Key Points
Question
Are regulatory T-cells associated with a slower rate of disease progression in amyotrophic lateral sclerosis?
Findings
In this study, the expansion of endogenous regulatory T-cells in a mouse model of amyotrophic lateral sclerosis significantly prolonged survival time and was associated with preserved motor neuron soma size, marked suppression of glial cell immunoreactivity, and increased neuroprotective gene-expression profiles. Regulatory T-cells were also shown to correlate with a slower rate of disease progression in patients with amyotrophic lateral sclerosis.
Meaning
Therapies aimed at modulating regulatory T-cells in patients with amyotrophic lateral sclerosis may prove therapeutically beneficial.
This human and animal study assesses the role of regulatory T-cells in the pathophysiology of amyotrophic lateral sclerosis and the therapeutic association with increasing regulatory T-cell activity in a mouse model of the disease.
Abstract
Importance
Neuroinflammation appears to be a key modulator of disease progression in amyotrophic lateral sclerosis (ALS) and thereby a promising therapeutic target. The CD4+Foxp3+ regulatory T-cells (Tregs) infiltrating into the central nervous system suppress neuroinflammation and promote the activation of neuroprotective microglia in mouse models of ALS. To our knowledge, the therapeutic association of host Treg expansion with ALS progression has not been studied in vivo.
Objective
To assess the role of Tregs in regulating the pathophysiology of ALS in humans and the therapeutic outcome of increasing Treg activity in a mouse model of the disease.
Design, Setting, and Participants
This prospective multicenter human and animal study was performed in hospitals, outpatient clinics, and research institutes. Clinical and function assessment, as well as immunological studies, were undertaken in 33 patients with sporadic ALS, and results were compared with 38 healthy control participants who were consecutively recruited from the multidisciplinary ALS clinic at Westmead Hospital between February 1, 2013, and December 31, 2014. All data analysis on patients with ALS was undertaken between January 2015 and December 2016. Subsequently, we implemented a novel approach to amplify the endogenous Treg population using peripheral injections of interleukin 2/interleukin 2 monoclonal antibody complexes (IL-2c) in transgenic mice that expressed mutant superoxide dismutase 1 (SOD1), a gene associated with motor neuron degeneration.
Main Outcomes and Measures
In patients with ALS, Treg levels were determined and then correlated with disease progression. Circulating T-cell populations, motor neuron size, glial cell activation, and T-cell and microglial gene expression in spinal cords were determined in SOD1G93A mice, as well as the association of Treg amplification with disease onset and survival time in mice.
Results
The cohort of patients with ALS included 24 male patients and 9 female patients (mean [SD] age at assessment, 58.9 [10.9] years). There was an inverse correlation between total Treg levels (including the effector CD45RO+ subset) and rate of disease progression (R = −0.40, P = .002). Expansion of the effector Treg population in the SOD1G93A mice was associated with a significant slowing of disease progression, which was accompanied by an increase in survival time (IL-2c–treated mice: mean [SD], 160.6 [10.8] days; control mice: mean [SD], 144.9 [10.6] days; P = .003). Importantly, Treg expansion was associated with preserved motor neuron soma size and marked suppression of astrocytic and microglial immunoreactivity in the spinal cords of SOD1G93A mice, as well as elevated neurotrophic factor gene expression in spinal cord and peripheral nerves.
Conclusions and Relevance
These findings establish a neuroprotective effect of Tregs, possibly mediated by suppression of toxic neuroinflammation in the central nervous system. Strategies aimed at enhancing the Treg population and neuroprotective activity from the periphery may prove therapeutically useful for patients with ALS.
Introduction
Neuroinflammation is a pathological hallmark of amyotrophic lateral sclerosis (ALS),1 a progressive and fatal neurodegenerative disorder caused by loss of upper and lower motor neurons.2 Accumulating evidence suggests that astrocytes, microglia, and T-cells actively contribute to neurodegeneration and disease progression in ALS.3,4 Reducing ALS-linked mutant superoxide dismutase 1 (SOD1) synthesis in microglia5 or astrocytes6 has been shown to slow disease progression, while elimination of CD4+ T-cells has been shown to accelerate symptom severity in mice with a mutant form of the SOD1 gene.7,8 Peripheral blood cells and astrocytes generated from fibroblasts of patients with ALS are also toxic to motor neurons.9,10 Consequently, nonneuronal cell toxicity contributes to neurodegeneration in ALS through non-cell autonomous mechanisms.11
During early disease stages in mice with a mutant form of SOD1, CD4+Foxp3+ regulatory T-cells (Tregs) accumulate in blood and lymph nodes, while spinal cord messenger RNA (mRNA) expression of Foxp3, a key transcriptional regulator of Tregs,12 is induced. In concert, anti-inflammatory cytokines are upregulated, resulting in microglia exhibiting a neuroprotective M2 (alternatively activated) phenotype that provides neurotrophic support.13 With disease progression, proinflammatory cytokine levels increase and microglia transition to a neurotoxic M1 (classically activated) phenotype, while Treg levels decline.13 In patients with ALS, there are reduced levels of CD4+ T-cells,14 increased proportions of proinflammatory effector T-cells,15,16 increased macrophage activation,17 and upregulated costimulatory pathways,18 while reduced Treg numbers are associated with rapid disease course.19 Interestingly, adoptive transfer of Tregs in mice with a mutant form of SOD1 induces M2 microglia in the spinal cord and prolongs survival time,13,20 suggesting that Tregs contribute to neuroprotection by modulating microglial activation.
It is now apparent from studies in mouse models that Tregs can undergo further differentiation in the peripheral lymphoid organs into a population termed effector Tregs, which express a distinct set of molecules important for Treg function.21 To determine whether enhancement of Tregs frequency and effector function was associated with a slower rate of disease progression in ALS, this study initially undertook comprehensive Treg immunophenotyping that, to our knowledge, has not previously been undertaken in patients with ALS: both CD4+CD25hiCD127lo and CD4+CD25hiCD127loFoxp3+ phenotypes22 and functionally defined subsets of these (resting [CD45RA+] Tregs, recent thymic emigrant-enriched [CD45RA+CD31+] Tregs,23 and activated effector [CD45RO+] Tregs12). Subsequently, effector Tregs were expanded in SOD1G93A mice through infusions of interleukin 2c.21,24,25 The SOD1G93A mouse is a transgenic murine model with a G93A mutant form of SOD1 that results in motor neuron degeneration analogous to human amyotropic lateral sclerosis. The aim of this study is to better understand the pathophysiological processes by which Treg expansion mediated neuroprotective effects in ALS.
