This cohort study evaluates the prevalence of domain-specific cognitive impairment and its association with overall survival among older patients with blood cancer.
Key Points
Question
How prevalent and predictive is domain-specific cognitive impairment among older patients with hematologic cancers?
Findings
In this cohort study of 360 patients with blood cancer, screening results were positive for executive dysfunction in 127 (35.3%) and for impairment in working memory in 62 (17.2%). Impairment in working memory was associated with increased overall mortality (median survival, 10.9 vs 12.2 months), whereas impairment in executive function was not.
Meaning
Domains of cognitive dysfunction may be prevalent among patients with blood cancer and may have differential predictive value for survival.
Abstract
Importance
As the population ages, cognitive impairment has promised to become increasingly common among patients with cancer. Little is known about how specific domains of cognitive impairment may be associated with survival among older patients with hematologic cancers.
Objective
To determine the prevalence of domain-specific cognitive impairment and its association with overall survival among older patients with blood cancer.
Design, Setting, and Participants
This prospective observational cohort study included all patients 75 years and older who presented for initial consultation in the leukemia, myeloma, or lymphoma clinics of a large tertiary hospital in Boston, Massachusetts, from February 1, 2015, to March 31, 2017. Patients underwent screening for frailty and cognitive dysfunction and were followed up for survival.
Exposures
The Clock-in-the-Box (CIB) test was used to screen for executive dysfunction. A 5-word delayed recall test was used to screen for impairment in working memory. The Fried frailty phenotype and Rockwood cumulative deficit model of frailty were also assessed to characterize participants as robust, prefrail, or frail.
Results
Among 420 consecutive patients approached, 360 (85.7%) agreed to undergo frailty assessment (232 men [64.4%] and 128 women [35.6%]; mean [SD] age, 79.8 [3.9] years), and 341 of those (94.7%) completed both cognitive screening tests. One hundred twenty-seven patients (35.3%) had probable executive dysfunction on the CIB, and 62 (17.2%) had probable impairment in working memory on the 5-word delayed recall. Impairment in either domain was modestly correlated with the Fried frailty phenotype (CIB, ρ = 0.177; delayed recall, ρ = 0.170; P = .01 for both), and many phenotypically robust patients also had probable cognitive impairment (24 of 104 [23.1%] on CIB and 9 of 104 [8.7%] on delayed recall). Patients with impaired working memory had worse median survival (10.9 [SD, 12.9] vs 12.2 [SD, 14.7] months; log-rank P < .001), including when stratified by indolent cancer (log-rank P = .01) and aggressive cancer (P < .001) and in multivariate analysis when adjusting for age, comorbidities, and disease aggressiveness (odds ratio, 0.26; 95% CI, 0.13-0.50). Impaired working memory was also associated with worse survival for those undergoing intensive treatment (log-rank P < .001). Executive dysfunction was associated with worse survival only among patients who underwent intensive treatment (log-rank P = .03).
Conclusions and Relevance
These data suggest that domains of cognitive dysfunction may be prevalent in older patients with blood cancer and may have differential predictive value for survival. Targeted interventions are needed for this vulnerable patient population.
Introduction
Dementia affects as many as 14% of individuals older than 70 years in the United States, and its incidence will likely triple by the year 2050.1,2,3 Milder forms of cognitive impairment, also highly prevalent, are characterized by changes in memory and thinking that do not overtly impede function but can manifest under conditions of stress.4 These forms of impairment can affect specific cognitive domains and have different clinical implications. For example, Alzheimer dementia and cognitive impairment of multiple sclerosis are characterized by early deficits in working memory and delayed recall, which are cognitive domains that have been amenable to rehabilitation.5,6,7 Other forms of cognitive impairment are characterized by early deficits in executive function and visuospatial abilities.8,9 Although associated with higher rates of toxic effects of treatment and mortality in patients with solid tumors,10,11 cognitive impairment often goes undetected in the oncology clinic.
Intact cognition is especially important for patients with hematologic or blood cancers because cure is often still possible in advanced disease, and treatment regimens are complex and frequently include inpatient components. Moreover, routine treatment regimens and blood transfusion protocols often include drugs that further impair cognition, such as corticosteroids, benzodiazepines, and anticholinergic medications. Finally, all cancer treatment may exacerbate existing health deficits; in a large study of older patients with solid tumors, deaths among the cognitively impaired were most often due to nononcologic causes.10 Indeed, patients with blood cancer are also at risk of nononcologic causes of death.
