This multinational cohort study examines the clinical phenotypes, treatment responses, and outcomes of children with relapsing myelin oligodendrocyte glycoprotein antibody–associated disease.
Key Points
Question
What is the disease course and treatment response in children with relapsing myelin oligodendrocyte glycoprotein antibody–associated disorders?
Findings
In this multinational European cohort study of 102 children, neuromyelitis optica spectrum disorder (43.1%) was the predominant relapsing phenotype. In this cohort, in which more frequently relapsing patients were treated, azathioprine, mycophenolate mofetil, rituximab, and particularly intravenous immunoglobulins were effective in managing relapses but not multiple sclerosis disease-modifying drugs.
Meaning
More studies are required to evaluate how we optimally manage relapsing myelin oligodendrocyte glycoprotein antibody–associated demyelinating disorders.
Abstract
Importance
Myelin oligodendrocyte glycoprotein antibodies (MOG-Abs) are consistently identified in a range of demyelinating disorders in adults and children. Current therapeutic strategies are largely center specific, and no treatments have been formally evaluated.
Objective
To examine the clinical phenotypes, treatment responses, and outcomes of children with relapsing MOG-Ab–associated disease.
Design, Setting, and Participants
This study prospectively collected demographic, clinical, and radiologic data from 102 patients from 8 countries of the EU Paediatric Demyelinating Disease Consortium from January 1, 2014, through December 31, 2016. Patients were treated according to local protocols.
Main Outcomes and Measures
Annualized relapse rates (ARRs) and Expanded Disability Status Scale (EDSS) scores before and during treatment with disease-modifying drugs (DMDs).
Results
A total of 102 children were identified (median [range] age, 7.0 [1.5-7.9] years; male to female ratio, 1.0:1.8; white to other race/ethnicity ratio, 3.6:1.0). Original diagnoses were neuromyelitis optica spectrum disorder (44 patients [43.1%]), acute disseminated encephalomyelitis followed by optic neuritis (20 [19.6%]), multiphasic disseminated encephalomyelitis (20 [19.6%]), and relapsing optic neuritis (18 [17.6%]). In all, 464 demyelinating events were reported. Treated patients had more relapses (median, 3.0; range, 1.0-17.0) than untreated patients (median, 1.0; range 1.0-7.0) (P = .009) and higher EDSS scores (median, 1.5; interquartile range, 0-2.5) than untreated patients (median, 1.0; interquartile range, 0-1.5) (P < .001). Fifty-two children (51.0%) received DMDs: 28 (53.8%) were treated with 1 DMD, 17 (32.7%) with 2, and 7 (13.5%) with 3 or more sequential DMDs. Patients relapsed during all treatments, with a total of 127 relapses on treatment reported. No changes in median ARR and EDSS score were observed between the preinitiation and postinitiation phases of interferon beta and glatiramer acetate treatment (n = 11). The median ARR was reduced from 1.84 to 1.0 with azathioprine (n = 20, P < .001), 1.79 to 0.52 with mycophenolate mofetil (n = 15, P = .003), and 2.12 to 0.67 with rituximab (n = 9, P < .001), although the median EDSS score remained unchanged. An improvement in ARR (from 2.16 to 0.51, P < .001) and EDSS score (from 2.2 to 1.2, P = .01) was observed in the 12 patients treated with regular intravenous immunoglobulins.
Conclusions and Relevance
Although commonly used to treat patients with multiple sclerosis, DMDs were not associated with clinical improvement in children with MOG-Ab–associated disease, whereas azathioprine, mycophenolate mofetil, rituximab, and particularly intravenous immunoglobulins were associated with a reduction in relapse frequency. A correct diagnosis of relapsing MOG-Ab–associated disorders is therefore important to optimize immune treatment.
Introduction
Myelin oligodendrocyte glycoprotein antibodies (MOG-Abs) are consistently identified in a range of acquired demyelinating syndromes (ADSs) in adults and children and in up to 50% of children at first presentation of ADSs. Although MOG-Abs were initially reported in predominantly monophasic disease, a recent report of 210 children with ADSs who were followed up for at least 2 years observed that 22 of 65 MOG-Ab–positive children (33.8%) experienced clinical relapse and were diagnosed with multiphasic disseminated encephalomyelitis (MDEM), recurrent optic neuritis (RON), acute disseminated encephalomyelitis followed by optic neuritis (ADEM-ON), or neuromyelitis optica spectrum disorder (NMOSD). Two recent reports identified MOG-Abs in 22 of 35 children (62.8%) and 26 of 48 children (54%) with non–multiple sclerosis (MS) relapsing demyelination, which is more than 3 times more common than the aquaporin 4 antibody (AQP4-Ab) (4 of 35 patients and 8 of 48 patients). The MOG-Ab–positive children had distinctive clinical and magnetic resonance imaging (MRI) features different from MS and AQP4-Ab NMOSD.