Methods
Ethics Statement
Human studies were approved by the Western Sydney Local Health District human research ethics committee, and all patients provided written informed consent prior to recruitment into the study. All mouse experiments conformed to the Australian National Health and Medical Research Council Code of Practice39 and were approved by the Howard Florey Institute animal ethics committee.
Data Collection From Human Participants
Venous blood was collected in EDTA with informed consent from 33 patients who had been diagnosed as having possible, probable, or definite ALS according to the revised El Escorial criteria,26 as well as 38 age-matched healthy control participants recruited consecutively from the ALS clinic at Westmead Hospital in Westmead, Australia, between February 1, 2013, and December 31, 2014. (No participants declined to be involved in the study.) The sample size was based on a calculation of 80% power necessary to detect a correlation of whole-blood levels of Foxp3 with disease progression.19 At the time of blood collection, all patients were clinically staged using the revised ALS Functional Rating Scale (ALSFRS-R),27 which correlates well with survival time.28
Full blood cell counts were performed at the Westmead Hospital diagnostic laboratory for determination of lymphocyte counts per milliliter. Peripheral blood mononuclear cells were isolated on Ficoll-Paque Plus media (VWR International), washed in phosphate-buffered saline, and cryopreserved in Roswell Park Memorial Institute 1640 medium (Life Technologies) containing 2000μM glutamine, 10% heat-inactivated fetal bovine serum, 10% dimethyl sulfoxide, 50 units/mL of penicillin, and 50 μg/mL of streptomycin.
The details of flow cytometry and antibodies used in human samples are outlined in the eMethods in the Supplement. Briefly, antibodies to human antigens used were monoclonal antibodies to CD25 (clone BC96), CD4 (RPA-T4), CD31 (WM59), and a corresponding isotype control (IgG1; all from BioLegend); CD45RA (clone HI100) and CD45RO (clone UCHL1; both from BD Pharmingen); and CD127 (clone eBioRDR5) and FoxP3 (clone 236A/E7; both from eBioscience). Peripheral blood mononuclear cells were thawed, washed in Roswell Park Memorial Institute medium with 2% fetal bovine serum, and incubated for 30 minutes in the medium with 10mM HEPES, 1mM magnesium chloride, and 100 units/mL of DNase I (Roche Pharmaceuticals). Cells were blocked with mouse IgG (33 µg/mL) and stained for extracellular antigens (or, in the case of CD31, fluorescence minus 1 IgG control) followed by fixation, permeabilization, and staining for Foxp3 using the Foxp3 Staining Buffer Set (eBioscience). Cells were analyzed by flow cytometry on an FACS Canto II system (BD Biosciences).
Data Collection From Animal Participants
Given that the current clinical data demonstrated a correlation between Treg levels and disease progression in patients with ALS, we aimed to determine whether expansion of a murine-activated effector Treg phenotype (which was similar to the human Treg phenotype) was associated with a slower rate of disease progression and prolonged survival time in the SOD1G93A mouse model. Specifically, the endogenous Treg population in SOD1G93A mice was amplified by using the IL-2/IL-2 monoclonal antibody complexes (IL-2c) at 60 days of age.
Transgenic SOD1G93A mice derived from the B6SJL-TgN (SOD1*G93A) 1Gur/J line (Jackson Laboratory) were backcrossed 12 generations and maintained on a pure C57BL/6 genetic background. Male mice were divided into 3 groups with equal litter contribution: IL-2/IL-2c, 1 μg/5 μg, plus rapamycin, 1 mg/kg (Millipore); rapamycin alone; or vehicle (dimethyl sulfoxide or phosphate-buffered saline) alone.
Mouse IL-2 carrier-free recombinant protein and functional-grade purified IL-2 monoclonal antibody (of the JES6-1A12 clone) were both obtained from eBioscience (Jomar Life Research). Importantly, the IL-2/IL-2 monoclonal antibody complex increases the biological activity of the T-cell growth factor IL-2, and the JES6-1 monoclonal antibody clone confers specificity to cells expressing high-affinity αβγ IL-2R (CD25hiCD4+Foxp3 Tregs and activated effector T-cells) rather than those expressing low-affinity βγ IL-2R (memory CD8+ cells or natural killer cells).24 Combining the IL-2/anti-IL-2 monoclonal antibody JEA6-1A12 complex with rapamycin is known to specifically expand Treg cells with an effector phenotype and to exert immunosuppressive function.21,24,25 Cotreatment of IL-2c with rapamycin is essential because it inhibits proliferation of T effector cells, enabling selective expansion of Tregs.25
The IL-2c protein was prepared weekly, as previously described.24 Briefly, IL-2 and IL-2 monoclonal antibodies were mixed and incubated at 37°C for 30 minutes. Starting from a postnatal age of 60 days, mice were intraperitoneally injected with 100 μL of the mixture once a day for 3 days, followed by twice-weekly maintenance injections. All mice received 2 daily injections of rapamycin or 0.1% dimethyl sulfoxide (vehicle) in the morning, followed by either IL-2c or phosphate-buffered saline (vehicle), respectively, in the afternoon. (Injections occurred at least 5 hours apart.)
The numbers of Tregs in blood were determined by flow cytometry at 100 days of age. (Representative gating for Treg markers is shown in eFigure 1 in the Supplement.) In addition, we carried out immunohistochemical assessments of glucocorticoid-induced tumor necrosis factor receptor–related (GITR) protein, cytotoxic T-lymphocyte antigen 4 (CTLA4), and inducible T-cell costimulator (ICOS), which contribute to the suppressive phenotype of Tregs because IL-2c is known to induce expression of these immune checkpoint molecules.32,33,34
Age-matched wild-type mice of the C57BL/6 genetic background were used as controls for flow cytometry and immunohistochemistry. Mice were killed by an intraperitoneal lethal injection of sodium pentobarbitone, 100 mg/kg. (We observed mild thinning of coat fur around the injection site in all mice treated long term with IL-2c, and a subgroup of these mice developed spleen atrophy.)
