This cohort analysis assesses whether excessive daytime sleepiness at baseline is associated with longitudinal regional β-amyloid accumulation in elderly persons without dementia.
Key Points
Question
Is excessive daytime sleepiness associated with longitudinal regional β-amyloid accumulation in elderly persons without dementia?
Findings
In this cohort analysis that included 283 elderly participants without dementia, baseline excessive daytime sleepiness was associated with increased longitudinal β-amyloid accumulation in the cingulate gyrus and precuneus regions.
Meaning
Elderly individuals with excessive daytime sleepiness may be more vulnerable to β-amyloid accumulation.
Abstract
Importance
Aging is associated with excessive daytime sleepiness (EDS), which has been linked to cognitive decline in the elderly. However, whether EDS is associated with the pathologic processes of Alzheimer disease remains unclear.
Objective
To investigate whether EDS at baseline is associated with a longitudinal increase in regional β-amyloid (Aβ) accumulation in a cohort of elderly individuals without dementia.
Design, Setting, and Participants
This prospective analysis included participants enrolled in the Mayo Clinic Study of Aging, a longitudinal population-based study in Olmsted County, Minnesota. Of 2900 participants, 2172 (74.9%) agreed to undergo carbon 11–labeled Pittsburgh compound B positron emission tomography (PiB-PET). We included 283 participants 70 years or older without dementia who completed surveys assessing sleepiness at baseline and had at least 2 consecutive PiB-PET scans from January 1, 2009, through July 31, 2016, after excluding 45 (13.7%) who had a comorbid neurologic disorder.
Main Outcomes and Measures
Excessive daytime sleepiness was defined as an Epworth Sleepiness Scale score of at least 10. The difference in Aβ levels between the 2 consecutive scans (ΔPiB) in Aβ-susceptible regions (prefrontal, anterior cingulate, posterior cingulate-precuneus, and parietal) was determined. Multiple linear regression models were fit to explore associations between baseline EDS and ΔPiB while adjusting for baseline age, sex, presence of the apolipoprotein E ε4 allele, educational level, baseline PiB uptake, global PiB positivity (standardized uptake value ratio ≥1.4), physical activity, cardiovascular comorbidities (obesity, hypertension, hyperlipidemia, and diabetes), reduced sleep duration, respiratory symptoms during sleep, depression, and interval between scans.
Results
Of the initial 283 participants, mean (SD) age was 77.1 (4.8) years; 204 (72.1%) were men and 79 (27.9%) were women. Sixty-three participants (22.3%) had EDS. Baseline EDS was significantly associated with increased regional Aβ accumulation in the anterior cingulate (B coefficient = 0.031; 95% CI, 0.001-0.061; P = .04), posterior cingulate-precuneus (B coefficient = 0.038; 95% CI, 0.006-0.069; P = .02), and parietal (B coefficient = 0.033; 95% CI, 0.001-0.065; P = .04) regions. Association of EDS with longitudinal Aβ accumulation was stronger in participants with baseline global PiB positivity in the anterior cingulate (B coefficient = 0.065; 95% CI, 0.010-0.118; P = .02) and cingulate-precuneus (B coefficient = 0.068; 95% CI, 0.009-0.126; P = .02) regions.
Conclusions and Relevance
Baseline EDS was associated with increased longitudinal Aβ accumulation in elderly persons without dementia, suggesting that those with EDS may be more vulnerable to pathologic changes associated with Alzheimer disease. Further work is needed to elucidate whether EDS is a clinical marker of greater sleep instability, synaptic or network overload, or neurodegeneration of wakefulness-promoting centers. Early identification of patients with EDS and treatment of underlying sleep disorders could reduce Aβ accumulation in this vulnerable group.
Introduction
Excessive daytime sleepiness (EDS) has been defined as difficulty in maintaining desired wakefulness or as a complaint of an excessive amount of sleep.1 Aging has been associated with increased daytime sleepiness.2,3,4 The pooled prevalence of 24 studies estimated that 20% to 30% of older adults experience “falling asleep in the daytime and frequent sleep attacks.”2(p14) Excessive daytime sleepiness in this population has detrimental consequences.5,6,7,8,9,10,11,12 Several longitudinal studies have shown an association between EDS and an increased risk of dementia.6,7,8,9,10 However, the neurobiological mechanisms underlying this association remain unclear. In recent work, Carvalho et al13 reported an association between EDS and increased global cortical thinning in cognitively normal late middle–aged and older adults. The cortical thinning was more prominent in age-susceptible regions, suggesting accelerated brain aging, which could be influenced by pathologic changes associated with Alzheimer disease (AD).
A bidirectional association between sleep disturbance and neurodegeneration has been proposed.14,15 In this association, β-amyloid (Aβ) plays an important role. Because sleep has been proposed to participate in the clearance of soluble Aβ,16,17 disturbed sleep has been suggested to contribute to Aβ accumulation.18,19,20,21 Conversely, Aβ accumulation appears to further disrupt sleep or the sleep-wake cycle in animal models,22 which is corroborated by correlational studies in humans.23 Sleep disruption can increase synaptic activity, which also regulates Aβ production.24,25 Because Aβ accumulation is fundamentally involved in the pathophysiologic process of AD26,27,28 and manifests early in preclinical stages of AD,29 it is an important AD biomarker.29
In this exploratory work, we hypothesized that EDS in the elderly population may be associated with an increased vulnerability to Aβ accumulation. Because brain regions have different susceptibility to Aβ accumulation,30 we further hypothesized that region-level analyses of highly susceptible areas may be better suited for detecting associations with greater sensitivity to Aβ accumulation early in the AD process. Identifying whether EDS is associated with Aβ accumulation has important implications for developing early interventions that could reduce progression of cerebral amyloidosis and the possible eventual development of dementia. Therefore, the aim of this study was to assess whether EDS at baseline is associated with longitudinal regional Aβ accumulation in elderly persons without dementia.
Methods
Participant Selection
The participants in this study are from the population-based sample of Olmsted County, Minnesota, community-dwelling residents 70 years or older who were enrolled in the Mayo Clinic Study of Aging (MCSA). Details of the MCSA design have been published elsewhere.31 This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center, and written informed consent was obtained from all participants or their surrogates.