Treatment of MOG-Ab–associated disease has been influenced by protocols used for NMOSD with AQP4-Ab, although these 2 disorders are thought to be clinically and biologically different. The high proportion of monophasic courses in patients with MOG-Abs supports the decision against commencing maintenance immunosuppression after the first clinical event of MOG-Ab–associated disease. Furthermore, because some patients with MOG-Abs seem to have a milder NMOSD phenotype than patients with AQP4-Ab, with good short-term response to corticosteroids, many of the relapsing cases were also not treated with maintenance immunosuppression. Recent reports also highlight that patients with MOG-Abs continue to relapse and accrue disability, sometimes despite maintenance treatment, raising important questions about how patients with relapsing MOG-Ab–associated disease should be treated. Current therapeutic strategies are largely center specific, formal consensus guidelines are yet to be formulated, and no clinical trials have been performed. We therefore conducted this retrospective, multicenter study to describe the first attack features, paraclinical characteristics, disease course, and responses to different treatment strategies in children with MOG-Ab–associated relapsing demyelinating syndromes.
Methods
Participants
From January 1, 2014, through December 31, 2016, we collected demographic, clinical, and radiologic data from 102 patients from 8 countries of the EU Paediatric Demyelinating Disease Consortium (United Kingdom [n = 57], Germany/Austria [n = 18], the Netherlands [n = 12], France [n = 10], Turkey [n = 3], Switzerland [n = 1], and Israel [n = 1]), a component of the European Reference Network for Rare Immunodeficiency, Autoinflammatory, and Autoimmune Disease. Participants were retrospectively identified from those prospectively recruited into the respective national demyelination programs or centers and fulfilled the following inclusion criteria: (1) a diagnosis of relapsing demyelination syndrome, (2) presence of MOG-Abs detected at onset or at the time of a clinical relapse, and (3) age younger than 18 years at first presentation. Institutional review board and/or national research ethics approval was obtained at individual centers or national programs, respectively. Patients included in this study had been enrolled in national programs with respective review board/ethical committee approvals (France [Hôpital Bicêtre, Paris], the Netherlands [Medische ethische toetsings commissie Erasmus Medical Centre, Rotterdam], Germany and Austria [University of Innsbruck Ethics Committee], United Kingdom [West Midlands–South Birmingham Research Ethics Committee], and Turkey [Hacettepe University, Ankara]) or provided verbal and/or written informed consent to the respective referring physician. All data were deidentified.
Procedure
Clinical data were deidentified and entered by each participating investigator onto a unified case reporting form (CRF), detailing selected demographics, clinical findings, and laboratory results (MOG-Abs and AQP4-Ab, cerebrospinal fluid white blood cell count, protein level, number of oligoclonal bands, virologic test results, erythrocyte sedimentation rate, and Epstein-Barr virus serologic test results), first and subsequent attacks characteristics, and treatment information. All CRFs were initially reviewed by the respective national leads (5 of us, M.B., K.R., R.N., K.D., and M.L.) and subsequently analyzed by 2 of our investigators (Y.H., M.L.).
Demyelinating phenotype at onset was determined from the patient’s clinical features, according to established criteria. All patients had undergone brain and spinal cord MRI according to local MRI protocols (which do not routinely include orbital MRI).
Cases were assigned by participating investigator and subsequently confirmed by national leads based on clinical and radiologic information provided on the CRF to one of the following diagnostic categories: (1) MS, fulfilling the 2013 International Pediatric Multiple Sclerosis Study Group consensus criteria; (2) NMOSD, fulfilling the 2015 International Panel for NMO diagnosis criteria; (3) MDEM and ADEM-ON, fulfilling the 2013 International Pediatric Multiple Sclerosis Study Group consensus criteria; and (4) recurrent demyelination in a single central nervous system area without evidence of clinically silent disease, such as RON.
Annualized relapse rates (ARRs) were calculated as the number of relapses per year before treatment (excluding index event) and during treatment only in patients with at least 6 months of follow-up after initiation of treatment. Relapses were analyzed for up to 2 years before initiation of therapy and for the duration of the time undergoing therapy. Outcomes at last follow-up were retrieved from the patient’s medical records to represent the most contemporary assessment of disability. If unavailable, this assessment was obtained directly from the patient’s primary treating physician. The Expanded Disability Status Scale (EDSS) scores were documented at point of disease stability at least 3 months from acute or relapsing events.
MOG-Ab Testing
Within 1 month of an acute event (onset or relapse), clinically symptomatic children underwent testing for serum MOG-Abs, using a live cell-based assay optimized to reduce IgM cross-reactivity in the respective reference laboratories (detailed in the Acknowledgment) of the referring countries, as part of routine assessments of children with demyelinating diseases (antibody testing in the cerebrospinal fluid was not routinely performed).
Statistical Analysis
Parametric or nonparametric statistical tests (Mann-Whitney and Kruskal-Wallis tests) were used for continuous distributions, as appropriate, and χ2 or Fisher exact tests for nominal data to compare the demographics, presenting symptoms, demyelinating phenotypes, and radiologic and serologic characteristics across the different groups and between those who received or did not receive maintenance immunotherapy. Receiver operating characteristic analysis was used to identify the cutoff age associated with phenotype change. A paired 2-tailed t test was used to compare ARRs and EDSS scores before and during treatment. A 2-sided P < .05 was considered to be significant. Data were analyzed using GraphPad Prism 5 (GraphPad Software).
