Key Points
Question
Is preclinical Alzheimer disease associated with circadian rest-activity rhythm disturbances?
Findings
In this cross-sectional study, preclinical Alzheimer disease, as assessed by Pittsburgh Compound B imaging or increased cerebrospinal fluid phosphorylated tau to amyloid β 42 ratio in cognitively normal participants, was associated with increased rest-activity rhythm fragmentation. Older age and male sex were also associated with increased fragmentation and decreased amplitude of rest-activity rhythm, independent of Alzheimer disease pathology.
Meaning
Disturbances of the rest-activity rhythm are present in preclinical Alzheimer disease, even after accounting for effects of aging and sex, demonstrating that circadian dysfunction occurs very early in the course of Alzheimer disease and precedes cognitive symptom onset.
Abstract
Importance
Circadian rhythm disturbances occur in symptomatic Alzheimer disease (AD) and have been hypothesized to contribute to disease pathogenesis. However, it is unknown whether circadian changes occur during the presymptomatic phase of the disease.
Objective
To examine the associations between circadian function, aging, and preclinical AD pathology in cognitively normal adults.
Design, Setting, and Participants
This cross-sectional study was conducted using community volunteers from the Knight Alzheimer’s Disease Research Center at Washington University in St Louis. Cognitively normal participants (n = 205) underwent 7 to 14 days of actigraphy in their home environment between 2010 and 2012, in addition to clinical assessment, amyloid imaging with Pittsburgh Compound B (PiB), and cerebrospinal fluid biomarker collection. Data collected from 3 years before to 6 months after actigraphy were included. Sixteen participants were excluded owing to incomplete data collection.
Main Outcomes and Measures
Circadian rhythm analysis was performed on actigraphy data using 3 methods: cosinor, nonparametric, and empirical mode decomposition. Preclinical AD was assessed by longitudinal clinical assessment, amyloid imaging with PiB, and cerebrospinal fluid biomarker collection.
Results
Data from 189 participants were included in the analyses. The mean (SD) age was 66.6 (8.3) years, and 121 participants (64%) were women. Older age (β = .247; P = .003) and male sex (β = .170; P = .04), in the absence of amyloid pathology, were associated with a significant increase in intradaily variability, a nonparametric measure of rest-activity rhythm fragmentation, as well as decreased amplitude by several measures. After correction for age and sex, the presence of preclinical amyloid plaque pathology, assessed by positive PiB imaging (mean [SD], 0.804 [0.187] for PiB negative vs 0.875 [0.178] for PiB positive; P = .05) or increasing cerebrospinal fluid phosphorylated-tau to amyloid β 42 ratio (β = .231; P = .008), was associated with increased intradaily variability, indicating rest-activity rhythm fragmentation.
Conclusions and Relevance
Preclinical AD is associated with rest-activity rhythm fragmentation, independent of age or sex. Aging was also associated with circadian dysfunction independently of preclinical AD pathology, particularly in men. The presence of circadian rhythm abnormalities in the preclinical phase of AD suggests that circadian dysfunction could contribute to early disease pathogenesis or serve as a biomarker of preclinical disease.
This study examines the associations between circadian function, aging, and preclinical Alzheimer disease pathology in cognitively normal adults.
Introduction
The circadian system, controlled by the master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus, regulates 24-hour oscillations in a wide variety of biological processes, such as sleep-wake function and transcription of genes involved in metabolism, inflammation, and oxidative stress.1,2,3 Accordingly, circadian rhythm disturbances are associated with increased risk of chronic diseases, such as cancer and diabetes, in humans, suggesting that proper circadian function is required for optimal health.2,4,5,6 Circadian clock disruption7 causes neuroinflammation, oxidative stress, and neuronal damage in rodents,3,7 suggesting that circadian dysfunction in humans could promote neurodegeneration.8
Circadian function declines in aged animals and humans, and a variety of circadian deficits have been described in patients with symptomatic Alzheimer disease (AD) dementia.9,10,11,12,13,14 These include increased fragmentation of daily rhythms with increased nighttime and decreased daytime activity as well as delayed peak in daily activity (phase delay) and damped melatonin rhythms.12,13,14,15,16,17 Alterations in sleep timing are also observed in AD, including increased fragmentation of sleep-wake cycles.18,19,20,21 Ultimately, circadian dysfunction is a major source of morbidity for patients with AD and their caretakers and 1 of the major causes of institutionalization.22 Thus, circadian dysfunction in AD is a serious but poorly understood phenomenon.
