Key Points
Question
Can core Alzheimer disease cerebrospinal fluid biomarkers be used to select frontotemporal lobar degeneration (FTLD) subtypes?
Findings
In this case-control study, an algorithm that used different cutpoints of cerebrospinal fluid phosphorylated tau/amyloid β1-42 ratio and phosphorylated tau in a sporadic autopsy cohort first excluded Alzheimer disease and then provided a good discrimination of pure FTLD transactive response DNA-binding protein cases from the remaining FTLD-tau and mixed FTLD cases. This approach was confirmed in an independent cohort of sporadic living patients with likely FTLD pathology, but it showed reduced sensitivity when applied to a cohort of patients with frontotemporal generation with pathogenic mutations.
Meaning
Alzheimer disease cerebrospinal fluid core biomarkers can be reliably used for the in vivo identification of patients with pure FTLD transactive response DNA-binding protein and FTLD-tau when accounting for comorbid Alzheimer disease and genetic status.
Abstract
Importance
Cerebrospinal fluid (CSF) core Alzheimer disease (AD) biomarkers have shown an excellent capacity for the in vivo detection of AD. Previous studies have shown that CSF levels of phosphorylated tau (p-tau) also correlate with tau pathology in frontotemporal lobar degeneration (FTLD) after accounting for AD copathology.
Objective
To develop an algorithm based on core AD CSF measures to exclude cases with AD pathology and then differentiate between FTLD-tau and FTLD transactive response DNA-binding protein of approximately 43kDa (FTLD-TDP).
Design, Setting, and Participants
A case-control study at the University of Pennsylvania. Participants were selected from a database of 1796 patients included between 1992 and 2016 with different neurodegenerative diseases with available CSF. Three patient cohorts were included: a cohort of patients with sporadic, autopsy-confirmed FTLD and AD (n = 143); a cohort of patients with frontotemporal degeneration (FTD) with TDP-associated or tau-associated mutations (n = 60); and a living cohort of patients with syndromes highly predictive of FTLD (progressive supranuclear palsy and FTD–amyotrophic lateral sclerosis; n = 62).
Main Outcomes and Measures
Cerebrospinal fluid values of amyloid β1-42 (Aβ1-42), total tau (t-tau), and p-tau obtained using the INNO-BIA AlzBio3 (xMAP; Luminex) assay or INNOTEST enzyme-linked immunosorbent assay transformed using a previously validated algorithm. Sensitivities and specificities for differentiating AD from FTLD groups were calculated.
Results
This autopsy cohort included FTLD-tau (n = 27; mean [SD] age at onset, 60.8 [9.7] years), FTLD-TDP (n = 13; mean [SD] age at onset, 62.4 [8.5] years), AD (n = 89, mean [SD] age at onset, 66.5 [9.7] years); and mixed FTLD-AD (n = 14, mean [SD] age at onset, 70.6 [8.5] years).The p-tau/Aβ1-42 ratio showed an excellent diagnostic accuracy to exclude AD cases in the autopsy cohort with single neurodegenerative pathologies (area under the curve [AUC], 0.98; 95% CI, 0.96-1.00). Cerebrospinal fluid p-tau levels showed a good AUC (0.87; 95% CI, 0.73-1.00) for discriminating pure FTLD-TDP from pure FTLD-tau. The application of an algorithm using cutpoints of CSF p-tau to Aβ1-42 ratio and p-tau allowed a good discrimination of pure FTLD-TDP cases from the remaining FTLD-tau and mixed FTLD cases. The diagnostic value of this algorithm was confirmed in an independent cohort of living patients with progressive supranuclear palsy and FTD–amyotrophic lateral sclerosis (AUC, 0.9; 95% CI, 0.81-0.99). However, the algorithm was less useful in FTD cases carrying a pathogenic mutation (AUC, 0.58; 95% CI, 0.38-0.77) owing to elevated p-tau levels in TDP-associated mutation carriers.
Conclusions and Relevance
Alzheimer disease CSF core biomarkers can be used with high specificity for the in vivo identification of patients with pure FTLD-TDP and FTLD-tau when accounting for comorbid AD and genetic status.
This case-control study assesses the use of an algorithm based on core Alzheimer disease cerebrospinal fluid measures to exclude cases with AD pathology and then differentiate between frontotemporal lobar degeneration tau and frontotemporal lobar degeneration transactive response DNA-binding protein.