The investigators were blinded to the identities of treatment groups. The details of flow cytometry, quantitative reverse-transcription polymerase chain reaction, behavioral analysis, histology, and immunohistochemistry are outlined in the eMethods in the Supplement. Briefly, we correlated the expression of Foxp3, Th2 activation marker Gata3, M2 markers Retnla/Fizz1, and glial-cell–derived growth factor with Tregs in the spinal cords of IL-2c–treated SOD1G93A mice and vehicle-treated SOD1G93A mice. We further investigated Treg-induced modulation of disease through assessments of glial fibrillary acidic protein (GFAP) immunoreactivity and cluster of differentiation molecule 11b (CD11b) immunoreactivity in SOD1G93A mice and wild-type mice.
Statistical Analysis
Human data were analyzed with Mann-Whitney tests to compare nonparametric data on participants with ALS with control participants. The rate of disease progression was calculated as a change in ALSFRS-R,29 and Pearson correlations of disease progression and age with Treg frequency (or loge Treg for normalization) were calculated. After flow cytometry, CD4 subsets were determined as a percentage of all lymphocytes gated using forward and side scatter, and as a percentage of all leukocytes by dual-platform analysis using full blood cell counts from the same blood collection. Mouse flow cytometry data, motor neuron areas, and glial immunohistochemistry were analyzed using 1-way analysis of variance with Tukey multiple-comparison test. Disease onset and quantitative polymerase chain reaction data were analyzed using an unpaired t test. Survival data were analyzed using Kaplan-Meier survival analysis with the log-rank test. All analyses except flow cytometry were performed using GraphPad Prism software, version 6.0 (GraphPad Software Inc). Flow cytometry used FlowJo software (Treestar Inc).
Results
Comparison of Tregs in Patients With ALS and Control Participants
The clinical and demographic features of patients with ALS (n = 33) and healthy control participants (n = 38) are summarized in the Table. Briefly, the median disease duration at the time of cellular studies was 14 months (range, 4-84 months), and patients exhibited a moderate level of functional disability as indicated by a mean ALSFRS-R score of 43 (range, 18-48). Two phenotypes recently used to define the regulatory T-cell population were determined: CD4+CD25hiCD127lo and CD4+CD25hiCD127loFoxp3+.22 Both strongly correlated with epigenetic markers of suppressive function. Resting (CD45RA+) and activated effector (CD45RO+) subsets were gated separately to maximize gating accuracy,30 their Foxp3 levels were determined, and the total Treg population was calculated as the sum of resting and effector cells.22 Numbers of CD45RA+CD31+ Treg cells,12 enriched for recent thymic emigrants, were measured (eTable 1 in the Supplement).
Table. Clinical and Demographic Features of Patients With Amyotrophic Lateral Sclerosis and Control Participants.
| Characteristic | Participants With ALS | Healthy Control Participants |
|---|---|---|
| Total | 33 | 38 |
| Age, mean (SD), y | 58.9 (10.9) | 55.7 (11.1) |
| Sex, No. (%) | ||
| Male | 24 (73) | 17 (45) |
| Female | 9 (27) | 21 (55) |
| Amyotrophic lateral sclerosis diagnosis,a No. (%) | ||
| Definite | 12 (36) | NA |
| Probable | 9 (27) | NA |
| Possible | 12 (36) | NA |
| Site of disease onset No. (%) | ||
| Limb | 20 (61) | NA |
| Bulbar | 13 (39) | NA |
| ALSFRS-R score, median (range) | 43 (18-48) | NA |
| Disease duration, mo, median (range) | 14 (4-8) | NA |
| Disease progression rate, point/mo, median (range) | 0.36 (0-1.9) | NA |
Abbreviations: ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale; NA, not applicable.
Diagnosis of ALS was completed with the revised El Escorial criteria.26
There was a significant reduction in lymphocyte counts in patients with ALS compared with control participants (ALS group: mean [range], 1.5 × 109 cells/L [7 × 108 to 3.0 × 109 cells/L]; vs control participants: 1.8 × 109 cells/L [1.1 × 109 to 3.2 × 109] cells/L; P = .04). This reflected reduced CD4+ counts (ALS group: mean [range], 4.8 × 108 cells/L [2.1 × 109 to 1.4 × 109]; vs control participants: 6.0 × 108 cells/L [2.2 × 108 to 1.5 × 109]; P = .04) and particularly reduced levels of CD45RO+ subsets (ALS group: 2.9 × 108 cells/L [8.0 × 107 to 8.9 × 108]; vs control participants: 3.8 × 108 cells/L [1.5 × 108 to 1.2 × 109]; P = .01; eTable 1 in the Supplement). Total Treg (CD4+CD25hiCD127lo) counts in patients with ALS were comparable with controls, as were resting (CD45RA+) and effector (CD45RO+) subsets, Foxp3+ subsets, and the CD45RA+CD31+ Treg subset, which was enriched for recent thymic emigrants23 (eTable 1 in the Supplement). Treg subsets as a proportion of leukocytes were also determined, revealing no significant difference in total Tregs, CD45RO+ Tregs, or CD45RA+ Tregs in patients with ALS (eTable 1 in the Supplement).
In addition, there was no significant difference in Foxp3 expression levels between patients with ALS and control participants in the CD45RA+Foxp3+ subset (ALS group: Foxp3 median fluorescence intensity [MFI] [range], 712 [624-800]; vs control participants: 670 [570-798]; P = .63). There was also no significant difference in the CD45RO+Foxp3+ Treg subsets (ALS group: MFI [range], 1312 [1157-1485]; vs control participants: 1269 [1070-1527]; P = .47). However, effector (CD45RO+) FoxP3+ Tregs expressed significantly higher levels of Foxp3 protein (MFI [range], 1289 [1095-1490]) than resting (CD45RA+) Foxp3+ Tregs did (MFI [range], 680 [581-797]; P < .001).