Of all 2900 MCSA participants, 2172 (74.9%) agreed to undergo carbon 11–labeled Pittsburgh compound B positron emission tomography (PiB-PET) scans. For the present study, the inclusion criteria were the availability of the sleep assessment questionnaires at baseline and at least 2 amyloid imaging assessments (baseline and follow-up scans). Therefore, we initially identified 328 participants without dementia who completed all core questions of a sleep assessment measure and had at least 2 consecutive PiB-PET scans from January 1, 2009, through July 31, 2016. We excluded 45 participants (13.7%) who had a comorbid neurologic disorder (eg, mostly ischemic strokes) at baseline or during follow-up that could be associated with EDS. A total of 283 participants were included in our study.
Cognitive Assessment
The cognitive status of the participants was based on a collective agreement among the MCSA study coordinator, neuropsychologist, and examining physician, as previously described.31,32,33 The investigators evaluated the detailed history, neurologic examination findings, and a neuropsychological battery assessing 4 cognitive domains (executive, language, memory, and visual spatial) and took into account educational level, prior occupation, and visual or hearing deficits.
Sleep Assessment
Sleep-related symptoms were assessed using the Mayo Sleep Questionnaire.34 Collateral information was obtained when a bed partner was available. The questionnaire inquired whether participants had experienced (1) changes in their sleep duration, (2) dream enactment behavior (acting out of dreams), (3) snoring or choking during sleep, (4) stopping of breathing during sleep (witnessed apneas), (5) bedtime restlessness, (6) bedtime leg cramps, and (7) sleepwalking. The Epworth Sleepiness Scale (ESS) was used to assess daytime sleepiness.35 Excessive daytime sleepiness was defined as an ESS score of at least 10 (range, 0-24). Although no universally defined cutoff score exists for EDS, we preferred to be consistent with previously reported literature with similar populations.12,13,36 Continuous positive airway pressure (CPAP) was assessed solely in participants who reported witnessed apneas. The MCSA design was originally established for epidemiologic studies. Therefore, details on polysomnographic (PSG) data and CPAP adherence were not obtained.
Clinical Assessment
The history of medical comorbidities was abstracted by trained nurses from the Rochester Epidemiology Project medical records linkage system.37,38 Obesity (body mass index [calculated as weight in kilograms divided by height in meters squared] >30), history of tobacco use, and depression were acquired from the structured interview and measurements. Physical activity was measured as a mean of 6 components that assessed how often certain physical tasks and exercises were performed during midlife (ages 50-65 years).39 Depression was defined as a Beck Depression Inventory-II score of at least 13 (range, 0-63).40
Imaging Assessment
Accumulation of Aβ was measured by PiB-PET, which consisted of four 5-minute dynamic frames obtained 40 to 60 minutes after injection. We analyzed PiB-PET data cross-sectionally at each time point with our in-house, fully automated, image-processing pipeline.41 The main components of the image-processing pipeline include (1) coregistering the PiB to structural magnetic resonance imaging (atlas is warped to the magnetic resonance imaging space), (2) sharpening (exclusion of voxels with higher probability of being cerebrospinal fluid compared with the probability of gray and white matter combined), and (3) 2-compartment partial volume correction. Then we computed the regional PiB-PET standardized uptake value ratio (SUVR) by estimating the median uptake in 4 bilateral Aβ-susceptible30,42 regions of interest divided by the median uptake in the cerebellar gray matter.43 The 4 regions of interest chosen for analysis were the prefrontal, anterior cingulate (anterior and midcingulate), cingulate-precuneus (posterior cingulate and precuneus), and parietal areas. However, for global PiB estimation, the orbitofrontal and temporal regions were also included to calculate the mean SUVR. Global PiB positivity is defined by an SUVR of at least 1.4. A global PiB SUVR of at least 1.4 has been proposed to be a reliable cutoff to select participants in whom Aβ accumulation is more like likely to occur44 and corresponds to Thal amyloid phases of at least 1 to 2.45 Conversion of PiB was defined as global PiB SUVR at second scan of at least 1.4 in participants with a global PiB SUVR of less than 1.4 at baseline.
Statistical Analysis
For comparison of demographic, clinical, and imaging data, participants were categorized into groups without and with EDS. Normality was assessed by visual inspection of data frequency distribution and by the Kolmogorov-Smirnov test. Group comparisons for continuous data used the unpaired t test or the Mann-Whitney test, as appropriate. Categorical data were compared using the χ2 test or Fisher exact test. Nonparametric correlations were performed using the Spearman rank correlation.
To test the association between EDS and longitudinal Aβ accumulation for each brain region, multiple linear regression models were fit using the difference between the second and first PiB SUVR for each region (ΔPiB) as our dependent variable. Therefore, ΔPiB is a numerical continuous variable. Potential covariates were examined using the enter method (simultaneous entry as opposed to stepwise)46 and included baseline age, sex, presence of the apolipoprotein E ε4 allele, educational level, baseline PiB uptake, global PiB positivity, midlife physical activity, cardiovascular comorbidities (obesity, hypertension, hyperlipidemia, and diabetes), reduced sleep duration, respiratory symptoms during sleep (snoring, choking, or witnessed apneas), depression, and the interval between scans. Baseline global PiB positivity was included in the model to control for the potential independent effect that higher levels of PiB distributed in several regions (mean SUVR≥1.4) may have on regional PiB accumulation.44,47 We set P = .05 for 2-tailed significance levels. Owing to the exploratory nature of this work, adjustment for multiple comparisons was not performed. Statistical analyses were performed with SPSS software for Windows (version 20; IBM Corporation).
Results
Demographic
Of the 283 participants included in our study (204 men [72.1%] and 79 women [27.9%]; mean [SD] age, 77.1 [4.8] years), 63 (22.3%) had EDS at baseline. Participants with EDS were older than those without EDS (mean [SD] age, 78.7 [5.0] vs 76.7 [4.6] years; P = .003). A greater proportion of the EDS group were men (53 of 63 [84.1%] vs 151 of 220 [68.6%]; P = .02) (Table 1).