Chronological age, comorbidities, and performance status have limited utility in capturing the heterogeneity of the older population.12,13 The National Comprehensive Cancer Network recommends screening for geriatric syndromes, such as cognitive impairment and frailty, as an integral part of routine oncologic care for older patients.14,15 Cognitive impairment is highly correlated with frailty and associated functional loss; however, the Fried frailty phenotype scale,16 the most widely used measure of frailty,17 does not specifically consider cognitive function. The Rockwood cumulative deficit model is another approach that translates frailty into a conceptually simple score based on deficits, such as comorbidities and impaired activities of daily living, and can incorporate measures of cognition.18 In both measures of frailty, individuals may be cognitively impaired without meeting frailty criteria.
Preliminary data suggest that cognitive impairment may be associated with worse survival among patients with blood cancer19,20,21; however, many questions remain regarding the prevalence and influence of domain-specific cognitive impairment in this patient population. In other disease processes, such as stroke, subarachnoid hemorrhage, and chronic obstructive pulmonary disease, impairment in working memory has been associated with nonadherence to medication therapy and difficulties reporting complications,22 whereas executive dysfunction has been associated with increased delirium and difficulties with positive health behaviors, such as smoking cessation.23,24 Poor performance on different cognitive domains often suggests different interventions.25
Deficits in executive function suggest greater difficulties with planning, organization, and multitasking, all of which are cognitive skills important for independent living and functioning.8 Deficits in executive function are often due to underlying cerebrovascular disease and can be elucidated by specific testing such as the Clock-in-the-Box (CIB) test. Impaired working memory suggests greater difficulties with simple directions and retaining information. Working memory deficits can be elucidated by delayed recall and are more treatable with cognitive training.25
In this context, we performed cognitive and frailty screening on all patients with blood cancer 75 years and older who presented for an initial visit at our institution. We hypothesized that impairment in working memory and executive dysfunction would be correlated with overall frailty and that both would be associated with worse survival.
Methods
Study Design and Patients
From February 1, 2015, through March 31, 2017, all patients 75 years and older who presented for initial consultation in the leukemia, myeloma, or lymphoma clinics at the Dana-Farber Cancer Institute, Boston, Massachusetts, were approached to participate in a frailty assessment. Information about the screening was included in the new patient packet sent to each patient before their visit. The screening was performed by a nonphysician clinic assistant (W.F.J.) and took an average of 15 minutes. The assistant was trained in the methods of geriatric screening by a board-certified geriatrician (T.T.H. and J.A.D.) and observed closely for competency before performing the screening independently. To ensure independent clinical decision making, patients’ oncologists were blinded to the results of the frailty assessment for at least 48 hours; cognitive impairment screening results were not explicitly provided because they are not diagnostic. The study was approved by the Dana-Farber Cancer Institute–Harvard Cancer Center Office for the Protection of Human Research Subjects. All participants signed informed consent.
Measures of Frailty
The phenotype approach to frailty measurement by Fried et al16 involves the following 5 items: slow gait speed, poor grip strength, unintentional weight loss, low energy expenditure, and self-reported exhaustion. Patients with 3 or more factors were categorized as frail; with 1 to 2 factors, as prefrail; and with no factors, as robust.14 This approach has been well validated as a predictive marker of poor outcomes, including hospitalizations, falls, and mortality. Our assessment additionally measured frailty using the cumulative deficit method developed by Rockwood et al,18 which resulted in a 42-item frailty index (range, 0-1.0, with higher scores indicating increased frailty).
Measures of Cognitive Impairment
The Mini-Cog,26 which consists of a 3-word delayed recall and a clock drawing task, is commonly used as a cognitive screen but is generally not considered to be sensitive enough to detect milder forms of impairment.27 The Mini-Cog includes items that test executive function and memory, which are the domains of cognition most relevant to patient self-management and adherence with medical care. We therefore decided to use a variation on the Mini-Cog that has a more challenging combination of tests, the CIB plus the 5-word delayed recall from the Montreal Cognitive Assessment. All 3 tests are screening tools typically scored without adjustments made for age or educational level, although the reading and/or drawing abilities needed for the CIB test inherently require a vision and educational threshold.