Tregs and Rate of ALS Progression
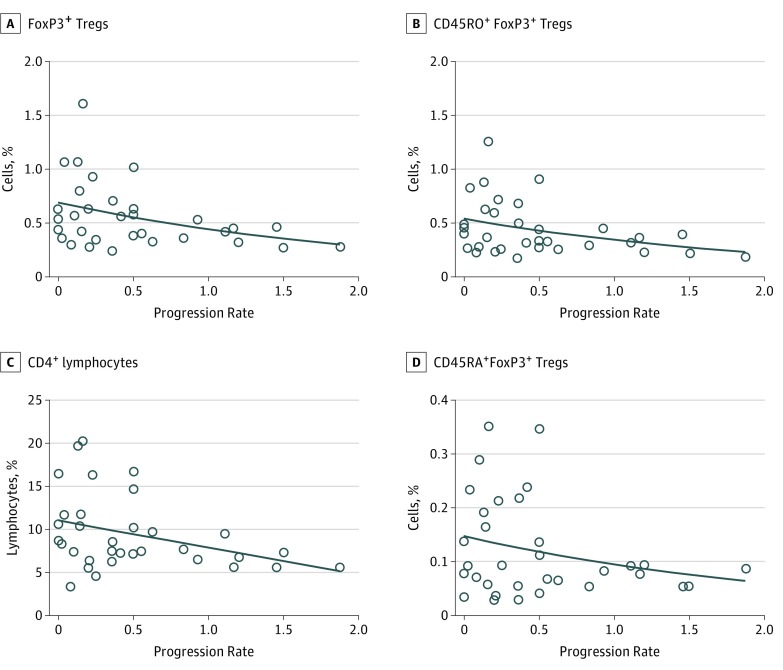
The rate of progression in patients with ALS was inversely correlated with total Treg counts (R = −0.40; P = .02; eTable 2 in the Supplement), total Foxp3+ Treg (R = −0.41; P = .02; Figure 1A) and effector (CD45RO+) Foxp3+ Tregs counts; (R = −0.39; P = .02; Figure 1B), as well as CD4+ lymphocyte counts (R = −0.36; P = .04; Figure 1C). There was a significant correlation of disease progression rate with absolute counts for each of these subsets (eTable 2 in the Supplement). In contrast, resting (CD45RA+) Foxp3+ Tregs were not correlated with the rate of disease progression (R = −0.20; P = .26; Figure 1D). Rather, resting (CD45RA+) Foxp3+ Tregs were inversely correlated with age in patients with ALS (R = −0.41; P = .02; eFigure 2 in the Supplement). Of these, the Foxp3+ Treg subsets enriched by recent thymic emigrants were more strongly correlated with age (CD45RA+Foxp3+CD31+ Treg: R = −0.52; P = .002; eFigure 2 in the Supplement) as is expected for recent thymic emigrant CD4 T-cells associated with age-related thymic involution.23,31 In contrast, the correlation between age and the CD45A+Foxp3+ Treg subset or the Foxp3+-recent thymic emigrant Treg subset (eFigure 2 and eTable 3 in the Supplement) did not reach statistical significance in healthy control participants.
Figure 1. Correlation of ALS Progression Rate With Peripheral Blood Regulatory T-Cells (Tregs).
A, Progression rate (change in Amyotrophic Lateral Sclerosis Functional Rating Scale score per month) was inversely correlated with frequency of total FoxP3+ Tregs, calculated as the sum of CD45RO+FoxP3+ Tregs (CD45RO+CD4+CD25hiCD127loFoxP3+) and CD45RA+FoxP3+ Tregs (CD45RA+CD4+CD25hiCD127loFoxP3+) in patients with ALS (n = 33; R = −0.41; P = .02). B, The frequency of CD45RO+FoxP3+ Tregs was inversely correlated with disease progression (R = −0.39; P = .02). C, The frequency of CD4+ was inversely correlated with disease progression (R = −0.36; P = .04). D, The presence of CD45RA+ Tregs was not correlated with disease progression (R = −0.20; P = .26). Frequencies were determined as a percentage of all leukocytes, and Pearson correlation was used on loge-transformed cell subsets.
Studies of SOD1 Mice
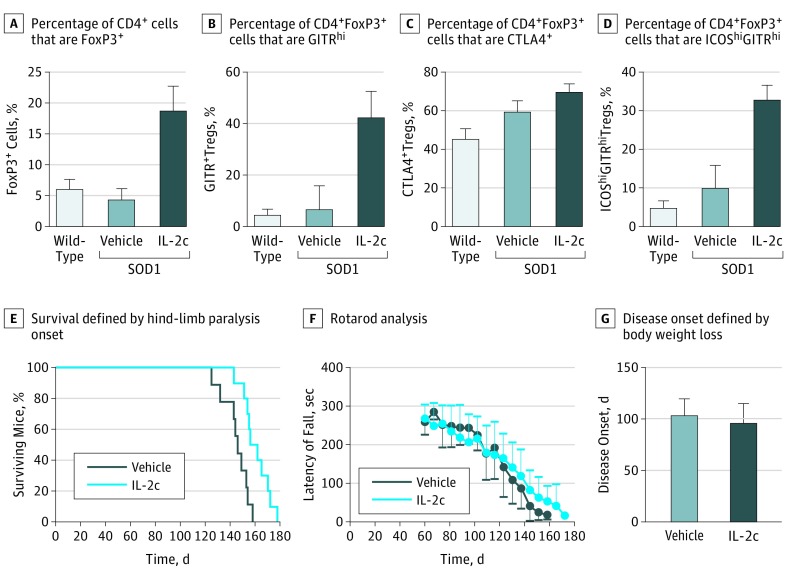
Treatment with IL-2c significantly increased the percentage of CD4+Foxp3+ Tregs in SOD1G93A mice (IL-2c–treated mice: mean [SD], 18.9% [3.8%]; vs vehicle-treated mice: 4.4% [1.9%]; P < .001) and age-matched wild-type mice (mean [SD], 6.2% [1.5%]; P < .001, Figure 2A). It is also known that IL-2c induces expression of glucocorticoid-induced tumor necrosis factor receptor–related protein (GITR), cytotoxic T-lymphocyte antigen 4 (CTLA4), and inducible T-cell costimulator (ICOS), which contribute to the suppressive phenotype of Tregs.32,33,34 There was a significant mean (SD) increase of GITRhi Tregs in IL-2c–treated SOD1G93A mice compared with mice injected only with vehicle preparations and with wild-type mice (IL-2c–treated mice: mean [SD], 42.6% [9.7%]; vs vehicle-treated mice: 6.9% [9.2%]; P < .001; vs wild-type mice: 4.8% [2.1%]; P < .001 compared with IL-2c–treated mice; Figure 2B). The same association appeared in measurements of CTLA4+ (IL-2c–treated mice: mean [SD], 69.5% [9.6%]; wild-type mice: 45.0% [11.3%]; P = .03; Figure 2C) and measurements of ICOShi (IL-2c–treated mice: 33.1% [8.0%]; vs vehicle-treated mice: 9.9% [13.4%]; P = .01; vs wild-type mice: 5.0% [3.3%]; P = .003 compared with IL-2c–treated mice; Figure 2D). Treatment with IL-2c did not affect the proportion of ICOS-CD62L+ Tregs, which suggests a selective expansion of effector Tregs rather than recruitment of the naive or memory populations (eFigure 3 in the Supplement). Rapamycin treatment alone did not affect T-cell frequencies, disease onset, or progression in SOD1G93A mice (eFigure 4 in the Supplement). These data demonstrate that IL-2c induces marked expansion of effector Tregs that possess a suppressor phenotype.