Table 1. Demographic, Clinical, and Imaging Characteristics.
| Characteristic | Patient Group | P Value | ||
|---|---|---|---|---|
| All (n = 283) | No EDS (n = 220) | EDS (n = 63) | ||
| Age, mean (SD), y | 77.1 (4.8) | 76.7 (4.6) | 78.7 (5) | .003a |
| Male, No. (%) | 204 (72.1) | 151 (68.6) | 53 (84.1) | .02b |
| APOE4 allele, No. (%) | 90 (31.8) | 69 (31.4) | 21 (33.3) | .77b |
| Educational level, median (IQR), y | 14 (12-16) | 14 (12-17) | 13 (12-16) | .10c |
| Physical activity, mean (SD), scored | 9.3 (4.7) | 9.4 (4.5) | 9 (5.5) | .69a |
| Cognitively normal, No. (%) | ||||
| At baseline PiB-PET | 250 (88.3) | 202 (91.8) | 48 (76.2) | <.001b |
| At follow-up PiB-PET | 227 (80.2) | 185 (84.1) | 42 (66.7) | <.001b |
| Dementia conversion | 9 (3.2) | 5 (2.3) | 4 (6.3) | .22e |
| PiB-PET data | ||||
| Interval between scans, mean (SD), y | 2.2 (1.1) | 2.2 (1.1) | 2.2 (1.2) | .78a |
| Baseline global PiB uptake, median (IQR), SUVR | 1.38 (1.31-1.62) | 1.38 (1.30-1.58) | 1.40 (1.32-1.85) | .06c |
| Baseline global PiB positivity, No. (%)f | 130 (45.9) | 97 (44.1) | 33 (52.4) | .24b |
| Global PiB conversion, No. (%)g | 37 (24.2) | 30 (24.4) | 7 (23.3) | .90b |
| Global ΔPiB, mean (SD), SUVR | 0.073 (0.099) | 0.066 (0.097) | 0.097 (0.100) | .03a |
| Baseline sleep screening | ||||
| ESS score, median (IQR)h | 7 (4-9) | 5 (3-7) | 12 (11-14) | NA |
| Bed partner, No. (%) | 247 (87.3) | 194 (88.2) | 53 (84.1) | .39b |
| Reduced sleep, No. (%) | 50 (17.7) | 41 (18.6) | 9 (14.3) | .43b |
| Snore or choke, No. (%) | 65 (23.0) | 46 (20.9) | 19 (30.2) | .12b |
| Witnessed apneas, No. (%) | 51 (18.0) | 31 (14.1) | 20 (31.7) | <.001b |
| Dream enactment, No. (%) | 26 (9.2) | 20 (9.1) | 6 (9.5) | .92b |
| Bedtime restlessness, No. (%) | 22 (7.8) | 17 (7.7) | 5 (7.9) | >.99e |
| Leg cramps, No. (%) | 106 (37.5) | 83 (37.7) | 23 (36.5) | .86b |
| Sleepwalking, No. (%) | 1 (0.4) | 0 | 1 (1.6) | .22e |
| Baseline comorbidities, No. (%) | ||||
| Obesity | 89 (31.4) | 71 (32.3) | 18 (28.6) | .58b |
| Dyslipidemia | 202 (71.4) | 160 (72.7) | 42 (66.7) | .35b |
| Hypertension | 179 (63.3) | 138 (62.7) | 41 (65.1) | .73b |
| Diabetes | 118 (41.7) | 90 (40.9) | 28 (44.4) | .62b |
| Current smoking | 5 (1.8) | 4 (1.8) | 1 (1.6) | >.99e |
| Depression | 12 (4.2) | 10 (4.5) | 2 (3.2) | >.99e |
| Use of hypnotics, No. (%) | ||||
| Benzodiazepines | 5 (1.8) | 5 (2.3) | 0 | .59e |
| Nonbenzodiazepines | 13 (4.6) | 11 (5) | 2 (3.2) | .74e |
Abbreviations: APOE4, apolipoprotein E ε4; ΔPiB, difference between the second and first Pittsburgh B compound (PiB) uptake measures; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; IQR, interquartile range; NA, not applicable; PET, positron emission tomography; SUVR, standardized uptake value ratio.
Calculated using the unpaired t test.
Calculated using the χ2 test.
Calculated using the Mann-Whitney test.
Measured as a mean of 6 components that assessed how often certain physical tasks and exercises were performed during midlife, with scores ranging from 0 to 21 and higher scores indicating more physical activity.
Calculated using the Fisher exact test.
Indicates SUVR of at least 1.4.
Includes 153 participants, 123 without EDS and 30 with EDS, who did not have baseline positivity.
Scores range from 0 to 24, with higher scores indicating more daytime sleepiness.
Clinical Assessment
At baseline, the EDS group had more participants with mild cognitive impairment than the group without EDS (15 of 63 [23.8%] vs 18 of 220 [8.8%]; P < .001). However, we found no difference in the conversion from cognitively normal or mild cognitive impairment to dementia between groups in this relatively short interval (Table 1).
No significant differences were found between groups regarding sleep-related symptoms other than witnessed apneas. Participants in the EDS group and their bed partners reported more witnessed apneas (20 of 63 [31.7%] vs 31 of 220 [14.1%]; P < .001), but CPAP use in participants with witnessed apneas was not significantly different between groups (6 of 20 [30%] vs 11 of 31 [35.5%]; P = .69). Because CPAP use was not assessed in participants without witnessed apneas, we were unable to determine any difference between the groups with and without EDS for this subset of participants. Both groups had similar profiles of medical comorbidities (Table 1).
Aβ Accumulation on PET
We found no difference between the groups with and without EDS regarding the interval between scans in years (mean [SD], 2.2 [1.2; range, 1.0-5.4] years vs 2.2 [1.1; range, 0.9-5.4] years; P = .78). Mean (SD) global ΔPiB was higher in EDS participants (0.097 [0.100] vs 0.066 [0.097]; P = .03). The ESS scores correlated with global ΔPiB (Spearman r = 0.129; P = .03) but not with PiB SUVR at baseline or follow-up. When analyzed regionally, ESS scores also correlated with ΔPiB in the anterior cingulate and cingulate-precuneus regions (Spearman r = 0.138; P = .02 for both) and the parietal region (Spearman r = 0.142; P = .02), but not the prefrontal regions.