The CIB test is a validated, easily administered modification of the Clock Drawing Test.28,29 Testing takes approximately 2 minutes and correlates well with performance on the more comprehensive Mini-Mental State Examination and measures of independent function in community-dwelling older persons.8 To administer the CIB test, patients are presented with 2 sheets of paper. The top sheet provides written instructions for the patient to read and briefly remember. The second sheet includes 4 colored boxes. The patient is asked to draw a clock in the blue box, setting the hands to 10 minutes past 11. Eight discreet items are included, designed to assess working memory (eg, placing the response in the blue box and including all clock numbers) and planning and organization (eg, clock size and number spacing), both of which are components of executive functioning. Consistent with previous studies,8 we defined a score of 7 to 8 as normal; 5 to 6, possible cognitive impairment; and less than 5, probable cognitive impairment. Interrater reliability for scoring on a sample of CIB tests between the clinical assistant and geriatrician was high (κ = 0.89). Sample CIB results and scores for 6 patients are available in eFigures 1 to 3 in the Supplement.
We used the 5-word delayed recall list from the Montreal Cognitive Assessment to screen for impairment in short-term memory.30,31 We asked patients to remember the words and repeat them immediately to ensure encoding, distracted them for approximately 5 minutes with the CIB test and a grip strength test, and then prompted recall. We defined a score of 3 of 5 words remembered spontaneously after 5 minutes as possible cognitive impairment and 2 or fewer words as probable cognitive impairment, as in prior studies.32,33,34 Administration of both screening tests takes an average of 8 minutes.
Additional Variables
Clinical characteristics such as sex, age, disease type, comorbidities (calculated using the Charlson Comorbidity Index [range, 0 to ≥5, with higher scores indicating greater comorbidity]35), and survival days were collected from the medical record. As in prior work with this population,36 patients were classified as having indolent disease (myelodysplastic syndromes, chronic lymphocytic leukemia, myeloproliferative neoplasms, follicular lymphoma, hairy cell leukemia, monoclonal gammopathy of unknown significance, smoldering myeloma, and Waldenstrom hypergammaglobulinemia) or aggressive disease (acute myeloid leukemia, multiple myeloma, acute lymphoblastic leukemia, mantle cell lymphoma, and diffuse large B-cell lymphoma). In terms of treatment, patients were divided into the following 3 groups: none, supportive or attenuated, and intensive. Patients were considered to have received supportive or attenuated treatment if they were receiving only erythropoiesis-stimulating agents, blood transfusions, or significantly dose-reduced chemotherapy. All patients included in our analysis had at least 3 months of follow-up from the initial assessment.
Statistical Analysis
We first characterized the prevalence of possible and probable cognitive impairment and covariates using means with SDs for continuous data and percentages for categorical data. To assess the rigor of our overall frailty screening process, we characterized the ability of phenotypic frailty scores to estimate mortality by using Kaplan-Meier analyses. We classified participants into 2 groups for cognitive performance (probable cognitive impairment vs other) for the CIB and delayed recall tests and estimated Spearman correlation coefficients for association with the Fried frailty phenotype.
The association of performance on each of the screening tests with overall survival was evaluated with survival analysis and repeated in analyses stratified by cancer type (indolent vs aggressive) and treatment type (none vs supportive or attenuated vs intensive). We also examined the association between the 2 domains of impaired cognition and survival with multivariable regression analysis, adjusting for age, comorbidity, and cancer aggressiveness (we chose to exclude treatment in these models given the colinearity with cancer aggressiveness). Data were analyzed using multivariable logistic regression analysis, with P < .05 indicating significance.
Results
Cohort Characteristics
Of 420 patients 75 years or older who were approached, 360 (85.7%) consented to frailty screening (Table 1). In the study population of 360 patients, 232 were men (64.4%) and 128 were women (35.6%). The mean (SD) age was 79.8 (3.9) years, and 195 (54.2%) were 75 to 79 years of age. One hundred seventeen patients (32.5%) were treated in the leukemia clinic; 123 (34.2%), in the lymphoma clinic; and 123 (34.2%), in the myeloma clinic (including a small group of patients seen in 2 clinics). Overall, 132 patients (36.7%) had aggressive disease and 228 (63.3%) had indolent disease; 70 (19.4%) were not offered treatment, 54 (15.0%) underwent supportive or attenuated care, and 236 (65.6%) underwent intensive treatment. The mean (SD) Charlson Comorbidity Index was 3.6 (1.8). The median overall survival of the cohort was 12.1 months (range, 0.1-22.8 months). One hundred twenty-four patients with indolent disease (54.4%) were alive at 1 year of follow-up compared with 58 (43.9%) with aggressive disease; 13 patients were lost to follow-up. In terms of frailty status, 104 patients (28.9%) were phenotypically robust; 211 (58.6%), prefrail; and 45 (12.5%), frail. The 1-year overall survival in each of these groups was 64%, 47%, and 34%, respectively.