Longitudinal studies of AD using cerebrospinal fluid (CSF) biomarkers and amyloid positron emission tomography (PET) imaging have revealed that amyloid plaque pathology in the brain precedes symptomatic cognitive impairment by 15 to 20 years23,24,25 and begins a period of progressive pathologic changes of AD without cognitive symptoms, termed preclinical AD.26,27,28 Increases in CSF levels of total and phosphorylated tau protein occur closer to the onset of cognitive impairment and are thought to reflect neurodegeneration.24,29,30
Circadian rhythm disturbances have been extensively described in symptomatic patients with AD with moderate to severe dementia.13,31,32 To our knowledge, only a single study has examined patients with mild cognitive impariment,33 and no previous studies have described circadian function in preclinical AD. Decreased robustness of circadian rhythms has been associated with increased risk of future dementia in an elderly cohort, while sleep fragmentation appears to impart a higher risk of subsequent AD.34,35 However, to our knowledge, no studies have used AD biomarkers to determine whether circadian dysfunction is present in preclinical AD, at what point circadian rhythm alterations occur in the cascade of AD pathogenesis, or whether preclinical AD pathology underlies age-related changes in circadian function. Thus, we examined circadian rest-activity rhythms and AD biomarkers in a large cohort of cognitively normal individuals, a subset of whom had preclinical AD, to elucidate the associations between circadian function, aging, and preclinical AD pathology.
Methods
Participants
All participants were research community volunteers in longitudinal studies of memory and aging at the Washington University Knight Alzheimer’s Disease Research Center, in St Louis, Missouri. All procedures were approved by the Washington University human research protection office. Written informed consent was obtained from each participant. Inclusion criteria included being older than 45 years, cognitively normal based on a Clinical Dementia Rating score of 0, and no abnormal movement of the nondominant arm. Clinical dementia rating was based on evaluation by experienced clinicians with expertise in dementia, including semistructured interviews with each participant and a knowledgeable collateral source.36 Of the 205 participants enrolled in the study, 5 were excluded for insufficient actigraphy data, 3 were excluded owing to clinical dementia rating greater than 0, and 8 did not have biomarker data; therefore, 189 participants were included in the analysis.
Biomarkers
Pittsburgh Compound B (PiB) PET imaging was performed in 142 participants and was considered to be positive for amyloid deposition if mean cortical binding potential was greater than 0.18.37 Cerebrospinal fluid was obtained by fasted lumbar puncture at 8 am and processed as previously described.38 Cerebrospinal fluid amyloid β 42 (Aβ42) and phosphorylated tau181 (pTau) were measured by the Alzheimer Disease Research Center Biomarker Core using enzyme-linked immunosorbant assay (INNOTEST; Innogenetics) in 155 participants. Cutoff values for Aβ42 were 500 or 600 pg/mL, based on enzyme-linked immunosorbent assay lot.37 Amyloid β 42 values less than the cutoff were considered to represent amyloid deposition.
Biomarker data from 3 years before to 0.5 years after actigraphy recording were included. Additionally, assuming irreversibility of AD pathology, if Aβ42 or PiB PET was amyloid positive more than 3 years prior to actigraphy, participants were considered to be amyloid positive at the time of actigraphy, and if Aβ42 or PiB PET imaging was negative more than 0.5 years after actigraphy, participants were considered to be amyloid negative.
Participants were defined as amyloid negative only if all available Aβ biomarkers (both CSF Aβ42 and PiB, if available) were negative at baseline. We used these criteria to maximize sensitivity for determining amyloid pathology. One hundred thirty-nine of 189 participants (74%) had no positive amyloid biomarkers (both PiB and CSF were negative if available). For the purposes of determining the effect of amyloid pathology on circadian variables, we used only PiB to define amyloid positivity, not CSF Aβ42, because PiB-positive (PiB+) imaging is more specific and detects fibrillar amyloid plaques.39 Finally, for the purposes of assessing the effect of AD-related neurodegeneration, we used the CSF pTau to Aβ42 ratio as a continuous measure because this ratio is a specific biomarker of preclinical AD and predicts conversion to symptomatic AD.40,41,42
APOE genotype was determined by the Knight Alzheimer’s Disease Research Center Genetics Core using quantitative polymerase chain reaction. Genotype was dichotomized as APOE ε4 carrier (heterozygote or homozygote) or noncarrier.
Circadian Rest-Activity Data
Rest-activity data were collected with a wrist-mounted actigraph (Actiwatch2; Philips Respironics) for 7 to 14 days in participants’ usual home setting. Participants were instructed to push a time stamp button on the side of the watch at bedtime and waketime and to complete a sleep diary each morning. Details of actigraphy data collection and analysis are in the eAppendix of the Supplement.
We tested several circadian analytic methods, as described in the subsequent paragraphs. We selected key variables for amplitude (the difference in magnitude of activity between active and rest phases), phase, robustness (how well the data fit a predicted pattern), and fragmentation (how scattered activity is across the 24-hour day). We used 3 different analysis methods.
Cosinor Analysis
The cosinor method fits a cosine function to the data and was derived using ClockLab, version 6.0.24 (Actimetrics). Mesor is a measure of amplitude; acrophase refers to time of peak activity and measures phase; and F, a statistic representing how well the data match the cosine function, is a measure of robustness.