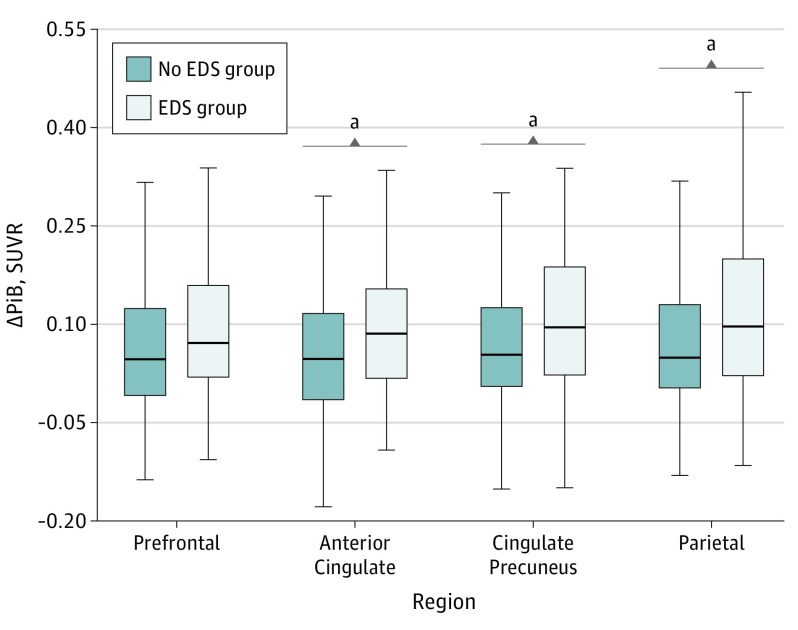
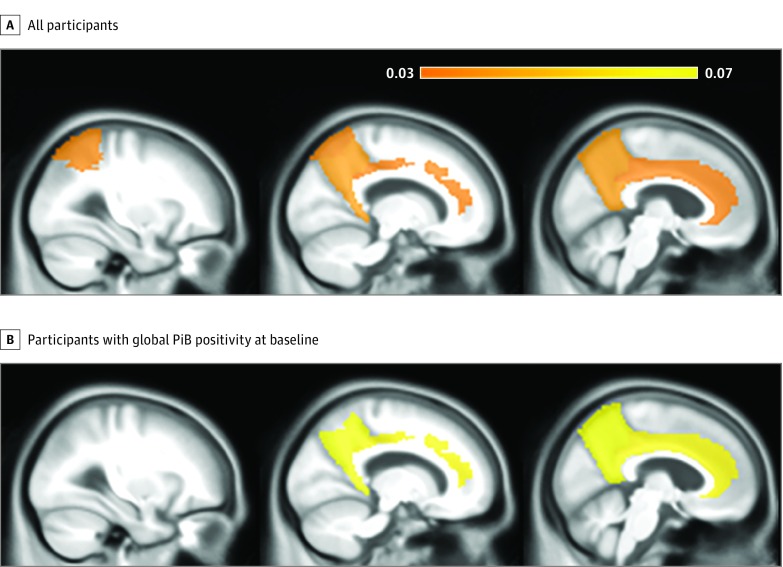
Figure 1 shows the distribution of longitudinal Aβ deposition dichotomized by EDS. We assessed regional ΔPiB associations with EDS using multiple linear regression models. First, with all participants included, we found that EDS was associated with a longitudinal increase in Aβ accumulation (ΔPiB) in the anterior cingulate (B coefficient = 0.031; 95% CI, 0.001-0.061; P = .04), cingulate-precuneus (B coefficient = 0.038; 95% CI, 0.006-0.069; P = .02), and parietal (B coefficient = 0.033; 95% CI, 0.001-0.065; P = .04) regions (Table 2 and Figure 2A). The ΔPiB increase estimated by EDS in the same regions was similar to that estimated by every additional year between scans for the anterior cingulate (B coefficient = 0.023; 95% CI, 0.012-0.034), cingulate-precuneus (B coefficient = 0.028; 95% CI, 0.016-0.040), and parietal (B coefficient = 0.027; 95% CI, 0.015-0.038) regions (P < .01).
Figure 1. Distribution of Longitudinal β-amyloid (Aβ) Deposition Dichotomized by Excessive Daytime Sleepiness (EDS) .
Deposition of Aβ is depicted as the unadjusted difference between the second and first Pittsburgh B compound (ΔPiB) standardized uptake value ratio (SUVR) for each region according to presence of EDS. Horizontal lines indicate median; boxes, first and third quartiles; and error bars, minimum and maximum.
aP < .05.
Table 2. Multiple Linear Regression Model Estimates for Associations Between EDS and ΔPiB in Aβ-Susceptible Regions of Interesta.
| Region of Interest | Modela | |||
|---|---|---|---|---|
| All Elderly Individuals Without Dementia | Individuals With Global PiB Positivityb | |||
| B Coefficient (95% CI) | P Value | B Coefficient (95% CI) | P Value | |
| Prefrontal | 0.025 (−0.005 to 0.055) | .10 | 0.048 (−0.007 to 0.104) | .08 |
| Anterior cingulate | 0.031 (0.001 to 0.061) | .04 | 0.065 (0.010 to 0.118) | .02 |
| Cingulate-precuneus | 0.038 (0.006 to 0.069) | .02 | 0.068 (0.009 to 0.126) | .02 |
| Parietal | 0.033 (0.001 to 0.065) | .04 | 0.054 (−0.008 to 0.116) | .12 |
Abbreviations: ΔPiB, difference between the second and first Pittsburgh B compound (PiB) uptake measures; EDS, excessive daytime sleepiness.
Models were controlled for baseline age, interval between scans, sex, apolipoprotein E ε4 allele, educational level, baseline regional PiB uptake, baseline global PiB positivity (all individuals model only), midlife physical activity, cardiovascular comorbidities (obesity, hypertension, hyperlipidemia, and diabetes), reduced sleep duration, respiratory symptoms during sleep (snoring, choking, or witnessed apneas), and depression.
Indicates standardized uptake value ratio of at least 1.4.
Figure 2. Regional Associations of Pittsburgh B Compound Uptake Changes (ΔPiB) With Excessive Daytime Sleepiness (EDS).
The prefrontal, anterior cingulate, posterior cingulate-precuneus and parietal regions are colored according to model estimates of regional difference between the second and first PiB scans (ΔPiB) by baseline EDS after controlling for multiple confounders in all participants (A) and participants who had global PiB positivity (standardized uptake value ratio [SUVR], ≥1.4) at baseline (B). The color scale indicates β-amyloid (Aβ) deposition increases in SUVR units as estimated by our models in every region of interest where EDS was significantly associated with ΔPiB. Regions of interest are demonstrated in a sagittal plane, from most lateral (left) to medial (right). An additional increase in Aβ accumulation is estimated in individuals with baseline global PiB positivity when compared with all individuals.