Table 1. Cohort Characteristics.
| Characteristic | Data (n = 360)a |
|---|---|
| Sex, No. (%) | |
| Male | 232 (64.4) |
| Female | 128 (35.6) |
| Age, mean (SD), y | 79.8 (3.9) |
| Age range, y, No. (%) | |
| 75-79 | 195 (54.2) |
| 80-84 | 116 (32.2) |
| 85-89 | 43 (11.9) |
| ≥90 | 6 (1.7) |
| Fried frailty phenotype, No. (%) | |
| Frail | 45 (12.5) |
| Prefrail | 211 (58.6) |
| Robust | 104 (28.9) |
| Blood cancer type, No. (%) | |
| Aggressiveb | 132 (36.7) |
| Indolentc | 228 (63.3) |
| Charlson Comorbidity Index, No. (%)d | |
| 0 | 5 (1.4) |
| 1 | 6 (1.7) |
| 2 | 110 (30.6) |
| ≥3 | 239 (66.4) |
Percentages have been rounded and may not total 100.
Includes acute myeloid leukemia, multiple myeloma, acute lymphoblastic leukemia, mantle cell lymphoma, and diffuse large B-cell lymphoma.
Includes myelodysplastic syndromes, chronic lymphocytic leukemia, myeloproliferative neoplasms, follicular lymphoma, hairy cell leukemia, monoclonal gammopathy of unknown significance, smoldering myeloma, and Waldenstrom hypergammaglobulinemia.
Comorbidities were assigned point values based on severity and then summed using Charlson Comorbidity Index criteria.
Prevalence of Cognitive Impairment
Of 360 patients who agreed to frailty screening, 19 declined at least 1 of the cognitive screening tests (Table 2). Among those who declined screening, 6 were robust, 8 were prefrail, and 5 were frail by the cumulative deficit index (excluding cognitive domains) and 2 were robust, 6 were prefrail, and 11 were frail on the Fried frailty phenotype scale. Survival among these 19 patients was not significantly different compared with the rest of the cohort (eFigure 4 in the Supplement).
Table 2. Cognitive Impairment Among Patients 75 Years or Older With Blood Cancer.
| Cognition Finding (Score) | No. (%) of Patients |
|---|---|
| Clock-in-the-Box test | |
| Normal (≥7/8) | 124 (34.4) |
| Possibly impaired (6/8) | 91 (25.3) |
| Probably impaired (≤5/8) | 127 (35.3) |
| Declined | 18 (5.0) |
| 5-Word delayed recall test | |
| Normal (≥4/5) | 219 (60.8) |
| Possibly impaired (3/5) | 69 (19.2) |
| Probably impaired (≤2/5) | 62 (17.2) |
| Declined | 10 (2.8) |
| Subjective measures | |
| Knows reason for presenting to clinic | |
| Yes, without help | 336 (93.3) |
| With help or unable | 24 (6.7) |
| Completes questionnaire independently | |
| Yes, without help | 304 (84.4) |
| With help or unable | 56 (15.6) |
In the overall cohort, deficits in executive function were more common than those in working memory. Probable cognitive impairment was found in 127 patients (35.3%) by the CIB and in 62 (17.2%) by delayed recall. Most patients with probable impairment in working memory measured by the 5-word delayed recall test (40 [64.5%]) also had probable impairment in executive function as measured by the CIB test.
We found a modest correlation between probable cognitive impairment and prefrail or frail status (CIB, ρ = 0.177; delayed recall, ρ = 0.170; P = .01 for both) but not for sex, comorbidity, or disease aggressiveness. Among patients classified as robust by the Fried frailty phenotype criteria (n = 104), the prevalence of probable cognitive impairment was 24 (23.1%) by the CIB test and 9 (8.7%) by the 5-word delayed recall test. Analyses of each clinical group for the 2 cognitive domains by aggressive and indolent disease presentation did not show a difference in prevalence except for the 5-word delayed recall test, which was more prevalent among patients with aggressive myeloma (12 of 58 patients [20.7%] vs 5 of 64 patients [7.8%]; P = .04).