Introduction
Frontotemporal lobar degeneration (FTLD) is a neuropathological umbrella term coined to describe a group of neurodegenerative disorders with prominent frontal and temporal lobe atrophy presenting with a wide spectrum of behavioral, language, and motor disturbances. Most FTLD cases can be classified in 2 main subtypes according to the protein that aggregates in the central nervous system: FTLD-TDP (approximately 50%), which is associated with aggregates composed of transactive response DNA-binding protein of approximately 43kDa (also known as TDP-43), and FTLD-tau (approximately 45%), which is associated with aggregates containing the microtubule-associated protein tau.1 Although most cases are considered sporadic, up to 25% of patients may have a pathogenic mutation, mainly in the MAPT, GRN, and C9orf72 genes.2 While genetic testing may enable a definite diagnosis in mutation carriers, the in vivo diagnosis of most FTLD cases with sporadic disease is challenging because there is no reliable correspondence between the clinical syndrome and the underlying neuropathology.3
Cerebrospinal fluid (CSF) markers have been studied in neurodegenerative diseases as a way to track different pathophysiological processes in the central nervous system. In Alzheimer disease (AD), levels of amyloid β1-42 (Aβ1-42), total tau (t-tau), and phosphorylated tau (p-tau), also named core AD biomarkers, have shown excellent diagnostic accuracy for the detection of AD at the prodromal and dementia stages.4 Core AD biomarkers are also useful in FTLD-related syndromes to exclude AD.5,6,7 In addition, core AD biomarkers could also be used to distinguish the different neuropathological subtypes of FTLD. In particular, previous studies have shown that low levels of p-tau or the ratio of p-tau to t-tau could be useful biomarkers for TDP-43 proteinopathies.7,8,9 Further, p-tau is more specific for tau pathology because t-tau also reflects nonspecific neuronal and axonal damage.10,11 Importantly, in 2017, 12 we have described an independent association of antemortem CSF p-tau levels with postmortem cerebral tau pathology in a large series of autopsy-confirmed FTLD, suggesting that low p-tau is a specific marker for TDP-43 proteinopathies.12
It is clear that specific markers for FTLD-TDP and FTLD-tau are needed, and some promising advances have been made.13 Unfortunately, many biomarker studies of FTLD-related syndromes may be confounded by co-occurring secondary AD pathology.7 It is possible that this secondary AD pathology confounds measurement of CSF analytes, with consequences for clinical trial outcomes that include CSF measurement of tau. In addition, most studies have grouped patients with frontotemporal degeneration (FTD) with and without pathogenic mutations, assuming that they all have a similar CSF biomarker profile. This study aimed to develop a 2-step algorithm where we first exclude cases with significant AD pathology and then use CSF tau analytes to differentiate between sporadic FTLD-tau and FTLD-TDP. This algorithm was tested in 3 different cohorts of patients with FTD: a sporadic autopsy cohort, a genetic cohort, and a living cohort with syndromes highly predictive of FTLD-tau and FTLD-TDP.
Methods
Patients
Participants were selected from a database of 1796 patients with different neurodegenerative diseases with available CSF included from May 1992 to April 2016 at the Center for Neurodegenerative Disease Research at the University of Pennsylvania.
Autopsy Cohort
We included data from patients with antemortem CSF and a neuropathological diagnosis of AD or FTLD who were followed longitudinally at the Frontotemporal Degeneration Center or Alzheimer Disease Core Center to autopsy establishment of their underlying neuropathology in the Center for Neurodegenerative Disease Research at the University of Pennsylvania.14 A total of 143 sporadic cases were included: 89 pure AD cases, 40 cases of FTLD (27 FTLD-tau and 13 FTLD-TDP), and 14 cases with AD plus FTLD (10 with FTLD-tau and 4 with FTLD-TDP, collectively known as FTLD-AD). All FTLD cases were screened for the 3 most common mutations (MAPT, GRN, and C9orf72) as previously described.2 Cases presenting as motor neuron disease, Lewy body dementia, and those with concurrent FTLD-Tau and FTLD-TDP (3 cases) were excluded.
Genetic Cohort
We included a group of 60 patients with FTD with pathogenic mutations and CSF available for analysis. This group was composed of 33 patients with mutations in C9orf72, 13 in GRN, 4 in TARDBP, and 10 in MAPT genes.
Replication Sporadic Cohort
We included a group of 62 living patients with clinical phenotypes that are highly predictive of FTLD-tau and FTLD-TDP: 39 patients with progressive supranuclear palsy (PSP) and 23 patients with amyotrophic lateral sclerosis (ALS) associated with FTD (ALS–mild cognitive impairment and FTD-ALS) diagnosed according to established diagnostic criteria.15,16 All individuals participated in a written informed consent procedure with their caregivers, when appropriate, that was approved by the institutional review board at the University of Pennsylvania. In the case of deceased patients, written consent was obtained from a family member. A subset of these patient samples has been previously published.5,6,7,12,14
Biofluid Collection and Analysis
Cerebrospinal fluid samples were obtained as described previously.5,14 We obtained data from Aβ1-42, t-tau, and p-tau levels previously analyzed using the enzyme-linked immunosorbent assay (INNOTEST) or the Luminex xMAP platform (INNO-BIA AlzBio3TM, for research use–only reagents) at the Center for Neurodegenerative Disease Research (enzyme-linked immunosorbent assay) and the Biomarker Core (xMAP) of the AD Neuroimaging Initiative at the University of Pennsylvania.17,18,19 Cerebrospinal fluid values from enzyme-linked immunosorbent assay were transformed to xMAP values using the validated formulas.5 Cerebrospinal fluid biomarker measures for Aβ1-42 and p-tau to Aβ1-42 ratio in the autopsy cohort were available for 123 of 143 patients (86%), a valid p-tau result was available for 122 of 143 patients (85.3%), and CSF biomarker measures for t-tau to Aβ1-42 ratio were available for all cases.