Because we noted that global PiB positivity at baseline in every region of interest was strongly associated with ΔPiB, an analysis restricted to subsets of individuals with global PiB positivity showed a stronger association between EDS and ΔPiB compared with the models with all individuals. Excessive daytime sleepiness was associated with further ΔPiB increase in the anterior cingulate (B coefficient = 0.065; 95% CI, 0.010-0.118; P = .02) and cingulate-precuneus (B coefficient = 0.068; 95% CI, 0.009-0.126; P = .02) regions (Table 2 and Figure 2B). The ΔPiB increase estimated by EDS was equivalent to that estimated by every additional year between scans for the anterior cingulate (B coefficient = 0.051; 95% CI, 0.029-0.074) and cingulate-precuneus (B coefficient = 0.072; 95% CI, 0.047-0.097) (P < .01).
We performed multiple sensitivity analyses to check for potential confounding effects that could not be controlled a priori in the regression. To rule out data overfitting in subsample analyses, we first limited the number of covariates to 9 (EDS, interval between scans, baseline PiB uptake, baseline age, sex, presence of apolipoprotein E ε4 allele, hypertension, diabetes, and depression). The association between EDS and ΔPiB persisted in the anterior cingulate (B coefficient = 0.056; 95% CI, 0.006-0.106) and cingulate-precuneus (B coefficient = 0.064; 95% CI, 0.010-0.118) regions of participants with PiB positivity (eTable 1 in the Supplement). In a subsample of cognitively normal individuals only to exclude a potential confounding effect of mild cognitive impairment status, the association of EDS with ΔPiB was again seen in the cingulate-precuneus region (B coefficient = 0.034; 95% CI, 0.001-0.068) but not in other regions (eTable 1 in the Supplement). To reduce a potential confounding effect of CPAP use, a model that excluded participants with witnessed apneas showed significant associations between EDS and ΔPiB in the anterior cingulate (B coefficient = 0.034; 95% CI, 0.002-0.067), cingulate-precuneus (B coefficient = 0.044; 95% CI, 0.009-0.078), and parietal (B coefficient = 0.038; 95% CI, 0.003-0.073) regions. Self-reported reduced sleep or respiratory symptoms (snoring, choking, or witnessed apneas) were not associated with ΔPiB, even when EDS was removed from the models (eTable 2 in the Supplement). Switching the compound variable respiratory symptoms to witnessed apneas did not result in significant associations with ΔPiB in any of the models (eTable 3 in the Supplement).
Discussion
We found that baseline EDS in individuals without dementia was significantly associated with longitudinal regional Aβ accumulation, primarily in the cingulate and precuneus regions. These results are consistent with those of a cross-sectional study20 that found increased Aβ burden in middle-aged participants without dementia with greater daytime somnolence in multiple regions, including the precuneus and anterior cingulate, using a different assessment of daytime sleepiness (the Sleep Scale from the Medical Outcomes Study).
EDS and Sleep Instability
In elderly persons, obstructive sleep apnea (OSA) often contributes to daytime sleepiness, especially when it is severe.48 Witnessed apnea was the only sleep-related symptom that was different between participants with and without EDS in our study, suggesting greater prevalence of OSA in those with EDS. In a small sample of adults without dementia,49 the apnea-hypopnea index and oxygen desaturation index were associated with greater PiB-PET Aβ burden measured globally and in the precuneus region. However, EDS was an exclusion criterion. Other authors50,51 also found associations between OSA severity and cerebrospinal fluid Aβ42 levels. In both studies, associations were restricted to PSG variables related to hypoxemia, corroborating previous findings in mice showing that chronic intermittent hypoxemia facilitates the production of Aβ.52
Witnessed apneas or the compound variable of self-reported sleep-related respiratory symptoms were not associated with Aβ accumulation in our study. This finding is in agreement with those of other studies that failed to identify an association between Aβ burden detected by PiB-PET or autopsy and self-reported OSA symptoms20 or by PSG data before death.53 Elderly persons may lack awareness of OSA symptoms,54 and a negative PSG finding several years before death does not exclude the later development of OSA.
However, EDS may have multiple determinants and is likely to be more than a surrogate for severe OSA. In the largest study assessing OSA and sleepiness in elderly persons (n = 835),36 sex, depressive symptoms, and body mass index were associated with sleepiness after controlling for multiple clinical and PSG variables. Although other authors55,56,57 also failed to identify an association between OSA severity and sleepiness in the general population, another study58 found an association restricted to non–rapid eye movement (NREM) sleep. Sleep fragmentation with increased N1 sleep (NREM stage 1 sleep, with a corresponding decrease in slow wave sleep [SWS]) appears to be more consistently associated with sleepiness in OSA.55,56,57,59,60 This finding suggests that sleepiness resulting from OSA may depend on an individual susceptibility to sleep instability. Total SWS time was found to be the best estimator of reduced cerebrospinal fluid Aβ42 levels, suggesting that reduced or fragmented SWS increases soluble Aβ initially, which may facilitate Aβ deposition over time.18 Therefore, EDS may be a marker of sleep instability, with fragmented sleep (increased N1 sleep and wakefulness after sleep onset) and reduced sleep depth (decreased SWS).
Moreover, the cingulate gyrus appears to participate in the propagation of slow waves to areas such as the precuneus.61 Impaired connectivity, secondary to increased Aβ burden in the cingulate gyrus in participants with EDS, could affect the propagation of slow waves, generating more sleep instability and reduced SWS. This hypothesis is consistent with recent findings associating Aβ burden in the medial prefrontal cortex (including the anterior cingulate) with reduced NREM slow wave activity in humans.23 However, this association may be mediated or at least contributed to by coexistent tau abnormalities.