Cognitive Impairment and Mortality
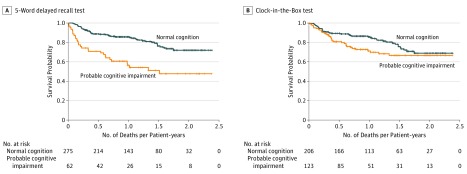
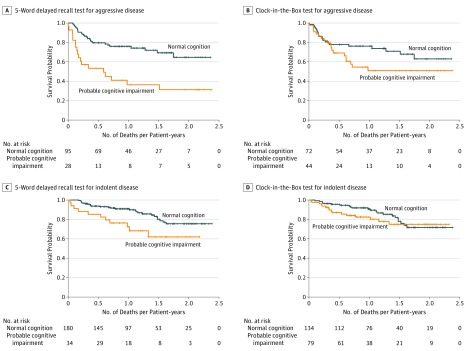
Among all patients, probable impairment on the 5-word delayed recall test was associated with an increased risk of overall mortality (median survival, 10.9 [SD, 12.9] vs 12.2 [SD, 14.7] months; log-rank P < .001) (Figure 1A), whereas probable impairment on the CIB test was not associated with survival (log-rank P = .10) (Figure 1B). Among patients with aggressive disease, decreased survival was seen among those with probable impairment on the 5-word delayed recall test only (log-rank P < .001) (Figure 2A). Among those with indolent disease, only impairment on the 5-word delayed recall test was associated with worse survival (log-rank P = .01) (Figure 2C). In multivariate analysis when adjusting for age, aggressiveness of disease, and comorbidity, patients with impairment in working memory had worse survival (odds ratio, 0.26; 95% CI, 0.13-0.50). Patients with executive dysfunction had survival odds similar to those without executive dysfunction (odds ratio, 1.11; 95% CI, 0.61-2.03).
Figure 1. Survival Curves for Patients With Normal Cognition vs Probable Cognitive Impairment.
Cognitive impairment was measured using the 5-word delayed recall and Clock-in-the-Box tests. Cross-hatch indicates censored data.
Figure 2. Survival Curves for Patients With Normal Cognition vs Probable Cognitive Impairment Stratified by Disease Aggressiveness.
Cognitive impairment was measured using the 5-word delayed recall and Clock-in-the-Box tests. Cross-hatch indicates censored data.
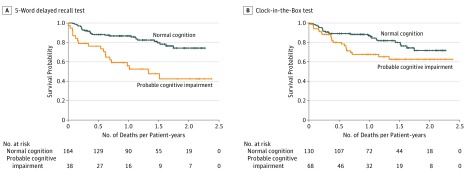
Among patients treated with intensive regimens, decreased survival was seen for probable cognitive impairment on the 5-word delayed recall test (log-rank P < .001) and CIB test (log-rank P = .03) (Figure 3). We found no significant difference in survival between patients with probable cognitive impairment who were untreated or underwent supportive or attenuated treatment (eFigure 5 in the Supplement). Finally, when stratified by phenotypic frailty status, impaired performance on the 5-word delayed recall test created distinct 90% survival (SD) (8.3 [0.81] vs 14.0 [0.55] months among robust patients) and 80% survival (SD) (4.0 [1.84] vs 16.5 [0.73] months among prefrail patients; 1.0 [1.47] vs 6.5 [2.02] months among frail patients) groups, although statistical significance was seen only among the frail (P = .001) and prefrail (P = .008) groups. The CIB results did not form distinct survival groups. All analyses were adjusted for age, which was not associated with worse survival.
Figure 3. Survival Curves for Patients With Normal Cognition vs Probable Cognitive Impairment Receiving Intensive Treatment.
Cognitive impairment was measured using the 5-word delayed recall and Clock-in-the-Box tests. Cross-hatch indicates censored data.
