81; 95% CI, 0.67-0.97) (Table 2).
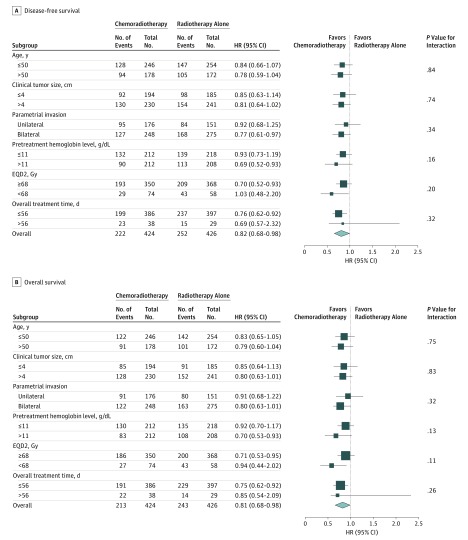
Post hoc analyses showed that there was no interaction between the effect of concurrent chemotherapy and subgroups defined by age, clinical tumor size, extent of parametrial invasion, pretreatment hemoglobin level, external beam RT doses, and overall treatment time for both DFS and OS (Figure 3). Concurrent chemoradiotherapy continued to be significantly superior to RT for DFS and OS after adjusting for covariates, including age, clinical tumor size, extent of parametrial invasion, pretreatment hemoglobin level (as a continuous variable), RT doses (combined external beam and brachytherapy), and overall treatment time. The clinical tumor size, pretreatment hemoglobin level, and RT doses were also significantly associated with DFS and OS (Table 2).
Figure 3. Forest Plots for Disease-Free Survival and Overall Survival.
A, Disease-free survival. B, Overall survival. EQD2 indicates dose equivalent of 2 Gy per fraction (to convert grays to rads, multiply by 100); HR, hazard ratio.
The patterns of first failure for the 2 arms are listed in detail in eTable 5 in Supplement 2. Locoregional-only failures were similar in both arms, while the distant-only plus both locoregional and distant failures were lower by 5 percentage points in the CT-RT arm.
The frequency of acute adverse effects of any grade was similar in both arms, but there were higher rates of grade 3 or 4 adverse effects in the CT-RT arm (eTable 5 in Supplement 2). Acute grade 3 or 4 neutropenia and thrombocytopenia were somewhat higher in the CT-RT arm, although there was no febrile neutropenia or bleeding. Hemoglobin level was maintained at 10 g/dL throughout the treatment as per the protocol with blood transfusions if necessary. Acute nonhematological grade 3 or 4 gastrointestinal tract adverse effects were higher in the CT-RT arm, but there was no difference between arms in renal and genitourinary tract adverse effects. There was no difference in the overall treatment time between the 2 treatment arms.
Moderate to severe symptomatic late adverse effects were comparable in both arms. Rectosigmoid adverse effects were higher in the CT-RT arm (6.8% [n = 29] vs 4.5% [n = 19]), while bladder adverse effects were similar (1.9% [n = 8] in the CT-RT arm vs 2.8% [n = 12] in the RT arm). The spectrum of late adverse effects is summarized in eTable 5 in Supplement 2.
Discussion
The final results of our randomized clinical trial demonstrate the benefit of concurrent CT-RT using weekly cisplatin compared with definitive RT in women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix, with an absolute benefit of 8.5 percentage points in DFS and 8 percentage points in OS. This benefit was consistent across patient subgroups. Although a meta-analysis of individual patient data concluded that CT-RT may benefit women with all stages of cervical cancer, a benefit of only 3% was shown in stage IIIB and stage IVA. A subsequent randomized clinical trial using the same chemotherapy regimen in 147 patients with FIGO stage IIIB squamous cell carcinoma showed a benefit in DFS but not OS.
Our study is the largest trial in a homogeneous group of women with advanced-stage (stage IIIB) squamous cell carcinoma of the uterine cervix to demonstrate the benefit of a simple and well-tolerated concurrent cisplatin regimen over adequately delivered RT. In keeping with the previous results, our analysis also confirms a modest gain in both locoregional (6%) and systemic (5%) control.