Nonparametric Analysis
This method does not fit a mathematical function to the data but instead is based on raw activity counts.11,43 Rest periods were manually defined as the times between mean bedtime and waketime, and active periods were defined as periods between adjacent rest periods. Alphacount, the mean activity count during active periods, was the primary measure of amplitude. Intradaily variability (IV) represents how consolidated the rest-activity rhythm is within each 24-hour period. The lowest possible IV results occur when there is 1 continuous period of high activity and 1 continuous period of low/no activity during the 24-hour period; higher IV indicates more fragmentation of the rest-activity pattern.11,43,44 Interdaily stability (IS), which measures how similar one 24-hour period is to the next, was another measure of robustness; higher values indicate more day-to-day stability.11
Empirical Mode Decomposition
The empirical mode decomposition (EMD) algorithm with a masking procedure45,46 was used to derive circadian amplitude (EMD amplitude) and period length (EMD period) using custom Matlab (IBM) scripts shared by the original authors. The EMD method does not make assumptions about the shape of the rest-activity rhythm and fits a curve to periodic data, given an approximate period (in this case, 24 hours).
Statistical Analysis
All continuous variables were examined for normal distribution by Kolmogorov-Smirnov test and visual inspection of histograms. Because pTau to Aβ42 ratio was not normally distributed, it was log transformed prior to using parametric methods. To compare variables between 2 groups, we used unpaired Student t tests for normally distributed continuous variables, Mann-Whitney U tests for nonnormally distributed continuous variables, and χ2 tests for categorical variables. Multivariate linear regressions were performed with circadian variable as the dependent variable, and age, sex, and either PiB status or log[pTau:Aβ42 ratio] as predictor variables were entered step-wise in this order. APOE genotype was not included in multivariate regressions because it was not found to have any significant effect on circadian variables on its own. For all tests, 2-sided tests were used, with α less than .05. All statistical analyses were performed using SPSS Statistics, version 24 (IBM).
Results
Demographics
Of 189 participants, 139 (74%) were amyloid negative. Overall, the participants were mostly women (64%), late middle age (mean [SD], 66.6 [8.3] years), and highly educated (Table 1). Pittsburgh Compound B–positive participants were older (mean [SD], 71.2 [6.1] years old vs 64.9 [0.6] for PiB− participants; P = .001), more often APOE-ε4 carriers (65% vs 28% of PiB− participants, P < .001), and had a slightly shorter interval between clinical evaluation and actigraphy compared with PiB− participants (mean [SD], 113 [154] days vs 267 [264] days).
Table 1. Demographic Characteristics of Participants Stratified by Amyloid Status.
| Characteristic | Mean (SD) | ||||
|---|---|---|---|---|---|
| All (N = 189), Mean (SD); Range | Amyloid Negative (n = 139) | PiB Negative (n = 116) | PiB Positive (n = 26) | P Value, PiB Positive vs PiB Negative | |
| Female, No. (%) | 121 (64) | 89 (64) | 74 (63) | 20 (77) | .18 |
| APOE-ε4 carrier | 72 (38) | 41 (29) | 33 (28) | 17 (65) | <.001 |
| Education, y | 16.1 (2.4) | 16.2 (2.4) | 16.3 (2.4) | 15.5 (2.6) | .12 |
| Age at actigraphy, y | 66.6 (8.3) | 65.3 (8.5) | 64.9 (.6) | 71.2 (6.1) | .001 |
| Time between clinical assessment and actigraphy, d | 228 (245) [0-957] | 252 (253) | 267 (264) | 113 (154) | .005 |
| MMSE | 29.32 (0.981) [24-30] | 29.4 (0.8) | 29.4 (0.8) | 29.1 (1.4) | .50 |
| CDR SB | 0.02 (0.088) [0-1] | 0.01 (0.08) | 0.02 (0.091) | 0.02 (0.098) | .92 |
| GDS | 0.96 (1.453) [0-10] | 1.01 (1.6) | 0.096 (1.5) | 0.69 (0.97) | .38 |
| Time between actigraphy and PiB, d | NA | NA | 641 (304)a | 664 (296)b | .75 |
Abbreviations: CDR SB, Clinical Dementia Rating Sum of Boxes; GDS, Geriatric Depression Scale; PiB, Pittsburgh Compound B; MMSE, Mini-Mental Status Examination; NA, not applicable.
n = 114.
n = 22.