Because self-reported sleep quality has been associated with excessive sleepiness in older adults,48 EDS can be interpreted as a manifestation of poor sleep quality due to sleep instability. Our results would then corroborate actigraphic findings of decreased cerebrospinal fluid Aβ42 levels in cognitively normal older adults with frequent napping or worse sleep efficiency.21 Our results were also consistent with previous findings of worse sleep quality associated with increased Aβ burden in the precuneus.19 The positive correlation between ESS scores and Aβ accumulation seen in the present work corroborates a possible dose-dependent association between sleep disruption and amyloid accumulation.21
EDS and Synaptic and/or Network Overload
Wakefulness and sleep deprivation promote soluble Aβ42 generation, which is reduced during sleep.16,17,62 The day-night patterns for Aβ42 appear to depend on Aβ42 clearance during sleep through a glymphatic pathway.16 However, Aβ42 generation is also influenced by synaptic activity,24,25,63 which is maximum during wakefulness and decreased after sleep, correlating with slow wave activity.64 Slow wave activity reduces cerebral cortex metabolism65 and also appears to promote downscaling of synaptic connections.66,67 Sleep disruption further increases Aβ expression, which can also increase neuronal excitability.68,69 Whole-brain glucose metabolism declines significantly from waking to NREM sleep.70 In particular, the default mode network, which includes the cingulate, precuneus, and parietal regions (especially the inferior parietal lobule), has high levels of metabolic activity during wakefulness71,72 and requires decoupling during sleep.73 This decoupling is more evident in the posterior cingulate and precuneus regions, where progressive changes in sleep depth are associated with decreased contribution to the default mode network.74 These areas were more susceptible to Aβ deposition30,42,71 and accumulated more Aβ in participants with EDS in our study. Therefore, sleep instability could lead to synaptic or default mode network overload, and EDS could be a clinical manifestation of this overload. Synaptic overload can increase oxidative stress and lead to neuronal death.75,76 Prior findings of global cortical thinning in EDS in cognitively normal individuals may be consistent with this process.13
EDS and Neurodegeneration
Finally, EDS in elderly persons without dementia may be secondary to neurodegeneration of wakefulness-promoting centers. Special consideration should be given to the cholinergic basal forebrain and noradrenergic locus coeruleus systems, which are involved very early in the pathologic processes of AD77,78 and are associated with Aβ accumulation in multiple ways.79,80 Orexin dysregulation and suprachiasmatic nucleus degeneration have also been proposed as mechanisms underlying sleep-wake cycle disruption in AD81,82,83 and may contribute to EDS.
Evidence supports that early tau abnormalities in these brainstem and subcortical nuclei occur before the deposition of cortical Aβ, driving sleep-wake cycle disruption and enabling Aβ toxicity.84,85 However, sleep disruption may also drive neurodegeneration. Neuronal count reductions of 50% and 25% were seen in the locus coeruleus and oxigenergic neurons, respectively, in a murine model of chronic sleep disruption.86 In addition, preliminary data from a murine model of chronic short sleep87 showed that tau-dependent neurodenegeration of the locus coeruleus depends on intraneuronal Aβ. Mice unable to produce Aβ through genetic or pharmacological manipulations were resistant to chronic short sleep–induced tau phosphorylation.
Limitations
The main limitation of our study was the lack of objective measures of sleep disturbance (eg, actigraphy or PSG) and treatment (eg, CPAP use) over time. Although the ESS has great internal consistency and has been recommended for group comparisons, not enough evidence is available to support individual-level comparisons longitudinally.88 Epworth Sleepiness Scale scores may be underestimated in elderly persons89 and have not been validated in individuals with mild cognitive impairment. With aging and progression of cognitive decline, the validity of the ESS could be further compromised, and therefore, longitudinal analysis of scores was not performed in the present study. Moreover, our assessment of reduced sleep did not include quantification of sleep time, which might have obscured its possible association with Aβ accumulation.
We were also unable to assess daytime sleepiness objectively (using the multiple-sleep latency test and maintenance of wakefulness test) in our study. However, the ESS has been proposed to represent a better assessment of average daytime sleepiness in real-life circumstances as opposed to a snapshot of sleepiness in a single day in a laboratory.90 The clinical utility of the multiple-sleep latency test and maintenance of wakefulness test has also been questioned.91,92 The ESS and objective measures of daytime sleepiness likely measure different components of daytime sleepiness and, therefore, have different utility. However, the ESS is a simple, inexpensive, and easily administered tool that has been widely used, allowing ready extrapolation and comparison of our findings to other populations.
Whether self-reported sleepiness measured by the ESS is more sensitive to detect Aβ burden than self-reported sleep symptoms or sleep quality remains uncertain.20,93 Because we tested a different outcome variable than prior studies (ΔPiB instead of baseline PiB uptake) and included a different set of covariates in our models, a direct comparison of Aβ burden associated with self-reported sleep variables could not be performed. However, daytime symptoms may be more noticeable to individuals and their relatives than sleep-related symptoms (recall bias). Moreover, daytime dysfunction as measured by EDS may represent an increased susceptibility to sleep disruption. If individuals react differently to sleep disorders, resultant daytime dysfunction may be more sensitive to differentiate individuals whose sleep disturbance is causing more deleterious effects in the brain.
Conclusions
Our study showed that EDS in elderly persons without dementia may be associated with longitudinal Aβ accumulation, particularly in the cingulate gyrus and precuneus. This finding supports previous literature suggesting that EDS is a risk factor for cognitive decline or dementia.6,8,10 It remains unclear whether EDS is a result of greater sleep instability, synaptic or network overload, or neurodegeneration of wakefulness-promoting centers. However, participants with EDS were more vulnerable to AD pathologic processes. Further investigations should assess determinants of EDS in elderly persons without dementia and whether early recognition of EDS and treatment of potential underlying sleep disorders can reduce amyloid accumulation in this vulnerable group.