Although the results of previous trials have suggested a benefit of concurrent chemotherapy, the findings have been less than definitive in stage IIIB because of concerns about sample size, chemotherapy regimen and schedule, RT doses, variable use of brachytherapy, and treatment time. The strengths of our study include an adequate sample size, homogeneous patient population (stage and histology), long-term follow-up period, and a simple, inexpensive, well-tolerated concurrent chemotherapy regimen that can be widely delivered in a variety of clinical settings. The toxicity of the chemotherapy regimen was manageable and did not lead to prolongation of treatment time in the experimental arm. Compliance with planned chemotherapy of at least 5 cycles was seen among approximately 69.1% (293 of 424) in the CT-RT arm. This is notable because our data also suggest that an optimal treatment time (<56 days) leads to a superior outcome in these women. Approximately less than one-third of patients with FIGO IIIB did not meet the inclusion criteria: older age (>65 years) accounted for 50%, while other factors included compromised renal functions (bilateral hydronephrosis, raised creatinine level, or severe comorbidities) or low body weight. These factors are key, especially in developing countries, where concurrent administration of cisplatin CT-RT may lead to increased acute adverse effects, poor compliance with completion of treatment, long overall treatment time, and compromised outcome.
In the CT-RT arm, we also noted a higher rate of moderate to severe late gastrointestinal tract adverse effect in the form of bleeding proctitis owing to telangiectasia and ulceration, which has been inadequately reported in earlier studies and may be clinically relevant. However, our study did not systematically evaluate the outcome of quality of life, which needs to be addressed in future studies.
Anemia at presentation has been a poor prognostic factor for local control rates, DFS, and OS. Also, maintaining an average nadir hemoglobin level with blood transfusions throughout the RT is associated with better outcome. One of the criticisms of the Canadian randomized clinical trial was failure to correct anemia in patients receiving CT-RT, which may have accounted for a decrement in survival of up to 8% to 10%. In our study, an entry criterion was a hemoglobin level of at least 10 g/dL. Also, patients with hemoglobin levels less than 10 g/dL were corrected with transfusions during the course of treatment. This may be one of the reasons for our better outcomes compared with individual patient data meta-analyses in advanced-stage cervical cancer (stage III to stage IVA).
Limitations
The limitations of our study include a long accrual period of 8 years, no additional RT boost to the residual nodes and parametrium, approximately 8% lost to follow-up after completion of treatment, suboptimal evaluation of pelvic and para-aortic nodal disease using ultrasonography or computed tomographic scan, and use of conventional RT techniques that include brachytherapy planning and prescription that includes lower EQD2 doses (median, 69 Gy EQD2 to point A). Also, the use of newer imaging modalities like magnetic resonance imaging and positron emission tomography may have identified pathological nodal disease and allowed for tailored treatment, but the effect on outcome is not robust. Compliance with follow-up regimens has always been a major challenge owing to long travel distances, other competing health and economic issues, and lower literacy rates in countries with low and middle incomes, including India. Despite these prevailing conditions, compliance with follow-up in our trial was reasonably good (>90%). Finally, these limitations hold true for both treatment arms in our study. Nevertheless, concurrent cisplatin CT-RT continued to independently improve outcomes.
Several single-institution series and the multi-institutional international Retrospective Study–International MRI-Guided Brachytherapy in Cervical Cancer (RetroEMBRACE) have reported excellent local pelvic control rates and late adverse effects with implementation of image-guided adaptive brachytherapy (IGABT) for all stages of cervical cancer with 80 Gy and above EQD2 doses to the target at the time of brachytherapy. Although the implementation of IGABT poses a major challenge in terms of availability, logistics, and economic viability, promising clinical outcomes have been reported for our institution. Furthermore, it appears that the use of a state-of-the-art brachytherapy approach in locally advanced cervical cancer may result in significant economic gain in our setting. Finally, mature results of EMBRACE I and ongoing prospective EMBRACE II (https://www.embracestudy.dk/) and randomized phase III study evaluating MR Image Based Brachytherapy (COMBAT) (https://clinicaltrials.gov/ct2/show/NCT03005743) will provide more insight into the influence of newer RT technology on treatment and outcome of cervical cancers.