Neuropathological Analysis
Autopsy was performed as previously described.3 Microscopic diagnosis was made by experienced neuropathologists (E.B.L. and J.Q.T.) using neuropathological diagnostic criteria.20,21,22,23,24 Cases were divided into those with 1 neuropathological diagnosis and those with multiple diagnoses using Braak and Consortium to Establish a Registry for Alzheimer’s Disease stages of AD pathology.20,21 Concurrent pathologies were registered as previously described.7 In patients with FTLD-tau, sections of the hippocampus were stained with thioflavin-S, as described,25 to distinguish comorbid AD neurofibrillary tangle pathology from primary FTLD tauopathy. We used pathological criteria of low AD to define secondary comorbid AD (either AD Braak tau stage ≥B2 or AD Braak tau stage B1 and Consortium to Establish a Registry for Alzheimer’s Disease ≥C2) in FTLD cases.23 We used the term pure FTLD for cases with a primary neuropathological diagnosis of FTLD and no comorbid AD and FTLD-AD for cases with FTLD and concomitant AD as in previous studies.7
Statistical Analysis
Variables were examined for normality. One-way analysis of variance or Kruskal-Wallis test were performed across the groups as appropriate. For group-wise comparisons and regression models, we used natural log (ln) transformation to obtain normally distributed CSF variables for analysis. Because the autopsy and validation cohorts differed in disease duration and age at which CSF samples were obtained, and because these factors may influence CSF analyte levels, we performed a logistic regression analysis for p-tau that included age and disease duration as covariates. These logistic regressions were completed in the autopsy cohort, and the probabilities then were entered into receiver operating characteristic curves. We calculated the optimal cutoff that was used to assess sensitivity and specificity and then applied this logistic regression model adjusted for age and disease duration to the genetic and replication sporadic cohorts.
Statistical significance for all tests was set at P less than .05. All P values were 2-sided. All analyses were performed using SPSS, version 20.0 (IBM Corp) or STATA, version 12.0 (STATA Corp).
Results
Demographic, Clinical, and Biomarker Data of the Autopsy Cohort
Demographic, clinical, and neuropathologic characteristics of the autopsy patient sample are summarized in Table 1. The FTLD-AD group had a later age at onset when compared with that of FTLD-tau. The age at death was higher in the AD group than in the FTLD-tau and FTLD-TDP groups. Age at CSF sampling was higher in the FTLD-AD group than in FTLD-tau and FTLD-TDP. APOE ε4 allele was overrepresented in the AD group when compared with the other groups.
Table 1. Demographic and CSF Biomarker Data of Patients of the Sporadic Autopsy Cohort by Neuropathological Group.
| Clinical and Biofluid Feature | Mean (SD) | |||
|---|---|---|---|---|
| Pure Cases | Mixed Cases; FTLD-AD (n = 14) |
|||
| AD (n = 89) | FTLD-Tau (n = 27) | FTLD-TDP (n = 13) | ||
| Age at onset, y | 66.5 (9.7) | 60.8 (8.9)a | 62.4 (8.5) | 70.6 (8.5)b |
| Age at death, y | 75.9 (10.1)b,c | 68.4 (8.8)a,d | 68.4 (9)d | 79.2 (11.3)b |
| Age at CSF measure, y | 70.2 (9.5) | 64.2 (9.4)a | 65.3 (7.6)a | 74.5 (10.1)b,c |
| Time from onset to CSF measure, y | 3.72 (2.4) | 3.41 (2) | 2.92 (1.7) | 3.9 (3.1) |
| Men, No. (%) | 50 (56.8) | 15 (65.2) | 4 (33.3) | 8 (57.1) |
| APOE ε4 positive, No. (%) | 56 (63.6) | 4 (17.4)d | 4 (33.3) | 5 (37.5) |
| CSF Aβ1-42 (n = 123) | 137.8 (50.6)b | 244.1 (46.1)a,d | 216.8 (63.3)a | 148.7 (29.7)b,c |
| CSF t-tau (n = 143) | 120.3 (88)b | 48 (22.7)d | 54.1 (39.1) | 65.6 (46.1) |
| CSF p-tau (n = 139) | 41.1 (30.3)b,c | 11.9 (3.8)a,c,d | 7.9 (5.4)a,b,d | 17.7 (8.6)b,c |
| CSF t-tau/Aβ1-42 (n = 143) | 1.01 (0.91)b,c,a | 0.20 (0.12)d | 0.26 (0.2)d | 0.44 (0.29)d |
| CSF p-tau/Aβ1-42 (n = 122) | 0.34 (0.26),a,b,c | 0.05 (0.02)a,d | 0.04 (0.03)a,d | 0.14 (0.06)b,c,d |
Abbreviations: Aβ1-42, amyloid β1-42; AD, Alzheimer disease; CSF, cerebrospinal fluid; FTLD, frontotemporal lobar degeneration; p-tau, phosphorylated tau; t-tau, total tau.
P < .05 compared with AD-FTLD.
P < .05 compared with FTLD-Tau.
P < .05 compared with FTLD-TDP.
P < .05 compared with AD.