Influences of Age and Sex on Circadian Function Independent of Cerebral Amyloid Pathology
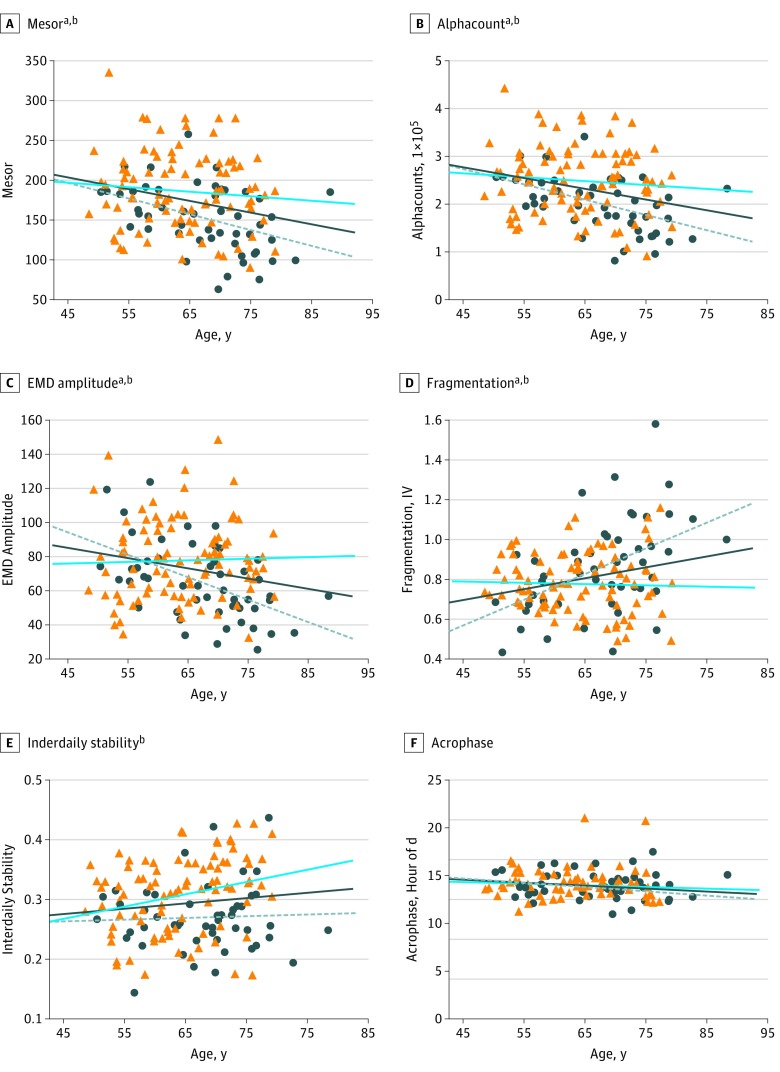
We examined the effect of age, sex, and APOE genotype on circadian function. When all participants were examined, increasing age was associated with decreased circadian amplitude by all tested measures (mesor [β = −.246; P = .001], alphacount [β = −.272; P < .001], and EMD amplitude [β = −.186; P = .01]). Older age was also associated with advanced phase (acrophase [β = −.149; P = .04]) and increased daily fragmentation as measured by IV (β = .212; P = .003) but more consistent day-to-day pattern by higher IS (β = .143; P = .05) (Table 2). To isolate the circadian changes of aging from any effect of AD pathology, we examined only amyloid-negative participants. In the absence of amyloid pathology, increasing age was again associated with decreased circadian amplitude by all measures (mesor [β = −.251; P = .003], alphacount [β = −.269; P = .001], and EMD amplitude [β = −.214; P = .01]) and increased fragmentation by IV (β = .247; P = .003), but there were no longer any age-associated changes with acrophase or IS (Table 2 and Figure 1).
Table 2. Effect of Age on Circadian Variables.
| Variable and Descriptiona | All (N = 189) | Amyloid Negative (n = 139) | |||
|---|---|---|---|---|---|
| Mean (SD) [Range] | βb | P Value | βb | P Value | |
| Cosinor | |||||
| Mesor, amplitude | 174 (49) [63-334] | −.246 | .001 | −.251 | .003 |
| Acrophase, timing of peak activity | 13.8 (2.1) [0.8-20.8] | −.149 | .04 | −.132 | .12 |
| F, goodness of fit to curve | 1089 (428) [345-2469] | .098 | .18 | .049 | .56 |
| Nonparametric | |||||
| Alphacount, amplitude | 230 056 (70 053) [81 415-441 424] | −.272 | <.001 | −.269 | .001 |
| IV, fragmentation | 0.824 (0.197) [0.434-1.581] | .212 | .003 | .247 | .003 |
| IS, day-to-day consistency | 0.294 (0.064) [0.145-0.540] | .143 | .05 | .119 | .16 |
| EMD | |||||
| EMD amplitude | 72.4 (23.7) [25.5-148.2] | −.186 | .01 | −.214 | .01 |
| EMD period of curve (approximately 24 h) | 24.1 (0.4) [22.0-25.5] | .087 | .23 | .152 | .07 |
Abbreviations: EMD, empirical mode decomposition; IS, interdaily stability; IV, intradaily variability; PiB, Pittsburgh Compound B.
Circadian variables are grouped by analysis method (cosinor, nonparametric, and EMD), and the parameter measured by each variable is detailed.
Standardized β values indicate magnitude and direction and are equivalent to correlation coefficient R, where negative β values indicate that increasing age causes a decrease in that circadian variable.
Figure 1. Association Between Age, Sex, and Circadian Variables in Amyloid-Negative Individuals.
A-C, Circadian amplitude (Mesor, alphacount, and EMD amplitude) declines with age. Circadian fragmentation, as measured by intradaily variability (IV) (D), and day-to-day consistency, as measured by interdaily stability (E), increase with age, while acrophase does not change (F). Men are indicated by dark blue circles and the dashed gray line, women by orange triangles and the blue line, and the dark blue line indicates linear trendline for all participants.
Magnitudes and P values of age effects are listed in Table 2. Magnitudes (in standardized β format) and P values for the effect of female sex on circadian variables (adjusting for age) are mesor, β = .292, P < .001; alphacount, β = .287, P = .001; empirical mode decomposition (EMD) amplitude, β = .226, P = .04; fragmentation, β = −.170, P = .04; interdaily stability, β = .320, P < .001; acrophase, β = .048, P = .16; cosinor F (goodness of fit, not shown), β = .233, P = .007; and EMD period (not shown), β = .035, P = .67.
aAge is a significant predictor of a circadian variable.
bSex is a significant predictor of a circadian variable after adjusting for age.