Results
Patients Group
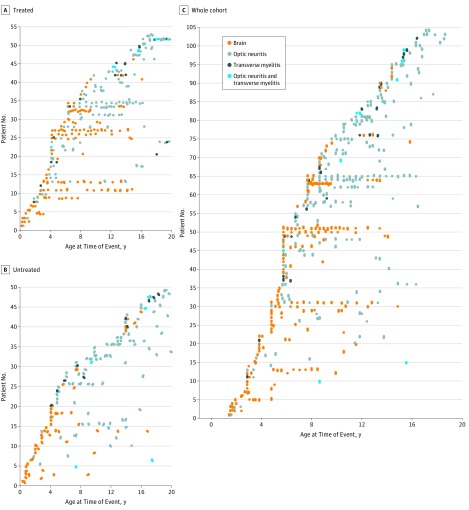
A total of 102 children with relapsing MOG-Ab–associated disease were studied (median [range] age, 7.0 [1.5-7.9] years; male to female ratio, 1.0:1.8; white to other race/ethnicity ratio, 3.6:1.0). All patients were tested for AQP4-Ab, and none were double positive. The median length of follow-up (from first clinical presentation) was 5 years (interquartile range [IQR], 3-9 years). The original diagnoses were NMOSD in 44 children (43.1%), MDEM in 20 (19.6%), ADEM-ON in 20 (19.6%), and RON in 18 (17.6%). None of the patients had a final diagnosis of relapsing-remitting MS. Patients presenting with ADEM were younger than patients presenting with other ADS (mean [SD] age, 5.6 [0.4] years vs 10.7 [0.6] years; P < .001). Mean (SD) age at onset was 3.8 (1.7) years in patients with MDEM, 6.9 (2.6) in patients with ADEM-ON, 9.1 (4.5) years in patients with NMOSD, and 11.7 (4.0) years in patients with RON. Clinical events and radiologic changes in patients 9 years or younger were more likely to affect the brain, whereas events in patients older than 9 years were more likely to affect the optic nerve (Figure 1). Patients’ demographic, clinical, and paraclinical features and EDSS scores according to each relapsing demyelination syndrome phenotype are summarized in Table 1.
Figure 1. Demyelinating Phenotypes of the First Attack and Subsequent Relapses.
A total of 464 demyelinating events were reported in 102 patients presenting with myelin oligodendrocyte glycoprotein antibody–associated relapsing demyelination syndrome. Receiver operating characteristic analysis identified the age of 9 years to be the best cutoff age associated with phenotype change. Clinical events in patients 9 years or younger were more likely to affect the brain, whereas events in patients older than 9 years were more likely to affect the optic nerve (P < .001). Brain magnetic resonance imaging abnormalities were also more common in the younger group (P < .001). There was no sex predisposition of differences (female to male ratio in patients ≤9 vs >9 years old was 1.0:1.64 vs 1.0:1.6, P > .99).
Table 1. Demographic, Clinical, and Paraclinical Features of Children According to Their Original Relapsing Demyelination Syndrome Diagnosisa.
| Variable | MDEM (n = 20) | ADEM-ON (n = 20) | NMOSD (n = 44) | RON (n = 18) | All Patients (N = 102) |
|---|---|---|---|---|---|
| Age, median (range), y | 3.6 (1.6-8.0) | 6.0 (3.9-15.0) | 8.0 (1.5-17.5) | 11.4 (3.8-17.9) | 7.0 (1.5-7.9) |
| Male to female ratio | 1.0:1.5 | 1.0:1.2 | 1.0:2.7 | 1.0:1.0 | 1.0:1.8 |
| White to other race/ethnicity ratio | 4.0:1.0 | 4.0:1.0 | 3.0:1.0 | 5.0:10 | 3.6:1.0 |
| Family history of autoimmunity | 2 (10.0) | 2 (10.0) | 5 (11.4) | 2 (11.1) | 11 (10.8) |
| Demyelinating phenotype at onset | |||||
| ADEM | 20 (100) | 20 (100) | 13 (29.5) | 0 | 53 (52.0) |
| Optic neuritis | 0 | 0 | 15 (34.1) (9 Bilateral) |
18 (100) (9 Bilateral) |
33 (32.4) (18 Bilateral) |
| Transverse myelitis | 0 | 0 | 6 (13.6) | 0 | 6 (5.9) |
| Optic neuritis and transverse myelitis | 0 | 0 | 8 (18.2) | 0 | 8 (7.8) |
| Brainstem syndrome | 0 | 0 | 2 (4.5) | 0 | 2 (2.0) |
| Symptoms at onset | |||||
| Vision | 3 (15.0) | 8 (40.0) | 26 (59.1) | 18 (100) | 55 (53.9) |
| Encephalopathy | 20 (100) | 20 (100) | 13 (29.5) | 0 | 53 (52.0) |
| Motor | 8 (40.0) | 11 (55.0) | 21 (47.7) | 0 | 40 (39.2) |
| Cerebellar syndrome | 11 (55.0) | 9 (45.0) | 9 (20.5) | 0 | 29 (28.4) |
| Seizures | 6 (30.0) | 8 (40.0) | 5 (11.4) | 0 | 19 (18.6) |
| Sensory | 0 | 4 (20.0) | 12 (27.3) | 0 | 16 (15.7) |
| Cranial nerve involvement | 3 (15.0) | 6 (30.0) | 4 (9.1) | 0 | 13 (12.7) |
| Autonomic features | 2 (10.0) | 5 (25.0) | 5 (11.4) | 0 | 12 (11.8) |
| Paraclinical features | |||||
| Intrathecal OCBs | 3/12 (25.0) | 1/10 (10.0) | 2/25 (8.0) | 0/7 | 6/54 (11.1) |
| CSF WBC count >10/μL | 13/17 (76.5) | 13/17 (76.5) | 15/27 (55.6) | 2/12 (16.7) | 43/73 (58.9) |