Cerebrospinal fluid Aβ1-42 levels were lower in both the pure AD and FTLD-AD groups compared with the FTLD-tau group (Table 1; eFigure 1 in the Supplement). Cerebrospinal fluid t-tau levels were higher in the pure AD group than in FTLD-tau. Finally, CSF p-tau levels were higher in the pure AD group than in pure FTLD groups. The FTLD-AD group showed intermediate values for tau analytes between pure AD and pure FTLD, highlighting the effect of comorbid AD on CSF biomarkers.7 As reported in previous studies,7,8,9 CSF p-tau levels were lower in FLTD-TDP than in FTLD-tau (Kruskal-Wallis H test, 7.43; P = .006). Total tau to Aβ1-42 and p-tau to Aβ1-42 ratios also showed clear differences among groups (Table 1).
Because of the AD-like CSF profile in the FTLD-AD cohort, we developed a 2-stage process for the biofluid-based diagnosis of FTLD spectrum disorders. First, we established cutpoints of CSF analytes for each form of pathology in the subset of patients with pure pathology. Then we applied these criteria to our entire cohort, which included individuals with mixed FTLD-AD pathology, to develop a 2-stage process for differentiating FTLD-tau and FTLD-TDP in individuals with sporadic FTLD; specifically, the first stage excludes individuals with primary or secondary AD pathology, and the second stage distinguishes between FTLD-TDP and FTLD-tau in individuals less likely to have primary or secondary AD pathology.
Establishing a Diagnostic Algorithm Based on AD Biomarkers in Patients With Single Neurodegenerative Pathologies
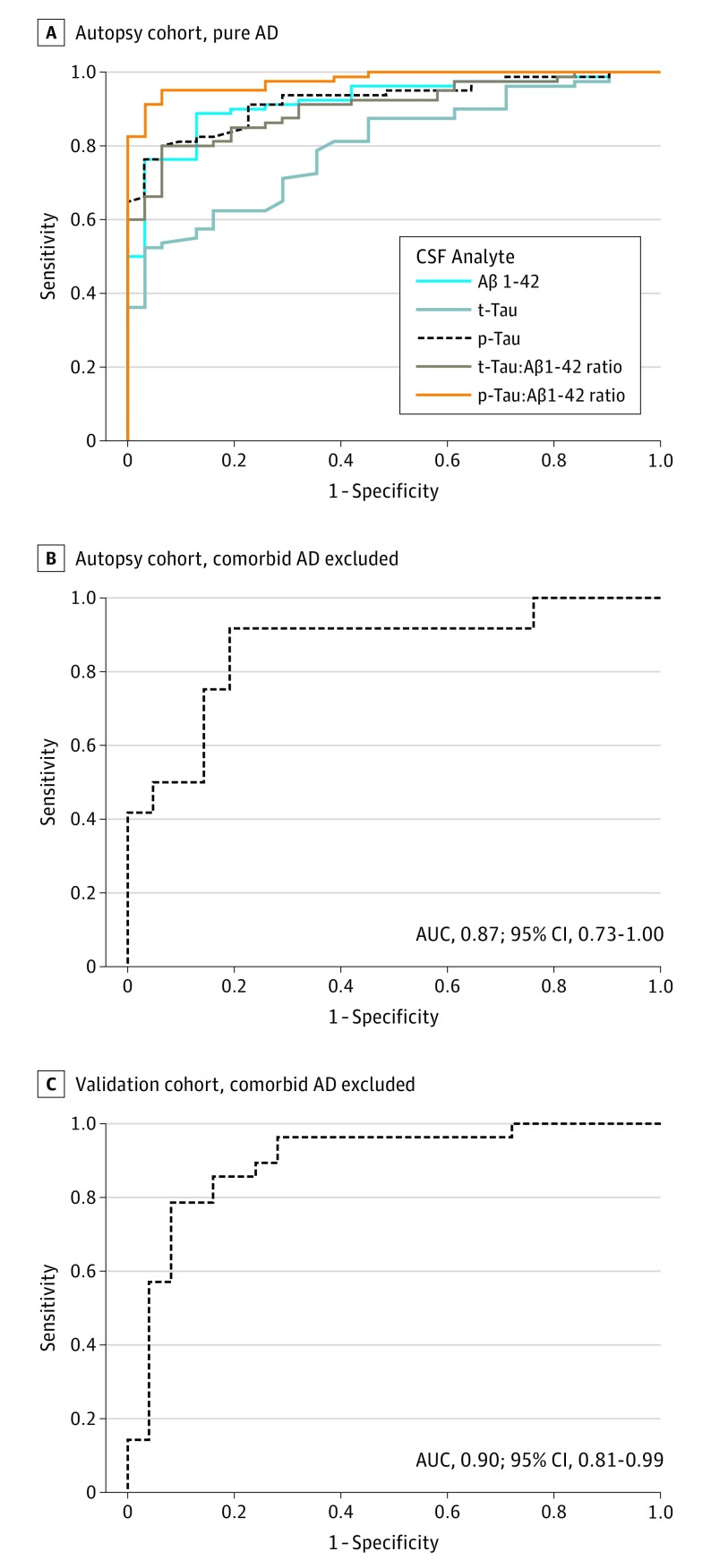
We performed receiver operating characteristic analyses for the differentiation between pure AD and pure FTLD (both FTLD-tau and FTLD-TDP), and results are shown in Figure 1A. The p-tau to Aβ1-42 ratio showed the best area under the curve (0.98; 95% CI, 0.96-1.00; P < .001) followed by the t-tau to Aβ1-42 ratio (0.91; 95% CI, 0.85-0.96; P < .001). A p-tau to Aβ1-42 ratio cutoff of 0.09 achieved a 91.3% sensitivity (95% CI, 82.8%-96.4%) and 96.8% specificity (95% CI, 83.3%-99.9%), with a likelihood ratio of 28.3.