Figure 2. Treatment With Interleukin 2c (IL-2c), Effector Regulatory T-Cell (Treg) Populations, and Disease Progression in SOD1G93A Mice.
A-D, Fluorescence-activated cell sorting analysis of Treg cell populations in peripheral blood isolated from wild-type mice (n = 4), vehicle-treated SOD1G93A mice (n = 5), and IL-2c–treated SOD1G93A mice (n = 5) at 100 days of age. A, Differences were significant between vehicle-treated and IL-2c–treated SOD1G93A mice with respect to the percentage of CD4+ cells that are Foxp3+ (IL-2c–treated mice: mean [SD], 18.9% [3.8%] vs vehicle-treated mice: 4.4% [1.9%]; P < .001) and between IL-2c–treated SOD1G93A mice and age-matched wild-type mice (18.9% [3.8%] vs 6.2% [1.5%]. respectively; P < .001). B, Differences were significant between all groups in the percentage of CD4+Foxp3+ Tregs that are GITRhi (IL-2c–treated mice: 42.6% [9.7%] vs vehicle-treated mice: 6.9% [9.2%]; vs wild-type mice: 4.8% [2.1%]; P < .001). C, In the percentage of CD4+Foxp3+ Tregs that are CTLA4+, differences were significant between IL-2c–treated SOD1G93A mice (69.5% [9.6%]) and wild-type mice (45.0% [11.3%]; P = .03). D, In respect to the percentage of CD4+Foxp3+ Tregs that are ICOShiGITRhi, differences were significant between IL-2c–treated SOD1G93A mice (33.1% [8.0%]), vehicle-treated SOD1G93A mice (9.9% [13.4%]; P = .01), and wild-type mice (5.0% [3.3%]; P = .003).E, Survival of IL-2c–treated SOD1G93A mice (n = 10) was significantly prolonged compared with the vehicle-treated group (n = 9). Survival was defined by onset of hind-limb paralysis. Significance was determined using Kaplan-Meier survival analysis with the log-rank test. F, Rotarod performance. G, Disease onset as determined by the onset of weight loss was not affected by IL-2c treatment.
We next determined the association of endogenous Treg expansion with disease onset and progression in SOD1G93A mice. Treatment with IL-2c resulted in significant prolongation of survival when compared with vehicle-treated mice (IL-2c–treated mice: mean [SD], 160.6 [10.8] days; vs vehicle-treated mice: 144.9 [10.6] days; P = .003, Figure 2E). There was a trend for improved motor function evidenced by rotarod analysis of SOD1G93A mice treated with IL-2c (Figure 2F). Disease onset, as determined by body weight decline, was not impacted by Treg expansion (Figure 2G). This is consistent with a role of Tregs in mediating disease course, but not initiation of ALS.
IL-2c and T-Cell Recruitment to the Spinal Cords of Mutant SOD1 Mice
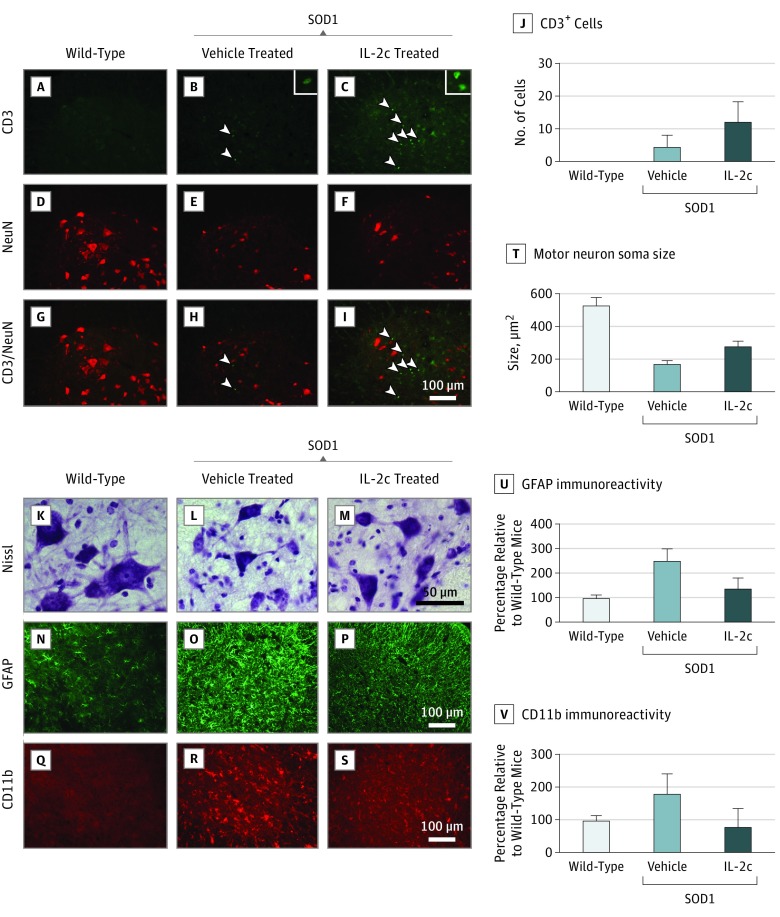
To confirm the presence of Tregs in the spinal cords of mice, we examined tissue using immunohistochemistry. We did not detect CD3+ T-cells in the lumbar spinal cords of wild-type mice as expected (Figure 3A, D, and G). Rather, CD3+ T-cells were occasionally observed in the ventral horns of vehicle-treated control SOD1G93A mice, as previously reported7 (Figure 3B, E, and H). In contrast, IL-2c treatment resulted in marked infiltration of T-cells into the ventral horns in SOD1G93A mice, particularly close to motor neurons identified by their size, location, and immunoreactivity to NeuN, a neuronal nuclear antigen (Figure 3C, F, and I). There was a significant increase of T-cell infiltration into the ventral horn by IL-2c therapy (IL-2c–treated mice: mean [SD], 12.2 [6.1] cells; vehicle-treated mice: 4.4 [3.7] cells, P = .03; Figure 3J). Consequently, elevated circulating Treg levels induced by IL-2c treatment were associated with T-cell recruitment to the spinal cord.