| CSF protein level >0.4 g/L | 7/15 (46.7) | 1/17 (5.9) | 12/30 (40.0) | 1/10 (10.0) | 21/72 (29.2) |
| ESR >10 mm/h | 7/9 (8.8) | 8/11 (72.7) | 6/12 (50.0) | 0/4 | 21/36 (58.3) |
| EBV IgG | 1/10 (10.0) | 0/10 | 10/19 (52.6) | 0/4 | 11/43 (25.6) |
| Abnormal brain MRI findings at onset | 20 (100) | 20 (100) | 18 (40.9) | 0 | 58 (56.9) |
| Outcome | |||||
| Follow-up duration, median (range), y | 6.3 (2.0-10.2) | 7.0 (3.6-9.2) | 5.0 (3.1-7.6) | 4.3 (3.0-6.7) | 5.5 (3.1-9.0) |
| TTFR, median (range), mo | 5.5 (3.5-28.2) | 10.0 (3.0-28.0) | 5.0 (2.0-19.0) | 12.0 (4.0-27.0) | 6.0 (3.0-22.0) |
| Total No. of relapses, median (IQR) | 2.5 (1.0-5.0) | 2.0 (2.0-4.0) | 2.0 (1.0-4.5) | 2.0 (1.0-4.0) | 2.0 (1.0-4.0) |
| EDSS score, median (range) | 1.5 (0-5.0) | 1.0 (0-4.0) | 1.2 (0-10.0) | 1.0 (0-2.0) | 1.0 (0-10.0) |
| Good recovery (EDSS score = 0 and no relapse >6 mo) | 5.0 (25.0) | 5.0 (25.0) | 14.0 (31.8) | 8.0 (44.4) | 32.0 (31.4) |
| Cognitive problems | 10.0 (50.0) | 6.0 (30.0) | 4.0 (9.1) | 0 | 20.0 (19.6) |
Abbreviations: ADEM-ON, acute disseminated encephalomyelitis followed by optic neuritis; CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; EDSS, Expanded Disability Status Scale; ESR, erythrocyte sedimentation rate; IQR, interquartile range; MDEM, multiphasic disseminated encephalomyelitis; MRI, magnetic resonance imaging; NMOSD, neuromyelitis optica spectrum disorder; OCBs, oligoclonal bands; RON, relapsing optic neuritis; TTFR, time to first relapse; WBC, white blood cell.
SI conversion factors: To convert WBCs to ×109/L, multiply by 0.001.
Data are presented as number (percentage) of patients unless otherwise indicated. Five patients had organisms identified in the CSF (polymerase chain reaction analysis: Enterovirus and Mycoplasma pneumoniae; CSF IgM positivity: cytomegalovirus, human herpesvirus 6, and Borrelia). Of these, 4 presented with ADEM and 1 presented with optic neuritis (human herpesvirus 6). All patients had negative serologic test results when retested at time of relapse.
First Attack Features
The most frequent demyelinating phenotype at onset was ADEM (53 [52.0%]) followed by optic neuritis (41 [40.2%]). Of the children presenting with optic neuritis, 18 (43.9%) had bilateral optic neuritis, 15 (36.6%) had unilateral optic neuritis, and 8 (19.5%) had simultaneous optic neuritis and transverse myelitis. Visual symptoms were reported in 55 patients (53.9%) and encephalopathy in 53 (52.0%). Of the 58 patients with abnormal brain MRI findings at onset, cerebellar symptoms were found in 29 (50.0%) and seizures in 19 (32.8%).
Paraclinical Features
Cerebrospinal fluid lymphocytosis was reported in 43 of 73 tested patients (58.9%) (lymphocyte count, 10-624/μL; to convert to ×109/L, multiply by 0.001). Intrathecal oligoclonal bands were seen in only 6 of 54 tested patients (11.1%) across the phenotypes (Table 1). Erythrocyte sedimentation rate was increased in 21 of 36 patients (58.3%),, and evidence of remote Epstein-Barr virus infection was seen in 11 of 43 (25.6%).
Disease Course
A total of 464 demyelinating events were reported in the cohort (Figure 1). No differences were found in time to first relapse, total number of relapses, and EDSS scores among the different original diagnoses. Despite no differences in EDSS scores detected in the different phenotypes, cognitive problems were seen more frequently in patients with MDEM and ADEM-ON (16 of 40 patients [40.0%]) vs NMOSD and RON (4 of 62 patients [6.5%], P < .001). Similarly, patients with abnormal intracranial MRI findings (18 of 65 patients [27.7%]) were more likely to have cognitive problems than patients with normal intracranial MRI findings (2 of 37 patients [5.4%], P = .008). Patients receiving immunotherapy had more clinical relapses and worse EDSSs than untreated patients (Table 2). One patient died. Good recovery, defined as an ARR of 0 at last follow-up (>6 months) and having no neurologic sequelae (EDSS score of 0), was reported in 32 of the 102 patients (31.3%; of these 10 were treated patients).