Figure 1. Receiver Operating Characteristic Curves.
A, Sensitivity and specificity of cerebrospinal fluid (CSF) amyloid β1-42 (Aβ1-42), total tau (t-tau), phosphorylated tau (p-tau), t-tau/Aβ1-42, and p-tau/Aβ1-42 in pure Alzheimer disease (AD) relative to pure frontotemporal lobar degeneration (FTLD) in the autopsy cohort. B, Sensitivity and specificity of CSF p-tau levels in FTLD-tau relative to FTLD-TDP in the autopsy cohort after excluding comorbid AD (at neuropathological evaluation); C, Sensitivity and specificity of CSF p-tau in the validation cohort after excluding comorbid AD (using p-tau/Aβ1-42). AUC indicates area under the curve.
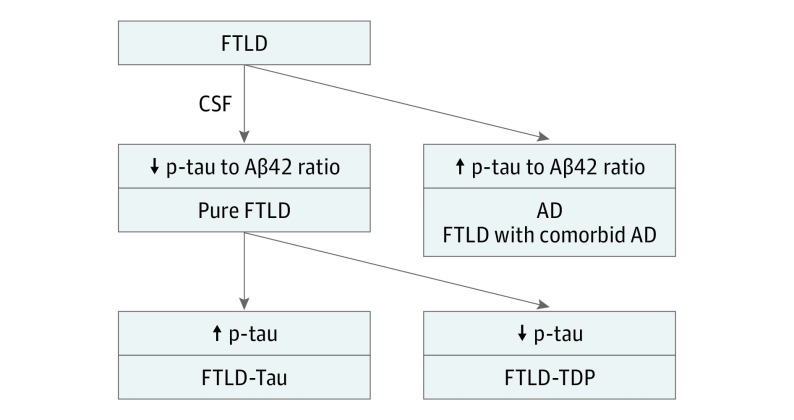
We next investigated the capacity of CSF p-tau levels to distinguish between pure sporadic FTLD-Tau and FTLD-TDP cases. We performed ROC analysis accounting for the differences in age and time from diagnosis at CSF sampling, and levels of p-tau showed a good capacity to discriminate between pure FTLD-tau and FTLD-TDP with an area under the curve of 0.87 (95% CI, 0.73-1.00; Figure 1B). Receiver operating characteristic analyses using raw values are shown in eFigure 2 in the Supplement. However, when we included all FTLD cases, including FTLD-AD, the area under the curve dropped to 0.69 (95% CI, 0.51-0.87), indicating that comorbid AD confounds the diagnostic value of p-tau. The optimal probabilistic cutoff for p-tau after adjusting for age at CSF sampling and time from diagnosis to CSF sampling achieved 81% sensitivity (95% CI, 74%-88%) and 92% specificity (95% CI, 85%-99%) for the differentiation between FTLD-tau and FTLD-TDP. Therefore, the best results were obtained when we applied a 2-step algorithm based on the application of the p-tau to Aβ1-42 ratio to exclude cases with any AD pathology (ie, primary AD or mixed FTLD-AD) and then the p-tau to distinguish between FTLD-tau and FTLD-TDP (Figure 2).
Figure 2. Cerebrospinal Fluid (CSF) Algorithm.
A 2-stage algorithm for the identification of frontotemporal lobar degeneration (FTLD). In a first step, cases with Alzheimer disease (AD) pathology are excluded by means of the application of phosphorylated tau (p-tau)/amyloid β1-42 (Aβ1-42) ratio, and subsequently, cases with FTLD-tau and FTLD-TDP are separated by means of p-tau cutoff in the subgroups of patients with a non-AD CSF biomarker profile.