After adjusting for age, female sex was associated with higher amplitude (higher mesor [standardized β = .292; unstandardized β = 29.8; 95% CI, 13.4 to 46.1; P < .001], alphacount [β = .287; unstandardized β = 41 735; 95% CI, 18 488 to 64 982; P = .001], and EMD amplitude [β = .226; unstandardized β = 11.1; 95% CI, 3.0 to 19.2; P = .04]), less fragmentation (lower IV [β = −.170; unstandardized β = −.066; 95% CI, −0.131 to −0.002; P = .04]), and more robustness (lower IS [β = .320; unstandardized β = .042; 95% CI, 0.020 to 0.063; P < .001] and F (β, .233; unstandardized β = 207; 95% CI, 57 to 357; P = .007]) (Figure 1). Thus, age and sex have marked effects on circadian function in the absence of preclinical amyloid pathology.
There were no significant differences in any circadian variables between APOE-ε4 carriers and noncarriers. Therefore, APOE genotype was not included as a covariate in multivariate analyses to preserve degrees of freedom.
Association of Preclinical Amyloid Plaque Pathology With Circadian Dysfunction
To examine the effect of amyloid pathology on circadian variables, we compared only PiB+ participants (n = 26) with PiB− participants (n = 116) because PiB is the most specific marker for fibrillar amyloid plaques.47 After adjusting for age and sex, PiB+ individuals had significantly more circadian fragmentation as measured by IV (mean [SD], 0.875 [0.178] for PiB+ participants vs 0.804 [0.187] for PiB− participants; P = .05) (Table 3). Unexpectedly, PiB+ individuals had very slightly higher circadian amplitude by cosinor (mean [SD] mesor, 176 [48] vs 174 [48]; P = .003) and nonparametric (mean [SD] alphacount, 235 920 [73 381] vs 231 829 [68 256]; P = .002) analyses but not by EMD. However, the magnitude of the differences in amplitude was extremely small, at 4% to 6% of the standard deviation (Table 3). Pittsburgh Compound B–positive status was also associated with increased IS (mean [SD], 0.307 [0.076] vs 0.294 [0.060]; P = .03), suggesting more consistent day-to-day activity patterns. Preclinical fibrillar amyloid plaque pathology was therefore associated with more fragmented circadian rhythm but with no negative effect on amplitude, day-to-day stability, or phase.
Table 3. Effect of Amyloid Deposition by PiB on Circadian Variables.
| Variable | Mean (SD) | P Value Adjusted for Age and Sex | |
|---|---|---|---|
| PiB Negative (n = 116)a | PiB Positive (n = 26)a | ||
| Mesor | 174 (48) | 176 (48) | .003 |
| Acrophase | 14.1 (1.8) | 13.6 (2.3) | .46 |
| F | 1068 (403) | 1214 (471) | .19 |
| Alphacount | 231 829 (68 256) | 235 920 (73 381) | .002 |
| IV | 0.804 (0.187) | 0.875 (0.178) | .05 |
| IS | 0.294 (0.060) | 0.307 (0.076) | .03 |
| EMD Amplitude | 75.1 (24.7) | 72.6 (23.8) | .13 |
| EMD Period | 24.1 (0.44) | 24.1 (0.29) | .40 |
Abbreviations: EMD, empirical mode decomposition; IS, interdaily stability; IV, intradaily variability; PiB, Pittsburgh Compound B.
Only participants with available PiB status were included (N = 142). P values are adjusted for age and sex.
Association of Increased CSF pTau to Aβ42 Ratio With Circadian Fragmentation in Preclinical AD
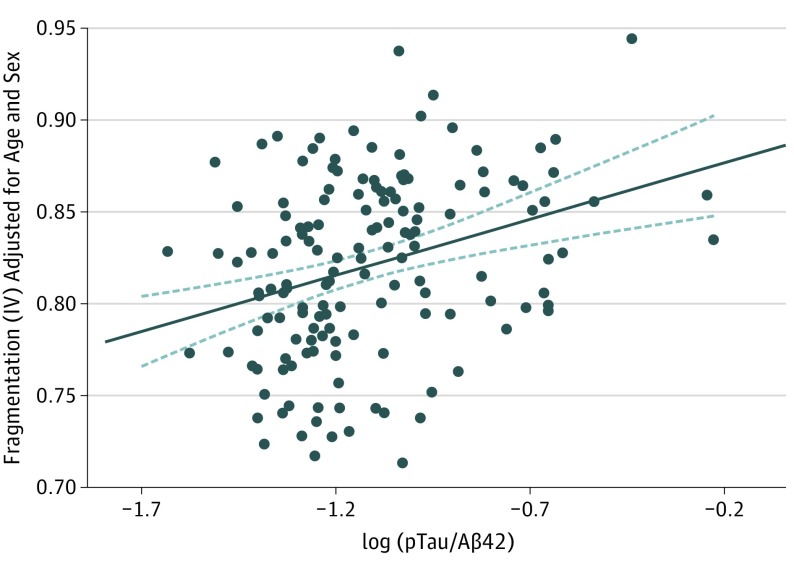
Increased CSF pTau to Aβ42 ratio is a sensitive and specific marker of AD-related neurodegeneration, indicating both neuronal injury (elevated pTau) and amyloid deposition (decreased Aβ42).40,41,42 Owing to the relatively small number of amyloid-positive and/or tau-positive individuals when applying a dichotomous approach with biomarker cutoffs, we examined the pTau to Aβ42 ratio as a continuous variable as it relates to circadian function in all participants with CSF biomarkers (n = 148). After adjusting for sex and age, increasing pTau to Aβ42 ratio (indicating more AD pathology) was associated with increasing circadian fragmentation as measured by IV (β = .231; P = .008) (Figure 2). Other circadian variables had no significant association with pTau to Aβ42 ratio (eTable 1 in the Supplement).