Table 2. Comparison Between Patients Who Were Treated and Not Treated With Disease-Modifying Drugsa.
| Variable | Treated (n = 52) | Untreated (n = 50) | P Value |
|---|---|---|---|
| Age, median (IQR), y | 6.0 (5.0-9.2) | 7.0 (5.0-13.0) | .22 |
| Female to male ratio | 2.0:1.0 | 1.5:1.0 | .84 |
| White to other race/ethnicity ratio | 1.9:1.0 | 1.4:1.0 | .54 |
| Demyelinating phenotype at onset | |||
| ADEM | 29 (55.8) | 23 (46.0) | .32 |
| Optic neuritis | 14 (26.9) | 19 (38.0) | .40 |
| Transverse myelitis | 3 (5.8) | 3 (6.0) | >.99 |
| Optic neuritis and transverse myelitis | 3 (5.8) | 5 (10.0) | .72 |
| Brainstem syndrome | 2 (3.8) | 0 | .24 |
| Original RDS diagnoses | |||
| MDEM | 10 (19.2) | 10 (20.0) | >.99 |
| ADEM-ON | 10 (19.2) | 10 (20.0) | >.99 |
| NMOSD | 25 (48.1) | 19 (38.0) | .32 |
| RON | 6 (11.5) | 12 (24.0) | .19 |
| Follow-up time, median (IQR), y | 5.0 (3.0-9.0) | 5.0 (3.0-8.0) | .78 |
| EDSS score at last follow-up, median (IQR) | 1.5 (0-2.5) | 1.0 (0-1.5) | .009 |
| Total No. of relapses throughout the follow-up, median (range) | 3.0 (1.0-19.0) | 1 (1.0-7.0) | <.001 |
Abbreviations: ADEM, acute disseminated encephalomyelitis; ADEM-ON, ADEM followed by optic neuritis; EDSS, Expanded Disability Status Scale; IQR, interquartile range; MDEM, multiphasic disseminated encephalomyelitis; NMOSD, neuromyelitis optica spectrum disorder; RDS, relapsing demyelination syndrome; RON, relapsing optic neuritis.
Data are presented as number (percentage) of patients unless otherwise indicated.
Response to Immunotherapy
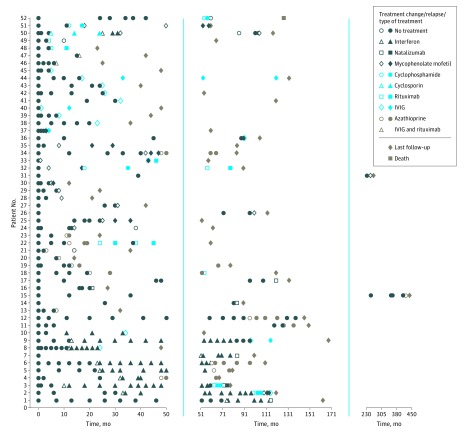
The short-term treatment for each of these patients at presentation and during subsequent episodes of relapses was directed by the treating pediatricians based on protocols influenced by their regional and/or national reference center for central nervous system demyelination, guided by severity and persistence of symptoms. Disease-modifying drugs (ie, all forms of maintenance immunomodulation or immunosuppression therapies) were given in 52 children (51.0%): 28 patients (53.8%) were treated with 1 DMD, 16 (30.7%) with 2, and 7 (13.5%) with 3 or more sequential DMDs, with only 2 patients receiving combinational treatment (intravenous immunoglobulin [IVIG] and rituximab) at any time point. All treatments were optimized at their respective regional or tertiary treating center. The clinical course and disease activity in patients who underwent therapy with maintenance treatment are illustrated in Figure 2. Median time from disease onset to DMD treatment was 1.64 years (IQR, 0.50-3.60 years). Patient relapsed while receiving various treatments, with a total of 127 relapses while receiving treatment reported in the cohort (Figure 2); Interferon beta and glatiramer acetate (total relapses, 71; 2.1 relapses during treatment), azathioprine (total relapses, 20.0; 0.5 relapse during treatment), mycophenolate mofetil (total relapses, 13.0; 0.5 relapse during treatment), rituximab (total relapses, 10.0; 0.7 relapse during treatment), IVIG (total relapses, 6.0; 0.1 relapse during treatment), cyclophosphamide (total relapses, 3.0; 2.0 relapses during treatment), cyclosporine (total relapses, 2.0; 2.0 relapses during treatment), and natalizumab (total relapses, 2.0; 0.3 relapse during treatment). The ARRs and EDSS scores before and during treatment are depicted in Figure 3.
Figure 2. Disease Course in Relation to Respective Therapies.
Each solid marker denotes a demyelinating event, with the color in the figure key denoting respective treatment, whereas an open marker denotes initiation of therapy. Patient relapsed while undergoing all treatments, with a total of 127 relapses during treatment reported in the cohort. All patients treated with first-line injectable multiple sclerosis treatment continued to relapse. Twenty-eight patients remained relapse free while receiving treatment; 7 of 15 (46.7%) treated with mycophenolate mofetil, 10 of 20 (50.0%) treated with azathioprine, 1 of 7 (14.2%) treated with rituximab alone, 6 of 10 (60.0%) treated with intravenous immunoglobulin (IVIG), and 2 of 2 (100%) treated with rituximab and IVIG together. Patient 52 presented initially with bilateral optic neuritis, relapsed 2 years later with transverse myelitis, experienced cognitive and psychiatric problems, and died at 20 years of age of progressive encephalopathy and respiratory failure. The clinical phenotypes of all treated patients are given in the eTable in the Supplement.