Performance of the Classification Algorithm in a Genetic FTD Cohort
We next applied this algorithm to a cohort of 60 patients with FTD carrying pathogenic mutations to test the hypothesis that mutation status may influence CSF biomarker profile.12 Most patients (50 [83.3%]) in this cohort had TDP-associated mutations (C9orf72, GRN, TARDBP, and VCP) while tau-associated mutations were less frequent ([16.6%]). Demographic and clinical characteristics of this sample are summarized in the eTable in the Supplement. There was no difference in p-tau levels between TDP-associated and tau-associated mutations (Mann-Whitney U, 121; P = .53) or between the different TDP-associated mutations (Kruskal-Wallis H test, 0.12; P = .94). After exclusion of patients with presumed AD pathology based on the p-tau to Aβ1-42 ratio, the area under the curve for p-tau was 0.58 (95% CI, 0.38-0.77) to discriminate between groups. These results indicate that the algorithm is not useful in cases with FTD-carrying pathogenic mutations. We also compared levels of p-tau in the group of TDP-associated mutations with those of the pure FTLD-TDP group in the autopsy cohort. We found higher p-tau levels in the group of TDP-associated mutations than in the sporadic FTLD-TDP (Mann-Whitney U, 399; P = .003). These findings, together with our previous observation of elevated p-tau levels in patients with the C9orf72 expansion, suggest higher levels of p-tau in TDP-associated mutation carriers.12
Validation of the 2-Stage CSF Algorithm in an Independent Cohort
To further confirm the performance of the algorithm in a clinically relevant scenario, we applied the CSF algorithm in an independent living cohort of 69 patients with clinical syndromes highly predictive of FTLD-tau and FTLD-TDP. We included 39 patients with a clinical diagnosis of PSP and 23 with FTD-ALS. Patients with FTD-ALS with C9orf72 mutations (n = 7) were excluded. Demographic and clinical characteristics of the validation sample are summarized in Table 2. Age at CSF sampling was higher in the PSP group (Mann-Whitney U, 696; P < .001) compared with the FTD-ALS group. Phosphorylated tau levels were lower in the FTD-ALS group (Mann-Whitney U, 611; P = .02; Table 2) compared with the PSP group. After the exclusion of patients with p-tau to Aβ1-42 greater than 0.09 (expected comorbid AD), p-tau CSF levels showed an area under the curve of 0.9 (95% CI, 0.81-0.99; P < .001; Figure 2C) for age-adjusted p-tau values. The probabilistic cutoff calculated in the autopsy cohort had a sensitivity of 89% (95% CI, 0.79%-0.99%) and a specificity of 73% (95% CI, 0.63- 0.83) for the detection of FTD-ALS.
Table 2. Demographic and CSF Biomarker Data of Patients of the Sporadic Living Cohort.
| Clinical and Biofluid Feature | Mean (SD) | |
|---|---|---|
| FTD-ALS (n = 23) | PSP (n = 39) | |
| Age at onset, y | 55.1 (10.9)a | 65 (7.7)b |
| Age at CSF measure, y | 57.5 (11.3)a | 68.4 (7.5)b |
| Time from onset to CSF, y | 2.7 (2.4)a | 3.7 (2.1)b |
| Men, No. (%) | 15 (38.5)a | 17 (73.9)b |
| APOE ε4 positive, No./total No. (%) | 4/12 (33.3) | 2/20 (10) |
| Expected comorbid AD, No. (%)c | 4 (17.4) | 9 (23.1) |
| CSF Aβ1-42 | 260.1 (72.2) | 254.7 (89.8) |
| CSF t-tau | 59.6 (28.6) | 47.7 (19.3) |
| CSF p-tau | 11.4 (7.4)a | 13.9 (5.9)b |
| CSF t-tau/Aβ1-42d | 0.26 (0.19) | 0.21 (0.11) |
| CSF p-tau/Aβ1-42 | 0.05 (0.05)a | 0.06 (0.04)b |
Abbreviations: Aβ1-42, amyloid β1-42; AD, Alzheimer disease; CSF, cerebrospinal fluid; FTD-ALS, frontotemporal dementia–amyotrophic lateral sclerosis; p-tau, phosphorylated tau; PSP, progressive supranuclear palsy; t-tau, total tau.
P < .05 compared with PSP.
P < .05 compared with FTD-ALS.
p-tau/Aβ1-42 at least 0.09
n = 61.
Discussion
The main finding of this study is that a 2-stage algorithm based on 3 frequently used CSF biomarkers can be applied to first exclude cases with AD pathology (as the primary or as a secondary neuropathological diagnosis) and to identify FTLD-tau and FTLD-TDP subtypes of FTLD in a cohort of sporadic FTLD. This algorithm may be a valuable tool for the enrichment of clinical trials and research studies on FTLD that require the diagnosis of FTLD subtypes.
Accurate diagnosis of the underlying pathology in FTLD spectrum disorders is a crucial step in developing a strategy for disease-modifying treatments in these conditions. The diagnosis of sporadic FTD is based on clinical criteria supported by the presence of anatomic markers (characteristic magnetic resonance imaging atrophy or 18fluoro-D-glucose-positron emission tomography [PET] hypometabolism).26,27 However, estimates of misdiagnosis suggest that up to 30% of patients with FTD receive another diagnosis, in particular AD, and that an equal number of AD cases are misdiagnosed as FTLD.3 Although the development of tau PET tracers represents an opportunity for detecting some subtypes of FTD, its clinical utility remains uncertain.28,29,30 Amyloid PET markers may be useful in distinguishing cases with or without AD pathology, but false-positive and false-negative findings often occur.31 Cerebrospinal fluid offers the possibility of detecting different pathophysiological changes in the central nervous system. Core CSF AD biomarkers are the most investigated biochemical markers in FTLD, and they have been mainly used for the identification of AD cases rather than as a confirmation of FTLD. Other markers, such as neurofilament light chain, have been investigated in FTLD. Levels of neurofilament light chain are elevated in FTD, and they correlate with disease progression.32,33 However, neurofilament light chain levels are also increased in AD, suggesting a lack of disease specificity. It is clear that novel and more specific markers of FTLD are needed, and some promising findings have been reported.13,34 Nonetheless, in this study, we present evidence that traditional CSF biomarkers for AD can be successfully used to improve accurate selection of sporadic cases with FTLD.