Figure 2. Association Between Phosphorylated Tau181 (pTau) to Amyloid β 42 (Aβ42) Ratio and Circadian Fragmentation Intradaily Variability (IV).
Scatterplot showing a significant positive association (P = .008) between the pTau to Aβ42 ratio (indicating increasing Alzheimer disease–related pathology) and circadian fragmentation (IV) for all participants with available cerebrospinal fluid biomarkers (n = 148), adjusted for age and sex.
Correlation of Circadian Fragmentation With Other Sleep Variables
We next examined the association between circadian end points and sleep-related variables calculated from both actigraphy data and sleep diaries. Previous analysis of sleep variables in a subset of this same cohort showed associations between decreased sleep efficiency and increased naps per week with preclinical amyloid pathology.48 While several circadian measures were correlated with various measures of sleep timing (eTable 2 in the Supplement), there was no correlation between sleep efficiency and any circadian variables. Circadian fragmentation (IV) did not correlate with any nocturnal sleep variables, although there was a significant correlation between number of naps and circadian fragmentation (r = 0.152; P = .04), suggesting that disrupted daytime rest-activity patterns may indicate preclinical AD.
Discussion
We found that disturbances of rest-activity rhythm are present in preclinical AD, even after accounting for effects of aging and sex, demonstrating that circadian fragmentation occurs very early in the course of AD pathogenesis and precedes cognitive symptom onset. When individuals with preclinical AD were excluded, increasing age was still associated with decreased circadian amplitude and increased fragmentation but not with advanced phase. The presence of amyloid plaques was associated with further increases in fragmentation, even after adjusting for age and sex. Lastly, increasing AD-related neurodegeneration, as measured by the CSF pTau to Aβ42 ratio, was associated with further fragmentation of circadian rest-activity rhythms. Altogether, our data suggest that aging and preclinical AD pathology have separate and additive negative effects on circadian rhythm fragmentation.
Advancing age has been associated with declining circadian function, both in rodents and humans, although the mechanisms remain unknown.9,46,49 While the prevalence of preclinical AD pathology increases dramatically with age,23 we found that increasing age was significantly associated with diminished amplitude and increased fragmentation of daily rhythms, even in the absence of preclinical AD (Table 2). Similarly, preclinical amyloid pathology was significantly associated with further circadian fragmentation, independent of age, but did not induce declines in amplitude (Table 3 and Figure 2). Thus, our results suggest that aging and AD pathology each separately drive circadian dysfunction, with both contributing to increased fragmentation. Mechanistically, loss of vasoactive intestinal peptide–expressing neurons in the SCN, which are critical to circadian pacemaking,50 has been described in postmortem AD studies51 and correlates with circadian rhythm dysfunction in both aging and AD.46 Thus, it is possible that both aging and preclinical AD influence circadian function by causing loss or dysfunction of vasoactive intestinal peptide–expressing neurons in the SCN. Alternative mechanisms of core clock disruption in AD, such as disrupted methylation of the BMAL1 promoter52 or direct effects of Aβ on clock gene homeostasis, have been suggested53,54 but, to our knowledge, have not been evaluated in preclinical AD. Disrupted light input to the SCN in AD, owing to loss of melanopsin-containing photoreceptors55 or inadequate light exposure,56 have been described in symptomatic AD and could potentially influence our findings, although it is unknown whether these are present in our otherwise healthy preclinical cohort. In mice, aging itself is associated with diminished neuronal synchronization in the SCN, leading to less robust electrical output.9 The longevity-associated deacetylase sirtuin 1 also may maintain clock gene expression in the SCN, with age-related declines in sirtuin 1 leading to circadian disruption.57 Thus, multiple potential mechanisms could explain the circadian dysfunction in preclinical AD, but, to our knowledge, none have been evaluated.