Figure 3. Interleukin 2c (IL-2c) Treatment, Infiltration of T-Cells, Motor Neurons, and Glial-Cell Immunoreactivity in Spinal Cords of SOD1G93A Mice.
A-C, Representative micrographs of the ventral horns of lumbar spinal cords stained for CD3+ cells in wild-type mice, vehicle-treated SOD1G93A mice, and IL-2c–treated SOD1G93A mice; arrowheads indicate T-cells and insets show magnified T-cells. D-F, Representative micrographs of the ventral horns of lumbar spinal cords stained for NeuN in all 3 groups of mice. G-I, Micrographs stained for CD3+ cells and NeuN in disease end stages in wild-type mice, vehicle-treated SOD1G93A mice, and IL-2c–treated SOD1G93A mice. J, Quantification of CD3+ cells localized to the ventral horns of spinal cord sections (IL-2c–treated mice: mean [SD], 12.2 [6.1] cells; vehicle-treated mice: 4.4 [3.7] cells; P = .03). K-M, Representative micrographs of the ventral horns of lumbar spinal cords stained for Nissl in wild-type mice, vehicle-treated SOD1G93A mice, and IL-2c–treated SOD1G93A mice. N-P, Representative micrographs of the ventral horns of lumbar spinal cords stained for glial fibrillary acidic protein (GFAP) in all 3 groups of mice. Q-S, Representative micrographs of the ventral horns of lumbar spinal cords stained for CD11b at disease end stage in all 3 groups of mice. T, Quantification of motor neuron soma size from Nissl-stained spinal cord sections of IL-2c–treated SOD1G93A mice (mean [SD], 279.8 [28.6] μm2) compared with vehicle-treated mice (mean [SD], 167.4 [21.9] μm2; P = .001). U, Quantification of GFAP from spinal cord sections of IL-2c–treated SOD1G93A mice (mean [SD], 137.3% [43.0%]) compared with vehicle-treated mice (248.7% [49.7%]; P = .002). V, Quantification of cluster of differentiation molecule 11b (CD11b) immunoreactivity from spinal cord sections of SOD1G93A mice receiving IL-2c therapy (mean [SD], 79.8% [55.0%]) compared with vehicle-treated mice (179.4% [61.1%]; P = .02).
IL-2c Treatment, Neuroprotection, and Neuroinflammation Mitigation in Mutant SOD1 Mice
We investigated the association of Treg-induced modulation of disease progression with preservation of motor neurons and attenuation of inflammatory glial cell activation within the central nervous system. Motor neurons maintained significantly larger soma areas in IL-2c–treated SOD1G93A mice (mean [SD], 279.8 [28.6] μm2) compared with vehicle-treated mice (mean [SD], 167.4 [21.9] μm2; P = .001; Figure 3K, L, M, and T). Pronounced activation of GFAP+ astrocytes (which encode glial fibrillary acidic protein) and CD11b+ microglia was observed in the spinal cords of vehicle-treated SOD1G93A mice, but not in wild-type control mice (Figure 3N, O, P, Q, R, and S). There was a 40% reduction in GFAP immunoreactivity in the spinal cords of IL-2c–treated SOD1G93A mice (mean [SD], 137.3% [43.0%]) compared with vehicle-treated mice (248.7% [49.7%]; P = .002; Figure 3U). Furthermore, cluster of differentiation molecule 11b (CD11b) immunoreactivity was reduced by 50% in the spinal cords of SOD1G93A mice receiving IL-2c therapy (mean [SD], 79.8% [55.0%]) compared with vehicle-treated mice (mean [SD], 179.4% [61.1%]; P = .02; Figure 3V). These data support the notion that endogenous effector Treg expansion reduces neuroinflammation, particularly microglial activation, and promotes neuroprotection in spinal cords of SOD1G93A mice.
IL-2c Treatment and Neurotrophic Response in the Spinal Cords and Peripheral Nerves of Mutant SOD1 Mice
We next correlated expression of Treg, Th2, and microglial transcripts in the spinal cord with increased lifespan in IL-2c–treated SOD1G93A mice, with data expressed as fold change of wild-type mice. There was a statistically significant increase in Foxp3 m RNA expression in IL-2c–treated SOD1G93A mice (IL-2c–treated mice: mean [SD], 2.8 [0.8]; vs vehicle-treated mice: 1.1 [0.3]; P = .003). Expression of the Th2 activation marker Gata3 was similar in the spinal cords of IL-2c–treated mice and vehicle-treated mice. In addition, transcription of M2 markers Retnla/Fizz135 were elevated in the spinal cords of SOD1G93A mice receiving IL-2c (mean [SD], 2.2 [0.9]; vs vehicle-treated mice: 1.0 [0.2]; P = .04), as was glial-cell–derived neurotrophic factor (GDNF), albeit nonsignificantly (IL-2c–treated mice: mean [SD], 2.5 [0.8]; vs vehicle-treated mice: 1.7 [0.2]; P = .05). However, it should be stressed that, in this instance, GDNF may not be solely a biomarker of M2 activation, but could also be derived from astrocytes. Separately, Foxp3 mRNA expression was elevated approximately 3-fold within the sciatic nerve of IL-2c–treated mice compared with vehicle-treated mice (mean [SD], 1.9 [1.01] vs 0.6 [0.3], respectively; P = .03), as was expression of Gata3 (IL-2c–treated mice: mean [SD], 4.2 [ 1.7]; vs vehicle-treated mice: 0.8 [0.4]; P = .003), and GDNF (IL-2c–treated mice: mean [SD], 7.6 [5.3]; vs vehicle-treated mice: 1.3 [0.5]; P = .03). (Details of each finding appear in eFigure 5 in the Supplement). Gata3 expression was increased in the sciatic nerve but not in the spinal cord. This would suggest a peripheral anti-inflammatory neuroprotective effect in the mouse model.