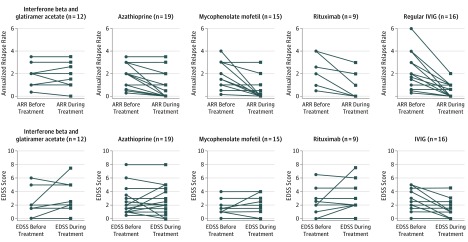
Figure 3. Efficacy of Various Disease-Modifying Therapies in Patients With Myelin Oligodendrocyte Glycoprotein Antibody–Associated Relapsing Demyelination Patients.
Only 2 patients receiving combinational treatment (intravenous immunoglobulin [IVIG] and rituximab) were included for both treatment analyses. No differences were detected in the pretreatment Expanded Disability Status Scale (EDSS) scores and annualized relapse rates among the different treatment groups.
Conventional MS treatment (interferon beta and glatiramer) was given as first-line treatment in 10 children and as a second-line treatment in 1 child and was discontinued in all in view of lack of response and ongoing treatment adverse effects. Two patients were initially switched to an alternative interferon preparation before changing treatment. All patients relapsed while receiving treatment. There was no change in the ARRs before and during treatment, with a mean difference of 0.02 (mean ARR before treatment, 2.40; mean ARR during treatment, 2.38; P > .99). There was no change in EDSS score (mean EDSS score before treatment, 2.2; mean EDSS during treatment, 3.0; P = .23). No severe or life-threatening relapses have been reported with conventional MS treatment. Three patients received natalizumab (2 with good response and 1 who continued to relapse), and no patients received fingolimod or alemtuzumab.
Eleven patients began therapy with mycophenolate mofetil of whom 3 were switched in view of treatment failure, with 1 having additional adverse effects. Four patients were switched to mycophenolate mofetil after cyclophosphamide (n = 2), azathioprine (n = 1), rituximab (n = 1), and cyclosporine treatment followed by interferon beta-1a (n = 1). Eight of 15 patients (53.5%) relapsed while receiving treatment. Mycophenolate mofetil treatment was associated with a mean reduction in the ARR of 1.27 (mean ARR before treatment, 1.79; mean ARR during treatment, 0.52; P = .003), with no change in EDSS score (mean EDSS score before treatment, 1.7; mean EDSS score during treatment, 1.9; P = .59).
Twelve patients began therapy with azathioprine of whom 2 were switched in view of treatment failure and 2 stopped treatment (1 because of treatment failure and 1 because of adverse effects). Eight patients received azathioprine as second-line treatment; first-line MS treatment failed in 5 (1 receiving mycophenolate mofetil and 2 after 1 year of corticosteroid treatment). Ten of 20 patients (50.0%) relapsed while receiving treatment. Azathioprine treatment was associated with a mean reduction in the ARR of 0.84 (mean ARR before treatment, 1.84; mean ARR during treatment, 1.0; P < .001), with no change in EDSS score (mean EDSS score before treatment, 2.5; mean EDSS score during treatment, 2.6; P = .74).
Rituximab was given as first-line treatment in 4 patients (with additional IVIG in 2), as second-line treatment in 4 patients, and as third-line treatment in 1 patient. Of the patients treated with rituximab as first-line treatment, no additional immunotherapy was used after treatment. Six of 9 patients (66.7%) relapsed during treatment. Two of 3 patients who did not relapse were additionally receiving maintenance IVIG. One child had a severe life-threatening relapse while receiving therapeutic doses of rituximab and had depleted B cells. Rituximab was associated with a mean reduction in the ARR of 1.61 (mean ARR before treatment, 2.12; mean ARR during treatment, 0.67; P < .001), with no change in EDSS score (mean EDSS before treatment, 2.4; mean EDSS during treatment, 3.2; P = .23).
Intravenous immunoglobulin (regular infusion every 4 weeks) was given as first-line maintenance treatment in 12 patients (2 received additional rituximab) and in 4 patients as a second-line treatment after revision of the diagnosis. All patients continued to receive IVIG, but in 2 the infusion was reduced to every 8 weeks. Four of 12 patients (33.3%) relapsed while undergoing treatment. The IVIG treatment was associated with a reduction in the ARR of 1.71 (2.16 to 0.51, P < .001). The EDSS was also reduced (mean EDSS before treatment, 2.2; mean EDSS during treatment, 1.2; P = .01).
A total of 8 patients received oral prednisolone for more than 6 months; 5 (62.5%) relapsed while receiving treatment (3 while weaning from corticosteroids), and 1 patient relapsed 1 week after treatment with corticosteroids was stopped. Two patients started treatment with cyclophosphamide and 1 with cyclosporine; all relapsed while receiving treatment. Overall, we did not identify any phenotype that was more responsive to any specific treatments (Figure 3).