Our results add important context to 2 previous studies that suggested that circadian alterations may precede symptom onset in AD.34,35 Tranah et al34 performed cosinor analysis on actigraphy data from cognitively normal elderly women and found that decreased amplitude, decreased robustness, and phase delay were all associated with increased future risk of developing cognitive impairment.34 However, no biomarkers, detailed cognitive analysis, or pathology were examined in this study to assess any contribution of preclinical AD to circadian changes. Lim et al35 developed a sleep fragmentation index (Kra), and found that dementia was more likely to develop in cognitively normal people with the worst Kra values, although this is not a true circadian end point.35 Considering the advanced age (mean older than 80 years) of the participants in both studies and the prevalence of preclinical AD with aging, 30% to 40% of participants likely had preclinical AD,23 suggesting that preclinical pathology could have contributed to the circadian and sleep dysfunction observed in these studies.
Among the several circadian end points examined, we found that the nonparametric IV index was most consistently sensitive to both aging and AD pathology. Intradaily variability was designed to detect fragmentation of rest-activity rhythm, suggestive of more periods of daytime rest (or sleep) and increased nighttime activity (or wake).11 Intradaily variability is elevated in patients with symptomatic AD11 and was found to be more sensitive for detecting circadian changes in patients with AD than other methods, presumably owing to the nonsinusoidal nature of rest-activity rhythms.43 Additionally, we note that in a previously reported overlapping cohort,48 amyloid deposition as assessed by CSF Aβ42 was associated with worse actigraphically measured sleep efficiency at night and increased reported napping frequency during the daytime48; the combination of the 2 are consistent with increased IV identified in this study. Accordingly, we found a positive correlation between napping frequency and IV. Our finding that IV correlates with increasing pTau to Aβ42 ratio suggests the IV might warrant further investigation as a noninvasive biomarker of disease progression in preclinical AD.
We also observed that IS, a marker of the consistency of rhythms day-to-day,11 increased with age and in PiB+ individuals. Intradaily stability has previously been found to increase with aging, possibly indicating voluntary adherence to a more rigid day-to-day routine.58 Finally, other groups have observed phase delays in patients with symptomatic AD and in mild cognitive impairment.13,33,59 We observed that increasing age altered phase only when considering both amyloid-positive and amyloid-negative participants (Table 2), suggesting that age and amyloid status may interact to influence phase.
This study has several strengths. First, to our knowledge, it is the only study to incorporate both circadian measures and AD biomarkers (both CSF and PiB PET imaging). Second, the cohort is large, and the detailed annual clinical assessments and clinical dementia ratings of all participants through the Knight Alzheimer’s Disease Research Center ensured consistent phenotyping. Third, actigraphy was collected for 14 days in combination with sleep diaries, providing excellent source data for analyses, and we performed a careful manual selection of 7 days’ valid data for each participant to avoid confounding or skewing effects of data quantity (duration actigraphs were worn) and missing data (when actigraphs were not worn). Finally, we have used 3 distinct circadian analysis methods because each method has its own strengths and weaknesses.
Limitations
There are some shortcomings of our approach. We cannot exclude the possibility that the age- or sex-related circadian changes observed in the amyloid-negative group are caused by nonamyloid pathologies, which are commonly seen in the aged brain.60 We did not have information on medications and comorbidities, specifically sleep disorders. Sleep apnea in particular was not assessed, although it is common and may influence amyloid burden.61 Finally, noncircadian factors, such as voluntary exercise, can influence rest-activity measurements. Replication using other circadian parameters (such as core body temperature) could be considered to confirm our findings.
Conclusions
Accumulating evidence supports the hypothesis that circadian clock dysfunction could promote neurodegeneration and perhaps contribute to AD pathogenesis.8 Circadian disruption in mice causes loss of dendritic arborization and impaired hippocampal neurogenesis,7,62,63 while genetic perturbation of the core clock causes astrogliosis, synaptic degeneration, and neuronal oxidative damage.3 Furthermore, circadian dysfunction could adversely influence sleep, which appears to independently promote amyloid deposition and neurodegeneration.64,65,66,67,68 Our findings suggest that circadian dysfunction could contribute to the earliest stages of AD pathogenesis, and that understanding this association could open the door to new diagnostic and therapeutic strategies.