Discussion
A growing body of evidence points to the immune response as a powerful modulator of disease activity in ALS, integral to neuroprotection.36 While this holds great promise as a therapeutic target, generalized immunosuppression has not exerted any effects on disease progression in ALS,37 thereby suggesting that targeted immunoregulation may be required. The findings in the present study establish an inverse correlation between comprehensively phenotyped Treg counts and rate of disease progression, suggesting the importance of Tregs in ALS pathogenesis.
Underscoring the pathogenic importance of Tregs in ALS are findings of prolonged survival in SOD1G93A mice treated with IL-2c that specifically expands the number of effector Tregs, with accompanying anti-inflammatory changes within the central nervous system. Consequently, modulation of the immune system in patients with ALS, particularly enhancement of regulatory T-cell function, may prove therapeutically useful in ALS treatment.
Regulatory T-Cells and ALS Pathogenesis
Tregs appear to be important in ALS pathogenesis, particularly in regulating the rate of disease progression.13 Specifically, an inverse correlation between disease progression rates and Treg levels was previously reported,13 and a follow-up study by the same group showed that levels of the Treg transcription factor Foxp3 in blood was predictive of progression rate and survival.19 Using a different functional scoring system (the ALSFRS-R27 rather than the Appel score13), and more comprehensive immunophenotyping, the present study provides independent replication of previous findings, confirming an inverse correlation between disease progression rate with Tregs13,19 and with total CD4+ as a proportion of leukocytes.13 Consistent with this, reconstitution studies in mice with a mutant form of SOD1 have demonstrated that CD4+ T-cells improve neurological function and prolong survival, but that passive transfer of Tregs conferred significantly greater protection than unfractionated CD4+ T-cells, suggesting a critical role for the Treg subsets in the neuroprotective effect.7,8,13,20
Of further relevance is a significant correlation of disease progression rate with the CD45RO+ effector subset of Tregs that expressed very high levels of Foxp3, but not the CD45RA+ resting subset that were Foxp3lo. This was a novel finding in the present study. Unlike CD45RO+ conventional T-cells, memory or recall response has not been demonstrated for Tregs, and CD45RO is a marker of activation and functional differentiation of Tregs that correlates with high expression of CTLA4, which mediates contact-dependent suppression, and GITR.12 These results suggest a correlation of reduced disease progression rate with the potent immunosuppressive activity of activated Tregs.12 Others have demonstrated that, in patients with ALS, with a rapid progression rate, reduction in peripheral Treg levels parallels the reductions in Foxp3 in the spinal cord,19 thereby suggesting that a systemic deficit of Tregs, rather than recruitment to the central nervous system, may explain the peripheral Treg deficits in rapidly progressing ALS.
Interestingly, CD45RO+Tregs are highly susceptible to apoptosis compared with CD45RA+ following activation and suppression.12 We hypothesize that activation-induced apoptosis may lead to the selective depletion of activated Tregs in rapidly progressive ALS, possibly because of greater Treg activation in a more inflammatory environment and greater disease-related cellular stress conferring a higher propensity to activation-induced cell death.
In contrast, resting CD45RA+ Treg subsets were not correlated with the rate of disease progression, a finding not previously reported. Instead, CD45RA+ Treg subsets were correlated with age of patients with ALS (an association that was stronger for the CD31+ subsets enriched for recent thymic emigrants),38 but naive CD45RA+ T-cells were not correlated with patient age. This is consistent with the previously reported age-related decline in CD45RA+ Tregs and could be attributed to thymic involution with age, a selective reduction in thymic output of Tregs with age, or reduced lifespan of Tregs in the periphery.31 Further studies are required to determine whether the exaggerated decline of resting Tregs with age in patients with ALS is of pathogenic importance.
Limitations
A potential limitation of this study pertains to the cross-sectional design. Although there was a correlation between disease progression and Treg activity in patients with ALS, which suggests a neuroprotective effect and is confirmed by animal studies, the correlation does not establish causality. Longitudinal studies in patients with ALS would be important because they could potentially shed light on effects of disease progression on the regulatory immune system and vice versa.
Conclusions
The present study provides strong support for a neuroprotective role of Tregs in patients with ALS, whereby enhanced Treg activity is associated with a slower rate of disease progression. Expansion and differentiation of endogenous Tregs in SOD1G93A mice by the biological agent IL-2c led to a significant reduction of glial activation, a shift to neuroprotective autoimmunity, and neurotrophic effects at the spinal cord level. The molecular and pathological changes were associated with significantly prolonged survival in the SOD1G93A mouse model, a finding likely explained by a slowing of disease progression. Strategies aimed at enhancing Treg function could prove therapeutically useful in patients with ALS.
eMethods. Supplementary Methods.
eTable 1. Immune cell subsets in ALS and healthy controls.
eTable 2. Correlation of disease progression rate with frequencies of CD4 T cell subsets expressed as proportions or absolute counts.
eTable 3. Correlation of age with frequencies of CD4 T cell subsets expressed as proportions or absolute counts.
eFigure 1. Regulatory T cell (Treg) subsets in WT and SOD1G93A mice.
eFigure 2. Resting peripheral blood regulatory T cells (Tregs) are inversely correlated with age in ALS patients.
eFigure 3. Effect of IL-2c treatment on naive/memory Tregs in SOD1G93A mice.
eFigure 4. Effect of rapamycin treatment on T cell frequencies, disease onset and survival in SOD1G93A mice.
eFigure 5. IL-2c treatment enhances Treg activation and neuroprotective gene expression in spinal cord and peripheral nerves of SOD1G93A mice.