We first applied the p-tau to Aβ1-42 ratio to exclude cases with AD irrespective of the clinical phenotype. As previously published,5,7,18 both tau to Aβ1-42 ratios performed better than single analytes for the prediction of AD pathology. This should be taken into account in future research criteria for both AD and FTLD syndromes because the use of independent Aβ1-42 and tau cutoffs may influence the diagnostic accuracy of the proposed criteria, especially for atypical AD phenotypes (eg, corticobasal syndrome and the behavioral variant of AD). The fact that the selected cutoff is based on a sample of pure AD cases has a consequence that the identification of mixed FTLD-AD cases is indeterminate. Specifically, because a small degree of concomitant AD pathology has a marked effect on core CSF biomarkers,7 cases with FTLD and comorbid AD may be excluded by the application of a strict cutpoint calculated based on cases with single neuropathologic conditions. Although cases with both FTLD and AD may represent a minority of all FTLD cases (<20%),7 concomitant AD pathology may interfere with treatments targeting FTLD-specific pathologies or may obscure clinical outcomes in a trial because this pathway may not be affected by the drug. Therefore, we believe that a classification algorithm for FTLD, such as the one proposed here, should aim at selecting cases with single neurodegenerative pathologies that are more likely to respond to therapies.
We next applied a p-tau cutpoint, building on previous evidence that this protein could be a useful biomarker for FTLD-TDP.7,8,9 Consistent with these prior reports, we observed that sporadic cases with FTLD-TDP had lower p-tau levels in CSF than cases with FTLD-tau. The more likely explanation of this finding is that p-tau in FTLD reflects more accurately pathologic tau, while t-tau also reflects nonspecific neuronal and axonal damage.5,10 This is supported by evidence showing that CSF p-tau levels are positively associated with cerebral tau burden in FTLD.12 Therefore, the data support the model that CSF p-tau levels in FTLD are lower in FTLD-TDP owing to the lack of tau pathology. However, this difference can be obscured by the existence of comorbid AD pathology that may be observed in a minority of FTLD cases. It is important to focus on excluding co-occurring AD pathology because we and others have observed that AD copathology in forms of FTLD is much more common than co-occurring FTLD-tau and FTLD-TDP. The 2-stage algorithm proposed in this study thus aims to identify cases with single neurodegenerative pathologies by first excluding cases with common dual pathologies such as co-occurring AD.
About 25% of clinical FTD cases are mutation carriers,2 and identification of the mutation can lead to a reliable prediction of the underlying histopathologic diagnosis. In this study, we found that the proposed algorithm was less useful in patients with FTD with pathogenic mutations. This is in agreement with our previous observation that the C9orf72 expansion is associated with higher CSF p-tau levels.12 These findings suggest that biomarker data and cutoffs cannot be equally applied to genetic and sporadic cases. This difference in biomarker profiles between genetic and sporadic disease has also been described in other neurodegenerative conditions such as AD.35,36 In addition, the utility of a diagnostic algorithm is likely to be more clinically relevant in sporadic FTD when pathology cannot be inferred from the clinical syndrome. The value of this algorithm was confirmed in an independent cohort of patients with FTD with syndromes highly predictive of FTLD-tau and FTLD-TDP. The value of p-tau in the living cohort showed a high sensitivity but modest specificity. The phenotypes in the living cohort (PSP and FTD-ALS) and in the autopsy cohort differed, and it is possible than the existence of motor neuron disease or the specific topographical pattern of aggregation in 4-repeated tauopathies may influence p-tau levels.
Strengths and Limitations
The main strength of this study is the use of a large autopsy-confirmed cohort with detailed neuropathologic data. This allowed us to establish a criterion-standard reference for CSF biomarkers and to consider concurrent pathologies known to affect CSF biomarker cutoffs.7 Limitations should be considered when evaluating our findings. We did not obtain cross-validation in an independent autopsy cohort because a comparable pathology-proven data set to replicate these findings is exceedingly rare. However, we replicated the ability of CSF p-tau for the discrimination of FTLD-tau from FTLD-TDP after excluding patients with expected comorbid AD in an independent living cohort. Further collaborative autopsy-proven studies are needed to refine and operationalize the proposed CSF algorithm. It is worth mentioning that we did not take into account the clinical phenotypes or imaging biomarkers (eg, amyloid or tau PET or magnetic resonance imaging); however, our methods suggest that CSF is a lower-cost alternative to PET imaging to exclude AD copathology in clinical FTD. For example, confidence in the diagnosis of FTLD-TDP may be improved if a low p-tau level is associated with clinical features of semantic variant primary progressive aphasia.37 Thus, it is likely that combinations of clinical features and CSF biomarkers can further improve diagnostic accuracy, and multimodal assessments should be further studied in patients followed up to autopsy.