eTable 1. Multiple Linear Regression Model Estimates for Predicted ΔPiB (SUVR)
eTable 2. Multiple Linear Regression Model Estimates for Baseline Self-reported Sleep Symptoms
eTable 3. Multiple Linear Regression Model Estimates for Witnessed Apneas
References
- 1.American Academy of Sleep Medicine The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Young TB. Epidemiology of daytime sleepiness: definitions, symptomatology, and prevalence. J Clin Psychiatry. 2004;65(suppl 16):12-16. [PubMed] [Google Scholar]
- 3.Hayley AC, Williams LJ, Kennedy GA, Berk M, Brennan SL, Pasco JA. Prevalence of excessive daytime sleepiness in a sample of the Australian adult population. Sleep Med. 2014;15(3):348-354. [DOI] [PubMed] [Google Scholar]
- 4.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90(8):4510-4515. [DOI] [PubMed] [Google Scholar]
- 5.Gooneratne NS, Weaver TE, Cater JR, et al. . Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51(5):642-649. [DOI] [PubMed] [Google Scholar]
- 6.Foley D, Monjan A, Masaki K, et al. . Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49(12):1628-1632. [DOI] [PubMed] [Google Scholar]
- 7.Jaussent I, Bouyer J, Ancelin ML, et al. . Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35(9):1201-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsapanou A, Gu Y, Manly J, et al. . Daytime sleepiness and sleep inadequacy as risk factors for dementia. Dement Geriatr Cogn Dis Extra. 2015;5(2):286-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elwood PC, Bayer AJ, Fish M, Pickering J, Mitchell C, Gallacher JE. Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health. 2011;65(9):820-824. [DOI] [PubMed] [Google Scholar]
- 10.Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13(7):886-892. [DOI] [PubMed] [Google Scholar]
- 11.Merlino G, Piani A, Gigli GL, et al. . Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010;11(4):372-377. [DOI] [PubMed] [Google Scholar]
- 12.Hayley AC, Williams LJ, Kennedy GA, et al. . Excessive daytime sleepiness and falls among older men and women: cross-sectional examination of a population-based sample. BMC Geriatr. 2015;15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho DZ, St Louis EK, Boeve BF, et al. . Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med. 2017;32:236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology: a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cedernaes J, Osorio RS, Varga AW, Kam K, Schiöth HB, Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev. 2017;31:102-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie L, Kang H, Xu Q, et al. . Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang JE, Lim MM, Bateman RJ, et al. . Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varga AW, Wohlleber ME, Giménez S, et al. . Reduced slow-wave sleep is associated with high cerebrospinal fluid Aβ42 levels in cognitively normal elderly. Sleep. 2016;39(11):2041-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spira AP, Gamaldo AA, An Y, et al. . Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprecher KE, Bendlin BB, Racine AM, et al. . Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36(9):2568-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju YE, McLeland JS, Toedebusch CD, et al. . Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roh JH, Huang Y, Bero AW, et al. . Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4(150):150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mander BA, Marks SM, Vogel JW, et al. . β-Amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cirrito JR, Yamada KA, Finn MB, et al. . Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913-922. [DOI] [PubMed] [Google Scholar]
- 25.Kamenetz F, Tomita T, Hsieh H, et al. . APP processing and synaptic function. Neuron. 2003;37(6):925-937. [DOI] [PubMed] [Google Scholar]
- 26.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353-356. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. [DOI] [PubMed] [Google Scholar]
- 28.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184-185. [DOI] [PubMed] [Google Scholar]
- 29.Jack CR Jr, Knopman DS, Jagust WJ, et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilgel M, Prince JL, Wong DF, Resnick SM, Jedynak BM. A multivariate nonlinear mixed effects model for longitudinal image analysis: application to amyloid imaging. Neuroimage. 2016;134:658-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts RO, Geda YE, Knopman DS, et al. . The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen RC, Roberts RO, Knopman DS, et al. ; The Mayo Clinic Study of Aging . Prevalence of mild cognitive impairment is higher in men. Neurology. 2010;75(10):889-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts RO, Geda YE, Knopman DS, et al. . The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78(5):342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boeve BF, Molano JR, Ferman TJ, et al. . Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med. 2013;9(5):475-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545. [DOI] [PubMed] [Google Scholar]
- 36.Sforza E, Pichot V, Martin MS, Barthélémy JC, Roche F. Prevalence and determinants of subjective sleepiness in healthy elderly with unrecognized obstructive sleep apnea. Sleep Med. 2015;16(8):981-986. [DOI] [PubMed] [Google Scholar]
- 37.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ III. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.St Sauver JL, Grossardt BR, Yawn BP, et al. . Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vemuri P, Lesnick TG, Przybelski SA, et al. . Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol. 2012;72(5):730-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588-597. [DOI] [PubMed] [Google Scholar]
- 41.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR Jr. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26(2):600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jack CR Jr, Lowe VJ, Senjem ML, et al. . 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131(pt 3):665-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR Jr, Wiste HJ, Vemuri P, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133(11):3336-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jack CR Jr, Wiste HJ, Lesnick TG, et al. . Brain β-amyloid load approaches a plateau. Neurology. 2013;80(10):890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray ME, Lowe VJ, Graff-Radford NR, et al. . Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain. 2015;138(pt 5):1370-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mundry R, Nunn CL. Stepwise model fitting and statistical inference: turning noise into signal pollution. Am Nat. 2009;173(1):119-123. [DOI] [PubMed] [Google Scholar]
- 47.Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci. 2015;16(2):109-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pack AI, Dinges DF, Gehrman PR, Staley B, Pack FM, Maislin G. Risk factors for excessive sleepiness in older adults. Ann Neurol. 2006;59(6):893-904. [DOI] [PubMed] [Google Scholar]
- 49.Spira AP, Yager C, Brandt J, et al. . Objectively measured sleep and β-amyloid burden in older adults: a pilot study. SAGE Open Med. 2014;2:2050312114546520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osorio RS, Ayappa I, Mantua J, et al. . Interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35(6):1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liguori C, Mercuri NB, Izzi F, et al. . Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40(5):zsx011. [DOI] [PubMed] [Google Scholar]