eAppendix. Participants and Actigraphy Analysis
eTable 1. Effect of pTau:Aβ42 Ratio on Circadian Variables
eTable 2. Correlations Between Circadian and Sleep Variables
References
- 1.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:582-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musiek ES, Lim MM, Yang G, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123(12):5389-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557-1562. [DOI] [PubMed] [Google Scholar]
- 5.Knutsson A, Kempe A. Shift work and diabetes: a systematic review. Chronobiol Int. 2014;31(10):1146-1151. [DOI] [PubMed] [Google Scholar]
- 6.Vetter C, Devore EE, Wegrzyn LR, et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA. 2016;315(16):1726-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108(4):1657-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura TJ, Nakamura W, Yamazaki S, et al. Age-related decline in circadian output. J Neurosci. 2011;31(28):10201-10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okawa M, Mishima K, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep. 1991;14(6):478-485. [DOI] [PubMed] [Google Scholar]
- 11.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27(6):563-572. [DOI] [PubMed] [Google Scholar]
- 12.Satlin A, Volicer L, Stopa EG, Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol Aging. 1995;16(5):765-771. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20(1):18-23. [PubMed] [Google Scholar]
- 14.Harper DG, Volicer L, Stopa EG, McKee AC, Nitta M, Satlin A. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry. 2005;13(5):359-368. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K, Okamoto N, Ohara K, Morita Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996;717(1-2):154-159. [DOI] [PubMed] [Google Scholar]
- 16.Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45(4):417-421. [DOI] [PubMed] [Google Scholar]
- 17.Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol. 2003;38(1-2):199-206. [DOI] [PubMed] [Google Scholar]
- 18.Vitiello MV, Prinz PN. Alzheimer’s disease: sleep and sleep/wake patterns. Clin Geriatr Med. 1989;5(2):289-299. [PubMed] [Google Scholar]
- 19.Prinz PN, Peskind ER, Vitaliano PP, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30(2):86-93. [DOI] [PubMed] [Google Scholar]
- 20.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29-38. [DOI] [PubMed] [Google Scholar]
- 21.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology: a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchetti A, Scuratti A, Zanetti O, et al. Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia. 1995;6(2):108-112. [DOI] [PubMed] [Google Scholar]
- 23.Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80(19):1784-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villemagne VL, Burnham S, Bourgeat P, et al. ; Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group . Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357-367. [DOI] [PubMed] [Google Scholar]
- 26.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358-368. [DOI] [PubMed] [Google Scholar]
- 28.Price JL, McKeel DW Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461(7266):916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain. 2004;127(Pt 5):1061-1074. [DOI] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50(2):282-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naismith SL, Hickie IB, Terpening Z, et al. Circadian misalignment and sleep disruption in mild cognitive impairment [published correction appears in J Alzheimers Dis. 2014;40(2):475]. J Alzheimers Dis. 2014;38(4):857-866. [DOI] [PubMed] [Google Scholar]
- 34.Tranah GJ, Blackwell T, Stone KL, et al. ; SOF Research Group . Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 37.Vlassenko AG, McCue L, Jasielec MS, et al. Imaging and cerebrospinal fluid biomarkers in early preclinical Alzheimer disease. Ann Neurol. 2016;80(3):379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306-319. [DOI] [PubMed] [Google Scholar]
- 40.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid beta-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382-389. [DOI] [PubMed] [Google Scholar]
- 41.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343-349. [DOI] [PubMed] [Google Scholar]
- 42.Tarawneh R, D’Angelo G, Macy E, et al. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011;70(2):274-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505-518. [DOI] [PubMed] [Google Scholar]
- 44.Gonçalves BS, Cavalcanti PR, Tavares GR, Campos TF, Araujo JF. Nonparametric methods in actigraphy: an update. Sleep Sci. 2014;7(3):158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang YH, Yeh CH, Young HWV, Hu K, Lo MT. On the computational complexity of the empirical mode decomposition algorithm. Physica A. 2014;400:159-167. doi: 10.1016/j.physa.2014.01.020 [DOI] [Google Scholar]
- 46.Wang JL, Lim AS, Chiang WY, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol. 2015;78(2):317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(pt 6):1630-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu K, Van Someren EJ, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: involvement of the circadian pacemaker. Proc Natl Acad Sci U S A. 2009;106(8):2490-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8(4):476-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16(4):571-576. [DOI] [PubMed] [Google Scholar]
- 52.Cronin P, McCarthy MJ, Lim ASP, et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement. 2017;13(6):689-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song H, Moon M, Choe HK, et al. Aβ-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol Neurodegener. 2015;10(13):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oyegbami O, Collins HM, Pardon MC, Ebling FJP, Heery DM, Moran PM. Abnormal clock gene expression and locomotor activity rhythms in two month-old female APPSwe/PS1dE9 mice. Curr Alzheimer Res. 2017;14(8):850-860. [DOI] [PubMed] [Google Scholar]
- 55.La Morgia C, Ross-Cisneros FN, Koronyo Y, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79(1):90-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell SS, Kripke DF, Gillin JC, Hrubovcak JC. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiol Behav. 1988;42(2):141-144. [DOI] [PubMed] [Google Scholar]
- 57.Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153(7):1448-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30(10):1223-1230. [DOI] [PubMed] [Google Scholar]
- 59.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry. 2001;158(5):704-711. [DOI] [PubMed] [Google Scholar]
- 60.Abner EL, Kryscio RJ, Schmitt FA, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol. 2017;81(4):549-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yun CH, Lee HY, Lee SK, et al. Amyloid burden in obstructive sleep apnea. J Alzheimers Dis. 2017;59(1):21-29. [DOI] [PubMed] [Google Scholar]
- 62.Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental “jet lag” inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5(12):e15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kott J, Leach G, Yan L. Direction-dependent effects of chronic “jet-lag” on hippocampal neurogenesis. Neurosci Lett. 2012;515(2):177-180. [DOI] [PubMed] [Google Scholar]
- 64.Ju YS, Ooms SJ, Sutphen C, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140(8):2104-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71(8):971-977. [DOI] [PubMed] [Google Scholar]
- 67.Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer’s disease. Brain Res. 2013;1529:200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Meco A, Joshi YB, Praticò D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging. 2014;35(8):1813-1820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Participants and Actigraphy Analysis
eTable 1. Effect of pTau:Aβ42 Ratio on Circadian Variables
eTable 2. Correlations Between Circadian and Sleep Variables