Conclusions
In conclusion, we show that core AD CSF biomarkers can be used to improve specificity for the in vivo identification of patients with sporadic FTLD-TDP and FTLD-tau. This involves a 2-stage algorithm that first excludes cases with likely AD pathology. We anticipate that this algorithm will be improved with the addition of novel pathway-specific biomarkers of FTLD that will undoubtedly increase the diagnostic accuracy in the FTLD-related syndromes.
eTable. Demographic and Clinical Characteristics of the Genetic Cohort
eFigure 1. Raw CSF Levels of CSF Aβ1-42, T-Tau, T-Tau/Aβ1-42 and P-Tau/Aβ1-42 in the Autopsy Cohort
eFigure 2. Sensitivity and Specificity of CSF Raw Values
References
- 1.Irwin DJ, Cairns NJ, Grossman M, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015;129(4):469-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood EM, Falcone D, Suh E, et al. Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol. 2013;70(11):1411-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59(6):952-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lleó A, Cavedo E, Parnetti L, et al. Cerebrospinal fluid biomarkers in trials for Alzheimer and Parkinson diseases. Nat Rev Neurol. 2015;11(1):41-55. [DOI] [PubMed] [Google Scholar]
- 5.Irwin DJ, McMillan CT, Toledo JB, et al. Comparison of cerebrospinal fluid levels of tau and Aβ 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms. Arch Neurol. 2012;69(8):1018-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70(19 Pt 2):1827-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toledo JB, Brettschneider J, Grossman M, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124(1):23-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman M, Elman L, McCluskey L, et al. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol. 2014;71(4):442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu WT, Watts K, Grossman M, et al. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology. 2013;81(22):1945-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382-389. [DOI] [PubMed] [Google Scholar]
- 11.Buerger K, Ewers M, Pirttilä T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129(pt 11):3035-3041. [DOI] [PubMed] [Google Scholar]
- 12.Irwin DJ, Lleó A, Xie SX, et al. Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration. Ann Neurol. 2017;82(2):247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teunissen CE, Elias N, Koel-Simmelink MJA, et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimers Dement (Amst). 2016;2:86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toledo JB, Van Deerlin VM, Lee EB, et al. A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement. 2014;10(4):477-84.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis: frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3-4):153-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höglinger GU, Respondek G, Stamelou M, et al. ; Movement Disorder Society-endorsed PSP Study Group . Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman M, Farmer J, Leight S, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer’s disease. Ann Neurol. 2005;57(5):721-729. [DOI] [PubMed] [Google Scholar]
- 18.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsson A, Vanderstichele H, Andreasen N, et al. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51(2):336-345. [DOI] [PubMed] [Google Scholar]
- 20.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095-1097. [DOI] [PubMed] [Google Scholar]
- 21.McKeith IG, Dickson DW, Lowe J, et al. ; Consortium on DLB . Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. [DOI] [PubMed] [Google Scholar]
- 22.Mackenzie IRA, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119(1):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montine TJ, Phelps CH, Beach TG, et al. ; National Institute on Aging; Alzheimer’s Association . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montine TJ, Monsell SE, Beach TG, et al. Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer’s disease. Alzheimers Dement. 2016;12(2):164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin DJ, Brettschneider J, McMillan CT, et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol. 2016;79(2):272-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMillan CT, Irwin DJ, Nasrallah I, et al. Multimodal evaluation demonstrates in vivo 18F-AV-1451 uptake in autopsy-confirmed corticobasal degeneration. Acta Neuropathol. 2016;132(6):935-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquié M, Normandin MD, Meltzer AC, et al. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol. 2017;81(1):117-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passamonti L, Vázquez Rodríguez P, Hong YT, et al. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain. 2017;140(3):781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherling CS, Hall T, Berisha F, et al. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol. 2014;75(1):116-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skillbäck T, Farahmand B, Bartlett JW, et al. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83(21):1945-1953. [DOI] [PubMed] [Google Scholar]
- 34.Alcolea D, Vilaplana E, Suárez-Calvet M, et al. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology. 2017;89(2):178-188. [DOI] [PubMed] [Google Scholar]
- 35.Pera M, Alcolea D, Sánchez-Valle R, et al. Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease. Acta Neuropathol. 2013;125(2):201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balasa M, Vidal-Piñeiro D, Lladó A, et al. PSEN1 mutation carriers present lower cerebrospinal fluid amyoid-β42 levels than sporadic early-onset Alzheimer’s disease patients but no differences in neuronal injury biomarkers. J Alzheimers Dis. 2012;30(3):605-616. [DOI] [PubMed] [Google Scholar]
- 37.Grossman M. Biomarkers in the primary progressive aphasias. Aphasiology. 2014;28(8-9):922-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Demographic and Clinical Characteristics of the Genetic Cohort
eFigure 1. Raw CSF Levels of CSF Aβ1-42, T-Tau, T-Tau/Aβ1-42 and P-Tau/Aβ1-42 in the Autopsy Cohort
eFigure 2. Sensitivity and Specificity of CSF Raw Values