Discussion
Using 2 validated measures in a population-based screening approach, we found domain-specific cognitive impairments to be prevalent among older patients with blood cancer, with 1 of 3 patients probably affected. Many patients who were robust on the frailty phenotype assessment also had probable cognitive impairment, a finding suggesting that cognitive screening adds important information to standard screening using the Fried criteria. Deficits in working memory were associated with worse survival in the overall cohort and after adjusting for age, comorbidities, and disease aggressiveness. However, deficits in executive function were predictive of mortality only in a subset of patients undergoing intensive treatment. Taken together, these data support the feasibility and potential utility of routine domain-specific cognitive screening among older patients with blood cancer.
Our findings are consistent with smaller prior studies. In a pilot study of older adults 65 years or older with hematologic cancers awaiting hematopoietic stem cell transplant (n = 62), cognitive assessment with the full Montreal Cognitive Assessment identified 24 with dementia (38.7%) and 14 with cognitive impairment (22.6%).37 Dementia detected as part of a geriatric evaluation was associated with poor survival among German patients with chronic lymphocytic leukemia (n = 97),20 and cognitive impairment was predictive of mortality among 107 ostensibly clinically fit older Belgian patients with different blood cancers (hazard ratio, 3.26; 95% CI, 1.04-10.19).19 In the United States, Klepin and colleagues21 found that inpatients with acute myelogenous leukemia with impaired cognition had worse overall survival (n = 74).
Cognitive impairment is associated with poor prognosis in patients with solid tumors,11 and our data suggest the same is true for blood cancers. In addition to the increased risk of delirium and nonadherence to medication therapy, impairment is likely also correlated with noncognitive health deficits. For example, poor cognitive function has been associated with higher levels of comorbidity for other populations such as long-term care facility patients.38
Understanding domain-specific cognitive impairment may assist hematologic oncologists in tailoring their explanations, assessments, and care delivery for older patients. Specifically, a poor CIB score with normal recall suggests deficits in executive functions such as planning, organization, and multitasking.8 These patients often seem cognitively intact but can have substantial difficulty following complex conversations and multistep commands, managing medications and appointments, and driving safely to the cancer center. Executive dysfunction most often reflects underlying cerebrovascular disease and can significantly influence independent function and quality of life.23 Patients may benefit from written instructions, repetition, and routine.25
In contrast, evidence of impaired working memory without executive dysfunction or delirium suggests Alzheimer disease or amnestic cognitive impairment (possibly from traumatic brain injury). These deficits can also be easily missed unless a family member is present to provide collateral information. Affected patients often have more difficulty following even simple directions and retaining educational information and may be labeled as unmotivated or not health literate. Fortunately, this form of impairment may be more modifiable by cognitive rehabilitation or training strategies.25,39,40 Probable impairment on both tests suggests more global involvement. Regardless of the type of impairment, the most important intervention may be to actively engage family members, case managers, or other allied health care professionals in the affected patient’s care.
Limitations
Our analysis has limitations. First, given the various socioeconomic and cognitive abilities needed to navigate the referral process and because our patients came from a single tertiary center, we probably underestimated the prevalence of cognitive impairment among new outpatients with blood cancer. Moreover, the high rate of intensive treatment among the elderly cohort (65.6%) is unlikely to be found in a community setting. Second, we were unable to perform criterion standard neurocognitive tests or to make specific cognitive diagnoses owing to resource limitations. However, we found an association with survival among patients found to be probably impaired on delayed recall testing, which argues for the underlying validity of that screening measure. Third, although a large proportion of patients agreed to participate (85.7%), some declined, which may have led to participation bias. Fourth, although we have conjectured that cognitive impairment may affect patients’ ability to make informed treatment choices or to manage their care, we acknowledge that we did not assess these constructs directly. Finally, we have not yet attempted to measure the association of cognitive impairment with other nonmortality outcomes important to older patients living with cancer including quality of life, mobility, socialization, and independent living.