References
- 1.Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50(1):30-36. [DOI] [PubMed] [Google Scholar]
- 2.Vucic S, Rothstein JD, Kiernan MC. Advances in treating amyotrophic lateral sclerosis: insights from pathophysiological studies. Trends Neurosci. 2014;37(8):433-442. [DOI] [PubMed] [Google Scholar]
- 3.Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson’s disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 2010;31(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beers DR, Zhao W, Wang J, et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight. 2017;2(5):e89530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boillée S, Yamanaka K, Lobsiger CS, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389-1392. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka K, Chun SJ, Boillee S, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11(3):251-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105(40):15558-15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu IM, Chen A, Zheng Y, et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105(46):17913-17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam L, Chin L, Halder RC, et al. Epigenetic changes in T-cell and monocyte signatures and production of neurotoxic cytokines in ALS patients. FASEB J. 2016;30(10):3461-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer K, Ferraiuolo L, Miranda CJ, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci U S A. 2014;111(2):829-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187(6):761-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490-500. [DOI] [PubMed] [Google Scholar]
- 13.Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134(pt 5):1293-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Feng W, Huang R, et al. Evidence for peripheral immune activation in amyotrophic lateral sclerosis. J Neurol Sci. 2014;347(1-2):90-95. [DOI] [PubMed] [Google Scholar]
- 15.Saresella M, Piancone F, Tortorella P, et al. T helper-17 activation dominates the immunologic milieu of both amyotrophic lateral sclerosis and progressive multiple sclerosis. Clin Immunol. 2013;148(1):79-88. [DOI] [PubMed] [Google Scholar]
- 16.Shi N, Kawano Y, Tateishi T, et al. Increased IL-13-producing T cells in ALS: positive correlations with disease severity and progression rate. J Neuroimmunol. 2007;182(1-2):232-235. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Gascon R, Miller RG, et al. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2005;159(1-2):215-224. [DOI] [PubMed] [Google Scholar]
- 18.Lincecum JM, Vieira FG, Wang MZ, et al. From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat Genet. 2010;42(5):392-399. [DOI] [PubMed] [Google Scholar]
- 19.Henkel JS, Beers DR, Wen S, et al. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med. 2013;5(1):64-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee R, Mosley RL, Reynolds AD, et al. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One. 2008;3(7):e2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 2013;34(2):74-80. [DOI] [PubMed] [Google Scholar]
- 22.Nettenstrom L, Alderson K, Raschke EE, et al. An optimized multi-parameter flow cytometry protocol for human T regulatory cell analysis on fresh and viably frozen cells, correlation with epigenetic analysis, and comparison of cord and adult blood. J Immunol Methods. 2013;387(1-2):81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2009;113(4):769-774. [DOI] [PubMed] [Google Scholar]
- 24.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311(5769):1924-1927. [DOI] [PubMed] [Google Scholar]
- 25.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206(4):751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299. [DOI] [PubMed] [Google Scholar]
- 27.Cedarbaum JM, Stambler N, Malta E, et al. ; BDNF ALS Study Group (Phase III) . The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1-2):13-21. [DOI] [PubMed] [Google Scholar]
- 28.Zinman L, Cudkowicz M. Emerging targets and treatments in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10(5):481-490. [DOI] [PubMed] [Google Scholar]
- 29.Labra J, Menon P, Byth K, Morrison S, Vucic S. Rate of disease progression: a prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry. 2016;87(6):628-632. [DOI] [PubMed] [Google Scholar]
- 30.Fazekas de St Groth B, Zhu E, Asad S, Lee L. Flow cytometric detection of human regulatory T cells. Methods Mol Biol. 2011;707:263-279. [DOI] [PubMed] [Google Scholar]
- 31.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummey SM, Ford ML. Braking bad: novel mechanisms of CTLA-4 inhibition of T cell responses. Am J Transplant. 2014;14(12):2685-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronchetti S, Ricci E, Petrillo MG, et al. Glucocorticoid-induced tumour necrosis factor receptor-related protein: a key marker of functional regulatory T cells. J Immunol Res. 2015;2015:171520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vocanson M, Rozieres A, Hennino A, et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J Allergy Clin Immunol. 2010;126(2):280-289, 289.e1-289.e7. [DOI] [PubMed] [Google Scholar]
- 35.Jablonski KA, Amici SA, Webb LM, et al. Novel markers to delineate murine m1 and m2 macrophages. PLoS One. 2015;10(12):e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooten KG, Beers DR, Zhao W, Appel SH. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12(2):364-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagani MR, Gonzalez LE, Uchitel OD. Autoimmunity in amyotrophic lateral sclerosis: past and present. Neurol Res Int. 2011;2011:497080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duszczyszyn DA, Williams JL, Mason H, Lapierre Y, Antel J, Haegert DG. Thymic involution and proliferative T-cell responses in multiple sclerosis. J Neuroimmunol. 2010;221(1-2):73-80. [DOI] [PubMed] [Google Scholar]
- 39.Australian Government National Health and Medical Research Council Australian code for the care and use of animals for scientific purposes, 8th edition (2013). https://www.nhmrc.gov.au/guidelines-publications/ea28/. Published September 27, 2017. Accessed January 29, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Methods.
eTable 1. Immune cell subsets in ALS and healthy controls.
eTable 2. Correlation of disease progression rate with frequencies of CD4 T cell subsets expressed as proportions or absolute counts.
eTable 3. Correlation of age with frequencies of CD4 T cell subsets expressed as proportions or absolute counts.
eFigure 1. Regulatory T cell (Treg) subsets in WT and SOD1G93A mice.
eFigure 2. Resting peripheral blood regulatory T cells (Tregs) are inversely correlated with age in ALS patients.
eFigure 3. Effect of IL-2c treatment on naive/memory Tregs in SOD1G93A mice.
eFigure 4. Effect of rapamycin treatment on T cell frequencies, disease onset and survival in SOD1G93A mice.
eFigure 5. IL-2c treatment enhances Treg activation and neuroprotective gene expression in spinal cord and peripheral nerves of SOD1G93A mice.