- 52.Shiota S, Takekawa H, Matsumoto SE, et al. . Chronic intermittent hypoxia/reoxygenation facilitate amyloid-β generation in mice. J Alzheimers Dis. 2013;37(2):325-333. [DOI] [PubMed] [Google Scholar]
- 53.Gelber RP, Redline S, Ross GW, et al. . Associations of brain lesions at autopsy with polysomnography features before death. Neurology. 2015;84(3):296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gooneratne NS, Vitiello MV. Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med. 2014;30(3):591-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guilleminault C, Partinen M, Quera-Salva MA, Hayes B, Dement WC, Nino-Murcia G. Determinants of daytime sleepiness in obstructive sleep apnea. Chest. 1988;94(1):32-37. [DOI] [PubMed] [Google Scholar]
- 56.Seneviratne U, Puvanendran K. Excessive daytime sleepiness in obstructive sleep apnea: prevalence, severity, and predictors. Sleep Med. 2004;5(4):339-343. [DOI] [PubMed] [Google Scholar]
- 57.Bennett LS, Langford BA, Stradling JR, Davies RJ. Sleep fragmentation indices as predictors of daytime sleepiness and nCPAP response in obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158(3):778-786. [DOI] [PubMed] [Google Scholar]
- 58.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep. 2002;25(3):307-314. [PubMed] [Google Scholar]
- 59.Punjabi NM, O’hearn DJ, Neubauer DN, et al. . Modeling hypersomnolence in sleep-disordered breathing: a novel approach using survival analysis. Am J Respir Crit Care Med. 1999;159(6):1703-1709. [DOI] [PubMed] [Google Scholar]
- 60.Heinzer R, Gaudreau H, Décary A, et al. . Slow-wave activity in sleep apnea patients before and after continuous positive airway pressure treatment: contribution to daytime sleepiness. Chest. 2001;119(6):1807-1813. [DOI] [PubMed] [Google Scholar]
- 61.Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106(5):1608-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucey BP, Mawuenyega KG, Patterson BW, et al. . Associations between β-amyloid kinetics and the β-amyloid diurnal pattern in the central nervous system. JAMA Neurol. 2017;74(2):207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bero AW, Yan P, Roh JH, et al. . Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14(6):750-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11(2):200-208. [DOI] [PubMed] [Google Scholar]
- 65.Wisor JP, Rempe MJ, Schmidt MA, Moore ME, Clegern WC. Sleep slow-wave activity regulates cerebral glycolytic metabolism. Cereb Cortex. 2013;23(8):1978-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324(5923):105-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143-150. [DOI] [PubMed] [Google Scholar]
- 68.Tabuchi M, Lone SR, Liu S, et al. . Sleep interacts with Aβ to modulate intrinsic neuronal excitability. Curr Biol. 2015;25(6):702-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kam K, Duffy AM, Moretto J, LaFrancois JJ, Scharfman HE. Interictal spikes during sleep are an early defect in the Tg2576 mouse model of β-amyloid neuropathology. Sci Rep. 2016;6:20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nofzinger EA, Buysse DJ, Miewald JM, et al. . Human regional cerebral glucose metabolism during non–rapid eye movement sleep in relation to waking. Brain. 2002;125(pt 5):1105-1115. [DOI] [PubMed] [Google Scholar]
- 71.Vlassenko AG, Vaishnavi SN, Couture L, et al. . Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ) deposition. Proc Natl Acad Sci U S A. 2010;107(41):17763-17767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horovitz SG, Braun AR, Carr WS, et al. . Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A. 2009;106(27):11376-11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sämann PG, Wehrle R, Hoehn D, et al. . Development of the brain’s default mode network from wakefulness to slow wave sleep. Cereb Cortex. 2011;21(9):2082-2093. [DOI] [PubMed] [Google Scholar]
- 75.Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural Regen Res. 2012;7(5):376-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7(4):278-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Theofilas P, Ehrenberg AJ, Dunlop S, et al. . Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: a stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 2017;13(3):236-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol Psychiatry. 2012;71(9):805-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schliebs R. Basal forebrain cholinergic dysfunction in Alzheimer’s disease: interrelationship with beta-amyloid, inflammation and neurotrophin signaling. Neurochem Res. 2005;30(6-7):895-908. [DOI] [PubMed] [Google Scholar]
- 80.Ross JA, McGonigle P, Van Bockstaele EJ. Locus coeruleus, norepinephrine and Aβ peptides in Alzheimer’s disease. Neurobiol Stress. 2015;2:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liguori C, Romigi A, Nuccetelli M, et al. . Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71(12):1498-1505. [DOI] [PubMed] [Google Scholar]
- 82.Slats D, Claassen JA, Lammers GJ, Melis RJ, Verbeek MM, Overeem S. Association between hypocretin-1 and amyloid-β42 cerebrospinal fluid levels in Alzheimer’s disease and healthy controls. Curr Alzheimer Res. 2012;9(10):1119-1125. [DOI] [PubMed] [Google Scholar]
- 83.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342(1):37-44. [DOI] [PubMed] [Google Scholar]
- 84.Theofilas P, Dunlop S, Heinsen H, Grinberg LT. Turning on the light within: subcortical nuclei of the isodentritic core and their role in Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2015;46(1):17-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karageorgiou E, Vossel KA. Brain rhythm attractor breakdown in Alzheimer’s disease: functional and pathologic implications. Alzheimers Dement. 2017;13(9):1054-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Y, Fenik P, Zhan G, Xin R, Veasey SC. Degeneration in arousal neurons in chronic sleep disruption modeling sleep apnea. Front Neurol. 2015;6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao ZZY, Zhao X, Zhan G, Fenik P, Veasey S. Chronic short sleep initiates an amyloid cascade in locus coeruleus neurons and tau-dependent neurodegeration. Sleep (Basel). 2017;40(suppl):A100. [Google Scholar]
- 88.Kendzerska TB, Smith PM, Brignardello-Petersen R, Leung RS, Tomlinson GA. Evaluation of the measurement properties of the Epworth Sleepiness Scale: a systematic review. Sleep Med Rev. 2014;18(4):321-331. [DOI] [PubMed] [Google Scholar]
- 89.Onen F, Moreau T, Gooneratne NS, Petit C, Falissard B, Onen SH. Limits of the Epworth Sleepiness Scale in older adults. Sleep Breath. 2013;17(1):343-350. [DOI] [PubMed] [Google Scholar]
- 90.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17(8):703-710. [DOI] [PubMed] [Google Scholar]
- 91.Bonnet MH. ACNS clinical controversy: MSLT and MWT have limited clinical utility. J Clin Neurophysiol. 2006;23(1):50-58. [DOI] [PubMed] [Google Scholar]
- 92.Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med. 2013;9(8):789-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown BM, Rainey-Smith SR, Villemagne VL, et al. ; AIBL Research Group . The relationship between sleep quality and brain amyloid burden. Sleep. 2016;39(5):1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Multiple Linear Regression Model Estimates for Predicted ΔPiB (SUVR)
eTable 2. Multiple Linear Regression Model Estimates for Baseline Self-reported Sleep Symptoms
eTable 3. Multiple Linear Regression Model Estimates for Witnessed Apneas