Conclusions
Our data suggest that routine domain-specific screening for cognitive impairment can be integrated into the care of older patients with blood cancer and that the prevalence of impairment is substantial. In our cohort, the CIB test detected more impairment than the 5-word delayed recall test; however, poor performance on the 5-word delayed recall test had better predictive value for mortality. Clinic staff can easily be trained to administer both screening tests, which take only a few minutes. Moreover, we found that many patients who appeared to be robust may have had a degree of cognitive impairment that could affect their survival. The median survival difference of greater than 1.3 months for patients with impaired delayed recall was arguably small, but in oncology, new treatments have been approved for even smaller gains.41 Further research should focus on tailored strategies to mitigate domain-specific cognitive dysfunction in this patient population.
eFigure 1. Examples of Clock-In-the-Box (CIB) Tests From Phenotypically Robust Patients 75 Years or Older With Blood Cancers
eFigure 2. Examples of Clock-In-the-Box (CIB) Tests From Phenotypically Prefrail Patients 75 Years or Older With Blood Cancers
eFigure 3. Examples of Clock-In-the-Box (CIB) Tests From Phenotypically Frail Patients 75 Years or Older With Blood Cancers
eFigure 4. Survival Among Older Patients With Blood Cancers Stratified by Previous Treatment
eFigure 5. Survival Among Older Patients With Blood Cancers and Probable Cognitive Impairment Stratified by Treatment Intensity
References
- 1.Plassman BL, Langa KM, Fisher GG, et al. . Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1-2):125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson RS, Weir DR, Leurgans SE, et al. . Sources of variability in estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN; U.S. Preventive Services Task Force . Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138(11):927-937. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Roberts RO, Knopman DS, et al. . Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam B, Middleton LE, Masellis M, et al. . Criterion and convergent validity of the Montreal Cognitive Assessment with screening and standardized neuropsychological testing. J Am Geriatr Soc. 2013;61(12):2181-2185. [DOI] [PubMed] [Google Scholar]
- 6.Marchetta ND, Hurks PP, Krabbendam L, Jolles J. Interference control, working memory, concept shifting, and verbal fluency in adults with attention-deficit/hyperactivity disorder (ADHD). Neuropsychology. 2008;22(1):74-84. [DOI] [PubMed] [Google Scholar]
- 7.Fleming JM, Shum D, Strong J, Lightbody S. Prospective memory rehabilitation for adults with traumatic brain injury: a compensatory training programme. Brain Inj. 2005;19(1):1-10. [DOI] [PubMed] [Google Scholar]
- 8.Chester JG, Grande LJ, Milberg WP, McGlinchey RE, Lipsitz LA, Rudolph JL. Cognitive screening in community-dwelling elders: performance on the Clock-in-the-Box. Am J Med. 2011;124(7):662-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller DC. Best Practices in School Neuropsychology: Guidelines for Effective Practice, Assessment, and Evidence-Based Intervention. Hoboken, NJ: Wiley; 2010. [Google Scholar]
- 10.Wongrakpanich S, Hurst A, Bustamante J, et al. . Prognostic significance of dementia in older adults with solid tumors. Dement Geriatr Cogn Disord. 2017;43(1-2):38-44. [DOI] [PubMed] [Google Scholar]
- 11.Raji MA, Kuo YF, Freeman JL, Goodwin JS. Effect of a dementia diagnosis on survival of older patients after a diagnosis of breast, colon, or prostate cancer: implications for cancer care. Arch Intern Med. 2008;168(18):2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy: a systematic review. Leuk Res. 2014;38(3):275-283. [DOI] [PubMed] [Google Scholar]
- 13.Dale W. “Staging the aging” when considering androgen deprivation therapy for older men with prostate cancer. J Clin Oncol. 2009;27(21):3420-3422. [DOI] [PubMed] [Google Scholar]
- 14.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denlinger CS, Ligibel JA, Are M, et al. ; National Comprehensive Cancer Network . Survivorship: cognitive function, version 1.2014. J Natl Compr Canc Netw. 2014;12(7):976-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156. [DOI] [PubMed] [Google Scholar]
- 17.Bouillon K, Kivimaki M, Hamer M, et al. . Measures of frailty in population-based studies: an overview. BMC Geriatr. 2013;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975-979. [DOI] [PubMed] [Google Scholar]
- 19.Dubruille S, Libert Y, Roos M, et al. . Identification of clinical parameters predictive of one-year survival using two geriatric tools in clinically fit older patients with hematological malignancies: major impact of cognition. J Geriatr Oncol. 2015;6(5):362-369. [DOI] [PubMed] [Google Scholar]