Conclusions
Our study demonstrates that concurrent weekly cisplatin-based CT-RT should be considered as the preferred standard of care in patients with stage IIIB squamous cell carcinoma of the uterine cervix. This study provides level 1 evidence in the largest clinical trial reported so far in favor of concurrent weekly cisplatin chemotherapy in this setting.
Trial Protocol.
eMethods. Supplemental Methods
eTable 1. Patient and Treatment Characteristics
eTable 2. Acute and Late Toxicities
eTable 3. Patterns of First Failure
eTable 4. Multivariate Analyses for Disease-Free Survival and Overall Survival
eTable 5. Acute and Late Toxicities, Patterns of First Failure
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed May 2017.
- 2.National Centre for Disease Informatics and Research (NCDIR) Time Trends in Cancer Incidence Rates 1982-2010. Bangalore: Indian Council of Medical Research; July 2013. [Google Scholar]
- 3.Shrivastava S, Mahantshetty U, Engineer R, Tongaonkar H, Kulkarni J, Dinshaw K. Treatment and outcome in cancer cervix patients treated between 1979 and 1994: a single institutional experience. J Cancer Res Ther. 2013;9(4):672-679. [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154-1161. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144-1153. [DOI] [PubMed] [Google Scholar]
- 6.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137-1143. [DOI] [PubMed] [Google Scholar]
- 7.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17(5):1339-1348. [DOI] [PubMed] [Google Scholar]
- 8.Peters WA III, Liu PY, Barrett RJ II, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606-1613. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. NCI issues clinical announcement on cervical cancer: chemotherapy plus radiation improves survival. http://www3.scienceblog.com/community/older/archives/B/nih478.html. Published 1999. Accessed December 15, 2017.
- 10.Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC) Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev. 2010;(1)(1):CD008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20(4):966-972. [DOI] [PubMed] [Google Scholar]
- 12.Nandakumar A, Kishor Rath G, Chandra Kataki A, et al. Concurrent chemoradiation for cancer of the cervix: results of a multi-institutional study from the setting of a developing country (India). J Glob Oncol. 2015;1(1):11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Toxic effects. In: WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization; 1979:14-22. Offset Publication 48.
- 14.Trotti A, Byhardt R, Stetz J, et al. Common Toxicity Criteria: version 2.0: an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47(1):13-47. [DOI] [PubMed] [Google Scholar]
- 15.Zuliani AC, Esteves SC, Teixeira LC, Teixeira JC, de Souza GA, Sarian LO. Concomitant cisplatin plus radiotherapy and high–dose-rate brachytherapy versus radiotherapy alone for stage IIIB epidermoid cervical cancer: a randomized controlled trial. J Clin Oncol. 2014;32(6):542-547. [DOI] [PubMed] [Google Scholar]
- 16.Grogan M, Thomas GM, Melamed I, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86(8):1528-1536. [DOI] [PubMed] [Google Scholar]
- 17.Sturdza A, Pötter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120(3):428-433. [DOI] [PubMed] [Google Scholar]
- 18.Mahantshetty U, Krishnatry R, Hande V, et al. Magnetic resonance image guided adaptive brachytherapy in locally advanced cervical cancer: an experience from a tertiary cancer center in a low and middle income countries setting. Int J Radiat Oncol Biol Phys. 2017;99(3):608-617. doi: 10.1016/j.ijrobp.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty S, Mahantshetty U, Chopra S, et al. Income generated by women treated with magnetic resonance imaging–based brachytherapy: a simulation study evaluating the macroeconomic benefits of implementing a high-end technology in a public sector healthcare setting. Brachytherapy. 2017;16(5):981-987. [DOI] [PubMed] [Google Scholar]
- 20.clinicaltrials.gov. Image Based Brachytherapy in Locally Advanced Cervical Cancers: a Randomized Controlled Trial (COMBAT-Cervix). NCT03005743. https://clinicaltrials.gov/ct2/show/NCT03005743?recrs=ab&cond=NCT03005743&rank=1. Accessed December 10, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eMethods. Supplemental Methods
eTable 1. Patient and Treatment Characteristics
eTable 2. Acute and Late Toxicities
eTable 3. Patterns of First Failure
eTable 4. Multivariate Analyses for Disease-Free Survival and Overall Survival
eTable 5. Acute and Late Toxicities, Patterns of First Failure