Fifty patients (49.0%) were not treated. The median number of relapses in the untreated group was 1.0 (range, 1.0-7.0), and the median EDSS score was 1.0 (IQR, 0-1.5). No differences were found in patient demographics and clinical symptoms at onset and final demyelinating phenotype between the patients who were treated and those who were not (Table 2). Overall, the treated patients had more relapses (median, 3.0; range, 1.0-17.0) and higher EDSS scores (median, 1.5; IQR, 0-2.5) than the untreated patients (median number of relapses, 1.0; range, 1.0-7.0; P = .009 and median EDSS score, 1.0; IQR, 0-1.5; P < .001).
Discussion
Although MOG-Ab–associated disease is now well recognized in children and adults, few comparative studies have been performed of their clinical and investigative features, treatment response, or outcomes. In this large multicenter study of 102 children with relapsing MOG-Ab–associated disease, the original diagnoses were various, and overall the treated patients had a more severe disease. Although treatments were heterogeneous, injectable MS drugs were not associated with improvement, and maintenance IVIG was found superior to other treatments.
We observed an age-dependent phenotype, with brain manifestation in younger children and optic neuritis and/or transverse myelitis with normal intracranial imaging findings in the older child. This finding is in keeping with the physiologic, age-dependent white matter maturation that occurs from infancy to adulthood and may suggest susceptibility of the uncompacted myelin to an antibody that targets the outermost layer of the myelin sheath. A progressive loss of tissue integrity occurs over time in patients with recurrent brain demyelination, which is likely to result in secondary neuroaxonal injury and could explain the poor cognitive outcome seen in this group and the reduced response to immunotherapies over time.
In this cohort, we observed the treatment paradox described in similar disorders, whereby the higher relapse rate and poorer outcome in the group receiving more therapy is simply reflected by the a priori threshold for initiating such treatments. In the 52 patients who were treated with DMDs, treatment was associated with a reduction in the ARR in patients treated with regular IVIG, rituximab, mycophenolate mofetil, and azathioprine in descending order. Care is also required when interpreting the ARR, which is susceptible to artifactual elevation, for example, when there is a short time to first relapse and a short time to treatment (increasing pretreatment ARR). Although we ensured therapeutic DMD doses by including treatment length of at least 6 months, lag time to therapeutic effect of specific treatments may lead to an artifactually elevated posttreatment ARR.
The unresponsiveness to conventional MS therapy is reminiscent of a report in AQP4-Ab NMOSD, although none of these children were reported to have life-threatening relapses after MS therapy as reported in some patients with NMOSD. None of the patients received alemtuzumab, which was reported to cause disease worsening in patients with NMOSD and MOG-Ab–associated disease. Interestingly, 6 of 7 patients (85.7%) who received rituximab alone continued to relapse despite B-cell depletion.
A key finding of our study is that IVIG as maintenance therapy was associated with the greatest improvements in ARR and EDSS score. Intravenous immunoglobulin is the only treatment that does not induce immunosuppression. Its mechanisms of action may go beyond the known immunomodulatory effect and may also be beneficial in patients with secondary inflammation. Interestingly, in a recent study using organotypic cerebellar section cultures from transgenic mice and MOG-Ab–induced demyelination, treatment with IVIG was protective from demyelination in a dose-dependent manner.
Limitations
A major limitation of this study is that disease was not systematically managed in all patients, with possible biases in treatment initiation and/or escalation. Because testing for MOG-Ab has only recently become available, the patients described in this article were frequently misdiagnosed with MS, viral encephalitis (in view of the cerebrospinal fluid leukocytosis), and central nervous system vasculitis (in view of the increased erythrocyte sedimentation rate), which resulted in heterogeneous treatment and management regimens across the multiple centers. Because a significant number of cases were retrospectively tested, with diagnosis only considered at relapse and often many years later, this study could not provide information on the utility of serial measurements and/or antibody titers in predicting disease course or directing DMDs.
This cohort was not adequately powered to evaluate potential differences of immunotherapy responses across the different relapsing phenotypes and was not optimal for a direct evaluation of an individual or sequence of treatment effect, which is better suited to a study design in which the lag phase of efficacy or washout period of specific therapies could be prospectively controlled. One particular treatment that deserves specific attention is the cumulative use of corticosteroids, often used in conjunction with DMDs and at low doses but also during relapses. Prolonged corticosteroid maintenance, which appears to be effective in adults with NMOSD, is less commonly used in the pediatric population in view of the adverse effects.
Conclusions
Despite the limitations, this post hoc evaluation and analysis of data previously collected and published allowed us to make important observations about the treatment responsiveness of patients with relapsing MOG-Ab–associated disease, which has to be carefully and pragmatically considered alongside the safety of many of these treatments. Importantly, because most children with MOG-Abs remain monophasic, the data reported here do not evaluate treatment for patients with monophasic ADS; hence, these treatment strategies should not be applied to children after the first clinical event. The important questions our study raises are whether the treatment-resistant group represents a selected group of patients who are biologically or immunologically different and whether earlier intervention with more specific maintenance immunotherapy would lead to a better neurologic outcome. However, to achieve this, studies must initially elucidate many key aspects of the MOG-Ab–associated disorders, such as disease heterogeneity, early biomarkers of relapsing and/or severe disease, and optimal outcome measures, after which controlled trials could be performed.