- 20.Goede V, Bahlo J, Chataline V, et al. . Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: results of the CLL9 trial of the German CLL study group. Leuk Lymphoma. 2016;57(4):789-796. [DOI] [PubMed] [Google Scholar]
- 21.Klepin HD, Geiger AM, Tooze JA, et al. . Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS Behav. 2011;15(8):1888-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer SA, Kreiter KT, Copeland D, et al. . Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology. 2002;59(11):1750-1758. [DOI] [PubMed] [Google Scholar]
- 24.Brega AG, Grigsby J, Kooken R, Hamman RF, Baxter J. The impact of executive cognitive functioning on rates of smoking cessation in the San Luis Valley Health and Aging Study. Age Ageing. 2008;37(5):521-525. [DOI] [PubMed] [Google Scholar]
- 25.Cleutjens FA, Franssen FM, Spruit MA, et al. . Domain-specific cognitive impairment in patients with COPD and control subjects. Int J Chron Obstruct Pulmon Dis. 2016;12:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The Mini-Cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021-1027. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Yan J, Jin X, et al. . Screening for dementia in older adults: comparison of Mini-Mental State Examination, Mini-Cog, Clock Drawing Test and AD8. PLoS One. 2016;11(12):e0168949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiwaki Y, Breeze E, Smeeth L, Bulpitt CJ, Peters R, Fletcher AE. Validity of the Clock-Drawing Test as a screening tool for cognitive impairment in the elderly. Am J Epidemiol. 2004;160(8):797-807. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S, Mogi N, Umegaki H, et al. . The Clock Drawing Test as a valid screening method for mild cognitive impairment. Dement Geriatr Cogn Disord. 2004;18(2):172-179. [DOI] [PubMed] [Google Scholar]
- 30.Ozer S, Young J, Champ C, Burke M. A systematic review of the diagnostic test accuracy of brief cognitive tests to detect amnestic mild cognitive impairment. Int J Geriatr Psychiatry. 2016;31(11):1139-1150. [DOI] [PubMed] [Google Scholar]
- 31.Vogel SJ, Banks SJ, Cummings JL, Miller JB. Concordance of the Montreal Cognitive Assessment with standard neuropsychological measures. Alzheimers Dement (Amst). 2015;1(3):289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc. 2014;62(4):679-684. [DOI] [PubMed] [Google Scholar]
- 33.Mai LM, Oczkowski W, Mackenzie G, et al. . Screening for cognitive impairment in a stroke prevention clinic using the MoCA. Can J Neurol Sci. 2013;40(2):192-197. [DOI] [PubMed] [Google Scholar]
- 34.Wong A, Nyenhuis D, Black SE, et al. . Montreal Cognitive Assessment 5-minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke. 2015;46(4):1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 36.Liu MA, Hshieh T, Condron N, Wadleigh M, Abel GA, Driver JA. Relationship between physician and patient assessment of performance status and survival in a large cohort of patients with haematologic malignancies. Br J Cancer. 2016;115(7):858-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards BJ-A, Holmes HM, Valladarez H, et al. . Prevalence of cognitive impairment in patients with hematologic cancers preceding stem cell transplantation: program for healthy aging. J Clin Oncol. 2016;34(3)(suppl):32. [Google Scholar]
- 38.Silay K, Yalcin A, Akinci S, et al. . Charlson Comorbidity Index, inappropriate medication use and cognitive impairment: Bermuda Triangle. Wien Klin Wochenschr. 2017;129:799-804. [DOI] [PubMed] [Google Scholar]
- 39.Barcelos N, Shah N, Cohen K, et al. . Aerobic and Cognitive Exercise (ACE) pilot study for older adults: executive function improves with cognitive challenge while exergaming. J Int Neuropsychol Soc. 2015;21(10):768-779. [DOI] [PubMed] [Google Scholar]
- 40.Anguera JA, Boccanfuso J, Rintoul JL, et al. . Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bach PB. Indication-specific pricing for cancer drugs. JAMA. 2014;312(16):1629-1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Examples of Clock-In-the-Box (CIB) Tests From Phenotypically Robust Patients 75 Years or Older With Blood Cancers
eFigure 2. Examples of Clock-In-the-Box (CIB) Tests From Phenotypically Prefrail Patients 75 Years or Older With Blood Cancers
eFigure 3. Examples of Clock-In-the-Box (CIB) Tests From Phenotypically Frail Patients 75 Years or Older With Blood Cancers
eFigure 4. Survival Among Older Patients With Blood Cancers Stratified by Previous Treatment
eFigure 5. Survival Among Older Patients With Blood Cancers and Probable Cognitive Impairment Stratified by Treatment Intensity