eTable. Clinical Phenotypes of All Treated Patients
References
- 1.Reindl M, Jarius S, Rostasy K, Berger T. Myelin oligodendrocyte glycoprotein antibodies: how clinically useful are they? Curr Opin Neurol. 2017;30(3):295-301. [DOI] [PubMed] [Google Scholar]
- 2.Tenembaum S, Chitnis T, Nakashima I, et al. Neuromyelitis optica spectrum disorders in children and adolescents. Neurology. 2016;87(9)(suppl 2):S59-S66. [DOI] [PubMed] [Google Scholar]
- 3.Ketelslegers IA, Van Pelt DE, Bryde S, et al. Anti-MOG antibodies plead against MS diagnosis in an acquired demyelinating syndromes cohort. Mult Scler. 2015;21(12):1513-1520. [DOI] [PubMed] [Google Scholar]
- 4.Hacohen Y, Absoud M, Deiva K, et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennes EM, Baumann M, Schanda K, et al. ; BIOMARKER Study Group . Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89(9):900-908. [DOI] [PubMed] [Google Scholar]
- 6.Baumann M, Hennes EM, Schanda K, et al. Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): Extending the spectrum of MOG antibody positive diseases. Mult Scler. 2016;22(14):1821-1829. [DOI] [PubMed] [Google Scholar]
- 7.Rostasy K, Mader S, Schanda K, et al. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol. 2012;69(6):752-756. [DOI] [PubMed] [Google Scholar]
- 8.Huppke P, Rostasy K, Karenfort M, et al. Acute disseminated encephalomyelitis followed by recurrent or monophasic optic neuritis in pediatric patients. Mult Scler. 2013;19(7):941-946. [DOI] [PubMed] [Google Scholar]
- 9.Lechner C, Baumann M, Hennes EM, et al. Antibodies to MOG and AQP4 in children with neuromyelitis optica and limited forms of the disease. J Neurol Neurosurg Psychiatry. 2016;87(8):897-905. [DOI] [PubMed] [Google Scholar]
- 10.Sepúlveda M, Armangue T, Martinez-Hernandez E, et al. Clinical spectrum associated with MOG autoimmunity in adults. J Neurol. 2016;263(7):1349-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014;71(3):276-283. [DOI] [PubMed] [Google Scholar]
- 12.Hacohen Y, Mankad K, Chong WK, et al. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology. 2017;89(3):269-278. [DOI] [PubMed] [Google Scholar]
- 13.Kitley J, Palace J. Therapeutic options in neuromyelitis optica spectrum disorders. Expert Rev Neurother. 2016;16(3):319-329. [DOI] [PubMed] [Google Scholar]
- 14.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reindl M, Rostasy K. MOG antibody-associated diseases. Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sepúlveda M, Armangué T, Sola-Valls N, et al. Neuromyelitis optica spectrum disorders: comparison according to the phenotype and serostatus. Neurol Neuroimmunol Neuroinflamm. 2016;3(3):e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montcuquet A, Collongues N, Papeix C, et al. Effectiveness of mycophenolate mofetil as first-line therapy in AQP4-IgG, MOG-IgG, and seronegative neuromyelitis optica spectrum disorders. Mult Scler. 2016;23(10):1377-1384. [DOI] [PubMed] [Google Scholar]
- 18.Krupp LB, Tardieu M, Amato MP, et al. ; International Pediatric Multiple Sclerosis Study Group . International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261-1267. [DOI] [PubMed] [Google Scholar]
- 19.Wingerchuk DM, Banwell B, Bennett JL, et al. ; International Panel for NMO Diagnosis . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dale RC, Brilot F, Duffy LV, et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology. 2014;83(2):142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters P, Woodhall M, O’Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468(7321):244-252. [DOI] [PubMed] [Google Scholar]
- 23.Reindl M, Di Pauli F, Rostásy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol. 2013;9(8):455-461. [DOI] [PubMed] [Google Scholar]
- 24.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis. Lancet Neurol. 2013;12(2):157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deiva K, Absoud M, Hemingway C, et al. ; UK Childhood Inflammatory Demyelination (UK-CID) Study and French Kidbiosep Study . Acute idiopathic transverse myelitis in children. Neurology. 2015;84(4):341-349. [DOI] [PubMed] [Google Scholar]
- 26.Marignier R, Cobo Calvo A, Vukusic S. Neuromyelitis optica and neuromyelitis optica spectrum disorders. Curr Opin Neurol. 2017;30(3):208-215. [DOI] [PubMed] [Google Scholar]
- 27.Azzopardi L, Cox AL, McCarthy CL, Jones JL, Coles AJ. Alemtuzumab use in neuromyelitis optica spectrum disorders: a brief case series. J Neurol. 2016;263(1):25-29. [DOI] [PubMed] [Google Scholar]
- 28.Wildemann B, Jarius S, Schwarz A, et al. Failure of alemtuzumab therapy to control MOG encephalomyelitis. Neurology. 2017;89(2):207-209. [DOI] [PubMed] [Google Scholar]
- 29.Kile S, Au W, Parise C, et al. IVIG treatment of mild cognitive impairment due to Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2017;88(2):106-112. [DOI] [PubMed] [Google Scholar]
- 30.Winter M, Baksmeier C, Steckel J, et al. Dose-dependent inhibition of demyelination and microglia activation by IVIG. Ann Clin Transl Neurol. 2016;3(11):828-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Clinical Phenotypes of All Treated Patients